Abstract
Quinolones were examined for rapid lethal activity against Mycobacterium smegmatis in the presence and absence of chloramphenicol, an inhibitor of protein synthesis. C-8 methoxy, C-6 fluorine, and particular C-7 ring substituents enhanced rapid killing. With the surprising exception of moxifloxacin, higher quinolone concentrations were required for lethal activity in the presence of chloramphenicol than in its absence. Moxifloxacin was also unusual in lacking the time lag characteristic of fluoroquinolone lethality. Several fluoroquinolone dimers, which represent quinolones with large C-7 substituents, showed modest bacteriostatic activity. Unlike other quinolones, the dimers failed to display lethal activity. The insensitivity of moxifloxacin to chloramphenicol has not been observed with other bacteria and may therefore reflect unique aspects of mycobacterial gyrase.
The fluoroquinolones are lethal, broad-spectrum antibacterial agents that are receiving increasing attention as potential antituberculosis agents. Two new C-8 methoxy derivatives, gatifloxacin and moxifloxacin, exhibit good activity against Mycobacterium tuberculosis, both in vitro (5, 12, 16) and in a murine model of tuberculosis (1, 14, 22). Moxifloxacin is now being used clinically (7). However, the lethal activity of the compounds may be reduced by cotreatment with rifampin (12), a first-line antituberculosis drug, and like many other antimycobacterial compounds, fluoroquinolones have marginal activity against nongrowing cells (20, 21). The latter feature may be particularly problematic because M. tuberculosis appears to enter a nongrowing, dormant stage shortly after infection (15, 17). Thus, understanding how the quinolones rapidly kill bacteria, especially in the absence of growth, is likely to be important.
The quinolones reversibly trap gyrase on DNA as complexes in which the DNA is broken (6, 18, 19). The complexes block DNA replication, thereby explaining the bacteriostatic action of the drugs. After long incubation, the absence of DNA replication leads to cell death through unspecified events. Lethal action can also be seen for short drug treatments if drug concentration is raised to roughly 5 to 10 times the MIC. This rapid lethal action is thought to be due to chromosome fragmentation arising from release of DNA breaks from protein-mediated constraints present in the drug-gyrase-DNA complexes (2). By expressing quinolone concentrations required for rapid death as multiples of bacteriostatic concentrations (fold of MIC), quinolones can be indirectly compared for chromosome fragmentation, independent of effects on processes such as drug uptake, drug efflux, and formation of quinolone-enzyme-DNA complexes.
In the present work, we examined Mycobacterium smegmatis for the effect of several quinolone substituents on rapid lethal action. Surprising activities were observed with moxifloxacin and quinolone dimers. Moxifloxacin killed cells equally well in the presence and absence of chloramphenicol, an agent widely known to interfere with quinolone lethality (2, 3). Dimer treatment of M. smegmatis is the first example of bacterial growth being blocked by a quinolone without rapid killing of growing cells.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
M. smegmatis mc2155 was obtained from S. Cole, Institut Pasteur, Paris, France. Liquid cultures were grown in Middlebrook 7H9 medium containing 10% albumin and 0.05% Tween 80 (9); colonies were grown at 37°C on 7H10 agar plates with or without fluoroquinolone. Bacterial doubling time was 4 h.
Fluoroquinolones.
Fluoroquinolones identified by PD numbers were products of Parke-Davis Pharmaceutical Division of Pfizer, Ann Arbor, MI. Fluoroquinolones identified by BMS numbers were obtained from Bristol-Myers-Squibb (Wallingford, CT). Fluoroquinolone dimers were prepared as described previously (11). Ciprofloxacin, Bay y3114, and moxifloxacin were obtained from Bayer Corp. (West Haven, CT). Fluoroquinolones were dissolved in 0.1 ml of 1 M NaOH (1/10 of the final volume), and then sterile water was added to obtain a final concentration of 10 mg/ml. Fluoroquinolone dimers were dissolved in dimethyl sulfoxide to 2 mg/ml. Stock solutions, divided into aliquots, were stored at −80°C. Dilutions were prepared with sterile distilled water. Solutions were kept at −20°C for several weeks during the course of experiments.
Measurement of fluoroquinolone susceptibility.
Inhibition of growth was defined as the minimal quinolone concentration required to inhibit colony formation by 99% [MIC(99)] after incubation on quinolone-containing agar for 3 days. Cultures contained about 5 × 108 CFU/ml prior to serial dilution and plating. For these determinations, fluoroquinolone concentration increments differed by no more than 25% per increment. MIC(99) was used rather than the standard MIC because it can be measured more accurately and because bactericidal effects are less likely to affect its value.
To measure lethal action, cells were grown in 7H9 liquid medium to mid-log phase at 37°C with shaking. Cells were split into 2-ml aliquots, and various concentrations of quinolone were added. For the measurement of killing in the absence of protein synthesis, chloramphenicol was added to a concentration of 20 μg/ml 1 h prior to addition of quinolone (15 μg/ml is sufficient to block growth within minutes; 20 μg/ml has no lethal effect and lowers protein synthesis to <1% in 20 min). After incubation for 5 or 18 h with shaking, cells were diluted in 7H9 medium and applied to 7H10 agar plates lacking drug. Growth was for 2 to 3 days to determine the fraction of surviving cells. A zero-time drug control was taken at the time of quinolone addition. Survival was calculated relative to bacterial CFU at zero time.
RESULTS
Effect of fluoroquinolone structure on bacteriostatic and bactericidal action. Susceptibility of M. smegmatis to a series of fluoroquinolones that differed at the C-8 position and in the C-7 ring system was determined (quinolone structures are shown in Table 1). In general, a C-8 methoxy moiety increased bacteriostatic activity [lower MIC(99)], except for derivatives with a large alkyl group attached to the C-7 piperazinyl ring (Table 2).
TABLE 1.
Quinolone structures employed in this studya 
|
| |||||
|---|---|---|---|---|---|
| Compound | Substituent at position indicated in structure | ||||
| 7 | 8 | X | Y | Z | |
| Ciprofloxacin | a | H | H | H | H |
| PD138032 | a | H | CH3 | H | H |
| PD158804 | a | H | CH3 | H | CH3 |
| PD160793 | a | H | C2H5 | H | H |
| PD160788 | a | H | H | C2H5 | H |
| PD161645 | a | H | H | C3H7 | H |
| PD135042 | a | O-CH3 | H | H | H |
| Gatifloxacinb | a | O-CH3 | CH3 | H | H |
| PD138926 | a | O-CH3 | CH3 | H | CH3 |
| PD161148 | a | O-CH3 | C2H5 | H | H |
| PD161144 | a | O-CH3 | H | C2H5 | H |
| PD162282 | a | O-CH3 | H | C3H7 | H |
| Moxifloxacin | b | O-CH3 | |||
| Bay y3114 | b | H | |||
| Garenoxacinc | c | O-CHF2 | |||
| BMS-340280 | c | O-CHF2 | |||
| BMS-433368 | c | O-CH3 | |||
The core structure of quinolones is shown at the top of the artwork. Substituents at position 7 are indicated (a to c).
Lacks a C-6 fluorine.
TABLE 2.
Effect of quinolone structure on bacteriostatic and bactericidal activities with M. smegmatis
| Compounda | C-8a | C-7 ringa | MIC(99) (μg/ml) | % Survivalb
|
|
|---|---|---|---|---|---|
| no Cm | + Cm | ||||
| Ciprofloxacin | H | None | 0.16 | 30 | 95 |
| PD135042 | O-Me | None | 0.048 | 1.2 | 55 |
| PD138032 | H | Methyl | 0.06 | 50 | 100 |
| Gatifloxacinc | O-Me | Methyl | 0.04 | 3 | 35 |
| PD158804 | H | Dimethyl | 0.075 | 50 | 100 |
| PD138926 | O-Me | Dimethyl | 0.044 | 5 | 40 |
| PD160793 | H | C-Ethyl | 0.12 | 60 | 100 |
| PD161148 | O-Me | C-Ethyl | 0.06 | 3 | 20 |
| PD160788 | H | N-Ethyl | 0.12 | 100 | 100 |
| PD161144 | O-Me | N-Ethyl | 0.05 | 50 | 100 |
| PD161645 | H | N-Isopropyl | 0.16 | 90 | 93 |
| PD162282 | O-Me | N-Isopropyl | 0.16 | 1.5 | 35 |
| Bay y3114 | H | Ring b | 0.05 | 20 | 97 |
| Moxifloxacin | O-Me | Ring b | 0.042 | 12 | 16 |
| BMS-433368 | O-Me | Ring c | 0.042 | 10 | 60 |
| BMS-340280 | O-CHF2 | Ring c | 0.05 | 40 | 50 |
| Garenoxacind | O-CHF2 | Ring c | 0.05 | 100 | 100 |
To assess rapid lethal activity, exponentially growing M. smegmatis was treated with various concentrations of each compound for slightly more than one doubling time (5 h). The fraction of surviving CFU was measured; in all cases, rapid killing required drug concentrations well above MIC(99). Quin-olone concentrations were then normalized to MIC(99) to correct for differences in drug uptake and formation of bacteriostatic quinolone-gyrase-DNA complexes. For six out of seven pairs of compounds differing in C-7 ring structure, the presence of a C-8 methoxy group increased lethal activity (Fig. 1). The exception occurred with compounds that contained an N-ethyl moiety on the C-7 piperazinyl ring: the C-8 methoxy compound PD161144 was not more active than its C-8-H derivative PD160788 when quinolone concentration was normalized to MIC(99) (Fig. 1). Moxifloxacin and BMS-433368, two compounds with large, complex C-7 ring systems, displayed no more lethality than some of the other C-8 methoxy compounds (Table 2; Fig. 1).
FIG. 1.
Bactericidal activity of C-8-H and C-8 methoxy fluoroquinolones in the presence or absence of chloramphenicol. Exponentially growing cultures of wild-type M. smegmatis were incubated with the indicated fluoroquinolone concentrations and normalized to MIC(99) for 5 h in the absence (open circles) or presence (filled circles) of chloramphenicol added 1 h prior to fluoroquinolone treatment. Aliquots were then removed, diluted, and plated on drug-free 7H10 agar for determination of viable cells. Names of compounds tested are indicated in each panel; structure information refers to Table 1. A replicate experiment gave results similar to those shown.
Effect of C-8 methoxy and C-7 ring substituents on quinolone-mediated lethal activity in the absence of protein synthesis.
Comparison of quinolone lethality in the presence of chloramphenicol revealed little activity for seven compounds that contained a hydrogen at position C-8 (Fig. 1). In contrast, the C-8 methoxy compounds displayed lethal activity that in some cases was influenced by moieties attached to the C-7 ring (Fig. 1). Moxifloxacin was insensitive to chloramphenicol and exhibited the greatest activity in the absence of protein synthesis (Fig. 1). BMS-433368, a C-8 methoxy compound that also contains a large, complex C-7 ring system, exhibited bacteriostatic and bactericidal activities that were similar to those observed with moxifloxacin (Table 2). However, BMS-433368 lethality was inhibited by chloramphenicol (Fig. 2) to an extent similar to that seen with compounds having a smaller C-7 ring system, such as PD135042 (Fig. 1). Thus, the unusual behavior of moxifloxacin is not readily explained by the size of its C-7 ring system.
FIG. 2.
Effect of C-6-F and C-8-OF2 groups on lethal action in the presence and absence of chloramphenicol. Exponentially growing cultures of wild-type M. smegmatis were incubated with increasing concentrations of quinolone for 5 h in the absence (open circles) or presence (filled circles) of chloramphenicol. Aliquots were removed, diluted, and plated on drug-free 7H10 agar for the determination of viable cell numbers. Names of compounds tested are indicated in each panel. A replicate experiment gave similar results.
Effect of C-6-F and C-8-OCHF2 groups on quinolone-mediated lethality in the presence and absence of chloramphenicol.
Fluoroquinolones are defined in part by a fluorine attached to position C-6. This substituent is absent from the newly developed agent garenoxacin (Table 1). To assess the effect of C-6 fluorine moiety, we compared lethal activity for garenoxacin with its C-6 fluorine derivative, BMS-340280 (Fig. 2). In the presence or absence of chloramphenicol, garenoxacin was less lethal, indicating that a C-6 fluorine is important for lethal events occurring after complex formation with gyrase and DNA. Compound BMS-340280, which has a difluoromethoxy moiety at the C-8 position, was slightly less active than its C-8 methoxy derivative, BMS-433368, in the absence of chloramphenicol; little difference was observed in the presence of chloramphenicol (Table 2; Fig. 2).
Rapid killing by moxifloxacin.
Moxifloxacin killed M. smegmatis equally well in the presence and absence of chloramphenicol (Fig. 1). It also killed cells without a detectable time lag (Fig. 3A), while a 60-min delay in lethal action was seen with PD161148, a compound that exhibited lethal activity at the same concentration [fold of MIC(99)] as moxifloxacin in the absence of chloramphenicol (Fig. 3B) but less activity in the presence of chloramphenicol (Fig. 3C). These data are consistent with moxifloxacin killing M. smegmatis by a pathway that does not require newly made protein.
FIG. 3.
Killing by moxifloxacin and PD161148. (A) Rate of killing. Exponentially growing cultures of wild-type M. smegmatis were incubated for the indicated times with 100-fold MIC(99) of PD161148 (open circles) or moxifloxacin (filled circles) in the absence of chloramphenicol. (B) Effect of fluoroquinolone concentration in the absence of chloramphenicol. M. smegmatis was incubated with the indicated concentrations of moxifloxacin (filled circles) or PD161148 (open circles) for 5 h. (C) Effect of fluoroquinolone concentration in the presence of chloramphenicol. M. smegmatis was treated with chloramphenicol for 1 h before the fluoroquinolones used in the experiments shown in panel B were added. Two replicate experiments gave similar results.
Bacteriostatic and bactericidal action of fluoroquinolone dimers.
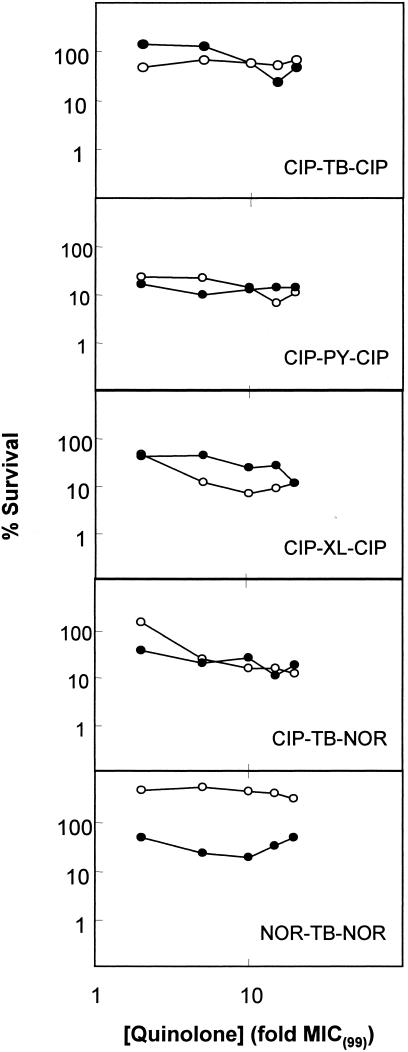
Fluoroquinolone dimers are known to block the growth of gram-positive bacteria, in some cases more effectively than monomers (8, 10, 11). Since dimers can be viewed as quinolones having large C-7 ring systems, we tested several with M. smegmatis in the presence and absence of chloramphenicol (see Fig. 4 for structures). Bacteriostatic activity [MIC(99)] varied considerably among the compounds (Table 3), with ciprofloxacin dimers exhibiting more activity than norfloxacin or gatifloxacin dimers. For this small sample of compounds, the butenyl linker appeared to confer the greatest activity. When dimer compounds were examined for lethality in the presence or absence of chloramphenicol, the four most active compounds showed only marginal killing after 18 h of exposure (Fig. 5); compounds with MIC(99) (>10 μg/ml) were not tested. The norfloxacin dimer (Fig. 5) was notable because it was unable to completely block growth in liquid medium at 20 times the concentration that inhibited growth on agar plates.
FIG. 4.
Structure of quinolone dimers.
TABLE 3.
Susceptibility of M. smegmatis to fluoroquinolone dimers
| Compounda | MIC(99) (μg/ml) |
|---|---|
| CIP (monomer) | 0.16 |
| NOR (monomer) | 0.60 |
| GAT (monomer) | 0.04 |
| CIP-TB-CIP | 0.3 |
| CIP-PY-CIP | 2.4 |
| CIP-XL-CIP | 2.8 |
| CIP-XL-NOR | 0.47 |
| NOR-TB-NOR | 2.8 |
| NOR-XL-NOR | 29 |
| GAT-PY-GAT | 15 |
| GAT-XL-GAT | 30 |
See Fig. 4 for structures. Abbreviations: CIP (ciprofloxacin), NOR (norfloxacin), GAT (gatifloxacin), TB (trans-butenyl), PY (pyridinyl), and XL (xylenyl).
FIG. 5.
Lethal activity of fluoroquinolone dimers in the presence and absence of chloramphenicol. Exponentially growing cultures of wild-type M. smegmatis were incubated with increasing concentrations of dimer fluoroquinolones. Names of compounds tested are indicated in each panel. Cells were treated for 18 h in the presence (filled circles) or absence (open circles) of chloramphenicol, which was added 1 h prior to fluoroquinolone treatment. Aliquots were then removed, diluted, and plated on drug-free 7H10 agar for determination of viable cells. A replicate experiment produced similar results.
DISCUSSION
Lethal activity is an important property of fluoroquinolones because it is expected to reduce the number of repeated treatments needed to control bacterial infections and thereby shorten treatment time. High concentrations of quinolone are required to rapidly kill M. smegmatis, in some cases 10 to 30 times the concentration needed to inhibit growth (Table 2). While these concentrations are higher than would be used clinically with pathogens, they provide a way to attribute specific features of quinolone structure to particular aspects of quinolone action. For example, C-8 methoxy and C-6 fluorine moieties enhanced killing when quinolone concentration was expressed as a multiple of MIC(99) (Fig. 1 and 2). With this normalization, lethal action is assumed to reflect chromosome fragmentation distinct from complex formation (with Escherichia coli, nalidixic acid lethality parallels chromosome fragmentation, which is seen as a loss of cell lysate viscosity in the absence of the protein denaturant sodium dodecyl sulfate; lethality does not parallel complex formation, which is seen as a loss of viscosity in the presence of sodium dodecyl sulfate [M. Malik, unpublished data]). The C-6 and C-8 substituents also enhanced lethality in the presence of chloramphenicol (Fig. 1 and 2), an agent that generally reduces rapid lethality (Fig. 1). In some cases the methoxy substituent also improved bacteriostatic activity (Table 2); in previous work, we reported that the methoxy group enhanced both bacteriostatic and bactericidal activity against gyrase resistance mutants (4, 13, 23). Thus, a C-8 methoxy group enhances many different aspects of quinolone action.
Moxifloxacin displayed two behaviors that were unusual among the compounds tested. First, its lethal action was comparable in the presence and absence of chloramphenicol (Fig. 1). Second, killing by moxifloxacin lacked a characteristic time lag (Fig. 3A). Examination of E. coli failed to show a similar behavior for moxifloxacin (Malik, unpublished). However, a lack of sensitivity to chloramphenicol was observed for nalidixic acid with an E. coli mutant expected to have an altered GyrA-GyrA dimer interface, and the mutation enhanced the ability of nalidixic acid to fragment chromosomes in the absence of protein synthesis (Malik, unpublished). Thus, it is likely that the unusual activity of moxifloxacin in mycobacteria also involves enhanced chromosome fragmentation.
Since moxifloxacin kills mycobacteria in the absence of protein synthesis, its activity should be less susceptible than that of other quinolones to interference by rifampin (12), an important component of tuberculosis therapy that indirectly inhibits protein synthesis. Experiments are now in progress to determine whether moxifloxacin behaves against M. tuberculosis as it does against M. smegmatis. If so, the compound may prove useful for exploring the effects of growth arrest generated in ways that are more likely to be relevant to tuberculosis (17, 21).
Quinolone dimers have been studied previously with Staphylococcus aureus and Streptococcus pneumoniae (8, 10, 11). With S. pneumoniae, resistance to a dimer mapped in gyrA, indicating that gyrase is an intracellular target (8). As with previous work, susceptibility of M. smegmatis to dimers depended on both the type of quinolone moiety and the type of cross-linker (Table 3). Surprisingly, even dimers that displayed bacteriostatic activity (Table 3) failed to rapidly kill M. smegmatis (Fig. 5). One possible explanation is that binding of one quinolone dimer to one GyrA subunit precludes binding of a second quinolone dimer to the other GyrA subunit, thereby allowing single-strand, rather than lethal double-strand, DNA breaks to be trapped in drug-enzyme-DNA complexes. We are now examining S. aureus to determine whether the lack of lethal activity by quinolone dimers is a general phenomenon.
Acknowledgments
We thank Marila Gennaro, Anthony Maxwell, and Richard Pine for critical comments on the manuscript.
This work was supported by NIH grant AI 35257.
REFERENCES
- 1.Alvirez-Freites, E., J. Carter, and M. Cynamon. 2002. In vitro and in vivo activities of gatifloxacin against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 46:1022-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen, C.-R., M. Malik, M. Snyder, and K. Drlica. 1996. DNA gyrase and topoisomerase IV on the bacterial chromosome: quinolone-induced DNA cleavage. J. Mol. Biol. 258:627-637. [DOI] [PubMed] [Google Scholar]
- 3.Deitz, W. H., T. M. Cook, and W. A. Goss. 1966. Mechanism of action of nalidixic acid on Escherichia coli. III. Conditions required for lethality. J. Bacteriol. 91:768-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong, Y., C. Xu, X. Zhao, J. Domagala, and K. Drlica. 1998. Fluoroquinolone action against mycobacteria: effects of C-8 substituents on bacterial growth, survival, and resistance. Antimicrob. Agents Chemother. 42:2978-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong, Y., X. Zhao, B. Kreiswirth, and K. Drlica. 2000. Mutant prevention concentration as a measure of antibiotic potency: studies with clinical isolates of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 44:2581-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gellert, M., K. Mizuuchi, M. H. O'Dea, T. Itoh, and J.-L. Tomizawa. 1977. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc. Natl. Acad. Sci. USA 74:4772-4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ginsburg, A., J. Grosset, and W. R. Bishai. 2003. Fluoroquinolones, tuberculosis, and resistance. Lancet Infect. Dis. 3:432-442. [DOI] [PubMed] [Google Scholar]
- 8.Gould, K., X. Pan, R. Kerns, and L. M. Fisher. 2004. Ciprofloxacin dimers target gyrase in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 48:2108-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs, W. R., G. V. Kalpana, J. D. Cirillo, L. Pascopella, S. B. Snapper, R. A. Udani, W. Jones, R. G. Barletta, and B. R. Bloom. 1991. Genetic systems in mycobacteria. Methods Enzymol. 204:537-555. [DOI] [PubMed] [Google Scholar]
- 10.Kerns, R., M. Rybak, G. Kaatz, F. Vaka, R. Cha, R. Grucz, and V. Diwadkar. 2003. Structural features of piperazinyl-linked ciprofloxacin dimers required for activity against drug-resistant strains of Staphylococcus aureus. Bioorg. Med. Chem. Lett. 13:2109-2112. [DOI] [PubMed] [Google Scholar]
- 11.Kerns, R. J., M. J. Rybak, G. W. Kaatz, F. Vaka, R. Cha, R. Grucz, V. Diwadkar, and T. Ward. 2003. Piperazinyl-linked fluoroquinolone dimers possessing potent antibacterial activity against drug-resistant strains of Staphylococcus aureus. Bioorg. Med. Chem. Lett. 13:1745-1749. [DOI] [PubMed] [Google Scholar]
- 12.Lu, T., and K. Drlica. 2003. In vitro activity of C-8-methoxy fluoroquinolones against mycobacteria when combined with anti-tuberculosis agents. J. Antimicrob. Chemother. 52:1025-1028. [DOI] [PubMed] [Google Scholar]
- 13.Lu, T., X. Zhao, and K. Drlica. 1999. Gatifloxacin activity against quinolone-resistant gyrase: allele-specific enhancement of bacteriostatic and bactericidal activity by the C-8-methoxy group. Antimicrob. Agents Chemother. 43:2969-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyazaki, E., M. Miyazaki, J. Chen, R. Chaisson, and W. Bishai. 1999. Moxifloxacin (Bay12-8039), a new 9-methoxyquinolone, is active in a mouse model of tuberculosis. Antimicrob. Agents Chemother. 43:85-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mogues, T., M. Goodrich, L. Ryan, R. LaCourse, and R. North. 2001. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 193:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez, H., M. Ruiz, M. Lopez, and G. Royo. 2002. In vitro activity of moxifloxacin, levofloxacin, gatifloxacin, and linezolid against Mycobacterium tuberculosis. Int. J. Antimicrob. Agents 20:464-467. [DOI] [PubMed] [Google Scholar]
- 17.Shi, L., Y. Jung, S. Tyagi, M. Gennaro, and R. North. 2003. Expression of Th1-mediated immunity in mouse lungs induces a Mycobacterium tuberculosis transcription pattern characteristic of nonreplicating persistence. Proc. Natl. Acad. Sci. USA 100:241-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snyder, M., and K. Drlica. 1979. DNA gyrase on the bacterial chromosome: DNA cleavage induced by oxolinic acid. J. Mol. Biol. 131:287-302. [DOI] [PubMed] [Google Scholar]
- 19.Sugino, A., C. Peebles, K. Kruezer, and N. Cozzarelli. 1977. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc. Natl. Acad. Sci. USA 74:4767-4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wayne, L. G. 1994. Dormancy of Mycobacterium tuberculosis and latency of disease. Eur. J. Clin. Microbiol. Infect. Dis. 13:908-914. [DOI] [PubMed] [Google Scholar]
- 21.Wayne, L. G., and L. G. Hayes. 1996. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 64:2062-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshimatsu, T., E. Nuermberger, S. Tyagi, R. Chaisson, W. Bishai, and J. Grosset. 2002. Bactericidal activity of increasing daily and weekly doses of moxifloxacin in murine tuberculosis. Antimicrob. Agents Chemother. 46:1875-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao, X., C. Xu, J. Domagala, and K. Drlica. 1997. DNA topoisomerase targets of the fluoroquinolones: a strategy for avoiding bacterial resistance. Proc. Natl. Acad. Sci. USA 94:13991-13996. [DOI] [PMC free article] [PubMed] [Google Scholar]