Abstract
Chronic antibiotic use has been reported to impair mitochondrial indices, hypothalamus-mediated metabolic function, and amygdala-regulated emotional processes. Natural substances such as black seed (Nigella sativa) oil could be beneficial in mitigating these impairments. This study aimed to assess the impact of black seed oil (NSO) on depression and sociability indices, redox imbalance, mitochondrial-dependent markers, and insulin expression in mice subjected to chronic ampicillin exposure. Forty adult male BALB/c mice (30 ± 2 g) were divided into five groups: the CTRL group received normal saline, the ABT group received ampicillin, the NSO group received black seed oil, the ABT/NSO group concurrently received ampicillin and black seed oil, and the ABT+NSO group experienced pre-exposure to ampicillin followed by subsequent treatment with black seed oil. The ampicillin-exposed group exhibited depressive-like behaviours, impaired social interactive behaviours, and disruptions in mitochondrial-dependent markers in plasma and hypothalamic tissues, accompanied by an imbalance in antioxidant levels. Moreover, chronic antibiotic exposure downregulated insulin expression in the hypothalamus. However, these impairments were significantly ameliorated in the ABT/NSO, and ABT+NSO groups compared to the untreated antibiotic-exposed group. Overall, findings from this study suggest the beneficial role of NSO as an adjuvant therapy in preventing and abrogating mood behavioural and neural-metabolic impairments of chronic antibiotic exposure.
Keywords: Antibiotic misuse, Depression, Social interaction, Mitochondrial function, Black seed oil, Insulin expression
Graphical Abstract
1. Introduction
Antibiotics are most widely used prescribed in both inpatient and outpatient settings (Lee, 2020). Although they are mostly well tolerated, antibiotics just like most medication are associated with side effects (Pancu et al., 2021). In addition to diarrhoea, the commonest side effect of antibiotic use, its adverse effect on the central nervous system (CNS) has been reported (Grill and Maganti, 2011, Vecchio et al., 2021). The neurological signs of toxicity could be mistaken for another neurological condition if clinicians and patients fail to recognize the neurotoxic effects of antibiotics (Dubey et al., 2022). Advancement in the production and use of antibiotics is a reflection of its almost invaluable role in the reduction of mortality globally. Nonetheless, its popularity and share availability has led to it overuse, misuse and abuse (Bonilla-Carrero et al., 2020). These culminate in the so-called antibiotic resistance which has made the work against infection almost counter-productive (Morgan et al., 2011). Antibiotic abuse/misuse is a widespread occurrence in Africa as medications are obtained without an official professional prescription as well as cases where they are prescribed but without follow-up supervision (Sacre et al., 2020; Long et al., 2022). Several studies reported that antibiotics disrupt the gut microbiome, resulting in different pathologies and the development of antimicrobial resistance (Kelly et al., 2021).
Regarding the neurotoxicity effect of antibiotics, studies have implicated different contributing factors including nutritional status, tissue vascularity, absorption rate, drug elimination dynamics, and the blood-brain barrier (BBB) integrity (Chow et al., 2005). In addition, antibiotic neurotoxicity can also be due to genetic subsceptibilty, impaired renal homeostasis, and impaired pharmacokinetics (Chow et al., 2004). The principal signs of antibiotic toxicity include allergic responses, hepatotoxicity, ototoxicity, phototoxicity, and neural impairment (Rehman et al., 2020). The neurotoxic effect is mediated by both more pronounced systemic pathways as well as local mechanisms (Helaly et al., 2019, Yang et al., 2021). However, the mechanism driving the neurotoxicity of antibiotics is not fully understood.
Acute treatment following bacterial infection has been shown to elicit behavioural deficits including depressive behaviours (Guida et al., 2018). According to a population-based study by Lurie et al. (2015), specific antibiotic exposure is linked to a higher risk of anxiety and depression. One of the established mechanisms of antibiotic toxicity is the alteration of the gut microbiome which drives systemic and neuroinflammation (Giau et al., 2018, Bairamian et al., 2022). Moreover, Morais et al. (2021) reported a notable disruption in mouse brain chemistry due to antibiotic exposure, leading to memory impairment and depressive-like behaviour. Depression is one of the prevalent psychiatric disorder responsible for the growing suicide rates (Korczak et al., 2023). Depressive disorders affect 264 million people worldwide and are the second leading cause of death for people between the ages of 15 and 29 (Fan et al., 2022). The onset of depression is influenced by several factors, including stress, poor diet, insufficient or no physical exercise, obesity, low-grade inflammation, smoking, atopy, poor sleep hygiene, dental care, alcohol/substance abuse, and nutritional deficiencies like vitamin insufficiency (mostly vitamin D) (Anand et al., 2023). Regarding the neurobiology of depression, the amygdala, striated nucleus, limbic system, and subcortical regions are distinctly separate parts of the brain where hyperactivity is present (Mithani et al., 2020). Depressive disorder is characterized by a lack of motivation, low mood, hopelessness, and morbid depression and anxiety, which have an impact on key life functions like low self-esteem, extreme exhaustion, frustration, intrusive thoughts, and restlessness (de Zwart et al., 2019, Anand et al., 2023). Another classical symptom of depression is anhedonia, which reflects the loss of interest, withdrawal, loneliness and social isolation (Wang et al., 2021). Disruptions in social conduct and social recognition can also be seen in other neuropsychiatric disorders, including schizophrenia, autism spectrum disorders, bipolar disorders, depression, and obsessive-compulsive disorders (Genovese and Butler, 2023).
Mitochondria are functional dependent for bioenergetics and cellular survival (Rossmann et al., 2021). In addition to adenosine triphosphate (ATP) generated by mitochondria, they are also vital for the efficient working of signaling pathways including the antioxidant system (Malek et al., 2018). Mitochondrial function and dysfunction have become important mediators of metabolic disease, cancer cell metabolism, ageing, and neurodegeneration (Seyfried & Shelton, 2010). In the pancreatic beta cells, mitochondria have been demonstrated as nutrition sensors and signaling systems for insulin release (Wiederkehr & Wollheim, 2012). The mitochondria signal in the beta cell mediates nutrient oxidation through nutrient metabolism (Liesa & Shirihai, 2013). Studies have shown that insulin deficiency results in impaired mitochondrial function (Ruegsegger et al., 2018). The hormone insulin and related peptides known as insulin-like growth factors (IGFs) exert a variety of effects (Al-Samerria and Radovick, 2021). It is well known that they play a critical part in growth and development. Although it is widely acknowledged that IGF-I has complementary effects on glucose counter-regulation, insulin is still the chief regulator of blood glucose. Insulin modulates neural processes of eating, and cognition (Schell et al., 2021). In addition, studies have shown that an increased insulin signal in the mediobasal hypothalamus mediates the overall catabolic response to the diverse metabolic signals and in the hippocampus, insulin acts to facilitate the memory processes (Woods et al., 2006). Numerous studies have examined the effects of insulin control on depressive symptoms and behaviours. The hypothalamic-pituitary-adrenal axis (HPA) balance, neurotrophic factor and neurotransmitter secretion regulation, interactions with the gastrointestinal microbiome, neuroprotective effects, and effects on depression are all influenced by insulin and activation of its receptor (Zou et al., 2020).
Nigella sativa (NS), sometimes known as black seed, is a member of the Ranunculaceae plant family. The seeds and oil of NS have long been used to treat a variety of illnesses throughout the world. Nigella sativa has a wide range of biological effects and therapeutic potential, including anti-diabetic, anti-cancer, immunomodulator, analgesic, antimicrobial, anti-inflammatory, spasmolytic, bronchodilator, renal protective, gastro-protective, antioxidant, and neuroprotective properties. (Tavakkoli et al., 2017). The potency of NS is attributable to the oil in the seeds which consist of several phytochemical ranging from polyphenols to alkaloids. The most pharmacologically active compound identified so far is thymoquinone (Kooti et al., 2016, Talaei et al., 2022). Several studies indicate that NS oil benefits brain health and cognitive function even while protecting against neurodegeneration (Sahak et al., 2016). However, the impact of black seed oil on depression and social interaction behaviour following chronic antibiotics exposure remains unknown. Hence the present study.
2. Materials and methods
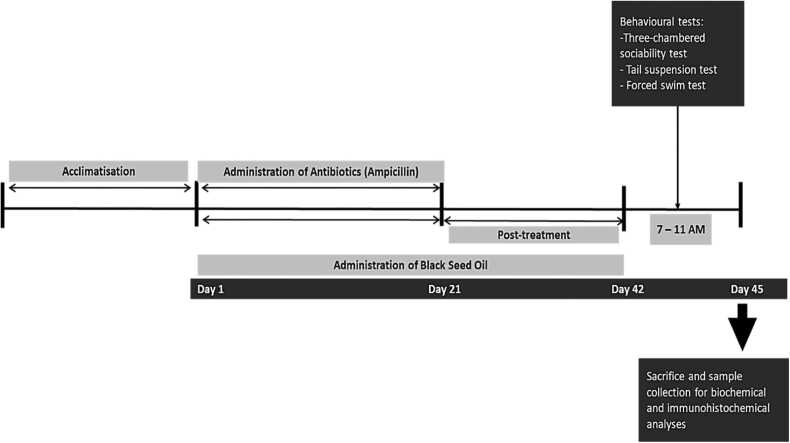
Forty adult male BALB/c mice weighing (30 ± 2 g) obtained from Afe Babalola University Animal Holdings were housed in standard plastic cages at room temperature under a 12/12-h light-dark cycle with rat chow and water ad libitum. The research was conducted following relevance guidelines as previously reported (Engwall et al., 2023). Due diligence was taken to reduced animal pain and discomfort. The study was approved by the Health Research Ethical Committee of Afe Babalola University Ado-Ekiti (ABUADHREC/13/04/2023/02).Fig. 1.
Fig. 1.
Experimental design.
2.1. Experimental design
Following a week of acclimatization, the animal was randomly assigned into 5 groups and treated as follows:
-
•
The control group (CTRL) was given standard chow and water throughout the experiment. Additionally, the CTRL group received 0.1 mL of normal saline once daily for 3 weeks via oral gavage.
-
•
The antibiotic toxicity (ABT) group received oral administration of ampicillin (Sagar vitaceutical, Nigeria) once daily for 3 weeks at a dose of 200 mg/kg body weight (b.w.) (Lee et al., 2020).
-
•
The black seed oil (NSO) group received the black seed oil once daily for 3 weeks at a dose of 2 mL/ kg b.w. (Ajao et al., 2016) via oral gavage.
-
•
The antibiotic toxicity plus black seed oil (ABT /NSO) group was treated with 200 mg/kg antibiotic simultaneously with 2 mL/kg b.w. NS oil once daily for 3 weeks via oral gavage.
-
•
The antibiotic toxicity plus black seed oil/post-treatment (ABT + NSO) group pre-exposure to 200 mg/kg antibiotic once daily for 3 weeks via oral gavage then followed by post-treatment of 2 mL/kg b.w. NS oil once daily for 3 weeks orally.
Note:
The drug was mixed with distilled water to the specified concentration, store refrigerated for stability, and follow experiment protocol.
The 5th group (ABT + NSO) was included to establish treatment effects and reduce bias. Additionally, it addresses ethical concerns by not subjecting animals to unnecessary treatment upfront and it is regarded versatile for investigating Black seed oil in various animal models and health conditions.
2.2. Behavioural studies
Before the actual test, the animals were trained to help them become accustomed to the environment and the tools needed for each type of test. When necessary, the animals were also given assistance to move, navigate, or climb to speed up learning and get them ready for the test itself. The behavioural test was conducted at the Animal Research Centre Unit of Afe Babalola University in Ekiti State, Nigeria, in a secure space with appropriate lighting and sound control. The animals were subjected to three-chamber sociability tests, tail suspension and forced swim tests.Fig. 2.
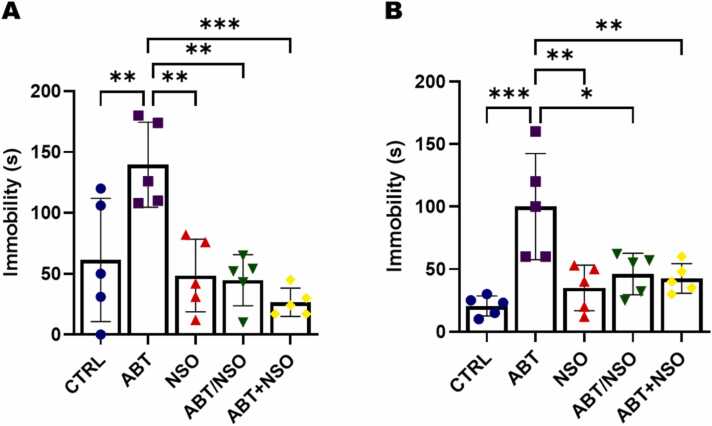
Fig. 2.
Effects of Nigella sativa oil on depressive-like behaviours following antibiotic exposure in mice from the TST (A) and FST (B) tests. Data are expressed as ± SD; n = 5 and analysed by one-way ANOVA followed by Tukey posthoc test. Legend: CTRL = Control; ABT = Antibiotics toxicity; NSO = Nigella sativa oil, ABT/NSO = Antibiotic toxicity and Nigella sativa oil simultaneously; ABT+NSO = Antibiotics toxicity with Nigella sativa oil post-treatment.
2.2.1. Social interaction behaviour using the three-chamber sociability test
The three-chambered tool was made of an opaque rectangular plexiglass box (80 cm long, 40.5 cm wide, and 40 cm tall), which was divided into three compartments (54 cm long, 30 cm broad) by transparent plexiglass walls with tiny, retractable entryways. The test was carried out as it had been described previously (Kim et al., 2014). For five minutes before the test, the subject mice were acclimated in the central compartment with the doors closed. The mouse is positioned in the centre of the compartment for the first session. The cage of either the left or the right compartment here referred to as the stranger zone 1, was then filled with the stranger mouse, with the opposite end of the compartment remaining vacant as an empty zone. A 10-minute sociability test was permitted for each mouse. A socialization index was calculated by comparing the amount of time spent in the stranger zone 1 with interaction from the new mouse to the amount of time spent in the empty zone.
2.2.2. Depressive-like behaviours
Tail suspension test (TST).
The method of Steru et al. (1985) was used to measure the overall length of immobility caused by tail suspension. The sticky tape was positioned about 1 cm from the tip of the tail and used to hang mice that were both visually and audibly separated 50 cm off the ground. Throughout a 5-minute test, immobility time was recorded.
Forced swim test (FST).
Briefly, each mouse was put into a clear swim cylinder (height: 36 cm, diameter: 20 cm) that contained water that was 30 cm deep and between 23 and 25 °C in temperature. A 5-minute immobility period was recorded. Throughout the final 4 min, each mouse's motion was timed. An antidepressant-like effect or a decrease in immobility are signs of depression, respectively. The groups being scored during the test session were hidden from the scorer (Can et al., 2012, Slattery and Cryan, 2012).
2.2.3. Euthanasia and tissue collection
The animals were deeply anesthetized before blood collection, euthanasia, and perfusion. The mice were euthanised with a single dose of ketamine hydrochloride (100 mg/kg; Overmyer et al., 2015). Blood was collected through cardiac puncture using 2 mL syringes, transferred into unmarked vials, and allowed to clot on the bench. Animals underwent transcardial perfusion with phosphate-buffered saline (PBS) (pH 7.4) to remove blood and prevent cell shrinkage, followed by 10% neutral buffer formal saline (NBF) as a pre-fixative. Only PBS was used to perfuse tissues for biochemical investigations before they were frozen for later examination.
2.3. Biochemical assays
2.3.1. Determination of MDA and SOD levels
Thiobarbituric acid reactive species (TBARs) concentrations were utilized to quantify the plasma lipid peroxidation levels, and malondialdehyde (MDA) production was used as an indicator of lipid peroxidation (Placer et al., 1966). The stock reagent (15%, w/v trichloroacetic acid in 0.25 N HCl and 0.375%, w/v thiobarbituric acid in 0.25 N HCl) and the test sample (1 vol each) were combined in a centrifuge tube. The solution was heated for 15 min in boiling water. After cooling, the precipitate was removed by centrifuging the mixture at 1500 g for 10 min. The supernatant's absorbance was then measured at 532 nm using a spectrophotometer (Shimadzu 2 R/UV–visible, Tokyo, Japan) against a blank that contained all the reagents except the test sample. The MDA concentration was given in nmol/mL or µM.
Superoxide Dismutase (SOD) assay was performed according to the manufacturer's instructions. The levels of SOD activity were determined as previously reported (Magnani et al., 2000, Łepecka et al., 2023). The values of SOD activity were expressed in U/mL.
2.3.2. Determination of lactate levels
A tissue disruptor (IKA-T10 basic Ultra-Turrax, IKA, Stauffen Germany) was used to homogenize samples of hippocampus and hypothalamus tissues while they were still frozen. The final ratio (which considers the expected water content of the samples) was 1.25:1 (Arriarán et al., 2015). All proteins were removed from the homogenate with the precipitate after centrifugation, and floating lipids that were insoluble in the diluted acetone were discarded. The kit 1001430 Spinreact, Sant Esteve d′en Bas, Spain, was used to estimate lactate using acetone tissue extract and plasma (10 µL samples), with sodium L-lactate (Sigma-Aldrich) serving as the reference.
2.3.3. Determination of creatinine kinase (CK) activity
For the assay of CK activity (Fortress Diagnostic Limited, Antrim, UK, Cat# BXC0111), numerous photometric, fluorometric, and coupled-enzyme techniques have been devised, using either the forward (Cr --> CRP) or the reverse (Cr --> CRP) reaction (Hrder et al., 1979). All commercial tests for total CK are now based on the reverse reaction, which moves along about six times more quickly than the forward reaction. Hexokinase (HK)/glucose-6-phosphate dehydrogenase (G6PD) coupled processes that ultimately convert NADP+ to NADPH, which is monitored spectrophotometrically at 340 nm, are used to assess the amount of ATP produced (Mohammadi, 2022). A measurement of the amount of CK activity in the specimen is the rate at which absorbance increases.
2.3.4. Evaluation of acetylcholinesterase (AChE) activity
AChE inhibitory activity assay was carried out using a slightly modified version of a spectrophotometric technique that had previously been reported (Ellman et al., 1961). Briefly, a total volume of 1 mL was incubated for 15 min at room temperature with 415 µL of Tris-HCl buffer (0.1 M, pH 8), 10 µL of various extract strengths diluted in methanol, and 25 µL of AChE solution (0.28 U/mL). AChI (1.83 mM) solution in a volume of 75 microliters was added, followed by 475 microliters of DTNB, and the final mixture was incubated for 30 min at room temperature.
A spectrophotometer was used to detect absorbance at 405 nm. Positive control was galantamine. As a negative control, bidistilled water was utilized as an alternative to EO or galanthamine. The following equation was used to compare the AChE activity inhibition rate (%) to the negative control:
where: Ac is the absorbance control (consisting of all reactants, excluding the AChE enzyme), and As is the experimental absorbance for each sample concentration.
2.4. Histological and immunohistochemical analyses
The fixed amygdala, and hypothalamus tissues were dehydrated in graded alcohol, cleared in xylene, embedded in paraffin, and sectioned at 5 µm. Tissue sections were stained with haematoxylin and eosin (Ankle and Joshi, 2011) and cresyl violet. For the immunohistochemical staining, sections of 5 µm thickness obtained from routine paraffin were deparaffinized and subjected to antigen retrieval by heating in a Tris/EDTA buffer (pH 9.0) for 30 mins in a steamer and allowed to cool at room temperature. 0.3% hydrogen peroxide in Phosphate Buffered Saline (PBS, pH 7.4) was used to block endogenous peroxidase activity. Sections were blocked in animal-free serum for 20 mins and later incubated in primary rabbit antibodies: Insulin (ab181547, Abcam, USA; 1/64000 dilution) overnight, followed by Anti-Rabbit HRP (ab97051, Abcam, USA; 1/500 dilution). Counter-stained with haematoxylin. All slides were scanned using Motic Easyscan Pro 6 (TX, USA) at 20X: 0.52 µm/pixel, and 40X: 0.26 µm/pixel with high precision. Motic Easyscan whole-slide images were viewed with Motic VM 3.0 – Motic Digital Slide Assistant.
2.5. Statistical analyses
This was done using one-way ANOVA (analysis of variance) or a mixed model, and differences between groups were evaluated by a post hoc turkey test with the aid of GraphPad Prism Software 9.0.0 (121). The outcome of the statistical analysis was represented using bar charts and graphs, with error bars representing the mean ± SD (standard deviation). The threshold for a significant difference was set at p < 0.05. Image J software (1.48 v/java 1.6.0_20) was used to quantify the cellular and connective tissue content.
2.6. Quantitative analyses
The qualitative information obtained from the histological and immunohistochemical section stained were translated into quantitative data. The quantitative data was generated by counting neurons in specific regions of interest (H&E and CFV).
To complement this, immunohistochemical staining with insulin antibody provided additional quantitative information. This involved measuring the intensity of insulin staining, often through Image J software (1.48 v/java 1.6.0_20), to quantify the presence of insulin in the tissue. This combined approach allows for comprehensive understanding of both structural changes (H&E and CFV) and immunohistochemical (insulin antibody staining) related to neuron loss.
The insulin percentage area occupied was quantified through Image J software (1.48 v/java 1.6.0_20) using a systematic approach. Initially, images were prepared by converting them to grayscale and applying a threshold to delineate insulin-stained regions, transforming the image into binary form. In the quantitative analysis phase, the "Analyse > Analyze Particles" function isolated relevant features, adjusting parameters for refined selection. The results window provided metrics, including insulin percentage area occupied, offering a quantitative representation of insulin distribution.
To assess insulin staining intensity using Image J software (1.48 v/java 1.6.0_20), the grayscale image was opened, and a region of interest (ROI) was selected for measurement. The "Analyse > Measure" function was utilized to determine the mean intensity within the chosen ROI. Optionally, contrast was enhanced by setting a threshold using "Image > Adjust > Threshold." If necessary, the image histogram was analyzed for threshold adjustment. The results displayed in the window were reviewed, including mean intensity data. For precise measurements, the image was calibrated using known standards with "Analyse > Set Scale.".
NB: Calibration enhanced precision, aligning pixel values with physical dimensions. Batch processing ensured consistent analysis across images, bolstering reliability and reproducibility of findings.
3. Results
3.1. Behavioural assessment
3.1.1. Effects of Nigella sativa oil on antibiotic-induced depressive-like behaviour in mice
Comparison between groups by one-way ANOVA followed by Tukey's multiple tests shows a significant increase in immobility time using the TST following exposure to antibiotics in the ABT group when compared with CTRL (**p < 0.01; F (4, 20) = 9.206, 0.0085). There is a significant difference in immobility time in the NSO (**p < 0.01; F (4, 20) = 9.206, 0.0021), ABT/NSO (**p < 0.01; F (4, 20) = 9.206, 0.0014) and ABT+NSO (***p < 0.001; F (4, 20) = 9.206, 0.0002) groups compared to the ABT group (see Fig. 3A). Similarly, the evaluation of FST shows a significant increase in immobility time following exposure to antibiotics in the ABT group when compared with CTRL (***p < 0.001; F (4, 20) = 8.750; 0.0002). There is a significant difference in immobility time in the NSO (**p < 0.01; F (4, 20) = 8.750, 0.0018), ABT/NSO (*p < 0.05; F (4, 20) = 8.750, 0.0104) and ABT+NSO (***p < 0.001; F (4, 20) = 8.750, 0.0060) groups compared to the ABT group (see Fig. 3B).
Fig. 3.
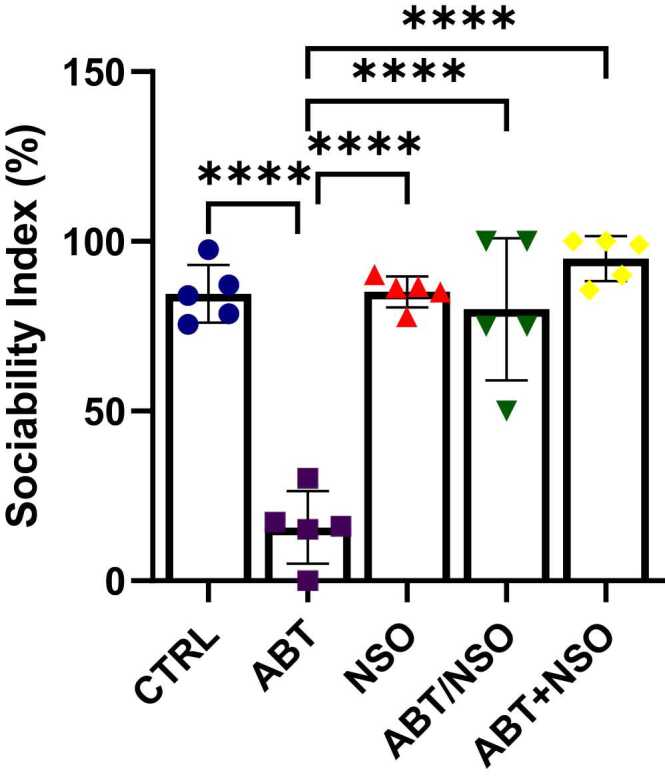
Evaluation of social interaction behaviour (sociability index from the three-chamber sociability test) following Nigella sativa administration and/or chronic antibiotic exposure. Data are expressed as ± SD; n = 5 and analysed by one-way ANOVA followed by Tukey posthoc test. Legend: CTRL = Control; ABT = Antibiotics toxicity; NSO = Nigella sativa oil, ABT/NSO = Antibiotic toxicity and Nigella sativa oil simultaneously; ABT+NSO = Antibiotics toxicity with Nigella sativa oil post-treatment.
3.1.2. Effects of Nigella sativa oil on antibiotic-mediated social interaction deficit in mice
Three-chamber sociability test shows a significant decrease in the sociability index in the ABT group compared to CTRL (****p < 0.0001; F (4, 20) = 37.04, < 0.0001). Treatment with Nigella sativa oil in the NSO (****p < 0.0001; F (4, 20) = 37.04, < 0.0001), ABT/NSO (****p < 0.0001; F (4, 20) = 37.04, < 0.0001) and ABT+NSO (****p < 0.0001; F (4, 20) = 37.04, < 0.0001) groups shows a significant increase in percentage alternation compared to ABT group (see Fig. 4).
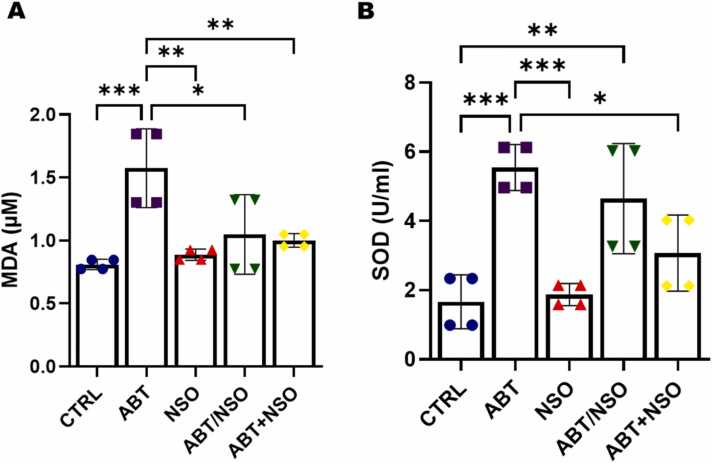
Fig. 4.
Effects of Nigella sativa oil on oxidative following antibiotic exposure in mice from the MDA (A) and SOD (B) estimation. Data are expressed as ± SD; n = 4 and analysed by one-way ANOVA followed by Tukey posthoc test. Legend: CTRL = Control; ABT = Antibiotics toxicity; NSO = Nigella sativa oil, ABT/NSO = Antibiotic toxicity and Nigella sativa oil simultaneously; ABT+NSO = Antibiotics toxicity with Nigella sativa oil post-treatment.
3.1.3. Nigella sativa oil improves oxidative stress following exposure to antibiotics in mice
Comparison between groups by one-way ANOVA followed by Tukey's multiple tests shows a significant increase in plasma malondialdehyde (MDA) activity following exposure to antibiotics in the ABT group when compared with CTRL (***p < 0.001; F (4, 15) = 8.802, 0.0007). There is a significant difference in MDA activity in the NSO (**p < 0.01; F (4, 15) = 8.802; 0.0019), ABT/NSO (*p < 0.05; F (4, 15) = 8.802, 0.0164) and ABT+NSO (**p < 0.01; F (4, 15) = 8.802, 0.0087) groups compared to the ABT group (see Fig. 5A). Evaluation of superoxide dismutase activity shows a significant increase in plasma SOD levels following exposure to antibiotics in the ABT (***p < 0.001; F (4, 15) = 11.84, 0.0005) and ABT/NSO (**p < 0.01; F (4, 15) = 11.84, 0.0053) when compared with CTRL group. There is a significant increase in plasma SOD levels in the NSO (***p < 0.001; F (4, 15) = 11.84, 0.0008), and ABT+NSO (*p < 0.05; F (4, 15) = 11.84, 0.0217) groups compared to the ABT group (see Fig. 5B).
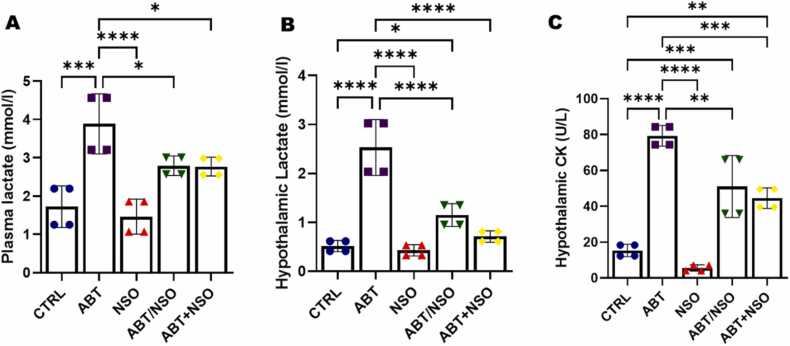
Fig. 5.
Effects of Nigella sativa oil on mitochondrial activity following antibiotic exposure in mice from the plasma lactate (A), hypothalamic lactate (B), and hypothalamic CK (C) evaluation. Data are expressed as ± SD; n = 4 and analysed by one-way ANOVA followed by Tukey posthoc test. Legend: CTRL = Control; ABT = Antibiotics toxicity; NSO = Nigella sativa oil, ABT/NSO = Antibiotic toxicity and Nigella sativa oil simultaneously; ABT+NSO = Antibiotics toxicity with Nigella sativa oil post-treatment.
3.1.4. Nigella sativa oil improves chronic antibiotic-induced mitochondrial dysfunction
Results from the plasma lactate analyses reveal a significant increase in plasma lactate levels in the ABT group when compared to CTRL (***p < 0.001; F (4, 15) = 15.09; 0.0002). Treatment with Nigella sativa oil produced significant decrease in plasma lactate levels in the NSO (****p < 0.0001; F (4, 15) = 15.09; < 0.0001), ABT/NSO (*p < 0.05; F (4, 15) = 15.09; 0.0484), and ABT+NSO (*p < 0.05; F (4, 15) = 15.09; 0.0435) groups compared to the ABT group (see Fig. 6A). Hypothalamic lactate activity reveals a significant increase in ABT (****p < 0.0001; F (4, 15) = 35.56; < 0.0001), and ABT/NSO (*p < 0.05; F (4, 15) = 35.56; 0.0488) compared to CTRL. Administration of Nigella sativa oil significantly reduced NSO (****p < 0.0001; F (4, 15) = 35.56; < 0.0001), ABT/NSO (****p < 0.0001; F (4, 15) = 35.56, < 0.0001), and ABT+NSO (****p < 0.0001; F (4, 15) = 35.56, < 0.0001) (see Fig. 6B). Additionally, Hypothalamic creatinine kinase (CK) activity reveals a significant increase in hippocampal CK levels in the ABT (****p < 0.0001; F (4, 15) = 45.52, < 0.0001), ABT/NSO (***p < 0.001; F (4, 15) = 45.52, 0.0003), and ABT+NSO (**p < 0.01; F (4, 15) = 45.52, 0.0022) compared to CTRL. Treatment with Nigella sativa oil produced significant decrease in hypothalamic CK levels in the NSO (****p < 0.0001; F (4, 15) = 45.52, < 0.0001), ABT/NSO (***p < 0.001; F (4, 15) = 45.52, 0.0029), and ABT+NSO (**p < 0.01; F (4, 15) = 45.52, 0.0004) (see Fig. 6C).
Fig. 6.
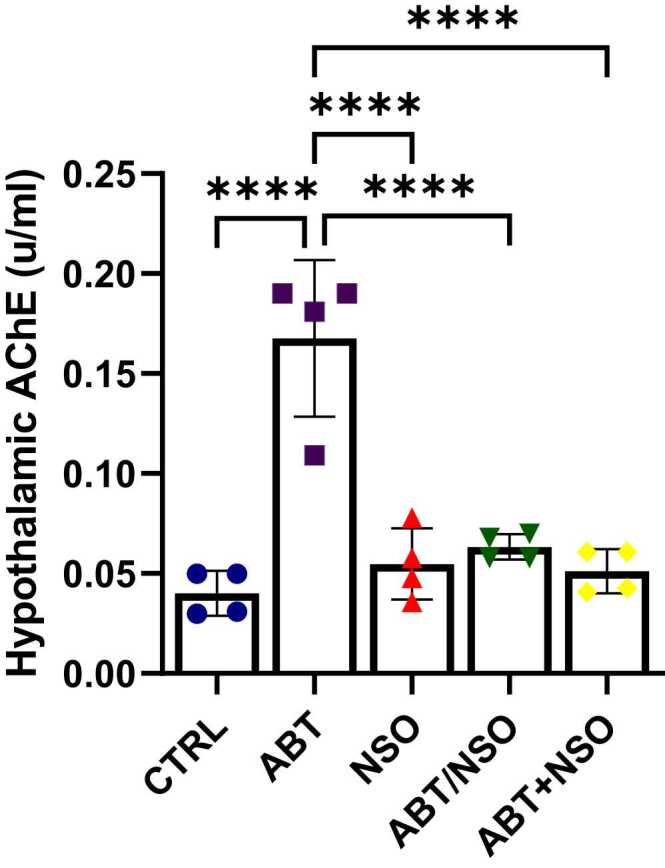
Effects of Nigella sativa oil on hypothalamic acetylcholinesterase activity following antibiotic exposure in mice. Data are expressed as ± SD; n = 4 and analysed by one-way ANOVA followed by Tukey posthoc test. Legend: CTRL = Control; ABT = Antibiotics toxicity; NSO = Nigella sativa oil, ABT/NSO = Antibiotic toxicity and Nigella sativa oil simultaneously; ABT+NSO = Antibiotics toxicity with Nigella sativa oil post-treatment.
3.1.5. Nigella sativa oil improves chronic antibiotic-induced acetylcholinesterase dysregulation
Comparison between groups by one-way ANOVA followed by Tukey's multiple tests shows a significant increase in hypothalamic acetylcholinesterase level following exposure to antibiotics in the ABT group when compared with CTRL (****p < 0.0001; F (4, 15) = 25.44, < 0.0001). There is a significant difference in immobility time in the NSO (****p < 0.0001; F (4, 15) = 25.44, < 0.0001), ABT/NSO (****p < 0.0001; F (4, 15) = 25.44, < 0.0001) and ABT+NSO (****p < 0.0001; F (4, 15) = 25.44, < 0.0001) groups compared to the ABT group (see Fig. 7).
Fig. 7.
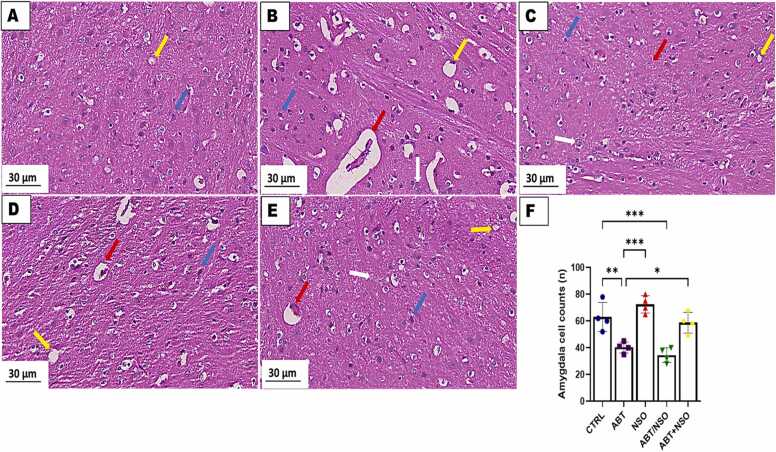
Photomicrograph of amygdala in mice following Nigella sativa administration and/or chronic antibiotic exposure. Group A = control (CTRL); Group B = Antibiotic toxicity (ABT); Group C = Nigella sativa oil (NSO); Group D = Antibiotic toxicity and Nigella sativa oil simultaneously (ABT/NSO); Group E = Antibiotics toxicity with Nigella sativa oil post treatment (ABT+NSO). Yellow arrow (vacuolation of the neuropil), blue arrow (viable cell), white arrow (degenerated cell) red arrow (pyknotic cell), F. Amygdala cell counts X800 (H&E).
3.1.6. Effect of NSO on histomorphology of the amygdala following chronic antibiotic exposure
The histological examination of the amygdala, using H&E staining techniques, revealed significant cell loss with extensive degenerative changes, including swollen cell bodies, loss of integrity, and shrunken cytoplasm in the amygdaloid complex following chronic antibiotic exposure. Treatment with NS oil revealed more viable cells with an improved morphological alteration in the amygdaloid complex. Comparison between groups by one-way ANOVA followed by Tukey multiple tests shows a significant decrease in cell population following exposure to antibiotics in the ABT (**p < 0.01; F (4, 15) = 18.90, 0.0041), and ABT/NSO (***p < 0.001; F (4, 15) = 18.90, 0.0005) groups compared with CTRL. There is a significant increase in cell population in the NSO (***p < 0.001; F (4, 15) = 18.90, 0.0001), and ABT+NSO (*p < 0.05; F (4, 15) = 18.90, 0.0200) groups compared to the ABT group (see Fig. 8).
Fig. 8.
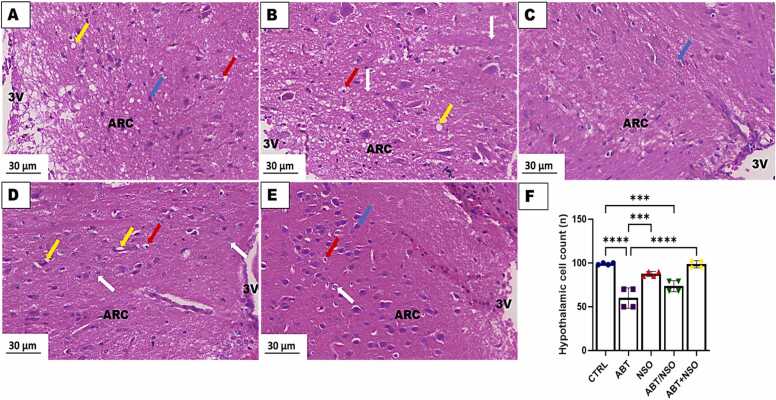
Photomicrograph of hypothalamus in mice following Nigella sativa administration and/or chronic antibiotic exposure. Group A = control (CTRL) – with numerous viable cells; Group B = Antibiotic toxicity (ABT) – with reduction in number of cells in the arcuate nucleus region of the hypothalamus; Group C = Nigella sativa oil (NSO) – numerous viable cells; Group D = Antibiotic toxicity and Nigella sativa oil simultaneously (ABT/NSO) – presenting viable cells more than ABT group; Group E = Antibiotics toxicity with Nigella sativa oil post treatment (ABT+NSO) – with numerous viable cells compared to ABT group, Yellow arrow (vacuolation of the neuropil), blue arrow (viable cell), white arrow (degenerated cell) red arrow (pyknotic cell), F. Hypothalamic cell counts X800 (H&E). X800 (H&E).
3.1.7. Effect of NSO on hypothalamic histomorphology following chronic antibiotic exposure
The histological examination of the hypothalamus, using H&E staining techniques, revealed significant cell loss accompanied by conspicuous vacuolated neuropil and nuclear pyknosis in the mediobasal hypothalamus following chronic antibiotic exposure. Treatment with NS oil revealed more viable cells with an improved morphological status in the mediobasal hypothalamus. Comparison between groups by one-way ANOVA followed by Tukey's multiple tests shows a significant decrease in cell population following exposure to antibiotics in the ABT (****p < 0.0001; F (4, 15) = 28.38, < 0.0001), and ABT/NSO (***p < 0.001, F (4, 15) = 28.38, 0.0003) groups when compared with CTRL. There is a significant increase in cell population in the NSO (***p < 0.0001; F (4, 15) = 28.38, 0.0002), and ABT+NSO (****p < 0.001; F (4, 15) = 28.38, < 0.0001) groups compared to the ABT group (see Fig. 9).
Fig. 9.
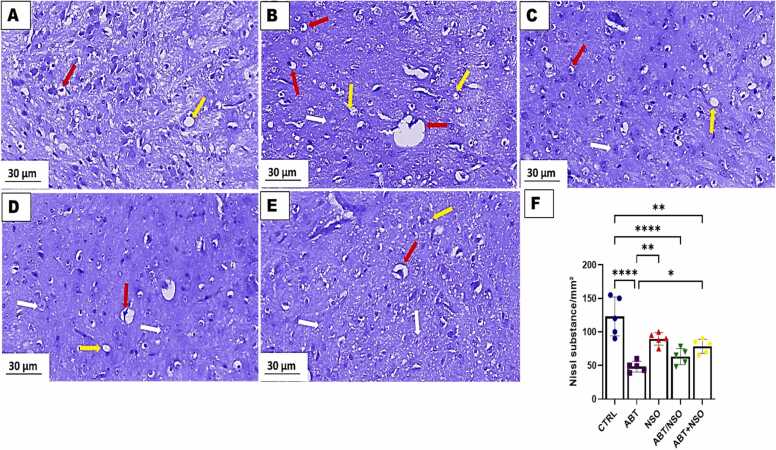
Photomicrograph of amygdala in mice following Nigella sativa administration and/or chronic antibiotic exposure. Group A = control (CTRL); Group B = Antibiotic toxicity (ABT); Group C = Nigella sativa oil (NSO); Group D = Antibiotic toxicity and Nigella sativa oil simultaneously (ABT/NSO); Group E = Antibiotics toxicity with Nigella sativa oil post treatment (ABT+NSO). Yellow arrow (vacuolated neuropil), white arrow (degenerated cell) red arrow (pyknotic cell), F. Amygdala Nissl substance area. X800 (CFV).
3.1.8. . Effect of NSO on amygdala histomorphology following chronic antibiotic exposure using the cresyl fast violet staining technique.
The histological examination of the amygdala, using cresyl fast violet staining techniques, revealed a relative decrease in Nissl substances of the amygdaloid complex following chronic antibiotic exposure, with evident nuclear pyknosis and degenerated neurons. Treatment with NS oil ameliorated the pathological alterations in the amygdaloid complex. Comparison between groups by one-way ANOVA followed by Tukey's multiple tests shows a relatively decrease in Nissl substances following exposure to antibiotics in the ABT (****p < 0.0001; F (4, 20) = 16.19, < 0.0001), NSO (*p < 0.05; F (4, 20) = 16.19, 0.0240), ABT/NSO (****p < 0.0001; F (4, 20) = 16.19, < 0.0001), and ABT+NSO (**p < 0.01; F (4, 20) = 16.19, 0.0020) compared to CTRL group. There is a significant increase in Nissl substances in the NSO (**p < 0.01; F (4, 20) = 16.19, 0.0041), and ABT+NSO (*p < 0.05; F (4, 20) = 16.19, 0.0471) groups compared to the ABT group (see Fig. 10).
Fig. 10.
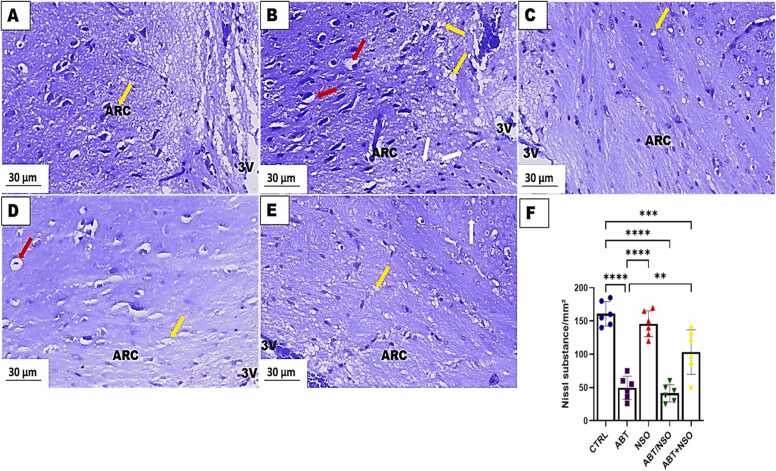
Photomicrograph of hypothalamus in mice following Nigella sativa administration and/or chronic antibiotic exposure. Group A = control (CTRL) – with numerous viable cells; Group B = Antibiotic toxicity (ABT) – with reduction in number of cells in the arcuate nucleus region of the hypothalamus; Group C = Nigella sativa oil (NSO) – numerous viable cells; Group D = Antibiotic toxicity and Nigella sativa oil simultaneously (ABT/NSO) – presenting viable cells more than ABT group; Group E = Antibiotics toxicity with Nigella sativa oil post treatment (ABT+NSO) – with numerous viable cells compared to ABT group. Yellow arrow (vacuolated neuropil), white arrow (degenerated cell) red arrow (pyknotic cell), F. Hypothalamic Nissl substance area. X800 (CFV).
3.1.9. Effect of NSO on hypothalamic histomorphology following chronic antibiotic exposure using the cresyl fast violet staining technique.
The histological examination of the hypothalamus using the cresyl fast violet staining technique revealed a relatively decrease in Nissl substances of the mediobasal hypothalamus accompanied by neuronal degeneration, nuclear pyknosis, and karryorhexis following chronic antibiotic exposure. Treatment with NS oil revealed the neuroprotective capacity of this oil with improved morphological status in the mediobasal hypothalamus. Comparison between groups by one-way ANOVA followed by Tukey's multiple tests shows a significant decrease in cell population following exposure to antibiotics in the ABT (****p < 0.0001; F (4, 25) = 38.28, < 0.0001), ABT/NSO (****p < 0.0001; F (4, 25) = 38.28, < 0.00001), and ABT+NSO (***p < 0.001; F (4, 25) = 38.28, 0.0008) groups when compared with CTRL. There is a significant increase in cell population in the NSO (****p < 0.0001; F (4, 25) = 38.28, < 0.0001), ABT+NSO (**p < 0.0001; F (4, 25) = 38.28, 0.0018) group compared to the ABT group (see Fig. 11).
Fig. 11.
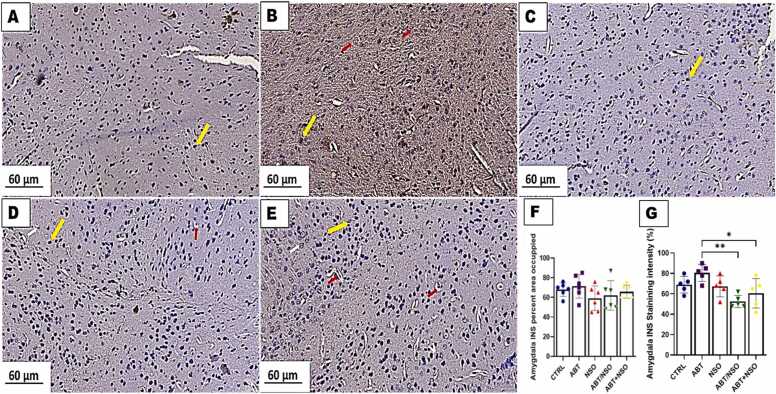
Photomicrograph of amygdala in mice following Nigella sativa administration and/or chronic antibiotic exposure. Group A = control (CTRL); Group B = Antibiotic toxicity (ABT); Group C = Nigella sativa oil (NSO); Group D = Antibiotic toxicity and Nigella sativa oil simultaneously (ABT/NSO); Group E = Antibiotics toxicity with Nigella sativa oil post treatment (ABT+NSO). Yellow arrow (insulin expression), white arrow (degenerated neuron) red arrow (pyknotic nucleus) F. Amygdala INS percent area occupied G. Amygdala INS staining intensity. X400 (Immunohistochemical staining techniques).
3.1.10. Effect of NSO on insulin expression in the amygdala following chronic antibiotic exposure
The immunohistochemical examination of insulin expression in the amygdala revealed a degenerated neuron with relative amount of insulin expression in the amygdaloid complex following chronic antibiotic exposure. Treatment with NS oil revealed the neuroprotective capacity of the oil accompanied by regeneration of neurons with relative amount of insulin in the amygdaloid complex. Comparison between groups by one-way ANOVA followed by Tukey's multiple tests shows a relative amount of insulin expression across the groups for INS area occupied (ns F (4, 25) = 1.125, P = 0.3672). Additionally, there is a significant decrease in amygdala insulin staining intensity in the ABT/NSO (**p < 0.01; F (4, 20) = 5.564, 0.0019) and ABT+NSO (*p < 0.05; F (4, 20) = 5.564, 0.0320) groups compared to ABT group (see Fig. 11).
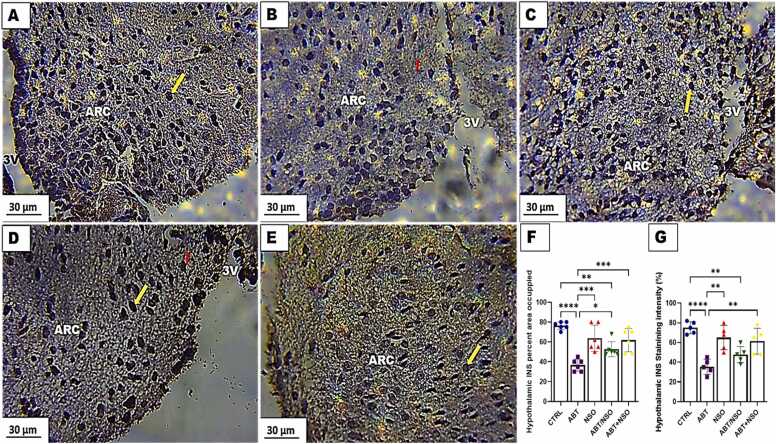
3.1.11. Effect of NSO on the insulin expression in the hypothalamus following chronic antibiotic exposure
The immunohistochemical examination of insulin expression in the hypothalamus revealed a significant downregulation of insulin expression accompanied by decrease in insulin with degenerated neurons in the mediobasal hypothalamus following chronic antibiotic exposure. Treatment with NS oil improves insulin dysregulation in the mediobasal hypothalamus. Comparison between groups by one-way ANOVA followed by Tukey's multiple tests shows a significant decrease in insulin area occupied following exposure to antibiotics in the ABT (****p < 0.0001; F (4, 25) = 15.35, < 0.0001), and ABT/NSO (**p < 0.01; F (4, 25) = 15.35, 0.0013) groups when compared with CTRL. There is a significant increase in insulin area occupied in the NSO (***p < 0.001; F (4, 25) = 15.35, 0.0003), ABT/NSO (*p < 0.05; F (4, 25) = 15.35, 0.0424), and ABT+NSO (***p < 0.001; F (4, 25) = 15.35, 0.0006) group compared to the ABT group. Additionally, comparison between groups by one-way ANOVA followed by Tukey's multiple tests shows a significant decrease in insulin intensity following exposure to antibiotics in the ABT (****p < 0.0001; F (4, 20) = 12.27, < 0.0001) and ABT/NSO (**p < 0.01; F (4, 20) = 12.27, 0.0025) groups when compared with CTRL. Treatment with NS oil reveals a significant increase in insulin intensity in the NSO (**p < 0.01; F (4, 20) = 12.27, 0.0011) and ABT+NSO (**p < 0.01; F (4, 20) = 12.27, 0.0039) groups compared to the ABT group (see Fig. 12).
Fig. 12.
Photomicrograph of hypothalamus insulin expression in mice following Nigella sativa administration and/or chronic antibiotic exposure. Group A = control (CTRL); Group B = Antibiotic toxicity (ABT); Group C = Nigella sativa oil (NSO); Group D = Antibiotic toxicity and Nigella sativa oil simultaneously (ABT/NSO); Group E = Antibiotics toxicity with Nigella sativa oil post treatment (ABT+NSO); 3 V = third ventricle; ARC = arcuate nucleus. Yellow arrow = (insulin expression); red arrow = (degenerated neuron). F. Hypothalamic INS percent area occupied G. Hypothalamic INS staining intensity. X800 Immunohistochemical staining techniques.
4. Discussion
Antibiotic abuse/misuse has been reported to drive social deficits as seen in neurodevelopmental disorders including autism spectrum disorder (ASD) and attention-deficit hyperactivity disorder (ADHD), limited data currently exist regarding the direct impact of antibiotic toxicity on social interaction. This study evaluated the effect of untherapeutic antibiotic exposure on the sociability index in mice using the three-chambered sociability test. Impaired social interaction profile is a marker of social recognition memory, a process mediated by complex interactions between neural pathways in the hippocampus (Wang and Zhan, 2022), amygdala (Gur et al., 2014, Tanimizu et al., 2017), and hypothalamus (Wang et al., 2022). In rodents, social recognition is made based on chemosensory cues present in the anogenital area, which collectively compose an “olfactory signature” (Markham and Juraska, 2007). The social recognition ability of rodents can be observed in the laboratory by measuring the reduction in the amount of time spent investigating the same individual across repeated exposures (Aspesi and Choleris, 2022; Paletta et al., 2023), as confirmed by the present results where antibiotic-exposed mice spent less time with the stranger animal signifying impaired social behaviour (Fig. 4). This impact could be a result of the disruption of oxytocin and arginine vasopressin in the amygdala-hypothalamic-hippocampal system. Findings from our histological evaluation of the amygdala-hypothalamic system revealed a significant loss of cells in the amygdaloid complex and mediobasal hypothalamus following antibiotic exposure which could result in amygdala hyperactivity and energy dyshomeostasis. Antibiotic-potentiated sociability deficit was ameliorated following treatments with Nigella sativa oil. While studies have shown numerous benefits of the oil on cognitive functions there are currently no specific reports on the effects of the oil on antibiotic-induced social interaction impairments. Treatments with the NS oil significantly increased the sociability index following exposure to 200 mg/kg of ampicillin antibiotic, indicating the social-interaction-improving ability of the oil (Fig. 4). Although the mechanism behind this improvement is unclear, it is highly plausible that synergistic interactions of different bioactive compounds including thymoquinone in the NS oil (Sahak et al., 2016) modulated the oxytocin and arginine vasopressin pathways. Also, black seed oil plays a neuroprotective role in the amygdala-hypothalamic system with a significant increase in the number of cells in the amygdaloid complex and mediobasal hypothalamus demonstrating the antidepressant and energy/metabolic regulatory capacity of the oil (Fig. 8, Fig. 9, Fig. 10, Fig. 11).
Recurrent antibiotic exposure is associated with increased risk for depression and anxiety as seen in major depressive disorders (Lee et al., 2021). Several studies have demonstrated an association between antibiotic use and subsequent depression. This study demonstrated the induction of depressive-like behaviours following antibiotic exposure from the tail suspension and forced swim tests with more time spent immobile (Fig. 3). The tail suspension and forced swim tests are behavioural testing paradigms for depressive-like behaviours in rodents, and in testing antidepressant products. Depression is mediated by neural processes in the amygdala-hippocampal pathway (Hiew et al., 2020). The tail-suspension test is useful for screening new antidepressant treatments and testing other manipulations that are likely to influence behaviours linked with depression (Amaghnouje et al., 2020). This impact could be a result of profound effects on neural structure and function including dysregulation of the neural plasticity in the hippocampus and prefrontal cortex. The present study observed an amelioration of antibiotic-induced depressive-like behavior with reduced immobility time following treatments with Nigella sativa oil, as depicted in Fig. 3. The findings demonstrated significant improvements in immobility time as assessed by the Tail Suspension Test (TST) and Forced Swim Test (FST). Notably, there is limited existing literature on the antidepressant activity of Nigella sativa oil specifically in the context of antibiotic toxicity. However, different studies have demonstrated the anxiolytic activity of Nigella sativa oil (Al-Snafi, 2021, Benkermiche et al., 2022). The polar extract of N. sativa has been reported to contain phytochemical and biological constituents’ quercetin-3-O-α-L rhamnopyranoside, quercetin-7-O-β-D glucopyranoside, tauroside E, and sapindoside B as the potential antidepressant constituents in the seed using extensive NMR analysis (Elkhayat et al., 2016). In addition, evidence shows the fixed oil of Nigella sativa contains thymoquinone with great impacts on depressive-like behaviours. This finding, therefore, suggests that NS oil can be a candidate adjuvant therapy for the management of mood/affective disorders associated with chronic antibiotic toxicity.
Antibiotics have been reported to induce oxidative damage to cellular tissues and DNA (Elgazzar et al., 2022). In every living system, the creation of cellular energy is maintained in large parts by oxygen. This is accomplished by oxidative phosphorylation, which is carried out by electron transporters in cellular mitochondria (Kuzmiak-Glancy et al., 2022). Reactive oxygen species (ROS), also known as free radicals, are produced throughout the body in an unbalanced manner by oxidative stress (OS), which impairs biomolecules functions resulting in the alteration of proteins, lipids, and amino acids as well as DNA (Juan et al., 2021). Our findings reveal impaired antioxidant defence following chronic exposure to ampicillin antibiotic evident in increased MDA and upregulation of SOD activity in plasma (Fig. 5). Upon treatment with NS oil, there was significant amelioration of this oxidative dysfunction (Fig. 5). One of the most significant problems affecting human health today is antibiotic resistance, which is largely attributable to antibiotic misuse or overuse (Ferri et al., 2017). Governments and significant funding organizations are therefore once again prioritizing research aimed at developing novel antibiotherapeutic approaches (Mourenza et al., 2020). An important approach that has attracted interest recently is the development of therapies based on the induction of oxidative stress to disrupt the redox defences of bacterial pathogens (Shandilya et al., 2022). Using this method, it is possible to find antimicrobials with the potential to be repurposed and employed in combinational chemotherapies that are intended to treat illnesses brought on by resistant bacterial pathogens. Important strides have also been made in the identification of novel secondary metabolites from bacteria and plants that may cause oxidative stress as a component of their antibacterial mechanism of action. In our findings, the administration of NS oil was able to alleviate antibiotic-induced oxidative damage demonstrating the antioxidant capacity of the oil (Fig. 5). This is in line with several studies (Mahi-Birjand et al., 2020, Balaha et al., 2023) on the antioxidant properties of the oil, including thymoquinone, carvacrol, t-anethole, and 4-terpineol (Ibrahim et al., 2022). Oxidative damage impairs mtDNA transcription and replication, which lowers mitochondrial function (Renaudin et al., 2021). Findings from this study reveal a systemic and central mitochondrial dysfunction (Fig. 6) following chronic antibiotic exposure signifying a dysfunction of the mitochondrial respiratory chain that could result in several disorders including hypoglycaemia and lactic acidosis (Babiker et al., 2020). Nigella sativa improves mitochondrial dysfunction, and this could be a result of thymoquinone (Fig. 6).
Several antibiotics, notably those in the fluoroquinolone class (such as ciprofloxacin and levofloxacin), have been found to inhibit the activity of acetylcholinesterase (Singh et al., 2013). In the present study, an assessment of hypothalamic AChE activity revealed that chronic antibiotic exposure upregulates hypothalamic AChE levels (Fig. 7). It is also plausible that the hypothalamic structural integrity of cholinesterase is disrupted by reactive oxygen species (ROS) and free radicals produced by chronic antibiotic exposure, which would result in decreased enzyme activity (Danaei et al., 2022). The main cholinesterase in the body, AChE, is crucial for controlling the central cholinergic systems. It has been demonstrated that neurobehavioural impairments associated with Alzheimer’s disease (AD) may be related to central cholinergic systems damage caused by AChE deficiency in the brain (Uddin et al., 2016). In addition to sleep, endocrine, and metabolic regulation the hypothalamus has also been implicated in the modulation of social, and affective behaviours including depression, and social interaction (Neumann, 2008, Sandi and Haller, 2015, Wang et al., 2022). Our result further shows that NS oil decreased the AChE level potentially via the activity of thymoquinone, a potent antioxidant that scavenges and deactivates free radicals and helps in improving the AChE inhibition (Danaei et al., 2022) (Fig. 7).
Furthermore, oxidative stress as observed in this study is a driver of several cellular dysregulations including impaired insulin signalling and synthesis (Kang et al., 2019; Hodson & Gunn, 2019). Immunohistochemical evaluation of insulin-producing cells in the amygdala and hypothalamus revealed a reduction of insulin-synthesized cells following chronic antibiotic exposure, revealing for the first-time insulin production-impairing potential of chronic antibiotic exposure (Fig. 11 & 12). While the mechanisms behind these significant cell loss upon antibiotic exposure are unclear, it can be said that chronic antibiotic exposure can potentially impact the supplemental control of insulin and glucagon secretion negatively by the hypothalamic arcuate nucleus neurons (Heiss et al., 2021). We report a decrease in the staining intensity of insulin protein following chronic antibiotic exposure in the hypothalamic arcuate nucleus region compared to the control group (Fig. 12). The NS oil was able to increase hypothalamic insulin expression signifying the role of the hypothalamus in insulin protein production by the energy/metabolic homeostasis, and insulin regulation within normal levels. The role of NS oil in modulating hypothalamus-mediated endocrine function vis-à-vis insulin synthesis and absorption has not been explored in the context of chronic antibiotic exposure. This, therefore, warrants more research to elucidate the mechanisms behind the effect of NS oil in modulating insulin regulation in the hypothalamic-pancreatic axis.
5. Conclusion
Overall, findings from this study suggest the beneficial role of black seed oil as an adjuvant therapy in preventing and abrogating mood behavioural and metabolic impairments of chronic antibiotic exposure.
Ethical statement
The study was approved by the Health Research Ethical Committee of Afe Babalola University Ado-Ekiti (ABUADHREC/13/04/2023/02).
Financial support
No external funding was obtained for this study.
CRediT authorship contribution statement
Mujeeb Adedokun: Validation, Investigation, Formal Analysis, Data Curation, Writing - Original Draft. Linus Enye: Conceptualization, Project Supervision. Elizabeth Akinluyi and Toheeb Ajibola: Investigation, Formal Analysis, Data Curation. Edem Edem: Conceptualization, Methodology, Project Administration, Writing - Review & Editing.
Declaration of competing interest
The authors declare none.
Contributor Information
Linus Anderson Enye, Email: enyelinus@abuad.edu.ng.
Edem Ekpenyong Edem, Email: ee.edem@abuad.edu.ng.
Data Availability
A reasonable request to the corresponding author for access to data from this study can be made.
References
- Ajao, M.S. , Imam, A. , Amin, A. , Abdulmajeed, W. I , Ajibola, M. I , Alli-Oluwafuyi, A. & Ibrahim, A. (2016). Black seed oil improves motor and anxiety-like behaviors and cerebellar cyto-architectonic in adult male wistar rats. Nigerian journal of Neuroscience, 8(1–2), 8–14.
- Al-Samerria S., Radovick S. The role of insulin-like growth factor-1 (IGF-1) in the control of neuroendocrine regulation of growth. Cells. 2021;10(10):2664. doi: 10.3390/cells10102664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Snafi A.E. A complementary and alternative natural antidepressant therapy with emphasis on their mechanisms of action. Int. J. Biol. Pharm. Sci. Arch. 2021;2(1):7–21. [Google Scholar]
- Amaghnouje, A., Mechchate, H., Es-Safi, I., Boukhira, S., S. Aliqahtani, A., M. Noman, O.,. & Bousta, D, 2020, Amaghnouje, A., Mechchate, H., Es-Safi, I., Boukhira, S., S. Aliqahtani, A., M. Noman, O.,. & Bousta, D (2020). Subacute assessment of the toxicity and antidepressant-like effects of Origanum majorana L. polyphenols in Swiss albino mice. Molecules, 25(23), 5653. [DOI] [PMC free article] [PubMed]
- Anand N., Gorantla V.R., Chidambaram S.B. The role of gut dysbiosis in the pathophysiology of neuropsychiatric disorders. Cells. 2023;12(1):54. doi: 10.3390/cells12010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankle M.R., Joshi P.S. A study to evaluate the efficacy of xylene-free hematoxylin and eosin staining procedure as compared to the conventional hematoxylin and eosin staining: an experimental study. J. Oral. Maxillofac. Pathol.: JOMFP. 2011;15(2):161. doi: 10.4103/0973-029X.84482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriarán S., Agnelli S., Sabater D., Remesar X., Fernández-López J.A., Alemany M. Evidences of basal lactate production in the main white adipose tissue sites of rats. Eff. Sex. a Cafe. Diet. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0119572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspesi D., Choleris E. Neuroendocrine underpinning of social recognition in males and females. J. Neuroendocrinol. 2022;34(2) doi: 10.1111/jne.13070. [DOI] [PubMed] [Google Scholar]
- Babiker, M.O.E., Abdalla, A.A., Kabiraj, M.M. (2020). Neurological Disorders in the Neonate. Clinical Child Neurology, 29–74.
- Bairamian D., Sha S., Rolhion N., Sokol H., Dorothée G., Lemere C.A., Krantic S. Microbiota in neuroinflammation and synaptic dysfunction: a focus on Alzheimer’s disease. Mol. Neurodegener. 2022;17(1):1–23. doi: 10.1186/s13024-022-00522-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaha M., Alahmari A., Kandeel S., Balaha M. Vinpocetine’s immunomodulating, anti-oxidant, anti-inflammatory, ant-ifibrotic, and PDE inhibiting potencies ameliorate bleomycin-induced pulmonary fibrosis. Iran. J. Basic Med. Sci. 2023;26(1):13. doi: 10.22038/IJBMS.2022.64175.14130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkermiche S., Djemli S., Haloui M., Benabed M.L., Tahraoui A. Preventive effects of ginger extract and nigella sativa oil on anxiety and depression behavior in wistar rats exposed to mercuric chloride. Pharmacog. Res. 2022;14:1. [Google Scholar]
- Can A., Dao D.T., Arad M., Terrillion C.E., Piantadosi S.C., Gould T.D. The mouse forced swim test. JoVE (J. Vis. Exp. ) 2012;59 doi: 10.3791/3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow K.M., Hui A.C., Szeto C.C. Neurotoxicity induced by beta-lactam antibiotics: from bench to bedside. Eur. J. Clin. Microbiol. Infect. Dis. 2005;24(10):649–653. doi: 10.1007/s10096-005-0021-y. [DOI] [PubMed] [Google Scholar]
- Chow K.M., Szeto C.C., Hui A.C.F., Li P.K.T. Mechanisms of antibiotic neurotoxicity in renal failure. Int. J. Antimicrob. Agents. 2004;23(3):213–217. doi: 10.1016/j.ijantimicag.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Danaei G.H., Amali A., Karami M., Khorrami M.B., Riahi-Zanjani B., Sadeghi M. The significance of thymoquinone administration on liver toxicity of diazinon and cholinesterase activity; a recommendation for prophylaxis among individuals at risk. BMC Complement. Med. Ther. 2022;22(1):1–8. doi: 10.1186/s12906-022-03806-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zwart P.L., Jeronimus B.F., de Jonge P. Empirical evidence for definitions of episode, remission, recovery, relapse and recurrence in depression: a systematic review. Epidemiol. Psychiatr. Sci. 2019;28(5):544–562. doi: 10.1017/S2045796018000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey A., Ghosh N., Saxena G.K., Purohit D., Patel S., Singh S. Management implications for neurotoxic effects associated with antibiotic use. NeuroQuantology. 2022;20(6):304–328. [Google Scholar]
- Elgazzar, D., Aboubakr, M., Bayoumi, H., Ibrahim, A.N., Sorour, S.M., El-Hewaity, M. & Abdeen, A. (2022). Tigecycline and gentamicin-combined treatment enhances renal damage: oxidative stress, inflammatory reaction, and apoptosis interplay. Pharmaceuticals, 15(6), 736. [DOI] [PMC free article] [PubMed]
- Elkhayat E.S., Alorainy M.S., El-Ashmawy I.M., Fat’hi S. Potential antidepressant constituents of Nigella sativa seeds. Pharmacogn. Mag. 2016;12(Suppl 1):S27. doi: 10.4103/0973-1296.176118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman G.L., Courtney K.D., Andres V., Jr, Featherstone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7(2):88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Engwall M.J., Baublits J., Chandra F.A., Jones Z.W., Wahlstrom J., Chui R.W., Vargas H.M. Evaluation of levocetirizine in beagle dog and cynomolgus monkey telemetry assays: Defining the no QTc effect profile by timepoint and concentration‐QTc analysis. Clinical and Translational. Science. 2023;16(3):436–446. doi: 10.1111/cts.13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X., Deng H., Qiu J., Ji H., Shen X. Antibiotics-induced depression in mice via the microbiota-gut-brain axis. J. Affect. Disord. 2022;318:152–158. doi: 10.1016/j.jad.2022.08.059. [DOI] [PubMed] [Google Scholar]
- Ferri M., Ranucci E., Romagnoli P., Giaccone V. Antimicrobial resistance: a global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2017;57(13):2857–2876. doi: 10.1080/10408398.2015.1077192. [DOI] [PubMed] [Google Scholar]
- Genovese A., Butler M.G. The Autism spectrum: behavioral, psychiatric and genetic associations. Genes. 2023;14(3):677. doi: 10.3390/genes14030677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giau V.V., Wu S.Y., Jamerlan A., An S.S.A., Kim S., Hulme J. Gut microbiota and their neuroinflammatory implications in Alzheimer’s disease. Nutrients. 2018;10(11):1765. doi: 10.3390/nu10111765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill M.F., Maganti R.K. Neurotoxic effects associated with antibiotic use: management considerations. Br. J. Clin. Pharmacol. 2011;72(3):381–393. doi: 10.1111/j.1365-2125.2011.03991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guida F., Turco F., Iannotta M., De Gregorio D., Palumbo I., Sarnelli G., Maione, S Antibiotic-induced microbiota perturbation causes gut endocannabinoidome changes, hippocampal neuroglial reorganization and depression in mice. Brain, Behav., Immun. 2018;67:230–245. doi: 10.1016/j.bbi.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Gur R., Tendler A., Wagner S. Long-term social recognition memory is mediated by oxytocin-dependent synaptic plasticity in the medial amygdala. Biol. Psychiatry. 2014;76(5):377–386. doi: 10.1016/j.biopsych.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Heiss C.N., Mannerås-Holm L., Lee Y.S., Serrano-Lobo J., Gladh A.H., Seeley R.J., Olofsson L.E. The gut microbiota regulates hypothalamic inflammation and leptin sensitivity in Western diet-fed mice via a GLP-1R-dependent mechanism. Cell Rep. 2021;35(8) doi: 10.1016/j.celrep.2021.109163. [DOI] [PubMed] [Google Scholar]
- Helaly A., El-Attar Y.A., Khalil M., Ahmed Ghorab D.S.E.D., El-Mansoury A.M. Antibiotic abuse induced histopathological and neurobehavioral disorders in mice. Curr. Drug Saf. 2019;14(3):199–208. doi: 10.2174/1574886314666190612130921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiew L.F., Poon C.H., You H.Z., Lim L.W. TGF-β/smad signalling in neurogenesis: Implications for neuropsychiatric diseases. Cells. 2021;10(6):1382. doi: 10.3390/cells10061382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim M.A., Mohammed B.A., El-Kamali H.H. GC/Ms profiling and antioxidant activity of Nigella sativa essential oil from Sudan. World J. Pharm. Life Sci. 2022;8(12):152–156. [Google Scholar]
- Juan C.A., Pérez de la Lastra J.M., Plou F.J., Pérez-Lebeña E. The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021;22(9):4642. doi: 10.3390/ijms22094642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G.G., Francis N., Hill R., Waters D., Blanchard C., Santhakumar A.B. Dietary polyphenols and gene expression in molecular pathways associated with type 2 diabetes mellitus: a review. Int. J. Mol. Sci. 2019;21(1):140. doi: 10.3390/ijms21010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S.A., Nzakizwanayo J., Rodgers A.M., Zhao L., Weiser R., Tekko I.A., Gilmore B.F. Antibiotic therapy and the gut microbiome: Investigating the effect of delivery route on gut pathogens. ACS Infect. Dis. 2021;7(5):1283–1296. doi: 10.1021/acsinfecdis.1c00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.W., Seung H., Kwon K.J., Ko M.J., Lee E.J., Oh H.A., Bahn G.H. Subchronic treatment of donepezil rescues impaired social, hyperactive, and stereotypic behavior in valproic acid-induced animal model of autism. PloS One. 2014;9(8) doi: 10.1371/journal.pone.0104927. e104927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooti W., Hasanzadeh-Noohi Z., Sharafi-Ahvazi N., Asadi-Samani M., Ashtary-Larky D. Phytochemistry, pharmacology, and therapeutic uses of black seed (Nigella sativa) Chin. J. Nat. Med. 2016;14(10):732–745. doi: 10.1016/S1875-5364(16)30088-7. [DOI] [PubMed] [Google Scholar]
- Korczak D.J., Westwell-Roper C., Sassi R. Diagnosis and management of depression in adolescents. CMAJ. 2023;195(21):E739–E746. doi: 10.1503/cmaj.220966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmiak-Glancy S., Glancy B., Kay M.W. Ischemic damage to every segment of the oxidative phosphorylation cascade elevates ETC driving force and ROS production in cardiac mitochondria. Am. J. Physiol. -Heart Circ. Physiol. 2022;323(3):H499–H512. doi: 10.1152/ajpheart.00129.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.R.U. Penicillin allergy delabeling can decrease antibiotic resistance, reduce costs, and optimize patient outcomes. Fed. Pract. 2020;37(10) doi: 10.12788/fp.0040. 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.W., Lee H., Kang H.Y. Association between depression and antibiotic use: analysis of population-based National Health Insurance claims data. BMC Psychiatry. 2021;21(1):1–10. doi: 10.1186/s12888-021-03550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.E., Kim J.K., Kim D.H. Orally administered antibiotics vancomycin and ampicillin cause cognitive impairment with gut dysbiosis in mice with transient global forebrain ischemia. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.564271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Łepecka A., Szymański P., Okoń A., Zielińska D. Antioxidant activity of environmental lactic acid bacteria strains isolated from organic raw fermented meat products. LWT. 2023;174 [Google Scholar]
- Liesa M., Shirihai O.S. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013;17(4):491–506. doi: 10.1016/j.cmet.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie I., Yang Y.X., Haynes K., Mamtani R., Boursi B. Antibiotic exposure and the risk for depression, anxiety, or psychosis: a nested case-control study. J. Clin. Psychiatry. 2015;76(11):825. doi: 10.4088/JCP.15m09961. [DOI] [PubMed] [Google Scholar]
- Mahi-Birjand M., Yaghoubi S., Abdollahpour-Alitappeh M., Keshtkaran Z., Bagheri N., Pirouzi A., Karimzadeh I. Protective effects of pharmacological agents against aminoglycoside-induced nephrotoxicity: a systematic review. Expert Opin. Drug Saf. 2020;19(2):167–186. doi: 10.1080/14740338.2020.1712357. [DOI] [PubMed] [Google Scholar]
- Magnani L., Gaydou E.M., Hubaud J.C. Spectrophotometric measurement of antioxidant properties of flavones and flavonols against superoxide anion. Anal. Chim. Acta. 2000;411(1-2):209–216. [Google Scholar]
- Malek M.H., Hüttemann M., Lee I. Mitochondrial structure, function, and dynamics: the common thread across organs, disease, and aging. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/1863414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham J.A., Juraska J.M. Social recognition memory: influence of age, sex, and ovarian hormonal status. Physiol. Behav. 2007;92(5):881–888. doi: 10.1016/j.physbeh.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithani K., Davison B., Meng Y., Lipsman N. The anterior limb of the internal capsule: anatomy, function, and dysfunction. Behav. brain Res. 2020;387 doi: 10.1016/j.bbr.2020.112588. [DOI] [PubMed] [Google Scholar]
- Mohammadi H. Sodium dithionate (Na2S2O4) induces oxidative damage in mice mitochondria heart tissue. Toxicol. Rep. 2022;9:1391–1397. doi: 10.1016/j.toxrep.2022.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais L.H., Schreiber H.L., IV, Mazmanian S.K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021;19(4):241–255. doi: 10.1038/s41579-020-00460-0. [DOI] [PubMed] [Google Scholar]
- Mourenza Á., Gil J.A., Mateos L.M., Letek M. Oxidative stress-generating antimicrobials, a novel strategy to overcome antibacterial resistance. Antioxidants. 2020;9(5):361. doi: 10.3390/antiox9050361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann I.D. Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J. Neuroendocrinol. 2008;20(6):858–865. doi: 10.1111/j.1365-2826.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- Overmyer K.A., Thonusin C., Qi N.R., Burant C.F., Evans C.R. Impact of anesthesia and euthanasia on metabolomics of mammalian tissues: studies in a C57BL/6J mouse model. PloS One. 2015;10(2) doi: 10.1371/journal.pone.0117232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paletta P., Bass N., Aspesi D., Choleris E. Sex differences in social cognition. Curr. Top. Behav. Neurosci. 2023;62:207–234. doi: 10.1007/7854_2022_325. [DOI] [PubMed] [Google Scholar]
- Pancu D.F., Scurtu A., Macasoi I.G., Marti D., Mioc M., Soica C., Dehelean C. Antibiotics: conventional therapy and natural compounds with antibacterial activity—a pharmaco-toxicological screening. Antibiotics. 2021;10(4):401. doi: 10.3390/antibiotics10040401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placer Z.A., Cushman L.L., Johnson B.C. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal. Biochem. 1966;16(2):359–364. doi: 10.1016/0003-2697(66)90167-9. [DOI] [PubMed] [Google Scholar]
- Rehman K., Haider K., Jabeen K., Akash M.S.H. Current perspectives of oleic acid: Regulation of molecular pathways in mitochondrial and endothelial functioning against insulin resistance and diabetes. Rev. Endocr. Metab. Disord. 2020;21(4):631–643. doi: 10.1007/s11154-020-09549-6. [DOI] [PubMed] [Google Scholar]
- Renaudin X., Lee M., Shehata M., Surmann E.M., Venkitaraman A.R. BRCA2 deficiency reveals that oxidative stress impairs RNaseH1 function to cripple mitochondrial DNA maintenance. Cell Rep. 2021;36(5) doi: 10.1016/j.celrep.2021.109478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann M.P., Dubois S.M., Agarwal S., Zon L.I. Mitochondrial function in development and disease. Dis. Models Mech. 2021;14(6) doi: 10.1242/dmm.048912. dmm048912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegsegger G.N., Creo A.L., Cortes T.M., Dasari S., Nair K.S. Altered mitochondrial function in insulin-deficient and insulin-resistant states. J. Clin. Investig. 2018;128(9):3671–3681. doi: 10.1172/JCI120843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacre, H., Hajj, A., Aoun, R., Hallit, S., Zeitoun, A., & Salameh, P. (2020). Drug safety in Lebanon. In Drug Safety in Developing Countries (pp. 449–470). Academic Press.
- Sahak M.K., Kabir N., Abbas G., Draman S., Hashim N.H., Hasan Adli D.S. The Role of Nigella sativa and Its Active Constituents in Learning and Memory. Evid. -Based Complement. Altern. medicine: 2016 doi: 10.1155/2016/6075679. eCAM, 2016, 6075679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C., Haller J. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat. Rev. Neurosci. 2015;16(5):290–304. doi: 10.1038/nrn3918. [DOI] [PubMed] [Google Scholar]
- Schell M., Wardelmann K., Kleinridders A. Untangling the effect of insulin action on brain mitochondria and metabolism. J. Neuroendocrinol. 2021;33(4) doi: 10.1111/jne.12932. [DOI] [PubMed] [Google Scholar]
- Seyfried T.N., Shelton L.M. Cancer as a metabolic disease. Nutr. Metab. 2010;7(1):1–22. doi: 10.1186/1743-7075-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shandilya S., Kumar S., Jha N.K., Kesari K.K., Ruokolainen J. Interplay of gut microbiota and oxidative stress: Perspective on neurodegeneration and neuroprotection. J. Adv. Res. 2022;38:223–244. doi: 10.1016/j.jare.2021.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M., Kaur M., Kukreja H., Chugh R., Silakari O., Singh D. Acetylcholinesterase inhibitors as Alzheimer therapy: from nerve toxins to neuroprotection. Eur. J. Med. Chem. 2013;70:165–188. doi: 10.1016/j.ejmech.2013.09.050. [DOI] [PubMed] [Google Scholar]
- Slattery D.A., Cryan J.F. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat. Protoc. 2012;7(6):1009–1014. doi: 10.1038/nprot.2012.044. [DOI] [PubMed] [Google Scholar]
- Steru L., Chermat R., Thierry B., Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85(3):367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Talaei S.A., Banafshe H.R., Moravveji A., Shabani M., Tehrani S.S., Abed A. Anti-nociceptive effect of black seed oil on an animal model of chronic constriction injury. Res. Pharm. Sci. 2022;17(4):383–391. doi: 10.4103/1735-5362.350239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimizu T., Kenney J.W., Okano E., Kadoma K., Frankland P.W., Kida S. Functional connectivity of multiple brain regions required for the consolidation of social recognition memory. J. Neurosci. 2017;37(15):4103–4116. doi: 10.1523/JNEUROSCI.3451-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakkoli A., Ahmadi A., Razavi B.M., Hosseinzadeh H. Black seed (Nigella sativa) and its constituent thymoquinone as an antidote or a protective agent against natural or chemical toxicities. Iran. J. Pharm. Res.: IJPR. 2017;16(Suppl):2. [PMC free article] [PubMed] [Google Scholar]
- Uddin M.S., Mamun A.A., Hossain M.S., Akter F., Iqbal M.A., Asaduzzaman M. Exploring the effect of Phyllanthus emblica L. on cognitive performance, brain antioxidant markers and acetylcholinesterase activity in rats: promising natural gift for the mitigation of Alzheimer's disease. Ann. Neurosci. 2016;23(4):218–229. doi: 10.1159/000449482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchio I., Sorrentino L., Paoletti A., Marra R., Arbitrio M. The state of the art on acetylcholinesterase inhibitors in the treatment of Alzheimer’s disease. J. Cent. Nerv. Syst. Dis. 2021;13 doi: 10.1177/11795735211029113. 11795735211029113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Wang S.C., Liu X., Jia S., Wang X., Li T., Wang Y.F. Neural functions of hypothalamic oxytocin and its regulation. ASN neuro. 2022;14 doi: 10.1177/17590914221100706. 17590914221100706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Leri F., Rizvi S.J. Anhedonia as a central factor in depression: Neural mechanisms revealed from preclinical to clinical evidence. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2021;110 doi: 10.1016/j.pnpbp.2021.110289. [DOI] [PubMed] [Google Scholar]
- Wang X., Zhan Y. Regulation of social recognition memory in the hippocampal circuits. Front. Neural Circuits. 2022;16:17. doi: 10.3389/fncir.2022.839931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederkehr A., Wollheim C.B. Mitochondrial signals drive insulin secretion in the pancreatic β-cell. Mol. Cell. Endocrinol. 2012;353(1-2):128–137. doi: 10.1016/j.mce.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Woods S.C., Benoit S.C., Clegg D.J. The brain-gut-islet connection. Diabetes. 2006:S114–S121. [Google Scholar]
- Yang L., Bajinka O., Jarju P.O., Tan Y., Taal A.M., Ozdemir G. The varying effects of antibiotics on gut microbiota. AMB Express. 2021;11:1–13. doi: 10.1186/s13568-021-01274-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X.H., Sun L.H., Yang W., Li B.J., Cui R.J. Potential role of insulin on the pathogenesis of depression. Cell Prolif. 2020;53(5) doi: 10.1111/cpr.12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A reasonable request to the corresponding author for access to data from this study can be made.