Abstract
Sewage sludge (biosolids) management represents a worldwide issue. Due to its valuable properties, approximately one half of the EU production is recovered in agriculture. Nevertheless, growing attention is given to potential negative effects deriving from the presence of harmful pollutants. It is recognized that a (even very detailed) chemical characterization is not able to predict ecotoxicity of a mixture. However, this can be directly measured by bioassays. Actually, the choice of the most suitable tests is still under debate. This paper presents a multilevel characterization protocol of sewage sludge and other organic residues, based on bioassays and chemical-physical-microbiological analyses. The detailed description of the experimental procedure includes all the involved steps: the criteria for selecting the organic matrices to be tested and compared; the sample pre-treatment required before the analyses execution; the chemical, physical and microbiological characterisation; the bioassays, grouped in three classes (baseline toxicity; specific mode of action; reactive mode of action); data processing. The novelty of this paper lies in the integrated use of advanced tools, and is based on three pillars:
-
•
the direct ecosafety assessment of the matrices to be reused.
-
•
the adoption of innovative bioassays and analytical procedures.
-
•
the original criteria for data normalization and processing.
Method name: Procedure for the evaluation of the effects deriving from the spreading of biogenic waste in agriculture
Keywords: Allium cepa, Ames test, Comet assay, Compost, Cucumis sativus, Endocrine disruption, Earthworms, Lepidium sativum, Manure, Zebrafish
Graphical abstract
Specifications table
| Subject area: | Environmental Science |
| More specific subject area: | The research is multidisciplinary and includes multiple areas: environmental engineering, applied ecology, human and environmental toxicology, analytical chemistry, agronomy, zoology, microbiology. Furthermore, the research has a strong applicative imprint, given that the results are directly transferable and usable by decision makers, politicians, control bodies and multi-utility managers. |
| Name of your method | Procedure for the evaluation of the effects deriving from the spreading of biogenic waste in agriculture |
| Name and reference of original method | All the references are promptly reported in the text. |
| Resource availability | All the details are specified and described in the text. |
Introduction
Sewage sludge (biosolids) is a typical by-product (waste) of our society and its recovery/disposal still represents a worldwide problem. In the EU, the application of the Urban Waste Water Treatment Directive 271/91 [1] introduced restrictive standards for effluent quality of municipal WWTPs (Waste Water Treatment Plants); as a consequence, a consistent increase of sewage sludge production has been recorded, up to the present amount of 7–8 million tonnes per year (dry solids) [2]. The upcoming new waste water directive (https://environment.ec.europa.eu/publications/proposal-revised-urban-wastewater-treatment-directive_en) will even emphasize this criticality, in that more restrictive standards for the effluents will be adopted and a greater number of plants will be involved (the 1000–2000 PE - Population Equivalent - WWTPs, which are not considered in the Directive in force, will be encompassed in the new one).
At present, due to its valuable properties (Fig. 1; for detailed calculation of mass balances, see [3]), approximately 50% of sewage sludge produced in the EU is spread on land for agricultural use, this option covering 70–80% in France and in UK [4]. This represents an optimal solution, in view of the waste management priorities and circular economy, which pose material recovery on the top of the goals to be pursued. Biosolids are indicated as improving soil properties, such as texture and water-holding capacity, because of their organic content, so favoring root growth and drought resistance. Phosphorus has raised at the attention in recent years due to the progressive depletion of mines and being included in the list of critical raw materials [5]. High-phosphorus iron ores could be depleted in 20–40 years according to Ofoegbu [6], even though more optimistic scenarios of up to almost 280 years have been also postulated [7]. The European Commission has estimated that, by recovering the sludge, about 20–30% of the need of P-fertilizers of the EU could be covered [8], thus making European farming much less dependent on imported mined and fossil raw materials, like phosphate rocks. The European Sustainable Phosphorus Platform, ESPP, has been recently issued to bring together companies and stakeholders to address the Phosphorus Challenge and its opportunities: https://phosphorusplatform.eu/. EUREAU (http://www.eureau.org/) underlines that sewage sludge is a material having great potential for phosphorus recycling.
Fig. 1.
Nutrients and pollutants in sewage sludge.
Nevertheless, great attention must be paid on potential negative effects on ecosystems, crops, livestock and human health (also along the food chain, including water), arising from the presence of (also unknown) harmful organic pollutants, metals and microbiological contaminants (Fig. 1). USEPA evidences that 352 pollutants and contaminants can be present according to literature (including pharmaceuticals, steroids and hormones, flame retardants, per- and poly-fluoroalkyl substances (PFASs)), of which 61 are designated as hazardous or as priority pollutants in other regulatory programmes (www.epa.gov/sites/production/files/2018–11/documents/_epaoig_20,181,115–19-p-0002.pdf). Moreover, it is recognized that the chemical characterization alone cannot take into account possible antagonistic, subtractive, additive or synergistic effects of a mixture of different substances [9].
Present regulations on the recovery of sludge in agriculture, worldwide, focus on a few chemical and microbiological indicators on sludge and soil, with some use limitations. An updated review on sewage sludge characteristics in Italy is reported in Domini et al. [10]. The debate on the suitability of these regulations is still open and controversial. This is clearly demonstrated by: (1) the lack of an updated directive at the European level. The one still in force was issued in 1986: 86/278/EEC [11]; in the 3rd Draft of Working document on sludge, more intensive pre-treatments finalized to pathogens reduction are required and new (organic pollutants) or more stringent (heavy metals) limits are proposed. (2) A completely different approach adopted in Europe (mainly focused on chemical pollution) and US (mainly focused on microbiological contamination). (3) Heterogeneity of national regulations.
The European Commission has recently revised the EU legislation on fertilizers, expanding its scope to secondary-raw-material-based fertilizing products, and resulting in the publication of the new EU Fertilizing Products Regulation ([12]/1009). The EC Joint Research Centre (JRC) has just published a report about the biogenic wastes and other secondary raw materials, aimed at defining their safe and effective use, as well as a possible legal framework [13]. Interestingly, there is a proposal of labelling for the EU fertilizing products, which also include wastewaters and sewage sludge from municipal wastewater treatment plants. The report underlines the possible occurrence of emerging trace pollutants and microplastics, for which risk assessment is often still lacking, and consequently, limit values have not been fixed yet.
Definitely, the reuse in agriculture of sludge and other organic residues (manure, digestate/compost, biochar etc.) is potentially a virtuous solution in the context of a circular economy. Nevertheless, there is still a lack of knowledge and experience to assess the real risk for ecosystems and humans: published works are based on theoretical models and assumptions for missing quality data (e.g. [14]). Moreover, the regulations to be applied (if any) for the characterization of different substrates, may not be coherent one each other.
On the other hand, though the attention of scientists, practitioners and decision makers is still mainly focused on chemical characterization rather than on bioassays [15], powerful tools for assessing toxicity of organic matrices have been developed in the last decades. Indeed, several studies suggest that the limitations of the chemical “single substances” approach can be overcome by carrying out multi-tiered biological assays, which can directly and more accurately measure the impact of a stream [[16], [17], [18], [19], [20], [21], [22], [23], [24]]. More recently, the adoption of bioassays within the protocol for the evaluation of the environmental footprint of wastewater treatment plants was proposed [[24], [25], [26], [27]]. Only bioassays, in fact, may be able to measure the actual effect of a matrix on the different components of the ecosystem, thus giving a more realistic answer to the question: is sludge (or another residue) reuse safe? In summary, a greater awareness of the real effect of the recovered materials on the surrounding environment may empower their reuse and circular economy with safety. Deeper substrate characterization and knowledge of its toxicological properties may also help people to understand real risks and, finally, to increase public acceptance, which is currently low due to people's concerns about odour emission and safety issues; it represents one of the major barrier to the use in agriculture of organic residues ([[28], [29], [30]]; Carraturo et al., 24).
This research was intended to develop a multilevel characterization protocol of sewage sludge and other organic residues, based on bioassays and chemical-physical-microbiological analyses. Since there are no threshold values to refer to, the principle of comparing the results with those obtained on reference substrates, such as manure and compost, has been adopted. An attempt has been made to cover the gap of knowledge caused by the missed application of (existing or to be adjusted) bioassays which showed to be a powerful tool for understanding the real interactions of matrices discharged into the environment. In effect, notwithstanding the general scientific consensus about the invaluable role of bioassays in the assessment of environmental toxicity, there is still an open debate about the choice of the most suitable tests to be performed [9]. The research proposes a paradigm shift in that a substrate suitability for agriculture use is evaluated through the direct measurement of possible toxic effects exerted on human health and ecosystems instead of being merely measured through the quantification of pollutants with a known toxicological profile. The main novelty, then, lies in the introduction of a concept - currently almost neglected - in the context of sludge and other residues recycling and re-use, which is the ecosafety assessment of the matrices. Moreover, innovative bioassays have been proposed as well as innovative analytical procedures for the determination of pollutants of recent concern (e.g. trace pollutants and microplastics). Particular attention was also given to the criteria for data processing, by proposing a normalization approach for homogenizing the numerical results describing each biological phenomenon.
A massive methodological frame was developed during this research. This is the reason why the authors decided to collect and describe the methods in the present paper. It is the ambition of the researchers that the outcomes of this work could represent a benchmark for the development of protocols and guidelines, which might be implemented in future national and international legislations and/or adopted for labelling and certification on a voluntary basis.
Samples that were processed following the proposed protocol
A total of nine samples of substrates were processed during this work. According to Directive 91/676/EEC [31] and Regulation [12]/1009 ([12]/1009), they belong to four different categories of products that can be spread on agricultural land: sewage sludge, liming materials, livestock manure, and soil improver. As described in the introduction, the main focus of the research was on sewage sludge. Thus, sewage sludge from different plants was collected, as well as liming material derived from sewage sludge. The other substrates (manure, digestate, and compost) were chosen as reference materials because they were intrinsically considered suitable for agricultural recovery. All the substrates were collected in facilities located in northern Italy.
Sewage sludge
Four kinds of sewage sludge were selected, in order to represent different situations:
-
•
Sewage sludge (S1) sample was collected at a WWTP with a pre-denitrification scheme and a capacity of 50,000 PE. The influent wastewater is mostly domestic, (industrial percentage < 5% in volume). The WWTP is equipped with a pre-treatment section for aqueous wastes. The dewatered sludge is sent to either composting or disposal.
-
•
Sewage sludge (S2) sample was collected at a WWTP with a post-denitrification scheme and a capacity of 100,000 PE. Leachate from a waste treatment facility (discharged into the sewer), aqueous waste, and sludge are also treated at the WWTP (transported by trucks).
-
•
A sample of sewage sludge (S3) was collected at a facility that composts treated sludge by mixing it with biodegradable wastes (European Waste Code – EWC 20.02.01).
-
•
Sewage sludge (S4) sample was collected at a sludge treatment platform with a capacity of 125,000 t/y. Mostly originating from urban WWTPs, sewage sludge is chemically stabilized by mixing it with lime to elevate its pH to greater than 12. High pH and rising temperature are intended to have a sanitizing effect and slow fermentation (thus preventing odour emissions).
Liming material
A sample of liming material obtained from sewage sludge (L) was collected in a sludge treatment platform with a capacity of 125,000 t/y. The sludge obtained from WWTPs is submitted to hydrolysis using lime and sulfuric acid, and subsequent precipitation of the calcium sulphate. According to the Italian Legislative Decree 75/2010 [32] and its changes, the final product is defined as "corrective."
Livestock manure
Two different kinds of livestock manure were selected:
-
•
A sample of manure (M1) was collected from a pig farm that specialized in the weaning and breeding of sows and pigs (750 heads). For long-term storage, the manure slurry is moved daily from the barn to a pond. An M1 sample was obtained from the pond used for stabilization.
-
•
A specimen of manure (M2) was procured from a dairy farm dealing exclusively in the breeding of dairy cows (290 heads). The slurry manure is temporarily kept at the end of the barn in a pit. The slurry is then transported daily to an anaerobic digestion (AD) facility. A sample of M2 was taken from the pit.
Moreover, a digestate sample (D) was taken from a pond receiving the output of the AD described above. This plant treats the cow manure M2 mixed with agricultural wastes (e.g. corn silage) and food residues.
Soil improver
Compost (C) sample was collected at a composting facility treating the organic fraction of the municipal solid wastes (50,000 t/y). Most of the treated wastes consist of biodegradable kitchen and canteen garbage (EWC 20.01.08) gathered mainly via door-to-door waste collection. The residual fraction consists of biodegradable waste (EWC 20.02.01) and wood free of hazardous substances (EWC 19.12.07).
All samples were taken in accordance with the UNI EN 10,802 [33] protocol. They were kept in glass mason jars with vacuum-sealing lids.
Samples pre-treatment
Due to their complexity, the chemical and biological characterization of the environmental matrices often requires a preliminary treatment phase, aimed at extraction/purification/concentration. Each analytical step inevitably involves an alteration of the sample (for example, extraction using solvents means that the final extract that will be tested in toxicological assays will not contain metals). Consequently, in the composition of the battery of tests, account was also taken of the need to have both the sample as they are, both aqueous extracts and organic extracts. Likewise, from time to time, account was taken of any available standards, as well as the state of the art of scientific literature and the previous experiences of research groups, which have been engaged for years in the monitoring of trace pollutants and related research (see, inter alia, these works within the sewage epidemiology, started by the Mario Negri Institute of Pharmacological Research: Zuccato et al. [34]; Riva et al. [35]; Riva et al. [36]; Kasprzyk-Hordern et al. [37].
Samples preparation procedure for trace pollutants quantification
Samples were extracted by accelerated solvent extraction (ASE) using an ASE 300 extractor (Dionex, USA) and cleaned up on different solid-phase extraction (SPE) cartridges according to the different substances investigated. In detail, a 0.5-g aliquot of each sample was mixed with 0.25 g of diatomaceous earth and poured into 34-mL stainless steel extraction cells that were previously filled with a GF/A filter (Whatman, Kent, UK) at the bottom. Samples were spiked with a mixture of deuterated labelled compounds (concentrations ranged from 20 to 200 ng/0.5 g) used as internal standards for quantification. The ASE extraction was performed with a solution of MilliQ water and methanol 1:1 v/v at 100 °C and a pressure of 100 bar for three subsequent cycles. Solvents were evaporated by rotavapor to reach a volume of about 45 mL. After centrifugation, the extracts were diluted to 200 mL with MilliQ water for the subsequent SPE extraction, which was performed after sample acidification (pH of 2.5–3.0) using two different cartridges (150 mg OASIS MCX and 60 mg OASIS HLB). Samples were passed through the cartridges according to specific protocols [36], and eluates were reconstituted for analysis in 500 µL of methanol/MilliQ water (10:90%) for MCX and in 500 µL of MilliQ water with EDTA for HLB.
Samples preparation procedure for plastic quantification
Due to the organic and inorganic particulate nature of the matrices, their extremely heterogeneous composition and morphology, and, most importantly, their much larger dimensions than those of the microplastics we wished to quantify, preliminary operations of separation and digestion of the undesired material were required.
Plastics were isolated from the collected matrices using a sodium chloride (NaCl) hypersaline solution, which allows the separation of plastics from the particulate matter by exploiting the generated density gradient. The hypersaline solution was prepared by adding NaCl to distilled water under magnetic stirring. Once the salt was dissolved, 1 mL of the solution was weighed until reaching a density of 1.2 g/cm3; if the density was not achieved, additional NaCl was added to the solution until the desired density was obtained. The solution was then filtered using glass fibre filters (Whatman GF/C 47 mm) with a mesh of 1.2 µm to eliminate any impurities and then stored in a glass beaker, covered with an aluminium foil, at 4 °C.
In case of sludge, manure and digestate, three samples of 10 mL were collected and weighted using a falcon tube of 15 mL. Samples were then stored into glass bottles. Subsequently, 10 mL of 30% v/v hydrogen peroxide were added in all the glass bottles, reaching the H2O2 concentration of 15% v/v, to allow initial digestion of the sample, and left overnight at 4 °C. The digested samples were transferred to graduated glass cylinders with a capacity of 250 mL and then 230 mL of NaCl hypersaline solution were added to separate plastics from the particulate matter. The glass cylinders were left for 3–4 days at 4 °C to guarantee an optimal separation of plastics from particulates. The supernatants were filtered using a membrane vacuum pump on 8 µm cellulose nitrate membrane filters (Sartorius™ 50 mm). The filters obtained from this procedure were then further treated with 15% v/v H2O2 to complete the digestion of the organic residues on the filter surface, keeping the samples under a laminar flow hood to avoid their potential contamination by microfibers.
In the case of compost, three samples (approximately 0.14 g each) were collected. Due to the presence of water-swelling materials in this matrix, the samples were neither treated with NaCl hypersaline solution, nor digested. Therefore, compost samples were directly analysed through a stereomicroscope to select the potential plastics (visual sorting). Collected particles were then placed on clean filters to perform the chemical characterization.
Samples preparation procedure for bioassays
Organic solvent extraction
The extracts to be submitted to the toxicological tests were prepared from all the fresh samples collected as described above and stored at 4 °C from sampling until processing (approximately two months). Sample S2 developed mold rapidly and was therefore stored frozen at −20 °C before preparation.
A 5 g aliquot of each sample was mixed with different amounts of diatomaceous earth (from 0 to 3 g) depending on the water content and poured into 34 mL stainless steel extraction cells that were previously filled with a GF/A filter (Whatman, Kent, UK) at the bottom. The extraction (using accelerated solvent extraction (ASE) with an ASE 300 extractor (Dionex, USA) was done using a mixture of hexane and acetone 1:1 v/v and performing three successive extraction cycles. The extraction conditions were temperature, 100 °C; pressure, 100 bar; static time, 5 min; flush volume, 60%; purge time, 90 s. The extracted volume (about 60–80 mL) was concentrated using a rotavapor (Büchi, Switzerland) to a volume of about 10 mL, and then the resulting extracts were evaporated to dryness under a gentle stream of nitrogen. Four 5 gs extracts of each sample were prepared for toxicological analysis. Dried extracts were finally stored at −20 °C until analysis.
Water extraction
Two different procedures of water extraction were followed in order to comply with the relative analytical protocols, one for the zebrafish test and the other for the toxicity and the genotoxicity tests.
The eluates tested on zebrafish embryos were prepared using the 1:4 (v/v ratio [38]. The matrices were weighted and the zebrafish artificial water (Instant Ocean® 0.1 g L−1, NaHCO3 0.1 g L−1, CaSO4 0.2 g L−1 and 0.1% methylene blue) added. Then, samples were mechanically shaken for 12 h at room temperature. The overlying water (elutriate) and settled material were separated by decanting. Elutriates were then passed through filter paper and filtered through a syringe filter (0.45 µm porosity) in order to remove suspended matter. Elutriates were immediately used for ecotoxicity tests.
The water extraction preliminary to the bioassays on bacteria, plants, and human cells (HepG2) was carried out depending on the initial water amount of the samples. The solid and semi-solid samples (C, S1, S2, S3, S4, L) were weighed in four aliquots of 100 g each; the liquid samples (M1, M2, D) were weighed in four aliquots of 100 mL (corresponding to 100 g). To proceed with the aqueous extraction (100 g/L and 100 mL/L, respectively), samples were mixed with distilled water and submitted to two cycles of sonication in an ultrasonic bath. Each 30-minute extraction cycle was conducted at room temperature (23–25 °C) [39]. At the end, the aqueous extracts were filtered on filter paper. The samples were kept in PE containers at −20 °C until they were used for the toxicity test in plants and the genotoxicity test in bacteria, plants, and human cells (HepG2).
Chemical, physical and microbiological characterisation
This work represents a proposal for an operational protocol for the characterization and quality assessment of biogenic wastes in relation to its spreading on the fields, in the context of the circular economy for agricultural practices. The disposal of the aforementioned wastes must necessarily have, as its founding element, the legal requirements, which must naturally consider the local regulations in force from time to time.
One aspect may be particularly critical (as happens in the European context, for example), given the different origins of the waste suitable for spreading in agriculture. In fact, the reuse of biogenic waste is far from being contemplated in an organic corpus legis; rather, it is regulated by numerous rules, which have stratified over time. In fact, they refer to completely different matrices as a sector of origin, such as sludge and liming materials (from wastewater treatment), manure (from the livestock sector), and compost (from the collection of the organic fraction of solid urban waste).
The regulations contemplated therein provide for the execution of (a) chemical and physico-chemical analyses, (b) microbiological analyses, and (c) phytotoxicity assays.
As regards the chemical parameters, they, although constantly updated and integrated, do not reflect the plethora of emerging pollutants that the academy has been dealing with for three decades now. For this reason, it was decided to also monitor trace contaminants, selected based on the frequency of detection and their intrinsic toxicity.
Finally, the analysis of the soil microbiome was also included in the integrated protocol, because it plays a crucial role in the provision of ecosystem services and could be modified following the application of matrices which, despite being microbiologically stabilized, could constitute vectors for the transfer of genes for resistance to antibiotic active principles.
Characterisation according to law requirements
The substrate was initially characterized in accordance with the current regulations. Emphasis was given on the reliability of the limits established in the regulations in relation to the substrates' actual toxicity. Sewage sludge and the other organic residues were first analyzed, as required by the following regulations: Legislative Decree 99/92 (Annex I B) [40] and seq. (enforcing the European Directive 86/278/EEC: [11]); Decree of the regional government of Lombardy 6665/2019 (table A) [41]; Decree of the regional government of Veneto 235/2009 (Annex B, table B1/1, Annex C table A) [42]; Legislative Decree 75/2010 et seq. (Annex 2) [32]; Nitrates Directive (91/676/EEC) [31].
The abovementioned regulations specify the methods to be adopted as well as the limits to be met.
Additional chemical analyses
Nitrogen and carbon agronomic characterization
Principle of the analysis. In addition to its immense relevance as a source of nutrients for the plant system, organic matter also plays an essential function as a carbon store. Consequently, it is necessary to have a comprehensive understanding of what organic stuff is, how it enters the soil and how it is changed, what variables impact mineralization and accumulation, and what its roles are in the agricultural soil system. Labile organic material consists of free-living organic components such as sugars, peptides, enzymatic proteins, and nucleic acids that may be found in soil. Humus is the stable organic material and can enhance the physical and structural, chemical and biological properties of soil, has nutritional effects, and stimulates certain metabolic, microbial, and other processes. The carbon-nitrogen ratio (C:N ratio) is crucial because it influences the breakdown of organic matter and the nitrogen cycle in soil. The higher the ratio, the longer it will take for the degradation. The ratio is also directly proportional to the amount of accessible nitrogen in the soil for plant development. 24:1 is the carbon-nitrogen ratio that decomposer microorganisms require to maintain their metabolic processes and carry out efficient breakdown of the organic material in the soil. Through the measurement and monitoring of the carbon-nitrogen ratio, it is possible to evaluate the quality of the soil and the production indicators, allowing for the selection of sustainable crops, crop rotation, and nitrogen fertilizer.
Execution. Fresh samples (n = 3, per organic amendment) were suspended with a 0.01 M CaCl2 solution in a 1:4 ratio (w/v), and gently agitated for 45 min. After passing through a Millex-HV 0.45-µm filter (Merck Millipore, USA), extracts were stored at −20 °C for later measurements. Total dissolved organic carbon (DOC), total dissolved nitrogen (DON), microbial biomass carbon (Cmic) and nitrogen (Nmic) were measured using a DIMA-TOC 100 Analyzer (DIMATEC Analysentechnik GmbH, Essen, Germany). Cmic and Nmic were quantified as the difference between the organic C or N extracted from chloroform-fumigated and non–fumigated samples, prior to the CaCl2 extraction step (DIN ISO 14,240–2:2011–09: [43]). These values were then normalised by efficiency coefficients KE (KEC = 0.45 and KEN = 0.54) ([44,45], respectively). Inorganic nitrogen compounds (ammonia, nitrites and nitrates) were quantified through continuous flow analysis using a photometric autoanalyzer (Skalar Continuous Flow Analyzer SA5100; Skalar Analytical B.V., NL).
Dilutions. The weight:volume ratio of 4:1 was considered.
Statistics. All data was expressed in mg/g dry weight (DW), and statistical analyses were conducted using one-way ANOVA, followed by the Holm-Sidak's multiple comparison test. All statistical analyses were performed using GraphPad Prism v8.
Trace organic pollutants
Principle of the analysis. Additional chemical analyses were then introduced in the protocol, to investigate the presence of organic pollutants that may induce known or suspected undesirable effects on humans and the ecosystems. Urban wastewater contains a huge number of chemical residues coming from people everyday life, agricultural and industrial activities. The presence of organic pollutants identified as emerging contaminants (ECs) was investigated in the different samples by developing specific analytical methods based on high-performance liquid chromatography coupled to mass spectrometry (HPLC-MS/MS).
The selection of a list of ‘priority’ ECs to be investigated was done among pharmaceuticals and hormones, personal care products, disinfectants, perfluoroalkyl compounds, alkylphenols, plasticizers (BPA). The following criteria were used for selection: i) presence in wastewater and sludge from previous investigations; ii) levels and frequency of detection; iii) potential to be absorbed into sludge according to specific properties such as the solid- liquid partition coefficient (Kd) and the octanol-water partition coefficient (Kow); iv) potential of persistence in the environment and sludge; v) potential of developing toxicological effects for humans and the ecosystem (i.e. antibiotics and resistance).
The list of the analytes (Table 1) included antibiotics belonging to different therapeutic categories and both for human and veterinary use, and other classes of pharmaceuticals mainly for human use (antihypertensive, anti-inflammatory, antidepressants, diuretics, estrogens, and anti-asthmatics). Among pharmaceuticals, some main antimycotics were also considered. The other ECs selected included disinfectants, plasticizers and perfluoroalkyl phenols.
Table 1.
List of the measured emerging pollutants.
| ANTIBIOTICS | OTHER PHARMACEUTICALS | OTHER EMERGING POLLUTANTS |
|---|---|---|
| Fluoroquinolones | Antihypertensives | Disinfectants |
| Ciprofloxacin | Irbesartan | Triclosan |
| Flumequine | Valsartan | Triclocarban |
| Ofloxacin | Analgesics/Anti-inflammatories | Surfactants - Plasticizers |
| Sulphonamides | Diclofenac | Bisphenol A |
| Sulfamethoxazole | Ibuprofen | Nonylphenol |
| Sulfadiazine | Antiepileptics/Antidepressants | |
| Sulfadimethoxine | Carbamazepine | |
| Macrolides | Desmethyldiazepam | |
| Clarithromycin | Diazepam | |
| Dehydro-erythromycin (Erythromycin) | Anti-asthmatics | |
| Oleandomycin | Salbutamol | |
| Spiramycin | Diuretics | |
| Tylosin | Hydrochlorothiazide | |
| Tilmicosin | Furosemide | |
| Vancomycin | Estrogens | |
| Lincosamides | Estrone | |
| Lincomycin | Progesterone | |
| Amphenicols | Antimycotics | |
| Florfenicol | Ketoconazole | |
| Thiamphenicol | Clotrimazole | |
| Diaminopyridines | ||
| Trimetoprim | ||
| Tetracyclines | ||
| Doxycycline | ||
| Chlortetracycline | ||
| Oxytetracycline |
Execution. A method previously developed in our laboratory to extract pharmaceuticals from sludge [36] was modified to include the new substances and the additional matrices investigated. Mass spectrometric analyses were done in both positive and negative ionization mode using the Selected Reaction Monitoring (SRM) mode. Analyses were performed choosing the two most abundant fragmentation products of the protonated pseudo-molecular ions for each analyte and one product for each deuterated/14C analog used as internal standard (IS): Carbamazepine-d10, Ciprofloxacin-d8, Salbutamol-d3, Ofloxacin-d3, Sulphamethoxazole-d4, Ramipril-d5, Valsartan-d3, Ramipril-d5, Valsartan-d3, Ketoprofen-d3, Ibuprofen-d3, Salbutamol-d3, Ibuprofen-d3, 17-β-Estradiol-d3, PFOA-14C, PFOS-14C. The quantification was done using the isotope dilution method. The analytical method was validated in the different matrices investigated and a detailed description with results will be reported in a separated publication (in preparation).
Plastics quantification and characterization
Principle of the analysis. WWTPs represent a major source of microplastic (MP) discharge in the environment, as they are not designed to remove this kind of emerging contaminants. A high percentage of MPs (up to 90%) settle with the sludge, so that it exits the plant with the surplus sludge [46]. Therefore, approximately 125–850 tons of MPs per million inhabitants are introduced every year in European soils [47], correlated with the possible re-use of WWTP sludge in agriculture. A recent study performed by the research group of UNIMI showed that 3400,000,000 MPs/day are transferred to the sludge phase at one of the biggest WWTPs in Northern Italy [48]. This aspect poses a potential threat not only for terrestrial ecosystems but also for water environments due to MPs leaching from soils to surface waters and groundwater [49]. Adverse effects on animals have been reported in the presence of MPs (e.g., [50,51] and references therein). Beside their toxicity per se, MPs could also interact with hydrophobic pollutants, generating further synergistic effects [52], and could also be vectors that favour the spread of pathogens [53]. Therefore, from a risk assessment perspective, the characterization of MPs should, of course, be included in a protocol for sludge ecotoxicological evaluation.
Execution. After the isolation step, described in previous paragraph, plastics were quantified and characterized using the Fourier Transform Microscope System (μFTIR; Spotlight 200i equipped with Spectrum Two).
The digested filters were visually analysed using a stereomicroscope (visual sorting) to select the suspected plastic particles from the remaining mineral and natural particles still present on the filters after the digestion process. These particles were transferred onto clean filters in order to carry out the chemical characterization to confirm their plastic nature. The FTIR spectrum of each particle was acquired in attenuated total reflectance (ATR) with 32 scans at wavelengths between 600 and 4000 cm−1 and analysed using the Spectrum 10 software with a comparison between standard spectra libraries. The similarity between the spectra of the samples and the standard ones was accepted only with a matching score of 0.70. Each plastic particle was also classified according to its shape (fragments, fibres, or pellets), size, and color. The size of the plastics was determined using the ImageJ software following the classification proposed by Hartmann et al. [54]: microplastics (1 –1000 µm), mesoplastics (1 mm–10 mm), and macroplastics (1 cm). It is worth noting that, to prevent any overestimation due to environmental contamination by atmospheric particles, especially microfibers, all analyses were carried out under a laminar flow hood, and, in addition, the operators always wore gloves and a cotton lab coat during the processing. Several filters were also processed as blanks in parallel to the samples (n = 17). The debris detected in these filters, which presented the same chemical composition, shape, and colour of plastics observed in the samples, was subtracted from the final count.
Biogas production potential
Principle of the analysis. The biogas production potential (measured by means of standardized methodologies) was introduced in the protocol to investigate the tendency of the substrates to undergo anaerobic fermentation, thus being responsible for odour emission. This aspect is not directly related to the ecotoxicity of the substrates, but it is quite relevant in practice for the social acceptance of land spreading. The Biochemical Methane Potential, which is also described as the maximum volume of methane produced per gram of VS (volatile solid) in the substrate, indicates the biodegradability of a substrate and its ability to produce methane via anaerobic digestion. Before analysing the substrate, its dry matter (total solids) and organic content were examined (volatile solids). The approach entails adding a pre-incubated standard inoculum to the test reactor. This examination was conducted in accordance with UNI EN ISO 17,734:2004 [55] and UNI/TS 11,703:2018 [56].
The Residual Biogas Potential test can determine the residual methane potential (RBP), which is the amount of biogas that could still be produced following anaerobic digestion. Before analysing the substrate, its dry matter (total solids) and organic content were assessed (volatile solids). This examination was conducted in accordance with UNI EN ISO 17,734:2004. The Residual Biogas Potential (RBP) test was used to evaluate sample D, which was the effluent of an anaerobic digestion process. Instead, the Biochemical Methane Potential (BMP) test was conducted on the remaining substrates (S1, S2, S3, M1, M2, and C).
Microbiome analysis
Principle of the analysis. Soil and plant microbiomes play an important role in plant growth and development and soil health; they provide the plant with a secondary genome that provides key ecological functions and benefits the host; they are able to influence plant health and productivity, improving stress tolerance and therefore yielding an adaptive advantage; they mediate several functional traits of plants; they influence the phenotypic plasticity of plants; and they are essential to ensuring the quality and safety of primary plant production, including fruits and related processed foods. The contribution of biological matrices, although stabilized, can potentially modify the soil microbiome: for this reason, the present research also included a characterization of the genetic material.
Execution. Prior to the phenol-chloroform DNA extraction protocol [57], the samples underwent a dual cleaning process (2x) involving NaCl (0.85%, 1:1). In detail, 0.5 g of the samples were subjected to DNA extraction within Lysing Matrix E tubes (MP Biomedicals, France). The extraction buffer consisted of 0.5 mL of hexadecyltrimethylammonium bromide coupled with 0.5 mL of phenol:chloroform:isoamyl alcohol (25:24:1) solution (pH 8). The samples were then lysed for 30 s employing a homogenizer (FastPrep-24 Classic, MP, California, USA) at a rate of 5500 strokes per minute. After the aqueous upper layer was separated, it was mixed with an equal volume of chloroform and isoamyl alcohol (24:1). After centrifugation (5 min at 4 °C), an equivalent volume of a precipitation solution (10% polyethylene glycol 6000 and 1.2 M NaCl) was combined with the aqueous upper phase. This mixture was left to incubate on ice for 2 h, allowing nucleic acid precipitation, followed by a 10-minute centrifugation at 4 °C. The nucleic acid pellet resulting from this process was subjected to a wash in ice-cold 70% ethanol, followed by another 10-minute centrifugation at 4 °C. Post-centrifugation, the pellet was air dried and then re-suspended in 20 L of TE buffer. To assess DNA extract quality and quantity, a Nanodrop 1000 Spectrophotometer (peqlab Biotechnologie GmbH, Germany) was utilized alongside the Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen, Life Technologies, USA). DNA sample concentrations were normalized to 10 ng/L before the initial PCR for 16S and ITS amplicon analyses.
Dilutions. For the target gene quantification (qPCR), serial DNA dilutions (1:4, 1:8, 1:16, 1:32, 1:64, and 1:128) were initially tested. The PCR reaction mixture (25 L) contained SYBR Green PCR Master Mix (12.5 L) (Thermo Fisher Scientific, Darmstadt, Germany), DEPC water (9 L), BSA 3% (0.5 L), each primer (0.5 L of each), and DNA template (2 L). The amplification was performed for 40 cycles. Results were then expressed as gene copy numbers per gram of dry weight mass using standard 8-point curves created with 10-fold dilutions with known copy numbers. The data was compared after log10 transformation and normalized by the 16S gene results.
Statistics. Statistical differences were assessed using one-way ANOVA, followed by the Dunn's Multiple Comparison Test (balanced data) or the Unequal N HSD (unbalanced data) (Statistica 7.0).
Bioassays
The bioassays were chosen based on their standardization, high reproducibility, automated protocol, sensitivity, adequacy, statistical robustness, biological representativeness, possibility of extending in vitro findings to possible risks in vivo, and possibility of cross-species extrapolation.
Then, by choosing endpoints that may be linked to short- and long-term impacts, acute and chronic toxicity were taken into consideration (up to transgenerational events). Once more, different biological complexity levels and levels of biological organization (organisms, tissues, and cells) were targeted (prokaryotes, eukaryotes, animals, plants, unicellular, and pluricellular). Finally, in case it is conceivable to link important events with biological outcomes, the bioassays should be able to identify both the so-called baseline toxicity (i.e., just a form of unspecific and generic toxicity) and specific modes of action (MoA).
Specifically, the experimental protocol proposed introduces the following novel elements:
-
-
new insights on the toxicological properties of well-known and new pollutants through multi-tiered biological assays that integrate the possible antagonistic, subtractive, additive, or synergistic effects of a mixture of different pollutants, overcoming the limitations of the chemical "single substances" approach;
-
-
the use of a battery of several biological models, representative of all environmental compartments, which could be affected by the toxic effects of sludge, including humans.
The selected assays are able to detect baseline toxicity (plant/earthworm/fish embryo), genetic toxicity (gene mutation in bacteria, structural and numerical chromosomal alterations in plant cells, and primary DNA damage on earthworm cells), endocrine disruption (mammary cells and yeasts), basal cytotoxicity (mammary, hepatic epithelial cells, plant cells), adaptive stress response (hepatic epithelial cells), inflammatory reaction, and neurotoxicity, oxidative stress and modulation of energetic metabolism (fish embryo). All of the proposed experimental techniques and tests are “alternative approaches” that do not include the use of animals (methods alternative to those based on the use of living animals). Therefore, this approach is consistent with the application of the 3R's principle of Russell & Burch (Reduce, Refine, Replace), which became the guiding principles for a more ethical use of animals in testing [58] and whose significance is well expressed by the European REACH Regulation on Registration, Evaluation, Authorization, and Restriction of Chemicals (EC 1907/2006). The toxicological tests were carried out according to two guiding criteria: (1) considering the matrices as complex mixtures of substances and working with possible progressive dilutions; and (2) considering the direct application in the field and therefore the agronomic regulatory constraints for the calculation of the quantities to actually spread in the field.
Non-specific mode of action (baseline toxicity)
Baseline or non-specific toxicity (also reverberated in apical effects) was evaluated on plant and animal organisms with increasing evolutionary complexity and with different trophic roles. In particular, the following biological models were used: Allium cepa bulbs (roots elongation), Lepidium sativum (seeds germination), Cucumis sativus (seeds germination), Allium cepa (seeds germination), Lactuca sativa (seeds germination, biomass growth), Lumbricus terrestris (acute and chronic reproductive toxicity), Danio rerio (acute embryo toxicity), lines of human cells (uptake of neutral red dye, gap junction intercellular communication -GJIC-, quasi in vivo (body-like) assay).
Roots elongation test in Allium cepa bulbs on aqueous extracts
Principle of the analysis. The test consists of dipping onion bulbs in the samples to be evaluated. By taking advantage of the high mitotic rate that characterizes the root meristem, potential impacts of mixtures on the cells of the developing tissue are observed.
Execution. To study the toxicity of aqueous extracts, 12 small onion bulbs (2.5–3.0 cm in diameter) were germinated in the samples at room temperature (21–25 °C) in the dark. After 24, 48 and 72 h, the length of the roots from each Allium cepa bulb was measured by a ruler [59], to calculate the average length of each bulb (cm, mean ± DS), the inhibition/increase index (%), and the ECx values. Other signs of toxicity such as changes in root consistency and color, and the presence of tumors, hooks, twisted roots, and flat/broken apices were considered (macroscopic parameters). Freshly prepared solutions were changed daily. Distilled water was used as a negative control.
Dilutions. The following solutions were tested: undiluted, 1:2, 1:10, 1:20, 1:100, 1:200, 1:1000.
Statistics. The statistical analysis of results was performed using the software STATA16, using Dunnett's and χ2 test, for root length and macroscopic parameters, respectively (at a significance level of 0.05).
Germination test in Lepidium sativum, Cucumis sativus and Allium cepa seeds on aqueous extracts
Principle of the analysis. Seedling growth tests involved exposing plant seeds to aqueous samples and measuring various growth parameters, including germination rate and root length [60,61].
Execution. Untreated Lepidium sativum, Cucumis sativus and Allium cepa seeds were subjected to a preliminary viability test in distilled water in the dark at 25 ± 1 °C (germination rates > 90%). Extracts solutions were tested at different dilutions and distilled water was used as a negative control. Three replicates per treatment were made by soaking a Whatman filter paper no. 1 with 2 mL of each solution. Ten seeds per replicate were spread on the filter. The three dishes of each replicate were placed in a tightly sealed plastic bag and incubated at 25 ± 1 °C in the dark for 72 h. At the end of the incubation period, complete shoots (≥1 mm) and root lengths were assessed. The results were expressed as percentage germination index (GI%). If IG% sample 〈 IG% control means that there is an inhibition of root growth; if IG% sample 〉 IG% control means that there is a biostimulation. A similar trend to negative control is considered when IG% values between 80 and 120 are obtained. When IG% 〈 80 means there is a germination inhibition effect while if IG% 〉 120 there is a biostimulation effect.
Dilutions. The following solutions have been considered: undiluted, 1:2, 1:10, 1:20, 1:100, 1:200, 1:1000.
Statistics. The statistical analysis of results was performed using the software STATA16, using Dunnett's test.
Lactuca sativa test
Principle of the analysis. Lettuce (Lactuca sativa) is chosen as the test organism due to its sensitivity and ease of cultivation. Lettuce is commonly used in bioassays because it has a relatively short life cycle, is readily available, and responds well to various environmental conditions and treatments. The bioassay is designed to expose lettuce seeds or seedlings to the substance/mixture of interest under controlled conditions. The response of lettuce plants is carefully monitored and measured as a quantitative or qualitative endpoint. Common endpoints in lettuce bioassays include germination rate, root and shoot length, leaf development, chlorophyll content, biomass accumulation, or other growth-related parameters.
Execution. Lettuce (Lactuca sativa L. var. capitata cv. ‘Tizian’, Syngenta, Bad Salzuflen, Germany) was grown for 21 days after germination in hydroponic systems in a phytochamber with 16/8 h light/dark cycle at 20/15 °C, and an average humidity of 50%.
The control group received only a commercial synthetic fertilizer (NPK 13:10:20); a total of 8 pots (1.2 L) were used per type of amendment. After 5 weeks, plantlets were gently washed, to remove the excess of soil, and then transplanted to 1.2 L pots, containing the respective amendment, and grown for 7 weeks until reaching a commercial size (ca. 60–70 g FW). After four weeks, half of the pots received an additional 70 kg Neff/ha of the same synthetic fertilizer. The plants were cultivated in a controlled phytochamber with a long-day cycle (16/8 h light/dark) at 20/15 °C and 50% average humidity, illuminated by a programmed LED lamp model LX601G (Heliospectra, Sweden). Pots were watered weekly until 80% of the maximum soil capacity of each condition. Soil, rhizosphere, plant leaves and roots were harvested separately, and the biological material was ground in liquid nitrogen to a fine powder for further analyses (molecular, analytical, enzymatic).
Dilutions. 2 g of fresh weight/each sample, were used to test the agronomic performance of the biogenic substrates as fertilizers. Each sample was blended with sandy field soil to reach an optimum nitrogen (N) level for lettuces, taking into account the maximum nitrogen supply for lettuce provided by the Lombardy Nitrates Action Plan 2020–2023 [62], (equal to 140 kg Neff/ha) but also respecting the maximum amount of N required by the European directive 91/676/CEE [31] (i.e., 170 kg N/ha in nitrate vulnerable zones).
Statistics. Physiological parameters, such as total leaves and root weight (g FW) and leaves/root ratio were averaged across replicates (n = 4). Statistical differences against the Control group were assessed using one-way ANOVA, followed by Dunnett's multiple comparison test (GraphPad Prism, ver. 6.01).
Acute and chronic reproduction toxicity on earthworms
Principle of the analysis. The assay with earthworms (Lumbricus terrestris) was conducted following the ISO standard ISO 11,268–1:2012 [63], which aims at assessing the potential toxicity of substances, particularly chemicals and contaminants, in soil. This standard provides guidelines for conducting toxicity tests with earthworms and is used to evaluate the effects of substances on the survival, reproduction, and behavior of earthworms. L. terrestris, commonly known as the common earthworm or nightcrawler, is selected as the test organism due to its ecological importance, sensitivity to soil contaminants, and ease of handling in laboratory settings.
Execution. Adult earthworms (>3 months old, with clitellum) weighing between 250 and 600 mg were selected for the experiment. The acute trial lasted for 14 days, and animals were sampled on day 2, 7, and 14 for analyses. Each treatment involved five glass pots (154 cm2, 5 cm deepness), with 10 worms in each pot, to evaluate the toxicity effects (mortality, DNA damage, and changes in enzymatic profile) of the chosen organic amendments. For the chronic study, the experiment lasted 56 days, and animals were samples on day 28 and 56 for analyses. Each treatment was composed of three pots (161 cm2, 13 cm of deepness), with 12 worms each, to evaluate the effects of long term-exposure through the mortality and reproduction rate, presence of nuclear abnormalities and bioaccumulation effects.
Both experiments were done in controlled chambers with controlled temperature (20±2 °C), air humidity (54±4%), and a light/dark cycle (16h:8 h). To maintain humidity and prevent animals from escaping, a perforated cellophane layer was placed in each pot. Humidity levels were monitored twice a week to ensure optimal conditions. The earthworms were fed once a week with a commercial feed (1.3 g of wormery mineral powder) to sustain their nutrition throughout the experiment.
Dilutions. The sample/LUFA soil ratio was equal to 23.09 g/363.11 g for sludge samples and 46.18 g/340.02 g for manures.
Statistics. After confirming the normality and homogeneity assumptions, the mortality and reproduction rate (%) was assessed using one-way ANOVA, followed by the post-hoc Tukey's test to identify any significant differences (p <0.05) between the treatments.
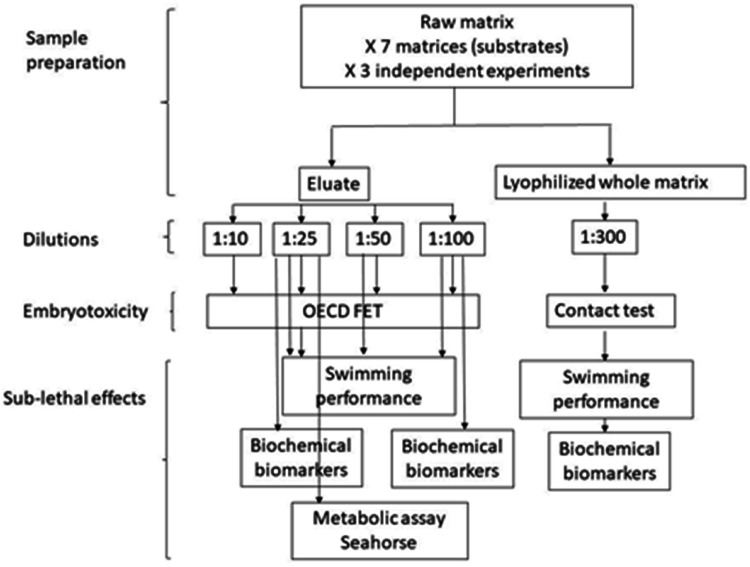
Fish (Danio rerio) embryo acute toxicity
Principle of the analysis. The assay was carried out on aqueous (eluates) samples following the Fish Embryotoxicity Test protocol OECD 236 (OECD TG 236), with slight modifications, and on solid samples according to the method optimized in our laboratory [64]. The test uses zebrafish (Danio rerio) embryos as the test organisms due to their transparency, rapid development, and well-characterized embryogenesis. Zebrafish embryos are also genetically similar to other fish species, making them a suitable model for assessing potential effects on fish early-life stages.
Execution. Adult zebrafish of the AB strain were bred at the Department of Biosciences, University of Milan (Italy, in flow-through conditions at water temperature of 28 °C, with a photoperiod of 14 h light and 10 h darkness. This facility strictly complies with the relevant Italian rules and regulations (Legislative Decree No. 116/92), as confirmed by the authorization issued by the municipality Milan (Art. 10 of Legislative Decree No. 116, dated 27.1.1992). Fish are fed three times a day with small granular food (ZM Fish and Food Equipment).
Eggs were collected within 3 h post fertilization (hpf) and observed under a stereomicroscope. For the exposure poor-quality embryos are discarded. To achieve a better robustness of the exposure results, each experiment was conducted with embryos obtained from three pairs of adults. Three independent exposure experiments were carried out.
For the assay on eluates, those were prepared one day before the assay, according to the procedure detailed in previous paragraph. At the beginning of the experiment dissolved oxygen concentration and pH were measured to assure that the parameters fulfil the requirements for test validity set by OECD (range of pH 6.5–8.5; O2 ≥80% of saturation). Twenty embryos per concentration (one embryo per well) were exposed to eluates in standard 24-well plates. Twenty embryos were kept in zebrafish water as negative control on one plate. In compliance with Italian law on welfare of animals subject to scientific purposes (D.L., 26/03/2014), exposure experiments were performed using embryos within 120 h post fertilization (hpf). Exposures run in an incubator at constant temperature of 27 °C. Every 24 h, embryos were observed under the stereomicroscope to assess the following lethal parameters: coagulated embryo, lack of somite formation, no detachment of the tail and lack of heartbeat. The following teratogenic endpoints were also considered: not completed epiboly, abnormal development of eyes, lack of spontaneous movement, edemas, reduced pigmentation, malformations, scoliosis, delayed growth [65].
Dead embryos were removed to avoid fungal/bacterial infections that might originate from degrading embryos. At the end of the exposure period, acute or embryo-toxicity was determined based on a positive outcome in any of the abovementioned observations recorded.
For the assays on the solid-phase samples, those were prepared 24 h before running the assay to promote the stabilization of the suspensions. The dry sewage sludges and other matrices were first mixed with standard reference soil with a final ratio of 1:300 up to a total wet weight of 3 g. Control group was exposed to 3 g of standard reference soil (Ostracodxkit F). Reference soil and samples were placed in 50 mL glass beakers, then filled with 15 mL of artificial water (Instant Ocean® 0.1 g L−1, NaHCO3 0.1 g L−1, CaSO4 0.2 g L−1 and 0.1% methylene blue). Dissolved O2 and pH of the waters in contact with soil were measured at the beginning of the exposure to assure that the parameters fulfil the requirements for test validity set by OECD (range of pH 6.5 - 8.5; O2 ≥80% of saturation). The test is carried out using 20 normally developed embryos placed in one beaker, each one representing an experimental group. The exposure run until 5 days post fertilization in an incubator at constant temperature 27 °C and embryotoxicity was screened as described for the FET OECD test.
Dilutions. Four dilutions of eluates (1:10 1:25 1:50 1:100) were prepared with zebrafish artificial water (Instant Ocean® 0.1 g L−1, NaHCO3 0.1 g L−1, CaSO4 0.2 g L−1 and 0.1% methylene blue).
Statistics. Data were analysed using various statistical tests through the program Graph Pad Prism 8.0.2. The EC50 was calculated using a non-linear regression curve. An ordinary one-way ANOVA followed by Dunnett's test was used to test whether exposure to solid phase resulted in statistically significant variations. Results were considered statistically significant only if they presented a p value ≤ 0.005.
The STATISTICA 7.0 software package was used; the one-way analysis of variance (ANOVA) was applied to assess any differences among samples and controls. The ANOVA assumptions were checked using the Kolmogorov-Smirnov test for data normality and Levene's test for variance homogeneity. Significant differences were identified by the Fisher LSD post-hoc test (p ≤ 0.05).
Mammal cells viability assessment: neutral red (NR) and MTT assays
Principle of the analysis. The neutral red (NR) uptake assay is based on the 3-amino-7-dimethyl-2-methylphenazine hydrochloride dye uptake by living cells [66]. The neutral red dye when integrated into cells mainly accumulates in lysosomes. In the in vitro toxicological approach, the neutral red assay is used to quantify the cytotoxicity of substances/molecules or mixtures. As a matter of fact, it is possible with a spectrophotometer, to determine the modification of the dye uptake in response to cell treatments.
Execution. Experiments were conducted on two different cell types to explore the potential cytotropism of the mixtures, in addition to assessing their basal cytotoxicity. We selected the IAR203 cell line, derived from rodent liver, due to its well established GJIC and known for its metabolic activity, and the MCF-7 cells, originating from human mammary adenocarcinoma, which are expected to exhibit lower sensitivity to xenobiotics.
Cells were seeded at the density of 20.000 cells/cm2 in 96-well plates in DMEM high glucose supplemented with 10% fetal bovine serum (FBS). Cells were let to adhere and acclimatize to these microenvironments for 24 h. Samples of organic extracts were dissolved in sterile DMSO to reach a final concentration of 1 g/mL. 24 h after seeding, samples were serially diluted in DMEM in order to test a wide range of sample concentrations. Cells were incubated in the presence of the sample for 24 or 48 h in the complete culture medium at 37 °C in the incubator in a 5% CO2 atmosphere. The day before the colorimetric reading, an RN solution was prepared by diluting (1:80) in DMEM medium with 5% FBS, the RN stock solution. This diluted solution was left overnight in an incubator at 37 °C. Before use, this solution was centrifuged twice at 3500 rpm for 10 min at room temperature. After the elimination of the culture medium, 200 µL of this solution were added to each well of the 96-well plates. Plates were let for 3 h at 37 °C (5% CO2). At the end of this incubation, a solution of formol-calcium was added (1 min contact time) to fix the cells that were finally lysed in an acetic acid/ethanol solution (1% acetic acid, 50,6% ethanol, 48,4% mQ water). After 5 min of stirring, the intensity of the reaction was measured with a microplate reader (Sunrise, Tecan Trading AG, Switzerland) at a wavelength of 540 nm.
Dilutions. The final sample concentrations varied from 0.1, 0.5, 1, 5, 10, 50, and 100 mg/mL.
Statistics. The statistical analysis of results was performed using the software STATA16, using Dunnett's test.
Principle of the analysis. Two assays employing the tetrazolium salts were carried out, namely the MTT test, based on [3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyl-2H-tetrazolium bromide] and the MTS test, based on [3-(4,5-dimethylthiazol-2-yl)−5-(3-carboxymethoxyphenyl)−2-(4-sulfophenyl)−2H-tetrazolium]. The primary difference lies in their chemical structures. MTS contains a 3-carboxymethoxyphenyl and a 4-sulfophenyl group, whereas MTT has two phenyl groups. This structural variation influences their solubility and the way they interact with cellular components. MTS is more soluble in aqueous solutions compared to MTT. This is largely due to the presence of the sulfophenyl group in MTS, which enhances its water solubility. In contrast, MTT requires solubilization steps post-assay due to its lower solubility. In MTT assays, the insoluble formazan product formed after reduction of MTT must be solubilized in a solvent like DMSO or isopropanol before quantification. On the other hand, the formazan product of the MTS assay is soluble in culture media, allowing direct quantification without additional solubilization steps. While both assays are used to measure cell viability, their sensitivity can vary depending on the cell type and experimental conditions. The choice between MTS and MTT often depends on factors like the nature of the experiment, the type of cells being used, and the required sensitivity and convenience of the assay. The common principle is the incorporation of the reagent into the cells and its conversion into water-insoluble formazan crystals [67]. This reductive reaction takes mainly place in mitochondria, even if, in a minor part, also in other organelles. The determination of cytotoxicity is allowed by spectrophotometric measures of the coloured suspension. This assay was exploited also to determine the sublethal doses to be used in a genotoxicity test, as detailed in the paragraph Comet assay protocol on HepG2 cells.
Execution. In order to investigate a possible cytotropism exerted by the samples (beside cytotoxicity), experiments were performed on three different cell types. Hence, the IAR203 cell line was chosen as a metabolically active cell type deriving from rodent liver, whereas the MCF-7 cells, derived from human mammary adenocarcinoma, were supposed to be less sensitive to xenobiotics.
Finally, the HepG2 cells were also employed: they derive from human hepatoma and are extensively employed in various assays to evaluate hepatotoxicity, drug metabolism, and enzyme induction. HepG2 cells are particularly valuable for assessing genetic damage, as they retain the ability to perform a range of liver-specific functions, including cytochrome P450 enzyme activity. This feature makes them suitable for genotoxicity testing, where they are used in assays like the Comet assay to detect DNA damage and repair, and in micronucleus tests for chromosomal damage assessment (see below).
IAR203 and MCF-7. Cells were seeded at the density of 20.000 cells/cm2 in 96-well plates in their classical medium (DMEM high glucose) supplemented with 10% fetal bovine serum (FBS). Cells were let to adhere and acclimatize to these microenvironments for 24 h. Samples of organic extracts were dissolved in sterile DMSO to reach a final concentration of 1 g/mL. 24 h after seeding, samples were serially diluted in DMEM in order to test a wide range of sample concentrations. Cell suspensions were incubated in the presence of the sample for 24 or 48 h in the complete culture medium at 37 °C in the incubator in a 5% CO2 atmosphere. The culture medium was removed from each well and was replaced with 200 µL of an MTT 0,2 mg/mL in Hank's/Hepes buffer. Cells were incubated for 2 h at 37 °C (5% CO2). Then after MTT solution was eliminated and, in order to dissolve the formazan crystals, an isopropanol acid solution (0,4 M HCl in isopropanol) was added to cell monolayers. After 5 min of stirring, the intensity of the reaction was measured with a microplate reader (Sunrise, Tecan Trading AG, Switzerland) at a wavelength of 620 nm.
HepG2. The exploitation of MTS assay as preliminary assessment before the genetic toxicity investigations, followed these steps: HepG2 cells were seeded in 96-well plates at a density of 1 × 104 cells/well (at least three wells per test condition) and incubated in complete medium for 24 h at 37 °C in a humidified atmosphere of 5% CO2. The culture medium was discarded and replaced with 100 µL of serum-free culture medium. Cells were treated with different amounts of aqueous or organic extracts and incubated for 3 h. At the end of the period, the treatment was discarded and replaced with 20% CellTiter 96® AQueous One Solution Reagent (Promega, Wisconsin, U.S.A.) in complete medium. The cells were incubated again at 37 °C to allow colour development and after 3 h the absorbance was recorded at 490 nm using a 96-well plate reader.
Dilutions. The tested doses were 0.1, 0.5, 1, 5, 10 mg/mL.
Statistics. The statistical analysis of results was performed using the software STATA16, using Dunnett's test.
Gap junction communication (GJC) assays
Principle of the analysis. Gap junctions are composed of six protein subunits (connexins) that form hemichannels on the cell membranes; hemichannels that can associate with others located on the membrane of neighbouring cells forming channels that allow the exchange of small molecules (molecular weight < 1000 Da). The intercellular communication mediated by gap junctions (GJIC) is fundamental in regulating cell homeostasis and response to the microenvironment changes in a well-orchestrated way. GJIC dysregulations can be observed in numerous pathologies regarding almost all systems, organs, tissues, and cells. Moreover, GJIC is also proposed as an interesting endpoint to be studied, since it could represent a target for tumour promoters. The scrape loading technique (Fig. 2) allows us to evaluate the GJIC, from a functional point of view. This technique involves the mechanical cut of the cell monolayer with a scalpel blade and the subsequent addition of a fluorescent tracer (Lucifer Yellow) which enters the cells damaged by the cut and spreads to the contiguous cells only if these have the ability to communicate through the GJs. The degree of communication is evaluated both with the measurement of the passage front of the tracer or with the count of fluorescent cells close to the injured one (notably more fluorescent); the choice between the two measurement methods is also made on the basis of the more or less marked intrinsic communication capacity of the cell line in use.
Fig. 2.
Cell monolayers after cutting: fluorescent dye transfer depends on the number of gap junctions and their functional state.
Execution. The technique of El-Fouly et al. [68] was modified as detailed below. The IAR203 hepatic cells were seeded in 24-well plates at the density of 300.000 cells/cm2 in which they were placed on a round cover-glass slide and were allowed to adhere for 24 h at 37 °C in an incubator with 5% CO2. They were treated for 6 h with each organic extract at the concentration of 0,1 mg/mL
Thereafter, this cell monolayer was washed twice with PBS containing Ca2+ and Mg2+ and it was injured with a scalpel. The fluorescent dye Lucifer Yellow solution was immediately added and incubated in the dark for 7 min at 37 °C. Lucifer Yellow was incorporated by cells all along the cut. The tracer trapped in the cytoplasm can spread to other adjacent cells only if cells communicate via Gap Junction channels. After washing cell monolayers with PBS, the cells were fixed for 5 min with formaldehyde at 4% and subsequently washed with PBS. The slides were mounted and rapidly observed under fluorescence microscopy (Olympus, Japan). Several photomicrographs were taken to quantify the effect of sludge samples on cellular communication mediated by gap junctions.
Dilutions. The final sample concentrations varied from 0.1, 0.5, 1, 5, 10, 50, and 100 mg/mL.
Body-like assays
Principle of the analysis. To investigate preclinical issues of potential new therapeutics, the in vitro approach represents an important alternative to the use of animal models. However, because the classical 2D in vitro models in static conditions could be limited in their ability to reproduce in vivo models; active investigations are in progress to better mimic the complexity of the whole body/or specific organ physiological behaviour. Nevertheless, several critical points need to be optimized. The shift from 2D to 3D models to better reproduce the natural tissue/organ architecture represents the main challenge. However, because the cell multi-layered tissues need a significant supply of oxygen and nutrients and waste removal, the shift from static to fluid dynamic microenvironments is also crucially required. Microfluidics were widely developed to allow the effective nutrient supply to engineered tissues and to sustain their physiological functions and cell response to microenvironmental stimuli. Even if these microfluidic systems allow the use of fewer chemicals and a lessened time of study, and moreover culture parameters can be finely controlled, they exhibit some defects. We believe that the passage from microfluidic to millifluidic system could allow the culture of well-defined 3D biological cultures. The greater dimension of the culture chamber can allow the formation of 3D structures that better mimic the architecture of the native tissue. In this part, we developed a multiorgan model based on the use of the IVTECH bioreactor (IVTECH, Italy). By the end, the ADME concept which means Absorption, Distribution, Metabolism, and Excretion, involves different tissues and their relative interactions. Thus, to better reproduce the in vivo context, the in vitro models should incorporate different tissue models. It is the reason why, we tried to develop an innovative system including the main organs involved in xenobiotic biotransformation: this model includes intestinal, hepatic, and renal models connected together as it is observed in the whole organism (see Fig. 3). To develop this “body like” model and evaluate its suitability, cells were treated with different treatments and cell viability was assessed.
Fig. 3.
How to reproduce the dynamic human body?
Execution. First, different experiments were performed to optimize the culture conditions (seeding density, culture medium characteristics). When such conditions were reached, cells were treated with all the samples.
Caco2 cell lines were chosen as the intestinal model, the HepG2 cell line for its capacity to reproduce the liver physiology, whereas Caki-1 cells were selected for their renal functions. Cells were seeded at the density of 150.000 cells/cm2; 100.000 cells/cm2 and 150.000 cells/cm2 respectively and let to adhere in the culture chamber. When cells were attached (24 h), the different chambers were connected and culture media with treatments were dynamically distributed in a serial way initiating from intestinal cells, secondly the hepatic cells, and at the end of the circuit the renal cells. The culture was maintained for 5 days. At the end of the experiment, cells were enzymatically detached and were counted with trypan blue in order to observe the eventual cytotoxicity of the sludge mixtures.
Dilutions. The final sample dilution (0.1 mg/mL) was chosen based on the results of the previous tests carried out on the same cell lines (see above).
Statistics. The statistical analysis of results was performed using the software STATA16, using Dunnett's test.
Specific mode of action
Specific modes of action in toxicity assessment involve understanding how toxicants interact with organisms at various stages, including adsorption, distribution, metabolism, and excretion (ADME). These processes are crucial in determining the toxic effects of pollutants. Distribution coefficients are used to assess the potential uptake of pollutants into specific organs or cell types. Metabolism involves enzymatic processes like activation, cleavage, or conjugation of parent compounds, while excretion deals with the removal of pollutants from the organism. In cases where clearance from the body is not achieved, the mode of action is used to describe the biological activity. However, reliable mode of action information is still lacking for many chemicals, and there is no standardized inventory or criteria for data-poor chemicals. Two approaches to assess toxicity are Toxicity Identification Evaluation (TIE), which considers bioavailability and baseline toxicity, and Effects-Directed Analysis (EDA), which focuses on specific modes of action through in vitro assays.
Endocrine disruption in yeast cells
Principle of the analysis. The endocrine activity elicited by the samples was quantified by utilizing genetically engineered yeast cells. The tests were performed on the organic extracts (see the specific Paragraph for the details). The A-YES (Arxula Yeast Estrogen Screen) is a reporter gene assay that can be used to measure the activation of the human estrogen receptor alpha (Er) in the presence of a sample containing compounds that cause estrogenic effects, including possible additive, synergistic, and antagonistic mixture effects. The activation of the estrogen receptor is measured by the induction of the reporter gene phyK, which encodes the enzyme phytase. The activity of the phytase is determined using an appropriate substrate, which is cleaved to a coloured reaction product. The reaction product can be measured photometrically. Attention must be paid to possible interference: coloured or turbid samples might interfere with photometric detection of the cell density and/or the detection of the cleaved substrate of the reporter enzyme phytase, and high salinity can cause toxic effects (strain Arxula adeninovorans yeast tolerates a conductivity of the sample up to 20% sodium chloride, which meets a conductivity of 180 mS/cm). The standard protocol ISO 19,040–2:2018 specifies a method for the determination of the estrogenic potential by means of a reporter gene assay with a genetically modified yeast strain, Arxula adeninovorans (a highly robust and salt- and temperature-tolerant test organism).
Execution. Test kits from new_diagnostics GmbH (Germany) were employed. Seven dilutions of 17β-estradiol) working solution (E2) were prepared by adding ultrapure water in order to obtain a dose-response curve from 1 ng/L to 80 ng/L; likewise, seven dilutions of 5-dihydrotestosterone (DH) working solution were prepared by adding ultrapure water in order to obtain a dose-response curve (from 1 ng/L to 350 ng/L). Freeze-dried yeast cells were reactivated by incubating at 31 °C and 450 rpm for 1 h. Ultrapure water (for HPLC use) was taken as a negative control (in duplicate). Four replicates of each dilution of the test samples were prepared. A process-undiluted field blank was tested in four replicates. A volume of 400 µL was added to each well. The inoculation with the reactivated yeast cells was done within 2 h after the plate setup. After inoculation, the multiwall plates were sealed with an air-permeable foil and centrifuged briefly. Afterward, they were placed in a shaking incubator and incubated the plate at 30 °C to 32 °C (± 2 °C) for 22 h. The substrate buffer and the developer were taken at room temperature before use. Multiwell plates were centrifuged for 10 min at 700 g. After centrifugation, 50 µL of the clear supernatant liquid from each well was transferred into a new microtiter plate, avoiding bubbles and disrupting the yeast pellet. 50 µL of a phytase substrate solution was added to each well. The plates were centrifuged briefly and transferred to an incubator for 1 h 5 min at 37 °C ± 0.2 °C. After 1 h 5 min of incubation, the absorbance was measured (with a wavelength of 405 nm 20 nm). Afterward, 100 µL of developer solution was added to each of the wells, and the absorbance was measured again. Depending on the growth of the yeast cells in the deep well plate and the linear range of the photometer for microtiter plates, a suitable dilution of the cell suspension in a whole volume of 300 µL has to be made. The yeast cell suspension was mixed thoroughly in the deep well plate by vigorous shaking. 10 µL of yeast cell suspension was immediately transferred into each well. The optical density at 630 nm ± 5 nm was measured. The final optical densities of the negative control and the reference compound solutions were calculated by multiplying the dilution factor by the corrected background optical density at 630 nm. The calculations are based on the measurement of reporter gene activity and cell density after the addition of the developer solution.
Dilutions: depending on the sample, the following dilutions (starting from the organic extracts) were considered: 1:2, 1:5, 1:10, 1:20, 1:50, 1:100.
Statistics: the standard deviation, the calibration curve and the compliance for the acceptability of the tests were carried out by using the software BioVAL® (new_diagnostics GmbH, Germany).
Endocrine disruption in mammal cells
Principle of the analysis. Steroid hormones can regulate the expression of a high number of specific genes. The typical mechanism involves the specific receptors: once they bind to their hormone and form dimers, they can recognize and bind specific DNA sequences, namely the Responsive Elements. In this research, transgenic mammary cells (MCF-7) expressing a reporter gene (luciferase) under the control of the ERE (Estrogen Responsive Sequences) were used. When luciferin is supplied, the enzymatic activity of luciferase is directly proportional to the degree of activation of estrogen-responsive sequences. (Fig. 4).
Fig. 4.
Evaluation of the endocrine disruption via the MCF-7 assay.
Execution. Mammary cells were seeded in 24-well plates at the density of 100.000 cells/cm2 in a complete culture medium (Phenol free DMEM) supplemented with 10% charcoal-stripped FBS, 1X antibiotic/antimycotic mix, 1X pyruvate, and 1X glutamine. Cells were allowed to adhere for 24 h at 37 °C in an incubator with 5% CO2 before being treated with organic extracts (0,1 mg/mL) for 24 h. In parallel, cells were treated with increasing doses of 17b-estradiol ranging from 1fM, 10 fM, 100 fM, 1 pM, 10pM, 100 pM, 1 nm, 10 nM in order to obtain a standard curve. Then, the cells were washed with PBS and left for 30 min, in ice, with 100 µL of lysis buffer including protease inhibitors (PMSF 100 mM, Aprotinin 2 mg/ml, Leupeptin 1 mg/mL). Cell lysates (20 µL) were added to 100 µl of luciferin, obtained by dissolving the lyophilized Reagent Luciferase Assay Substrate in the specific Luciferase Assay buffer, components of the kit in use supplied by the Promega (Wisconsin, U.S.A.). The plates were then incubated in the dark at room temperature for 20 min and subsequently the emitted photons were read using the LB961 luminometer (Berthold Technologies GmbH, Germany). Results were expressed as RLU (Relative Light Unit). To standardize results, proteins were quantified for each assay (BIO RAD, California, U.S.A.) and results were expressed as RLU/mg of proteins.
Dilutions. The final sample concentrations varied from 0.1, 0.5, 1, 5, 10, 50, and 100 mg/mL.
Statistics. The statistical analysis of results was performed using the software STATA16, using Dunnett's test.
Reactive mode of action
Reactive modes of action encompass various mechanisms of toxicity initiated by chemical interactions with organisms. This involves exposure, bioavailability, and the formation of bonds with ligands. These interactions can lead to either alterations or adaptations within organisms, driven by complex pathways. These modes of action describe how a toxicant affects an organism at the cellular, biochemical, or physiological level and can include: a) binding to receptors (many chemicals can bind to specific receptors on cells, interfering with normal cellular signalling and leading to various responses; for example, some chemicals may mimic the action of natural hormones by binding to hormone receptors, causing hormonal disruption); (b) enzyme inhibition (enzymes play critical roles in biochemical processes, and their inhibition can disrupt normal metabolic pathways); c) oxidative stress (some toxicants can induce oxidative stress by promoting the production of reactive oxygen species (ROS) and free radicals within cells. This oxidative damage can harm cell membranes, DNA, proteins, and other cellular components); (d) DNA damage (chemicals can directly damage DNA, leading to mutations, genetic instability, and potentially cancerous cell growth. DNA damage can result from chemical reactions that alter the structure of DNA molecules); (e) disruption of cellular structures (the structural integrity of cells and cellular organelles can be destroyed, leading to cell dysfunction and, possibly, cell death); (f) alteration of membrane function (cell membranes properties, like fluidity and permeability, can be affected, thus impairing various cellular functions, including the transport of molecules across the cell membrane); (g) interference with metabolism (metabolic pathways can be impaired by interfering with key biochemical reactions, such as those involved in energy production or nutrient utilization); (h) hormonal disruption (endocrine disruption can interfere with the endocrine system, affecting the regulation of hormones, causing a plethora of health effects, including reproductive, developmental, and metabolic dysregulation; (i) immune system modulation (the immune system can be modulated either by suppressing or overstimulating immune responses, making an organism more susceptible to infections or autoimmune diseases; (j) cellular signalling pathways (toxicants may interfere with cellular signalling pathways, disrupting communication between cells and affecting various physiological processes; k) alteration of normal behaviour, (neuroactive compounds can modify behaviour through different cellular mechanisms.
The proposed battery of tests includes different levels of biological organization targeted (organisms, tissues, cells) as well as different biological complexity (prokaryotes, eukaryotes; animals, plants; unicellular, multicellular). To specifically link crucial events with biological responses, several targeted assessments were carried out, including tests for both genetic toxicities and stress induction. The investigation on molecular biomarkers included the quantification of the catalase activity (CAT), ROS (reactive oxygen species), GST (Glutathione S-Transferase), Acetylcholinesterase activity (AChE), glycogen content (GLY), and lipid peroxidation level (LPO) in adult earthworms; the quantification of CAT, GST, ROS, and basal oxygen consumption in zebrafish embryos; the quantification of ROS, nitrous oxide concentration (NO), and glutathione content (GSH) in mammal cells. Reactive mode of actions study involved also the quantification of point reverse mutations in Salmonella typhimurium, the DNA damage using the Comet test in L. terrestris, on Lepidium sativum seeds (plant) and on HepG2 (human cells); the assessment of chromosomal aberrations in Allium cepa. The tests were chosen based on their standardization, high reproducibility and sensitivity, automated protocol, sensitivity, adequacy, statistical robustness. Swimming behaviour was also tested in zebrafish embryos as apical endpoint to screen the presence of neuroactive substances.
Biomarkers in L. terrestris
Principle of the analysis. Being, as was written above, L. terrestris a shared and standardized model in the field of soil ecotoxicology studies and applications [[69], [70], [71], [72]], on the same organisms tested to assess baseline toxicity, a number of biomarkers of specific toxicity, in this case, inherent to reactive modes of action, were also quantified.
ROS are highly reactive molecules and free radicals that contain oxygen. They are formed as natural by-products of various cellular processes, including metabolism, and can also be generated in response to external factors such as radiation and environmental toxins. Common ROS include, inter alia, the superoxide anion (O2•−), a radical formed by the addition of a single electron to molecular oxygen (O2) during processes like the electron transport chain in mitochondria; hydrogen peroxide, produced as a by-product of superoxide dismutation, where superoxide radicals are converted to hydrogen peroxide by the enzyme superoxide dismutase (SOD); the hydroxyl radical (•OH), formed when hydrogen peroxide reacts with transition metals such as iron (Fe) or copper (Cu) through a Fenton reaction. Singlet oxygen 1[O2] is an excited state of molecular oxygen (O2) with unpaired electrons. It can be generated during photosynthesis, where light energy is absorbed by pigments and transferred to oxygen molecules; peroxyl radical (ROO•) formed during lipid peroxidation, when ROS attack and damage lipids in cell membranes. ROS play both beneficial and harmful roles in the organisms. At low or moderate levels, they are involved in various cellular signalling processes, immune responses, and the regulation of physiological functions. However, excessive or uncontrolled ROS production can lead to oxidative stress, which can damage cellular components such as DNA, proteins, and lipids, contributing to various diseases and aging processes.
GSH is a molecule that serves as a primary antioxidant and plays various roles in cellular health, while GST is an enzyme that facilitates the conjugation of GSH with toxic substances, aiding in their detoxification and elimination. Together, GSH and GST are crucial components of the body's defence mechanisms against environmental toxins and oxidative stress. GST plays a crucial role in the detoxification and elimination of a wide range of xenobiotics, including carcinogens, environmental pollutants, and drugs. There are multiple isoforms of GST enzymes in the body, each with specific substrate preferences and functions; they are found in various tissues, including the liver, where they are highly active in detoxifying harmful substances.
Catalase is an enzyme predominantly found in the peroxisomes of eukaryotic cells and plays a crucial role in protection from oxidative damage caused by reactive oxygen species. It decomposes hydrogen peroxide, which is a toxic by-product of various metabolic processes and can cause cellular damage if it accumulates. Hence, catalase activity helps maintain cellular integrity and homeostasis.
Acetylcholinesterase activity (AChE) is a crucial indicator in bioassays primarily used to assess the presence and effects of neurotoxic compounds, particularly organophosphate and carbamate pesticides, as well as nerve agents. AChE is an enzyme found in the nervous system, including the brain and peripheral nerves, where it plays a vital role in terminating nerve signals by breaking down the neurotransmitter acetylcholine. In bioassays, a reduction in AChE activity can signify exposure to neurotoxic substances that inhibit the enzyme's function. This inhibition can lead to an accumulation of acetylcholine at nerve synapses, resulting in overstimulation of the nervous system and, in severe cases, paralysis or death. Monitoring AChE activity helps evaluate the toxicity of chemicals and serves as an early warning system for potential hazards to human and environmental health, making it a valuable tool in toxicology and environmental assessment.
Glycogen is a polysaccharide composed of glucose molecules and serves as a short-term energy storage molecule in animals, including humans. The glycogen content indicates the energy reserves within tissues or cells. The assessment of glycogenesis and glycogenolysis under various conditions, help understanding the regulatory mechanisms in metabolism. Overall, measuring glycogen content in bioassays is a valuable tool for understanding various aspects of metabolism, physiology, and disease.
Measuring lipid peroxidation in bioassays is important for several reasons, particularly in the fields of oxidative stress research, cell biology, and various disease investigations. Lipid peroxidation refers to the process in which reactive oxygen species (ROS) or free radicals attack and damage lipids (fats) in cell membranes and other cellular structures. Here are some of the reasons why measuring lipid peroxidation is valuable in bioassays:
Lipid peroxidation is linked with oxidative stress, which occurs when there is an imbalance between the production of ROS and the organism antioxidant defence mechanisms. LPO is a quantitative indicator of oxidative stress levels in cells or tissues; it can lead to the breakdown of cell membrane lipids, resulting in structural damage to cells. In addition, it can disrupt membrane integrity, alter membrane fluidity, and affect cellular functions.
Execution. Biochemical biomarkers were measured in individuals of L. terrestris after 28 days of exposure to the biogenic matrices. The measurements were carried out on the post-clitellar portion of each organism. Six individuals were analysed singly. Each analysis was carried out in triplicate. About 0.5 g of tissue were added to homogenization buffer (phosphate buffer 100 mM pH 7.4: 100 mM K2HPO4, 100 mM KH2PO4, 100 mM KCl, 1 mM EDTA, 1 mM DTT, protease inhibitors cocktail) at ratio 1:5 w/v. The samples were then homogenized using a TissueLyser II QIAGEN® in 3 cycles of 30 s at a frequency of 30 Hz. The samples were then centrifuged for 30 min at 4 °C at 10,000 x g. At the end, the supernatant was collected and kept at −80 °C until needed. The contnt of ROS, as well as the activities of CAT, GST and AChE were assessed as described for zebrafish embryos. On the same extract we also measured the content of glycogen (GLY), as the endpoint of energetic metabolism. The sulphuric acid method described by Dubois et al. [73] was followed, using glucose standards (0–2 mg/mL). The absorbance was read at λ = 492 nm, and the results were expressed as mg/g FW. Furthermore, the levels of lipid peroxidation (LPO) were determined to assess the potential occurrence of oxidative damage, according to the protocol developed by Buege and Aust [74]. For this assay, the supernatants were extracted using 20% (v/v) trichloroacetic acid (TCA) in a 1:2 proportion using TissueLyser II set at frequency 20 1/s., for 1.30 s and then centrifuge at 10,000 x g at 4 °C, for 20 min. Absorbance was read at λ = 532 nm and LPO levels were expressed as nmol of MDA formed per g ww−1.
Dilutions. See the paragraph Acute and chronic reproduction toxicity on earthworms.
Statistics. See the paragraph Acute and chronic reproduction toxicity on earthworms.
Molecular biomarkers in Danio rerio
Principle of the analysis. Chronic effects of matrices on zebrafish embryos were assessed by employing the robust and consolidated model of fish embryos, already considered for baseline toxicity assessment. The first biomarker assessed was the swimming behaviour of larvae, being the movement linked to neuroactivity and neurotoxicity. Swimming speed, distance travelled, and frequency of rest periods were registered, in order to get information about possible developmental or behavioural abnormalities. Behaviour like thigmotaxis (preference of swimming near the edges of the well) and circling and erratic movements or startle responses (e.g., escape after the exposure to sudden changes in light or mechanical stimuli) can suggest neurobehavioral issues possible due to the presence of toxic substances. The measurement of basal oxygen concentration helps establish a reference point for assessing the impact of these substances on zebrafish respiration. Changes in oxygen levels can be indicative of toxicity (e.g., regarding respiratory functions or developmental abnormalities).
Beside the abovementioned biomarkers (CAT, GST, and AchE), also the ROS concentrations, the basal oxygen consumption and the respiration rate were quantified.
Execution. Swimming performance was evaluated with the DanioVision™ apparatus. The protocol for measuring swimming performances consisted in a pre-test adaptation period (5 min dark and 5 min light), followed by 2 cycles of alternating periods of dark (20 min) and light (10 min) [75]. Light intensities ranged from 0 during the dark phases to 4400 lux during the light phases. A constant temperature of 28 °C was maintained. Video data were recorded at a sample rate of 30 frames/second via a high-speed infrared camera. EthoVision XT® software was used to analyse the distance moved by each individual embryo during the 60 min of dark/light cycles. The moved distance was binned into 1-minute intervals. To avoid erratic detection of the subject by the camera the Maximum Distance Moved smoothing method was applied with a threshold equal to 20 mm, and the Minimal Distance Moved smoothing method was also utilized to filter erroneous small movements caused by signal noise. Following exposures, a fixed number of embryos from each group was selected from those that did not exhibit any acute or sublethal effect to assess the swimming performance using the DanioVision™ apparatus. Lastly, all the embryos from each experimental group were collected and processed for biomarker analysis as depicted in Fig. 5.
Fig. 5.
Schematic representation of the exposures carried out on zebrafish embryos for each biogenic matrix.
At the end of the exposure all alive embryos from the same experimental group were pooled and kept at −80 °C. To each pool 250 µL of homogenization buffer (phosphate buffer 100 mM pH 7.4: 100 mM K2HPO4, 100 mM KH2PO4, 100 mM KCl, 1 mM EDTA, 1 mM DTT, protease inhibitors cocktail) were added. The samples were then homogenized using a TissueLyser II QIAGEN® in 3 cycles of 30 s at a frequency of 30 Hz. The samples were then centrifuged for 30 min at 4 °C at 10,000 x g. At the end, the supernatant was collected and kept at −80 °C until needed.
A set of biomarker quantification was performed on each homogenized sample to investigate the sub-lethal effect produced by the exposure of zebrafish embryos to these matrices, namely: CAT and ROS to estimate oxidative stress, GST to estimate detoxification; AChE for neurotoxicity, basal respiration and oxygen consumption as endpoints of effects on metabolism. Total protein content of each sample was measured to be used for data normalization. The measurement was carried out according to the Bradford assay [76], using a calibration curve of bovine serum albumin (BSA) (0–40 µg/mL).
ROS levels were measured using dichlorodihydrofluorescein-diacetate (DCFH-DA) as substrate [77]. In each well of a 96 multi-well plate the following reagents were added: 20 µL of homogenized sample, 100 µl PBS solution and 8.3 µL of DCFH-DA stock solution. The plate was incubated for 30 min at 37 °C. At the end of incubation, fluorescence was read at λex = 485 nm and λem = 530 in the Ensight™ multimode plate reader (Perkin Elmer). For each sample tree replicates were measured. Measured values were then normalized to the protein content of each sample and expressed as fluorescence units FU·mg proteins−1.
CAT and GST were measured according to the methods described in Della Torre et al. [78] using a 6715 UV/Vis spectrophotometer (Jenway). CAT was measured via direct spectrometry through the decrease of H2O2 at λ = 240 nm for 1 min. The activity was expressed as mmol·min−1·mg proteins−1. GST activity was measured using the conjugation of dinitrochlorobenzene (DNCB) with the thiol group of glutathione (GSH), at λ = 340 nm for 1 min. The activity was expressed as mmol·min−1·mg protein−1. For each sample three replicates were measured.
AChE activity was measured according to the method of Ellman et al. [79] The reaction mixture consisted of a potassium phosphate buffer (100 mM, pH 7.4) containing acetylthiocholine chloride (1 mM) and 5,5′ dithiobis-2-nitrobenzoic acid (0.5 mM). The reaction was run for 15 min at λ = 412 nm. The activity was expressed as μmoles min−1 mg protein−1.
Basal oxygen consumption and maximal respiration were measured using the Agilent Seahorse XFe24 Analyzer. This analysis was carried out only for embryos exposed to selected elutriates and concentrations, namely the swine manure, the compost and the digestate samples (at 1:25 dilution) and sludge S1 (at 1:50 dilution). Eluates (prepared as described above) and the required dilutions were prepared using E3 medium instead of fish water. E3 medium was prepared by mixing 17.4 g NaCl, 0.8 g KCl, 2.9 g CaCl2·2H2O and 4.9 g MgCl2·6H2O and 100 µl of 1% methylene blue in 1 L of deionized water. If needed, pH was adjusted to 7.2 using NaOH. Exposure of the embryos was performed as described in previous paragraph, but it was terminated at 48 hpf.
About 24 h prior to the assay the sensor cartridge was hydrated by filling each well with 700 µL of XF Calibrant fluid, and incubated in a non-CO2 incubator overnight at 28 °C. On the day of the assay 75 µL of each pharmaceutical working solution were loaded at the drug ports of the sensor cartridge reaching the following final solution: oligomycin 20 mM, FCCP 8 mM and sodium azide 20 mM. Then the sensor cartridge was loaded into the Analyzer and the Analyzer was left to calibrate for 45 min. Zebrafish embryos at 48 hpf were washed once with E3 medium and transferred to islet plate (one embryo per islet, 4 wells were left empty for temperature control). 525 µL of E3 medium were added to each islet and the plate was placed on the Analyzer tray to run the assay following the instrument protocol described in Table 2. Each cycle was composed of a mixing phase (2 min), a pause (1 min) and a measuring phase for O2 levels and pH (2 min).
Table 2.
Steps of the Seahorse assay.
| Phase | Measured parameter | Pharmaceutical | Number of cycles |
|---|---|---|---|
| 1 | Basal respiration | 10 | |
| 2 | ATP-coupled respiration | Oligomycin | 18 |
| 3 | Maximal respiration | FCCP | 8 |
| 4 | Non-mitochondrial respiration | Sodium azide | 24 |
Dilutions. Zebrafish embryos were exposed to four different elutriate dilutions (v/v): 1:10, 1:25, 1:50 and 1:100.
Statistics. Data were analysed using various statistical tests through the program Graph Pad Prism 8.0.2.An ordinary one-way ANOVA followed by Dunnett's test was used to test whether exposure to elutriates at different dilutions resulted in statistically significant variations in swimming performance, biochemical biomarkers and metabolic endpoints. Results were considered statistically significant only if presenting a p value ≤ 0.05.
Molecular biomarkers in mammal cells
Principle. Biomarkers of oxidative stress, such as ROS, nitric oxide (NO), and glutathione (GSH), play crucial roles in assessing the delicate balance between the body's antioxidant defences and the harmful effects of oxidative damage. Nitric oxide (NO) is an important, small, ubiquitous, and gaseous signalling molecule that regulates many biological functions either in physiological (immune system) or pathological contexts (cardiovascular, pulmonary, and renal diseases, diabetes, neurological disorders, inflammation, neoplastic pathologies, aging etc. [80]; consequently, the estimation of NO levels in tissues and body fluids assumes great relevance. NO can be degraded into different sub-products, nitrite (NO2–) presents a noteworthy interest since it is relatively stable and non-volatile. Thus, nitrite concentrations can be determined. GSH, a three-amino-acid peptide (Glu-Cys-Gly), is a powerful antioxidant, helps protect cells from oxidative damage by scavenging harmful free radicals and supporting various enzymatic antioxidant pathways. GSH is a ubiquitous endogenous thiol presenting a high antioxidant potential. It plays a fundamental role in redox homeostasis and more especially it participates in the detoxification processes, and the protection of cell membranes, and it is involved in cell signalling, proliferation, apoptosis, gene expression, etc. Reactive chemical species, mainly ROS, can cause a change in GSH levels. GSH can be increased to counteract ROS presence in order to protect cells. But if reactive species are in excess, the GSH level can be reduced either by oxidation or reaction with the thiol group. Thus, a GSH depletion could reflect excessive oxidative stress and could represent a predictive marker for cytotoxic or pathological processes.
Monitoring these biomarkers is essential in understanding oxidative stress implications in various diseases and conditions, enabling the development of targeted interventions to maintain a healthy oxidative balance in the body.
Execution. Regarding the quantification of the Reactive Oxygen Species, the ROS-Glo™ H2O2 Assay by Promega (Wisconsin, U.S.A.) was employed. It is based on the measurement of the H2O2 molecules that also represent the converted product of different ROS. A peculiar H2O2 substrate is added to cells/media that reacts directly with H2O2 in order to produce a luciferin precursor. Upon addition of ROS-Glo™ Detection Reagent containing Ultra-Glo™ Recombinant Luciferase and d-Cysteine, this precursor is converted to luciferin that reacts with Ultra-Glo™ Recombinant Luciferase to generate a bioluminescent signal that can be measured with a luminometer. RLU is proportional to the H2O2 concentration. Cells were seeded in 35 mm Petri dishes and were allowed to adhere for 24 h at 37 °C in an incubator with 5% CO2. They are treated for 6 h with each organic extract at the concentration of 0,1 mg/mL in the presence of H2O2 substrate. Organic extract and H2O2 substrate were diluted in the chilled H2O2 substrate dilution buffer. After 6 h, for each treatment assay, 10.000 cells (in triplicate) were transferred to 96-well plates specially created for bioluminescence assays. Cells were incubated with the ROS-Glo detection reagent (ratio v:v). This freshly prepared solution was a mix of Luciferin Detection Reagent (1 mL), d-Cysteine (10 µL), and Signal Enhancer Solution (10 µL). The plate was then incubated in the dark at room temperature for 20′ and subsequently the emitted photons were read using a luminometer (Berthold Technologies GmbH, Germany). Results were expressed as RLU (Relative Light Unit). To standardize results, proteins were quantified for each assay and results were expressed as RLU/mg of proteins.
Regarding the nitrous oxide quantification, the Griess Reagent System (Promega, Wisconsin, U.S.A.) was utilized. The reaction mixture contains sulfanilamide and N-1-naphthylethylenediamine dihydrochloride (NED) under acidic conditions (phosphoric acid). In such conditions after diazotization reactions (between nitrite and sulfanilamide), and the subsequent reaction of this diazonium salt with the N-1-naphthylethylenediamine, a reddish-purple azo dye is formed and can be quantified with visible spectrophotometry. Cells were seeded in 35 mm dishes at the density of 100.000 cells/cm2 and were allowed to adhere for 24 h at 37 °C in an incubator with 5% CO2. They were treated for 24 h with each organic extract at the concentration of 0,1 mg/ml. For each treatment, 50 mL of culture medium were transferred into a well of 96-well plates. Each treatment experiment was performed in triplicate. 50 mL of sulphanilamide solution were added to each well and incubation was performed for 10 min at room temperature in a dark container. Then 50 µL of the NED solution were dispensed to all wells. After 10 min of incubation at room temperature in a dark container, that corresponds to the formation of a reddish-purple colour. Within 30 min absorbance was read at 540 nm with the Sunrise microplate reader (Sunrise, Tecan Trading AG, Switzerland). Results were expressed as absorbance/ 10.000 cells.
As far as GSH activity is concerned, in order to individuate the potential involvement of redox stress unbalance in cells treated with our different source of samples, the GSH-Glo™ Glutathione Assay (Promega, Wisconsin, U.S.A.) which is a luminescence-based assay developed to measure glutathione (GSH) was employed. In the presence of glutathione, the enzyme glutathione S-transferase (GST) catalyses the conversion of a luciferin derivative (Luciferin-NT) into luciferin. The GSH concentration is proportional to the bioluminescent signal. Cells were seeded in 35 mm dishes at the density of 100.000 cells/cm2 and were allowed to adhere for 24 h at 37 °C in an incubator with 5% CO2. They were treated for 24 h with each organic extract at the concentration of 0,1 mg/ml. At the end of treatments, for each assay, 10.000 cells were harvested, centrifuged, resuspended in a maximum of 50 µL PBS, and dispensed in a well of white 96-well plates. 50 µL of GSH-Glo™ Reagent 2X were added to this cell suspension. Experiments were performed in triplicate. After a rapid shake of microplates, cells were incubated for 30 min at room temperature. To allow the measure of bioluminescence, 100 µL of the Luciferin Detection Reagent was added to each well. After a rapid shaking of microplates and 15 min of incubation, bioluminescence was quantified with the CentroLIA LB961 luminometer (Berthold Technologies GmbH, Germany). Results were expressed as RLU (Relative Light Unit). To standardize results, proteins were quantified for each assay and results were expressed as RLU/mg of proteins. A calibration curve was built using GSH at concentrations ranging from 0 to 5 mM.
Dilutions. The final sample concentrations varied from 0.1, 0.5, 1, 5, 10, 50, and 100 mg/mL.
Statistics. The statistical analysis of results was performed using the software STATA16, using Dunnett's test.
Ames test: conventional and miniaturized
The Ames test is a widely used method to assess the mutagenic potential of chemical compounds. Ames and his colleagues standardized the typical Ames assay protocol in the 1970s and conducted a re-evaluation of it in the 1980s [81]. This in vitro bacterial bioassay allows to assess the potential of a wide range of environmental toxins and carcinogens. At its core, this test is based on the observation that exposure to mutagenic substances can cause genetic mutations in the DNA of organisms.
Principle. Several strains of S. typhimurium have been used in Ames assay which requires histidine synthesis to highlight the mutagenicity. In the histidine operon, each tester strain contains a different mutation. These mutations render the bacteria unable to grow on a medium lacking histidine. These histidine-dependent strains are exposed to the chemical substance under investigation. The test can be conducted with or without a liver extract (S9 mix), which contains metabolic enzymes. The S9 mix is used to mimic the metabolic activation of compounds that occurs in mammals, thus, to show the possible effects of conjugates (transformation of pro-mutagens).
The principle of Ames test relies on detecting 'reverse mutations' that restore the bacteria's ability to synthesize histidine. In addition to the histidine mutation, the standard tester strain of S. typhimurium contains other mutations that greatly enhance their ability to detect the mutations. The newly formed mutant cells are allowed to grow in the absence of histidine and form colonies, hence this test is also called ‘reversion assay’. The degree of mutagenicity is assessed by comparing the number of colonies in the presence of the test substance to the number in the controls. A significantly higher number of colonies in the test substance group indicates mutagenic potential. The Ames test is valuable for its simplicity, cost-effectiveness, and ability to rapidly screen substances for mutagenic potential. However, it is worthy to note that it does not replicate the complexity of mammalian metabolism and genetic systems, and therefore, positive results in the Ames test are not definitive proof of carcinogenicity in humans or other animals.
Conventional Ames test protocol
Execution. The organic extracts dissolved in DMSO, as well as the aqueous extracts were tested at increasing doses. The Ames plate incorporation test [81] was conducted in accordance with the Standard Methods for the Examination of Water and Wastewater [82]. The Ames test detects gene mutations in bacteria. TA98 and TA100 strains of S. typhimurium were utilized, to evidence frameshift and base-substitution mutations, respectively. All the tests were carried out with and without the in vitro exogenous metabolic activation (±S9 mix). Pure DMSO and distilled water were used as negative controls for organic and aqueous extracts, respectively. Positive controls were strain-specific: 2-nitrofluorene for the TA98-S9 strain (10 µg/plate); sodium azide for the TA100-S9 strain (10 µg/plate); and 2-aminofluorene for both the strains with S9 (20 µg/plate). All the tests were performed in triplicate and repeated. The results of Ames test were expressed as revertants/plate and mutagenicity ratio (MR), by dividing the revertants/plate (mean number of revertant colonies per plate for each samples) by the spontaneous mutation rate (mean number of revertant colonies per plate in the negative control). The test responses were considered positive if two consecutive dose levels or the highest non-toxic dose level produced a response at least twice that of the solvent control, with mutagenicity ratio (MR) >2, and at least two of these consecutive doses showed a dose-response relationship [82].
Dilutions. The following mg equivalent/plate were tested for organic extracts: 0.1, 0.5, 1, 5, 10, 50, 100. Likewise, aqueous extracts were assayed at the following doses: 0.01, 0.1, 1, 10 mg equivalent/plate.
Statistics. In case of positive results, the net revertants/g could be obtained by means of the dose-response curve (linear regression).
Miniaturized Ames test protocol
Execution. The MutaChromoPlate™ (EBPI (Environmental Bio-Detection Products Inc., Canada) procedure is a simplified version of the traditional Ames test, employing the fluctuation assay method. The test solution is directly mixed with the bacterial strains and the reaction mixture. The protocol allows to overcome the traditional agar growth, since it employs multiwell plates and is based on a colorimetric response to mutation events. When a test compound induces a reverse mutation in the bacteria, the newly functional enzymes metabolize the chromogenic substrate, leading to a color change. This colorimetric response provides a visual indication of mutagenicity. The intensity of the color change can be quantitatively measured, often using spectrophotometry, providing an indication of the mutagenic potential of the test compound.
The test was carried out following the instructions of the producer (available at its website: https://www.biotoxicity.com/index.php/ebpi-toxicity-tests/ames-tests/ames-modified-isotest). Briefly, the lyophilized bacterial were resuspended in the nutrient broth, overnight (12–16 h at 37 °C, in a shaking incubator). The organic extracts were diluted with DMSO, without exceeding the final DMSO concentration of 10%. Positive controls consisted in sodium azide and 2-aminofluorene (TA100 strain), again 2-nitrofluorene and 2-aminoanthracene (TA98 strain). Negative control was the dilution medium. After checking the bacterial density by reading the absorbance at the wavelength of 600 nm, a final suspension was realized, with a value of 0.05 absorbance units. Serial dilutions (in triplicate) of the samples were carried out and the bacterial suspension solution was added. In parallel, identical multiwell plates were run with the addition of the S9 mix. After a 100 min incubation at 37 °C, samples were transferred to conical tubes and vortexed. Aliquots were then introduced in a multiwell plate, where the reversion mix had previously added. After an incubation of hours at 37 °C for 48–72 h. A visual score was prescribed: yellow and partial yellow wells were scored as positive, while purple wells were scored negative. The protocol also indicates validity criteria to be meet.
Dilutions. The following mg equivalent/plate were tested for organic extracts: 0.1, 0.5, 1, 5, 10, 50, 100.
Statistics. The statistical analysis was performed using ANOVA univariate and Dunnett's multiple comparison test.
Single cell gel electrophoresis assay (SCGE)/Comet test
Principle. The Comet Test, also known as single cell gel electrophoresis (SCGE), is a sensitive and versatile technique for assessing DNA damage (strand breaks and alkali-labile sites) at the individual eukaryotic cell level. The procedure involves embedding cells in a low-melting agarose gel on a microscope slide, lysing (either chemically, with surfactants or mechanically) the cells to form nucleoids (cell membranes and proteins are removed) and subjecting them to electrophoresis under neutral or alkaline conditions. Damaged DNA strands migrate away from the nucleus during electrophoresis, forming a tail that resembles a comet [83]. The degree of migration depends on the extent of DNA breakage. After the electrophoresis, the DNA is stained using a fluorescent dye. Then, under a fluorescence microscope, intact DNA appears as a round or oval structure (the 'head'), while damaged DNA forms a 'tail', resembling the comet [84]. This rapid and sensitive technique requires only a small number of cells, yet it provides information regarding the DNA damage and potential repair at the individual cell level [85].
Single cell gel electrophoresis assay (SCGE)/Comet test on Lepidium sativum
Execution. The test was performed on Lepidium sativum (watercress) seeds [86,87]. Three replicates per treatment were arranged by wetting Whatman filter paper No. 1 with 2 mL of sample solution, corresponding to EC50 and ½EC50. Ten seeds for each replicate were spread on the filter. The three dishes of each replicate were packed in a tightly closed plastic bag and incubated at 25 ± 1 °C in the dark for 72 h. Negative and positive controls were performed using distilled water and methyl methanesulfonate (MMS, 10 mg/L), respectively. At the end, seedlings were collected in an ice-cooled dish. The tips were finely minced with a scalpel and 500 µL of cold isolation buffer (200 mM Tris, 4 mM MgCl2·6H2O, 0.5% Triton-X) was added. The suspension was allowed to sediment on ice for several minutes. The supernatant (180 µL) was resuspended in low‐melting agarose (0.35% agarose final concentration). The suspension was applied to a glass slide pre-coated with agarose (NMA), immediately covered with a coverslip and left at 4 °C for 30 min to allow the agarose to solidify. The coverslips were then gently removed and the slides were placed in the electrophoresis tank containing cold buffer (Na4EDTA 1.5 mM, NaOH 30 mM, pH=12.3) for 1 h of unwinding and 20 min of electrophoresis (0.8 V/cm and 25 V at the limit). At the end, the slides were neutralized in distilled water, dehydrated in cold absolute ethanol and air-dried overnight. The slides were stained with 75 µL of GelRed Nucleic Acid Gel Stain (Biotinum) and examined under a fluorescence microscope (Olympus CX 41RF, Japan) equipped with a BP 515–560 nm excitation filter and an LP 580 nm barrier filter. DNA damage levels were highlighted by considering the comet parameter "tail intensity" (percentage of DNA migrated into the tail) out of 100 nuclei detected by automatic image analysis software (Komet 5, Kinetic Imaging Ltd, UK). The entire test was performed in duplicate.
Dilutions. The following doses have been considered EC50 and ½EC50.
Statistics. The statistical analysis was performed by using ANOVA univariate and Dunnett's multiple 1476 comparison test, where p < 0.05 was considered significant.
Single cell gel electrophoresis assay (SCGE)/Comet test in earthworms
Execution. The assay was performed in alkaline conditions (pH ≥13) using epidermal cells from earthworms (L. terrestris) tested for acute toxicity following the experimental conditions of ISO 11,268–1:2012 [63]. Therefore, the individuals submitted to the acute and chronic toxicity test (described in the paragraph Acute and chronic reproduction toxicity on earthworms) were collected after two and seven days of the examination. Negative (LUFA soil, type 2.2) and positive controls (75 µM of hydrogen peroxide, H2O2) were submitted to the analyses. The extraction protocol for coelomocytes followed an adapted version of Sforzini et al. [88]. Briefly, after removing dirt and soil residue by rinsing the earthworms, each animal was placed in a 50 mL propylene tube filled with 1.8 mL of cold Extrusion Buffer (7.4 mM Na2EDTA and 50.5 mM Guaiacol Glyceryl Ether, pH 7.3) for approximately 30 s. The earthworms were then immediately rinsed with distilled water, dried, and placed in a separate container with soil. After that, the extracted cell solution was centrifuged at 400 x g for 5 min at 4 °C and the supernatant was discarded.
The cells pellet was resuspended in 300 µL of Phosphate Buffered Saline (PBS, 137 mM NaCl, 2.7 mM KCl, 8.5 mM Na2HPO4 and 1.5 mM KH2PO4, pH 7.2–7.4) with gentle mixing. Cells were kept on ice while checking for viability and density using the Trypan Blue Method. Hence, a 1:5 dilution was made (10 µL cell solution, 40 µL PBS) and 12 µL were added to 12 µL of Trypan Blue. After gently mixing the solution, 10 µL were loaded on both sides of an improved Neubauer counting chamber. Cells inside each of the four squares at the corners in each chamber were then counted under a microscope, discarding those on top of the right and bottom lines. Later, stock cell density was normalised to 1.5–3.0 × 104 and suspended in 0.5% low melting agarose (1:20 v/v). The cell suspension was aliquoted (20 µL each) onto microscope slides, pre-coated with 1% normal-melting agarose and let to solidify for 20 min at 4 °C. For each animal, four slides were prepared (for assessing possible direct DNA damages and the H2O2-induced effects, respectively. Cell lysis and electrophoresis protocol were followed as mentioned in Ivorra et al. [85]. Briefly, cells were lysed by incubating the slides in a lysis solution (2.5 M NaCl, 100 mM Na2EDTA, 10 mM Tris base, 1% Triton-X pH 10) for 2 h at 4 °C in the dark. After incubation, the slides were rinsed with cold distilled water (1x) in electrophoresis buffer (3 M NaOH, pH ≥13) (1x). In case of positive controls, slides were immersed for 5 min (4 °C) in H2O2 solution, then rinsed gently twice with PBS. All the slides were then placed for 40 min at 4 °C in a horizontal electrophoresis chamber, filled with cold electrophoresis buffer to allow DNA unfolding. After that, electrophoresis was performed for 20 min at 24 V (1 V/cm). Slides were washed by immersion with distilled water (3x, 5 min each) and then with ethanol (C2H6O) (2x, 5 min each), and left to dry overnight. The slides, stained with GelRed Nucleic Acid Gel Stain (Biotinum), were examined under a fluorescence microscope (Olympus CX 41RF, Japan) equipped with a BP 515–560 nm excitation filter and an LP 580 nm barrier filter. Levels of DNA damage were evaluated by the comet parameter ‘tail intensity’ (TI) (percentage of DNA migrated in the tail) detected by an automatic image analysis software (Komet 5, Kinetic Imaging Ltd, UK).
Dilutions. The sample/soil ratios were detailed in the paragraph Acute and chronic reproduction toxicity on earthworms.
Statistics. The statistical analysis was performed by using ANOVA univariate and Dunnett's multiple comparison test, where p < 0.05 was considered significant.
Single cell gel electrophoresis assay (SCGE)/Comet test on HepG2 cells
Execution. The human hepatoma cell line HepG2 was used to assess the genotoxicity of aqueous and organic sludge extracts [89]. HepG2 cells were seeded in 6-well plates at a density of 2.5 × 105 cells/well and incubated in complete medium for 24 h at 37 °C in a humidified atmosphere of 5% CO2. The culture medium was discarded and replaced with serum-free culture medium. Cells were treated with two increasing doses of aqueous or organic extract of each sample, for 3 h at 37 °C. Negative and positive controls were performed using distilled water or DMSO and ethyl methanesulfonate (EMS, 2 mM), respectively. After treatment, the medium was removed, and cells detached with trypsin-EDTA solution. Cells were centrifuged at 5000 rpm for 5 min and resuspended in 400 µL of PBS. 10 µL of cell suspension was taken and mixed with 10 µL of Trypan Blue solution to assess cell viability. Then 20 µL of cell suspension was resuspended in low‐melting agarose (0.6% agarose final concentration) and rapidly applied to a glass slide pre-coated with agarose (NMA), immediately covered with a coverslip and left at 4 °C for 30 min to allow the agarose to solidify. The coverslips were then gently removed, and the slides were immersed in active lysis solution at 4 °C for 1 h. After that, slides were washed twice with distilled water for 10 min and placed in the electrophoresis tank containing cold buffer (Na4EDTA 1 mM, NaOH 300 mM, pH>13) for 20 min of unwinding and 30 min of electrophoresis (0.7 V/cm and 300 mA). At the end, the slides were neutralized in distilled water, dehydrated in cold absolute ethanol and air-dried overnight. The slides were stained with 75 µL of GelRed Nucleic Acid Gel Stain (Biotinum) and examined under a fluorescence microscope (Olympus CX 41RF, Japan) equipped with a BP 515–560 nm excitation filter and an LP 580 nm barrier filter. DNA damage levels were highlighted by considering the comet parameter "tail intensity" (percentage of DNA migrated into the tail) out of 100 nuclei detected by automatic image analysis software (Komet 5, Kinetic Imaging Ltd, UK). The entire test was performed in duplicate.
Dilutions. Undiluted and 1:2 diution.were used to test water extraxcts. EC50 and 1/2EC50 doses were used to test organic extracts.
Statistics. The statistical analysis was performed by using ANOVA univariate and Dunnett's multiple comparison test, where p < 0.05 was considered significant.
Chromosomal aberrations in Allium cepa
Principle. The Allium cepa test, commonly known as the onion root tip assay, is a simple, efficient, and cost-effective method for assessing cytotoxicity and genotoxicity of environmental samples and chemical substances. This bioassay utilizes onion (Allium cepa) root tips to detect chromosomal aberrations, mitotic activity, and other cytogenetic effects caused by toxic agents. In this test, onion bulbs are exposed to the test substance, and then the root tips are harvested, fixed, and stained to visualize the chromosomes under a microscope. Scientists assess parameters like the rate of cell division, the incidence of chromosomal aberrations, and micronucleus formation, providing insights into the potential genotoxicity of the tested substance. Due to its simplicity and the high correlation of its results with those obtained in more complex organisms, the Allium cepa test is widely used in ecotoxicological studies and environmental monitoring. The Mitotic index (MI), expressed as the total number of dividing cells in cell cycle, is used as a parameter to assess the cytotoxicity of several agents [90]. The MI of the samples should be similar to the MI of the negative control. MI significantly lower than the negative control can indicate alterations, deriving from the chemical action in the growth and development of exposed organisms. On the other hand, a MI higher than the negative control is the result of an increase in cell division, which can be harmful to the cells, leading to a disordered cell proliferation and even to the formation of tumour tissues. However, both the MI reduction and the MI increase indicate a toxic/cytotoxic effect. Chromosomal aberrations (CA) are characterized by changes in either chromosomal structure or in the total number of chromosomes. Several types of CA are considered in the different phases of cell division (prophase, metaphase, anaphase and telophase). The CA, such as chromosome bridges and breaks, are indicators of a clastogenic action, whereas chromosome losses, delays, adherence, multipolarity and C-metaphases result from aneugenic effects [91].
Execution. The Allium cepa test for the assessment of chromosomal aberrations (CAs) was performed on six onion bulbs per sample. After pre-germination for 48 h in Rank's solution, the bulbs were exposed for 24 h to undiluted or diluted samples. Positive and negative controls were performed using maleic hydrazide 10−2 M (6 h exposure) and Rank's solution (72-h exposure), respectively [92].
After the exposure, the roots were cut, fixed in 1:3 acetic acid–ethanol for 24 h, and stored in 70% ethanol. Five roots of each sample were considered for microscopic analysis after staining with 2% acetic orcein: 5000 cells were scored for the MI, the measure of the cell division rate. Samples with MI <1% were not included [90]. Then, 1000 cells in mitosis were scored for the CA frequency. The experiments were performed in duplicate (two independent assays).
Dilutions. Based on the results of the preliminary assay described in the paragraph Roots elongation test in Allium cepa bulbs on aqueous extracts, the dilutions corresponding to EC50, EC20 and EC10 values were prepared using distilled water, since the test must be performed at non-toxic doses.
Statistics. The statistical analysis of results was performed using χ2 tests for MI and CA.
Datawarehouse
The project is characterized by a multidimensional approach to assess all the matrices considered. Each substratum has been analysed by aspects related to physical, chemical, microbiological, and (eco)toxicological characteristics. Therefore, the results of all the experimental investigations and the bibliographic research led to the definition of very wide tables (few observations and a large number of variables). Furthermore, the assessment factors considered in the project involved both quantitative and qualitative variables, making integrated comparison and evaluation complex. In this context, the data warehouse aimed to provide a unified view of large amounts of data, typically represented in a multidimensional form. The flexible structure of the data warehouse made it possible to manage these complexities to avoid loss of information and have computer-based support.
The primary characteristics of the data warehouse are data integration and coherence, as it draws from multiple sources of heterogeneous data extracted from various internal information systems or external sources (scientific literature, economic data).
The methodology for data warehouse construction is composed of four levels (Fig. 6), adapted from Golfarelli and Rizzi [93]:
-
(1)
Source Level: Responsible for data collection. Data were acquired from the different analytical outcomes described in chapters 4 and 5, survey and external data sources (scientific literature).
-
(2)
Power Level: the principal aim of this level was to define a common schema for the diverse sludge matrices to amalgamate heterogeneous input data. To enhance the efficiency of this preprocessing phase, the ETL (Extraction, Transformation, and Loading) approach was adopted [94]. This approach enabled the integration of heterogeneous schemas and facilitated the extraction, transformation, cleansing, validation, filtering, and loading of data from CSV/XLSX files and other data sources into the warehouse.
-
(3)
Metadata level: a metadata repository has been implemented to describe the sources, values, uses, and functions of the data stored within the warehouse.
-
(4)
Warehouse Level: it had the function of an information repository and served as the central global container for synthesized data, and it was composed of different data marts. Data marts are optimized data structures (subsets of the warehouse) for expedited access, tailored to specific WP domains, and depicted in distinct Excel spreadsheets. This approach streamlined efficient storage, thus enhancing the quality of actual data analysis processes.
Fig. 6.
Data warehouse system architecture. Source: authors’ elaboration from Golfarelli and Rizzi [93].
From the laboratory to the field: data processing and results representation
At the moment, the regulation frame does not require an extensive use of bioassays for assessing the suitability of the studied residues for land spreading; however, this legislative gap is beginning to be acknowledged with a moderate openness to ecotoxicological tests (JRC). On a broader perspective, considering the whole water-waste domain, only very few of them are included in the regulations, together with the respective standards to be met. Consequently, for most of the tests proposed in this work, safety thresholds are not available. Thus, only a direct comparison of the results obtained for different substrates can be conducted. This comparison represents indeed a crucial step of the overall evaluation. In particular, it is important to consider that some of the tested materials (i.e. manure and compost) are commonly used in agriculture and socially well accepted. Thus, their behaviour and properties can be assumed as a reference for comparison. Moreover, in order to get meaningful conclusions, a contextualization process is required, that means to convert raw experimental results in such a way that they are representative of real field conditions (in particular with reference to the applied dosages). Depending on the analysed matrix (either the raw substrate, or the aqueous phase, or the organic extract, for instance) and based on the experimental procedure (e.g. dilution of the sample, addition of reagents or buffer etc.) and results representation mode (e.g. EC50 or comparison with a control sample) different approaches shall be used, as described in the following paragraph.
The direct comparison of toxicity measured on tested materials leads to a reliable assessment of the suitability of the substrates, under the assumption that the reference ones (manure and compost) are indeed suitable. Notwithstanding this, at this stage of the work, a scenario-based multi-dimensional impact assessment was also planned. The impact assessment takes into consideration the environmental, health and economic impact related to the use of the tested substrates in agriculture. Moreover, the agro-food sector is very sensitive towards the need of assessing and certifying products and processes of the supply chain. In this context the results of the impact assessment can successfully fill the knowledge gap and provide the basis for a better definition of communication strategy amongst stakeholders.
Integrated analysis of physical, chemical, microbiological and ecotoxicological results
As previously stated, the criteria for data processing depend on several factors, one of the most relevant being the matrix used for performing the bioassay, namely either the raw substrate or an extract (aqueous or organic). In any case, the dosages of substrates in the common agricultural practice must be considered for a sound evaluation. For these reasons, particular attention was given to this aspect, and the typical dosage was labelled as “reference dosage”. Following this principle, the results of all bioassays were then normalized, by adopting a proper procedure, which was defined, test by test, as described below.
Calculation of substrate dosages for field application in the common agricultural practice
As stated above, the results of all bioassays were normalized with respect to a “reference dosage”, typically adopted in the agriculture practice. For defining a sound “reference dosage” for the scope of this work, three different crops were selected (corn, lettuce and wheat), and, for each one, three different dosages were calculated, based on the chemical-physical properties of the tested substrates, the nutrient need of plants and limitations set by the regulations in force, concerning, for instance, the maximum allowable nitrogen load, the soil characteristics, the type of substrate etc. [11,31,40,41]. Among the three calculated dosages, the average one was assumed as the reference. When a plant was used in a bioassay (e.g. lettuce), its specific reference dosage (i.e. calculated for the same plant, e.g. lettuce) was adopted. Otherwise, the average dosage for corn was selected as representative, corn being the most widely diffused crop in the Po valley and those of corn being the highest dosages among the three cases (corn, lettuce and wheat). In the common agriculture practice, yearly dosages are calculated and expressed as kgSS/ha. These values were converted into kgSS/m3, by assuming a medium soil depth of 30 cm [95]. This was needed for allowing a proper comparison with bioassays conditions: here, the tested substrate was placed in a vessel or a well (of variable width, normally lower than 30 cm) and mixed with other substances (i.e. suspending solutions, cellular suspension, standard soil or other). The toxicity exerted by the pollutants depend on their concentration in the matrix where the target organism is placed. For this reason, in most cases, the assessment of the bioassay results has to take into account the concentration of toxics in the experimental environment, with respect to the concentration that is expected to be found in the soil where the substrate is spread (according to the common agriculture practice). When appropriate, in addition, the dosages used in the bioassays were also reported as mass of substrate per unit area, and this value was compared to the reference dosage expressed in the same measurement units (kgSS/ha).
Tests carried out on raw substrates
This represents the simplest situation to manage, in that the bioassay is conducted on the matrix as it is supposed to be spread on land. As described above, some bioassays were carried out at a specific dosage of the substrate, while others were carried out at different dosages, and the mode of expression of results may be different (e.g. percent of mortality of the test organism, EC50 etc.). Notwithstanding this, it is crucial to compare the tested dosages with the actual dosages adopted in the common agricultural practice. In effect, very high doses (respect to those typically adopted) will likely always cause toxic effects, thus leading to misleading conclusions. As reported above, the recommendation is that for all the substrates and bioassays, the ratio between the dosage used in the test and the average actual dosage (according to the typical agriculture practice) is calculated.
Tests carried out on the aqueous extracts or elutriates
Some bioassays were conducted by placing the target organism within an aqueous extract. This can be simply considered to be the liquid phase of the matrix. The experimental dosage was compared to the reference dosage, by considering that different conditions could be obtained either by diluting the aqueous extract (thus simulating conditions, in the agriculture practice, where the substrate is also diluted by rainwater or other added liquid substances) or, at the contrary, by a concentration process (e.g. evaporation, which can indeed occur in the storage tanks where the matrices may be stored even for long periods before spreading).
Tests carried out on the organic extract
For the bioassays carried out on the organic extract, the same principle was adopted, with minor adaptations, depending on the type of bioassay. Typically, these tests were carried out by dosing a volume of a mother solution into a medium containing the target organism, as described in detail in the previous paragraphs. The mother solution had a defined concentration (grade) of the organic extract. This grade, for sake of simplicity, was referred to the mass of raw substrate (e.g. 1 geq/mL indicates that the organic extract obtained from 1 g of raw substrate was resuspended in 1 mL of solvent). Predetermined volumes of the mother solution (depending on the dosage to be tested) were then taken and added to a constant volume medium, where the target organism was placed. The dosage was then calculated and expressed as the ratio between the mass of the organic extract (expressed as equivalent mass of raw substrate) and the total volume of the medium. Finally, the ratio between this dosage and the reference one (corresponding to the typical agricultural practice) was calculated.
In case a test was executed at a number of different dosages (see, for instance, the baseline toxicity assays), the dose-response curve was plotted. Among the relevant information obtainable from this curve, two were considered to be particularly important: the EC50 and the effect corresponding to the reference dosage. The latter was considered relevant for a sound comparison among the substrates.
In case only one dosage of organic extract was used, even if this was the same for all the substrates, after the normalization with respect to the reference dosage (which may be different for different substrates), slight differences in the normalized doses resulted. Consequently, the comparison of the measured effect among the various substrates was correlated to slightly different dosages (with respect to field application), thus inducing a degree of uncertainty in the comparison itself, unless the effects measured on the substrates were markedly different from each other. It was possible to avoid this risk of misleading comparison in case a dose-response curve with a model substance (calibration curve) was available (e.g. estrogenicity test, with E2 as the model compound). In this situation, a further data processing step was adopted to achieve an even better and more reliable comparison among the substrates. The toxicity measured during the bioassay was given by the adopted normalized dosage of the organic extract (say 5%) and an equivalent concentration of the model compound giving the same toxicity was identified on the calibration curve (say 1 nM). Within the range of normalized doses adopted for all the substrates (say 4–8%) one value was chosen for further normalization (say 6%). The predicted effect at this dosage (at this point, the same for all the substrates) was obtained by assuming the linearity of the dose-response curve within a narrow range of concentrations of the model compound. Following the example, the toxicity was measured at an organic extract (normalized) dosage of 5%, and the model compound concentration exerting the same (equivalent) effect was identified on the calibration curve (1 nM). The predicted effect of a 6% dosage of the organic extract was read on the calibration curve at a concentration of the model substance equal to 1,2 nM (= 1nM: 5 × 6). In case the tested normalized dosage of organic extract was close to the reference one, the effect corresponding to the latter could also be estimated with the same procedure. Conversely, if the tested dosage were much different from the reference one, this procedure could not be adopted since the slope of the dose-response curves of the model substance and the organic extract may be different.
Risk assessment
The presence of organic and inorganic pollutants, metals, and pathogens that are currently considered significant pollutants in the Sewage Sludge Directive (86/278/EEC; SSD) can affect human health through indirect environmental exposure, via the consumption of fish, root and leaf crops, meat, milk, beverages, and water (exposure routes) (Fig. 7). The environmental or health risk associated with potential contaminants present in the sludge matrix is primarily influenced by its application rate on agricultural soils, its stability, its physicochemical properties within the atmosphere-soil-plant-water continuum, and its (eco)toxicology, which indicates the sensitivity of biota and humans to exposure.
Fig. 7.
Exposure routes after use of sewage sludge on agricultural soils.
The first step of the risk assessment was the identification of relevant pollutants present in sewage sludge, which may pose risks to the environment or human health when applied to agricultural lands. Specifically, the methodology enabled the characterization of the risk related to sewage sludge applications for soil organisms and for humans through the consumption of crops cultivated on lands amended with sewage sludge.
The environmental exposure assessment was based on the Local Environment Tool (LET) model developed by the European Crop Protection Association (ECPA) and further adapted and validated by the Joint Research Centre [14]. The model estimates the predicted environmental concentrations (PEC) for various environmental compartments, as well as risk characterizations through risk characterization ratios (RCR) for various environmental receptors (such as aquatic and terrestrial organisms) (Technical Guidance Document on Risk Assessment, JRC 2002). The model has been adapted to project conditions and to the rate of use of sewage sludge according to Italian legislation (Fig. 8).
Fig. 8.
Adaptation of the ECPA-LET model applied to risk assessment.
Note: Wastewater Treatment Plants (WWTP); Predicted No-Effect Concentration of the matrix (PNECmix); Risk Characterization Ratio (RCR); Nitrogen (N); Phosphorus (P)
To assess the risk to soil organisms, the ECPA-LET model has been used to calculate average concentrations of contaminants in the upper soil layer (20 cm depth), computed over a 30-day period following the application of sludges. The model takes into consideration the losses of contaminants through volatilization, leaching, and biodegradation in the soil to estimate PEC of individual pollutant. To evaluate the impacts on soil organisms, the concentrations are compared with the Predicted No-Effect Concentrations (PNEC) integrating experimental tests conducted at multiple trophic levels (blue boxes). In detail, to assess the potential risk posed by the pollutants presents in the analyzed matrices, the calculation of a PNECmix was proposed by dividing the ECx/ LC50/ NOEC value by an appropriate assessment factor (AF) based on the recommendation of the European Medicines Agency (EMEA, 2006). The set of analytical assays performed on soil trophic levels enabled the selection of the Assessment Factor (AF) for the derivation of the PNECmix. A value of 1000 was assigned when at least one short-term EC50 from each of the three trophic levels was utilized, 100 was assigned when a long-term EC10 from one trophic level was employed, 50 was assigned when two long-term results (e.g., EC10) from species representing two trophic levels were considered, and 10 was assigned when long-term results (e.g., EC10) from at least three species representing three trophic levels were considered. Several uncertainties have been addressed to extrapolate from laboratory data of a single species to values suitable for a multispecies ecosystem. These include intra- and inter-laboratory variation in toxicity data; intra- and inter-species variation (biological variance); and short- to long-term toxicity extrapolation. RCRsoil was then calculated using Eq. (1), based on the relative contribution of individual compounds to the overall mixture:
| (1) |
where PECi is the predicted environmental concentration for the individual pollutant.
For the characterization of human risk, the values of Tolerable Daily Intake (TDI) resulting from indirect exposure by the environment are estimated. The concentrations estimation of the contaminant in fish, root and leaf crops, meat, milk, drinking water, and water, was based on a standard diet (Bio-transfer models) using the average concentrations in soil and surface waters over a 180-day period following sludge application. This allowed to consider biodegradation, volatilization, and leaching in soil, as well as biodegradation and water turnover in surface waters. The RCR was calculated as the relationship between the ratio of TDI to body weight and the Safe Limit for Human Consumption (SLHC). RCR greater than one indicates the presence of a potential human health and environmental risk, after which the identified contaminant data are verified and potentially updated based on a literature search. It should be emphasized that the "human to environment" risk rate assesses the impact of exclusive and continuous intake of products from soils amended with sewage sludge as a worst-case scenario.
The adaptation of the model highlighted in Fig. 8 (yellow boxes), pertained to the different analysed scenarios influencing the matrix's application rate. It also addressed the impact of wastewater treatment plant (WWTP) technologies on enhancing sludge quality, and the resultant concentration of pollutants within the matrix.
Lastly, the model included the evaluation of the effects of spreading treated sludge on the N and P cycles in soils and the social acceptability of the use of sewage sludge in agriculture. This assessment has been included to consider socio-economic impacts, such as the enhancement of productivity and the degree of synthetic fertilizer substitution, complementing the risk analysis with a broader evaluative framework.
Overall assessment
In order to put all the information together and give an overall evaluation of the suitability of sludge (and other matrices) for reuse in agriculture, a decision matrix can be proposed. This encompasses regulatory standards on chemical-physical and microbiological characteristics, the results of a battery of bioassays, and risk analysis based on chemical composition. As shown in Table 3, conditions for acceptability are set, depending on the combined evaluation of all three types of information. As for the regulation standards, only two options are considered: either compliance or not compliance. For the bioassays, three conditions are defined, depending on the number of assays (namely, less than 20%, between 20 and 50%, and more than 50%) that show the tested matrix to exert a greater ecotoxicity with respect to the reference one. Three levels are also assumed as possible outcomes of the risk analysis (low, medium, and high, respectively). Finally, the combination of the different factors leads to the judgement of suitability for reuse in agriculture: suitable, suitable with limitations (e.g., on the amounts to be spread on land or type of crops), not recommended. Indeed, some circumstances may not be likely to occur (e.g., “not compliant”; “≥50″; “low”). These less probable situations are put within brackets in the table.
Table 3.
Proposed decision matrix for evaluating the suitability of sludge (and other matrices) for reuse in agriculture. Between brackets those circumstances which are not likely to occur.
 |
The proposed approach gives greater importance to the bioassays, which - it is a whish of the authors - should soon gain the trust of the stakeholders. A similar schema could be proposed for product certification.
Flow-scheme
For sake of clarity, Fig. 9 reports the workflow of the proposed procedure, with indications of paragraphs where the different steps are described.
Fig. 9.
Flow scheme of the proposed procedure. Numbers between brackets refer to the paragraphs where different steps are described. (Rectangles show operations/actions, while other boxes show materials/products/results. White boxes refer to laboratory activities, while grey boxes refer to data processing).
Ethics statements
Zebrafish fertilized eggs were obtained by the facility of the Department of Earth and Environmental Sciences of the University of Milan Bicocca, according to the Italian laws, rules and regulations (Legislative Decree no. 116/92; authorization n. 0020984–12/02/2018).
CRediT authorship contribution statement
Giorgio Bertanza: Conceptualization, Methodology, Writing – original draft, Supervision, Project administration, Funding acquisition. Alessandro Abbà: Validation, Formal analysis, Data curation. Carlotta Alias: Investigation. Achille Amatucci: Investigation. Andrea Binelli: Conceptualization. Sara Castiglioni: Conceptualization, Investigation, Validation. Marco Fossati: Conceptualization, Investigation, Validation. Catarina Cruzeiro: Investigation, Methodology. Camilla Della Torre: Conceptualization, Investigation, Validation. Marta Domini: Resources, Data curation, Visualization. Donatella Feretti: Conceptualization, Investigation, Validation. Gianni Gilioli: Resources, Conceptualization. Stefano Magni: Investigation. Giovanna Mazzoleni: Conceptualization. Michele Menghini: Software, Validation, Formal analysis, Data curation. Roberta Pedrazzani: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. Peter Schroeder: Conceptualization. Anna Simonetto: Software, Validation, Formal analysis, Data curation. Nathalie Steimberg: Conceptualization, Investigation. Vera Ventura: Investigation, Formal analysis, Data curation. Simona Vezzoli: Investigation. Ilaria Zerbini: Investigation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was funded by the CARIPLO Foundation (Milan, Italy) within the call “Circular Economy 2020”. The funded project's title is: “SLURP - SLUdge Recovery in agriculture: environment and health Protection”. Grant number: 2020–1029. https://sites.unimi.it/slurp_project/en/home_en/.
Data availability
Data will be made available on request.
References
- 1.European Council Directive Council directive 91/271/EEC of 21 May 1991 concerning urban waste-water treatment. Off. J. Eur. Communities. 1991;L 135 Official Journal of the European Communities 30 May 1991. [Google Scholar]
- 2.European Commission (2023). COMMISSION STAFF WORKING DOCUMENT EVALUATION - Council Directive 86/278/EEC of 12 June 1986 on the protection of the environment, and in particular of the soil, when sewage sludge is used in agriculture. SWD(2023) 157 final. Brussels (Belgium), 22 May 2023. Available online: https://ec.europa.eu/transparency/documents-register/detail?ref=SWD(2023)157&lang=en (accessed on 22 August 2023).
- 3.Mininni G., Laera G., Bertanza G., Canato M., Sbrilli A. Mass and energy balances of sludge processing in reference and up-graded wastewater treatment plants. Environ. Sci. Pollut. Res. 2015;22:7203–7215. doi: 10.1007/s11356-014-4013-2. [DOI] [PubMed] [Google Scholar]
- 4.B. Vallet (2018). Sewage sludge destination in Europe. Presentation at the European Sustainable Phosphorus Platform (ESPP) General Assembly, Brussels (Belgium), 4 December 2018. Available online: http://www.eureau.org/resources/past-events/water-events-docs/3430-sewage-sludge-destination-in-europe/file (accessed on 12 January 2023).
- 5.European Commission . Memo; Brussels (Belgium): 2014. The European Critical Raw Materials review.http://europa.eu/rapid/press-release_MEMO-14-377_en.htm 26 May 2014 Available online: accessed on 22 August 2023. [Google Scholar]
- 6.Ofoegbu S.U. Technological challenges of phosphorus removal in high-phosphorus ores: sustainability implications and possibilities for greener ore processing. Sustainability, 2019;11(23):6787. doi: 10.3390/su11236787. [DOI] [Google Scholar]
- 7.USGS. (1996–2022). Mineral commodity summaries [YEAR] – phosphate rock
- 8.European Commission (2016). Proposal for a REGULATION OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL laying down rules on the making available on the market of CE marked fertilising products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009. COM/2016/0157 final - 2016/084 (COD). Brussels (Belgium), 17 March 2016. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2016%3A0157%3AFIN (accessed on 11 January 2023).
- 9.Pedrazzani R., Bertanza G., Brnardić I., Cetecioglu Z., Dries J., Dvarionienė J., García-Fernández A.J., Langenhoff A., Libralato G., Lofrano G., Škrbić B., Martínez-López E., Meriç S., Mutavdžić Pavlović D., Papa M., Schröder P., Tsagarakis K.P., Vogelsang C. Opinion paper about organic trace pollutants in wastewater: toxicity assessment in a european perspective. Sci. Total Environ. 2019;651:3202–3221. doi: 10.1016/j.scitotenv.2018.10.027. [DOI] [PubMed] [Google Scholar]
- 10.Domini M., Bertanza G., Vahidzadeh R., Pedrazzani R. Sewage sludge quality and management for circular economy opportunities in Lombardy. Appl. Sci. 2022;12:10391. doi: 10.3390/app122010391. [DOI] [Google Scholar]
- 11.European Council Directive Council directive 86/278/EEC of 12 June 1986 on the protection of the environment, and in particular of the soil, when sewage sludge is used in agriculture. Off. J. Eur. Communities. 1986;L 181 4 July 1986. [Google Scholar]
- 12.EU (2019/1009) Regulation of the European Parliament and of the Council of 5 June 2019 laying down rules on the making available on the market of EU fertilising products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003 (Text with EEA relevance)
- 13.Huygens D., Saveyn H.G.M., Tonini D., Eder P., Sancho L.Delgado. Publications Office of the European Union; Luxembourg: 2019. Technical proposals for selected new fertilising materials under the Fertilising Products Regulation (Regulation (EU) 2019/1009) - Process and quality criteria, and assessment of environmental and market impacts for precipitated phosphate salts & derivates, thermal oxidation materials & derivates and pyrolysis & gasification materials. EUR 29841 EN, JRC1178562019, ISBN 978-92-76-09888-1. [DOI] [Google Scholar]
- 14.Huygens D., García-Gutierrez P., Orveillon G., Schillaci C., Delre A., Orgiazzi A., Wojda P., Tonini D., Egle L., Jones A., Pistocchi A., Lugato E. Publications Office of the European Union; Luxembourg: 2022. Screening Risk Assessment of Organic Pollutants and Environmental Impacts from Sewage Sludge Management - Study to Support Policy Development On the Sewage Sludge Directive (86/278/EEC) [DOI] [Google Scholar]
- 15.Pistocchi H.R., Andersen, Bertanza G., Brander A., Choubert J.M., Cimbritz M., Drewes J.E., Koehler C., Krampe J., Launay M., Nielsen P.H., Obermaier N., Stanev S., Thornberg D. Treatment of micropollutants in wastewater: balancing effectiveness, costs and implications. Sci. Total Environ. 2022;850 doi: 10.1016/j.scitotenv.2022.157593. ISSN 0048-9697. [DOI] [PubMed] [Google Scholar]
- 16.Bertanza G., Papa M., Pedrazzani R., Repice C., Mazzoleni G., Steimberg N., Feretti D., Ceretti E., Zerbini I. EDCs, estrogenicity and genotoxicity reduction in a mixed (domestic + textile) secondary effluent by means of ozonation: a full-scale experience. Sci. Total Environ. 2013;458-460:160–168. doi: 10.1016/j.scitotenv.2013.03.108. [DOI] [PubMed] [Google Scholar]
- 17.Bertanza G., Pedrazzani R., Dal Grande M., Papa M., Zambarda V., Montani C., Steimberg N., Mazzoleni G., Di Lorenzo D. Effect of biological and chemical oxidation on the removal of estrogenic compounds (NP and BPA) from wastewater: an integrated assessment procedure. Water Res. 2011;45:2473–2484. doi: 10.1016/j.watres.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 18.Bertanza G., Steimberg N., Pedrazzani R., Boniotti J., Ceretti E., Mazzoleni G., Menghini M., Urani C., Zerbini I., Feretti D. Wastewater toxicity removal: integrated chemical and effect-based monitoring of full-scale conventional activated sludge and membrane bioreactor plants - Sci. Total Environ. 2022;851 doi: 10.1016/j.scitotenv.2022.158071. ISSN 0048-9697. [DOI] [PubMed] [Google Scholar]
- 19.Escher B.I., Allinson M., Altenburger R., Bain P.A., Balaguer P., Busch W., Crago J., Denslow N.D., Dopp E., Hilscherova K., Humpage A.R., Kumar A., Grimaldi M., Jayasinghe B.S., Jarosova B., Jia A., Makarov S., Maruya K.A., Medvedev A., Mehinto A.C., Mendez J.E., Poulsen A., Prochazka E., Richard J., Schifferli A., Schlenk D., Scholz S., Shiraishi F., Snyder S., Su G., Tang J.Y.M., van der Burg B., van der Linden S.C., Werner I., Westerheide S.D., Wong C.K.C., Yang M., Yeung B.H.Y., Zhang X., Leusch F.D.L. Benchmarking organic micropollutants in wastewater, recycled water and drinking water with in vitro bioassays. Environ. Sci. Technol. 2014;48:1940–1956. doi: 10.1021/es403899t. [DOI] [PubMed] [Google Scholar]
- 20.Escher B.I., Leusch F. IWA Pub; 2012. Bioanalytical Tools in Water Quality Assessment.https://books.google.it/books?hl=it&lr=&id=5CgiS3yNNbAC&oi=fnd&pg=PR13&dq=Bioanalytical+Tools+in+Water+Quality+Assessment&ots=W-ssIWkk0K&sig=yn2w752b2VU-yy6HD49VlWGxzGk&redir_esc=y#v=onepage&q=BioanalyticalToolsinWaterQualityAssessment&f=false (accessed June 7, 2018) [Google Scholar]
- 21.Gonzalez-Gil L., Papa M., Feretti D., Ceretti E., Mazzoleni G., Steimberg N., Pedrazzani R., Bertanza G., Lema J.M., Carballa M. Is anaerobic digestion effective for the removal of organic micropollutants and biological activities from sewage sludge? Water Res. 2016;102:211–220. doi: 10.1016/j.watres.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 22.Papa M., Ceretti E., Viola G.C.V., Feretti D., Zerbini I., Mazzoleni G., Steimberg N., Pedrazzani R., Bertanza G. The assessment of WWTP performance: towards a jigsaw puzzle evaluation? Chemosphere. 2016;145:291–300. doi: 10.1016/j.chemosphere.2015.11.054. [DOI] [PubMed] [Google Scholar]
- 23.Papa M., Pedrazzani R., Bertanza G. How green are environmental technologies? A new approach for a global evaluation: the case of WWTP effluents ozonation. Water Res. 2013;47:3679–3687. doi: 10.1016/j.watres.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 24.Pedrazzani R., Ziliani E., Cavallotti I., Bollati E., Ferreri M., Bertanza G. Use of ecotoxicology tools within the environmental footprint evaluation protocols: the case of wastewater treatment plant. Desalin. Water Treat. 2019;172:2–14. doi: 10.5004/dwt.2019.24344. [DOI] [Google Scholar]
- 25.Bertanza G., Boniotti J., Ceretti E., Feretti D., Mazzoleni G., Menghini M., Pedrazzani R., Steimberg N., Urani C., Viola G.C.V., Zerbini I., Ziliani E. Environmental footprint of wastewater treatment: a step forward in the use of toxicological tools. Int. J. Environ. Res. Public Health. 2021;18:6827. doi: 10.3390/ijerph18136827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menghini M., Pedrazzani R., Feretti D., Mazzoleni G., Steimberg N., Urani C., Zerbini I., Bertanza G. Beyond the black box of life cycle assessment in the wastewater treatment plants: which help from the bioassays? Water. 2023;15:960. doi: 10.3390/w15050960. (Basel) [DOI] [Google Scholar]
- 27.Pedrazzani R., Cavallotti I., Bollati E., Ferreri M., Bertanza G. The role of bioassays in the evaluation of ecotoxicological aspects within the PEF/OEF protocols: the case of WWTPs. Ecotoxicol. Environ. Saf. 2018;147:742–748. doi: 10.1016/j.ecoenv.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 28.Carraturo F., Siciliano A., Giordano A., Di Capua F., Barone F., Casaletta E., Cicotti F., Guida M., Adani F. Ecotoxicological assessment of waste-derived organic fertilizers and long-term monitoring of fertilized soils using a multi-matrix and multi-species approach. Sci. Total Environ. 2024;912 doi: 10.1016/j.scitotenv.2023.169341. [DOI] [PubMed] [Google Scholar]
- 29.Hébert M. Public acceptance and independent certification of biosolids in Canada. Water Pract. Technol. 2007;2(4) doi: 10.2166/wpt.2007.084. [DOI] [Google Scholar]
- 30.Segrè Cohen A., Love N.G., Nace K., Árvai J. Consumers’ acceptance of agricultural fertilizer derived from diverted and recycled human urine. Environ. Sci. Technol. 2020;54(8):5297–5305. doi: 10.1021/acs.est.0c00576. [DOI] [PubMed] [Google Scholar]
- 31.European Council Directive Council Directive 91/676/EEC of 12 December 1991 concerning the protection of waters against pollution caused by nitrates from agricultural sources. Off. J. 1991;L 375 31 December 1991. [Google Scholar]
- 32.Government of Italy (2010). Decreto Legislativo 29 aprile 2010, n. 75 - Riordino e revisione della disciplina in materia di fertilizzanti, a norma dell'articolo 13 della legge 7 luglio 2009, n. 88. [Legislative Decree n. 75 of April 29, 2010 - Reorganization and Revision of the Regulation on Fertilizers, Pursuant to Article 13 of Law no. 88. of 7 July 2009]. Ordinary supplement to the "Official Gazette" n. 121 of May 26, 2010 - General series. Rome (Italy). Available online: https://www.gazzettaufficiale.it/eli/id/2010/05/26/010G0096/sg (accessed on 1 August 2023).
- 33.UNI EN (2013): UNI, E. N. 10802, 2013. Waste–manual sampling, sample preparation and analysis of eluates. UNI–Ente nazionale italiano di unificazione (Italian unification authority). Standard Number: UNI, 2013, 10802: 2013.
- 34.Zuccato E., Calamari D., Natangelo M., Fanelli R. Presence of therapeutic drugs in the environment. Lancet. 2000;355(9217):1789–1790. doi: 10.1016/S0140-6736(00)02270-4. [DOI] [PubMed] [Google Scholar]
- 35.Riva F., Zuccato E., Castiglioni S. Prioritization and analysis of pharmaceuticals for human use contaminating the aquatic ecosystem in Italy. J. Pharm. Biomed. Anal. 2015;106:71–78. doi: 10.1016/j.jpba.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Riva F., Zuccato E., Pacciani C., Colombo A., Castiglioni S. A multi-residue analytical method for extraction and analysis of pharmaceuticals and other selected emerging contaminants in sewage sludge. Anal. Methods. 2021;13(4):526–535. doi: 10.1039/D0AY02027C. [DOI] [PubMed] [Google Scholar]
- 37.Kasprzyk-Hordern B., Béen F., Bijlsma L., Brack W., Castiglioni S., Covaci A.…Thomas K.V. Wastewater-based epidemiology for the assessment of population exposure to chemicals: the need for integration with human biomonitoring for global One Health actions. J. Hazard. Mater. 2023;450 doi: 10.1016/j.jhazmat.2023.131009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saiki P., Mello-Andrade F., Gomes T., Rocha T.L. Sediment toxicity assessment using zebrafish (Danio rerio) as a model system: historical review, research gaps and trends. Sci. Total. Environ. 2021;793 doi: 10.1016/j.scitotenv.2021.148633. [DOI] [PubMed] [Google Scholar]
- 39.Roig N., Sierra J., Nadal M., Martí E., Navalón-Madrigal P., Schuhmacher M., Domingo J.L. Relationship between pollutant content and ecotoxicity of sewage sludges from Spanish wastewater treatment plants. Sci. Total. Environ. 2012;425:99–109. doi: 10.1016/j.scitotenv.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 40.Government of Italy (1992). Attuazione della Direttiva n. 86/278/CEE concernente la protezione dell'ambiente, in particolare del suolo, nell'utilizzazione dei fanghi di depurazione in agricoltura. [Implementation of Directive 86/278/EEC on the protection of the environment, and in particular of the soil, when sewage sludge is used in agriculture]. Ordinary supplement to the Official Gazette n. 38 of February 15, 1992 - General series. Rome (Italy). Available online: https://www.gazzettaufficiale.it/eli/id/1992/02/15/092G0139/sg (accessed on 1 August 2023).
- 41.Lombardy Region (2014). Deliberazione del Consiglio Regionale X/2031, 1 Luglio 2014 - Disposizioni regionali per il trattamento e l'utilizzo, a beneficio dell'agricoltura, dei fanghi di depurazione delle acque reflue di impianti civili ed industriali in attuazione dell'art. 8, comma 8, della Legge Regionale 12 luglio 2007, n. 12. Conseguente integrazione del punto 7.4.2, comma 6, n. 2) della D.G.R. 18 aprile 2012, n. IX/3298, riguardante le linee guida regionali per l'autorizzazione degli impianti per la produzione di energia elettrica da fonti energetiche rinnovabili, 2014 [Deliberation of Regional Council X/2031 July 1, 2014 containing regional dispositions for treatment and use, for the benefit of agriculture, of sewage sludge from urban and industrial wastewater treatment plants in implementation of art. 8, paragraph 8 of Regional Law July 12, 2007, n. 12. Consequent integration of point 7.4.2, paragraph 6, n. 2 of D.G.R. April 18, 2012, n. IX/3298, regarding regional guidelines for the authorization of plants for the production of electricity from renewable energy sources]. Lombardy Region Official Gazette, Ordinary Serie n. 28 of July 10, 2014. Available online: https://www.regione.lombardia.it/wps/wcm/connect/e81d0f54-bedf-4918-886f-ad4db4c04911/D.g.r.+2031_2014.pdf?MOD=AJPERES&CACHEID=ROOTWORKSPACE-e81d0f54-bedf-4918-886f-ad4db4c04911-mH4K-bC (accessed on 2 February 2024)
- 42.Veneto Region (2009). Delibera della Giunta n. 235/2009. Utilizzo in agricoltura di fanghi di depurazione e di altri fanghi e residui non tossico e nocivi di cui sia comprovata l'utilità ai fini agronomici; impianti di recupero e di trattamento delle frazioni organiche dei rifiuti urbani ed altre matrici organiche mediante compostaggio, biostabilizzazione e digestione anaerobica. Modifiche al disposto della DGRV n. 2241/05 e DGRV n. 568/05 [Deliberation of the Regional Government n. 235/2009, Use in agriculture of sewage sludge and other sludge non-toxic and harmful, with agronomic usefulness; recovery and treatment plants for the organic fractions of municipal waste and other organic matrices through composting, biostabilisation and anaerobic digestion. Amendments to DGRV no. 2241/05 and DGRV no. 568/05]. Veneto Region Official Gazette n. 19 of March 3, 2009. Available online: http://assets.gestione-rifiuti.it/legislazione-regionale/Veneto/VENETO-DGR-10-febbraio-2009,n.235.pdf (accessed on 2 February 2024).
- 43.DIN ISO . German Institute for Standardization; Berlin (Germany): 2011. Soil quality - Determination of soil microbial biomass - Part 2: Fumigation-extraction method (ISO 14240-2:1997) DIN EN ISO 14240-2:2011-09. [Google Scholar]
- 44.Joergensen R.G. Quantification of the microbial biomass by determining ninhydrin-reactive N. Soil Biol. Biochem. 1996;28(3):301–306. doi: 10.1016/0038-0717(95)00141-7. [DOI] [Google Scholar]
- 45.Joergensen R.G., Mueller T. The fumigation-extraction method to estimate soil microbial biomass: calibration of the kEN value. Soil Biol. Biochem. 1996;28(1):33–37. doi: 10.1016/0038-0717(95)00101-8. [DOI] [Google Scholar]
- 46.Carr S.A., Liu J., Tesoro A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016;91:174–182. doi: 10.1016/j.watres.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 47.Nizzetto L., Futter M., Langaas S. S. Are agricultural soils dumps for microplastics of urban origin? Environ. Sci. Technol. 2016;50:10777–10779. doi: 10.1021/acs.est.6b04140. [DOI] [PubMed] [Google Scholar]
- 48.Magni S., Binelli A., Pittura L., Avio C.G., Della Torre C., Parenti C.C.…Regoli F. The fate of microplastics in an Italian Wastewater Treatment Plant. Sci. Total Environ. 2019;652:602–610. doi: 10.1016/j.scitotenv.2018.10.269. [DOI] [PubMed] [Google Scholar]
- 49.Hurley R.R., Nizzetto L. Fate and occurrence of micro (nano) plastics in soils: knowledge gaps and possible risks. Curr. Opin. Environ. Sci. Health. 2018;1:6–11. doi: 10.1016/j.coesh.2017.10.006. [DOI] [Google Scholar]
- 50.Huerta Lwanga E., Gertsen H., Gooren H., Peters P., Salánki T., Van Der Ploeg M.…Geissen V. Microplastics in the terrestrial ecosystem: implications for Lumbricus terrestris (Oligochaeta, Lumbricidae) Environ. Sci. Technol. 2016;50(5):2685–2691. doi: 10.1021/acs.est.5b05478. [DOI] [PubMed] [Google Scholar]
- 51.Rist S., Hartmann N.B. Aquatic ecotoxicity of microplastics and nanoplastics: lessons learned from engineered nanomaterials. Freshw. Microplastics. 2018:25–49. doi: 10.1016/j.jpba.2014.10.003. Emerging environmental contaminants? [DOI] [Google Scholar]
- 52.Wang T., Wang L., Chen Q., Kalogerakis N., Ji R., Ma Y. Interactions between microplastics and organic pollutants: effects on toxicity, bioaccumulation, degradation, and transport. Sci. Total Environ. 2020;748 doi: 10.1016/j.scitotenv.2020.142427. [DOI] [PubMed] [Google Scholar]
- 53.Franzellitti S., Canesi L., Auguste M., Wathsala R.H., Fabbri E. Microplastic exposure and effects in aquatic organisms: a physiological perspective. Environ. Toxicol. Pharmacol. 2019;68:37–51. doi: 10.1016/j.etap.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 54.Hartmann N., Hüffer T., Thompson R.C., Hassellöv M., Verschoor A., Daugaard A.E., Rist S., Karlsson T.M., Breenholt N., Cole M., Herrling M.P., Heß M., Ivleva N.P., Lusher A.L., Wagner M. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ. Sci. Technol. 2019;53(3):1039–1047. doi: 10.1021/acs.est.8b05297. [DOI] [PubMed] [Google Scholar]
- 55.UNI EN ISO (2004): UNI EN ISO 17734:2004. Evaluation of the “ultimate” anaerobic biodegradability of organic compounds in digested sludge. (In Italian).
- 56.UNI Ente italiano di normazione . Ente Nazionale Italiano di Unificazione; Rome, Italy: 2018. Metodo per la misura della produzione potenziale di metano da digestione anaerobica ad umido - Matrici in alimentazione [Method for the assessment of potential production of methane from anaerobic digestion in wet conditions - Matrix into foodstuffs] UNI/TS 11703:2018. [Google Scholar]
- 57.Griffiths R.I., Whiteley A.S., O'Donnell A.G., Bailey M.J. Rapid method for coextraction of DNA and RNA from natural environments for analysis of ribosomal DNA-and rRNA-based microbial community composition. Appl. Environ. Microbiol. 2000;66(12):5488–5491. doi: 10.1128/AEM.66.12.5488-5491.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Russell W.M.S., Burch R.L. Methuen & Co. Limited; 1959. The Principles of Humane Experimental Technique; p. 1959. [Google Scholar]
- 59.Fiskesjö G. The Allium test as a standard in environmental monitoring. Hereditas. 1985;102(1):99–112. doi: 10.1111/j.1601-5223.1985.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 60.APAT, (2004). Guida tecnica su metodi di analisi per il suolo e i siti contaminati. Utilizzo di indicatori biologici ed ecotossicologici. (In italian)
- 61.UNI (2010): UNI 11357, 2010. Water quality - determination of the inhibition of the seed germination and root elongation of Cucumis sativus L. (cucumber), Lepidium sativum L. (water cress), Sorghum saccharatum Moench (sorghum) Short-chronic toxicity test
- 62.Lombardy Region (2020). Programma d'azione nitrati 2020-23 [Nitrates Action Plan 2020-2023]. Lombardy Region Official Gazette n. 10 of March 6, 2020. Available at: https://www.regione.lombardia.it/wps/portal/istituzionale/HP/DettaglioRedazionale/servizi-e-informazioni/Imprese/Imprese-agricole/direttiva-nitrati/normativa-nitrati-in-regione-lombardia/normativa-nitrati-in-regione-lombardia (accessed on 8 February 2024).
- 63.ISO (2012): ISO 11268-1:2012 Soil quality. Effects of pollutants on earthworms. Part 1: determination of acute toxicity to Eisenia fetida/Eisenia andrei.
- 64.Della Torre C., Liberatori G., Ghilardi A., Del Giacco L., Puccini M., Ferraro F., Corsi I. The zebrafish (Danio rerio) embryo-larval contact assay combined with biochemical biomarkers and swimming performance in sewage sludge and hydrochar hazard assessment. Environ. Pollut. 2022;302 doi: 10.1016/j.envpol.2022.119053. [DOI] [PubMed] [Google Scholar]
- 65.Schiwy S., Bräunig J., Alert H., Hollert H., Keiter S. A novel contact assay for testing aryl hydrocarbon receptor (AhR)-mediated toxicity of chemicals and whole sediments in zebrafish (Danio rerio) embryos. Environ. Sci. Pollut. 2015;22:16305. doi: 10.1007/s11356-014-3185-0. –1. [DOI] [PubMed] [Google Scholar]
- 66.Borenfreud E., Puerner J.A. A simple quantitative procedure using monolayer cultures for cytotoxicity assays (HTD/NR-90) J. Tissue Cult. Methods. 1984;9:7–9. doi: 10.1007/BF01666038. [DOI] [Google Scholar]
- 67.Bernard-Beaubois K., Hecquet C., Hayem G., Rat P., Adolphe M. vitro study of cytotoxicity of quinolones on rabbit tenocytes. Cell Biol. Toxicol. 1988;14:283–292. doi: 10.1023/A:1007435025616. [DOI] [PubMed] [Google Scholar]
- 68.El-Fouly M.H., Trosko J.E., Chang C.C. Scrape-loading and dye transfer. A rapid and simple technique to study gap junctional intercellular communication. Exp. Cell Res. 1987;168(2):422–430. doi: 10.1016/0014-4827(87)90014-0. [DOI] [PubMed] [Google Scholar]
- 69.Jeyanthi V., Paul J.A.J., Selvi B.K., Karmegam N. Comparative study of biochemical responses in three species of earthworms exposed to pesticide and metal contaminated soil. Environ. Process. 2016;3:167–178. doi: 10.1007/s40710-016-0131-9. [DOI] [Google Scholar]
- 70.Qiao Z., Li P., Tan J., Peng C., Zhang F., Zhang W., Jiang X. Oxidative stress and detoxification mechanisms of earthworms (Eisenia fetida) after exposure to flupyradifurone in a soil-earthworm system. J. Environ. Manag. 2022;322 doi: 10.1016/j.jenvman.2022.115989. [DOI] [PubMed] [Google Scholar]
- 71.Yatoo A.M., Ali M.N., Zaheen Z., Baba Z.A., Ali S., Rasool S.…Hamid B. Assessment of pesticide toxicity on earthworms using multiple biomarkers: a review. Environ. Chem. Lett. 2022;20(4):2573–2596. doi: 10.1007/s10311-022-01386-0. [DOI] [Google Scholar]
- 72.Zhao W., Teng M., Zhang J., Wang K., Zhang J., Xu Y., Wang C. Insights into the mechanisms of organic pollutant toxicity to earthworms: advances and perspectives. Environ. Pollut. 2022;303 doi: 10.1016/j.envpol.2022.119120. [DOI] [PubMed] [Google Scholar]
- 73.Dubois M., Gilles K.A., Hamilton J.K.J., Rebers P.A., Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28(3):350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- 74.Buege J.A., Aust S.D. Microsomal lipid peroxidation. Meth. Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 75.Leuthold D., Klüver N., Altenburger R., Busch W. Can environmentally relevant neuroactive chemicals specifically Be detected with the locomotor response test in zebrafish embryos? Environ. Sci. Technol. 2019;53(1):482–493. doi: 10.1021/acs.est.8b04327. [DOI] [PubMed] [Google Scholar]
- 76.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 77.Parenti C.C., Ghilardi A., Della Torre C., Mandelli M., Magni S., Del Giacco L., Binelli A. Environmental concentrations of triclosan activate cellular defence mechanism and generate cytotoxicity on zebrafish (Danio rerio) embryos. Sci. Total Environ. 2019;650:1752–1758. doi: 10.1016/j.scitotenv.2018.09.283. [DOI] [PubMed] [Google Scholar]
- 78.Della Torre C., Maggioni A., Ghilardi A., Parolini M., Santo N., Landi C., Madaschi L., Magni S., Tasselli S., Ascagni M., Bini L., La Porta L., Del Giacco L., Binelli A. The interactions of fullerene C60 and Benzo(a)pyrene influence their bioavailability and toxicity to zebrafish embryos. Environ. Pollut. 2019;241:999–1008. doi: 10.1016/j.envpol.2018.06.042. [DOI] [PubMed] [Google Scholar]
- 79.Ellman G.L., Courtney K.D., Andres V., Featherstone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 80.Jomova K., Raptova R., Alomar S.Y., Alwasel S.H., Nepovimova E., Kuca K., Valko M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch. Toxicol. 2023;97(10):2499–2574. doi: 10.1007/s00204-023-03562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maron D.M., Ames B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983;113(3–4):173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- 82.APHA . 23rd edition. American Public Health Association/American Water Works Association/Water Environmental Federation; Washington D.C., USA: 2017. Standard Method for the Examination of Water and Wastewater. [Google Scholar]
- 83.Dhawan A., Anderson D. The Royal Society of Chemistry; Cambridge, UK: 2009. The Comet Assay in Toxicology. [Google Scholar]
- 84.Pedrazzani R., Baroni P., Feretti D., Mazzoleni G., Steimberg N., Urani C.…Bertanza G., Roy K. Ecotoxicological QSARs. Methods in Pharmacology and Toxicology. Humana; New York, NY: 2020. Methodological protocol for assessing the environmental footprint by means of ecotoxicological tools: wastewater treatment plants as an example case. [DOI] [Google Scholar]
- 85.Ivorra L., Cruzeiro C., Ramos A., Tagulao K., Cardoso P.G. How can environmental conditions influence dicofol genotoxicity on the edible Asiatic clam, Meretrix meretrix? Environ. Pollut. 2022;293 doi: 10.1016/j.envpol.2021.118467. [DOI] [PubMed] [Google Scholar]
- 86.Alias C., Piovani G., Benassi L., Abbà A., Sorlini S., Gelatti U., Zerbini I., Feretti D. Evaluation of toxicity and genotoxicity of concrete cast with steel slags using higher terrestrial plants. Environ. Toxicol. Chem. 2023 doi: 10.1002/etc.5709. [DOI] [PubMed] [Google Scholar]
- 87.Passatore L., Pietrini F., Carloni S., Massimi L., Giusto C., Zacchini M., Iannilli V. Morpho-physiological and molecular responses of Lepidium sativum L. seeds induced by bismuth exposure. Sci. Total. Environ. 2022;831 doi: 10.1016/j.scitotenv.2022.154896. [DOI] [PubMed] [Google Scholar]
- 88.Sforzini S., Boeri M., Dagnino A., Oliveri L., Bolognesi C., Viarengo A. Genotoxicity assessment in Eisenia andrei coelomocytes: a study of the induction of DNA damage and micronuclei in earthworms exposed to B [a] P-and TCDD-spiked soils. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2012;746(1):35–41. doi: 10.1016/j.mrgentox.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 89.Hong Y.H., Jeon H.L., Ko K.Y., Kim J., Yi J.S., Ahn I., Kim T.S., Lee J.K. Assessment of the predictive capacity of the optimized in vitro comet assay using HepG2 cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018 Mar;827:59–67. doi: 10.1016/j.mrgentox.2018.01.010. 10.1016/j.mrgentox.2018.01.010 [DOI] [PubMed] [Google Scholar]
- 90.Leme D.M., Marin-Morales M.A. Allium cepa test in environmental monitoring: a review on its application. Mutat. Res. 2009;682(1):71–81. doi: 10.1016/j.mrrev.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 91.Cabaravdic M. Induction of chromosome aberrations in the Allium cepa test system caused by the exposure of cells to benzo(a) pyrene. Med. Arh. 2010;64:215–218. [PubMed] [Google Scholar]
- 92.Rank J. The method of Allium anaphase-telophase chromosome aberration assay. Ekologija. 2003;1(1):38–42. [Google Scholar]
- 93.Golfarelli M., Rizzi S. McGraw-Hill, Inc; 2009. Data Warehouse design: Modern principles and Methodologies. ISBN: 978-0071610391. [DOI] [Google Scholar]
- 94.Jarke M., Lenzerini M., Vassiliou Y., Vassiliadis P. Multidimensional data models and aggregation. Fundam. Data Wareh. 2000:87–105. doi: 10.1007/978-3-662-04138-3_5. [DOI] [Google Scholar]
- 95.Lombardy Region (2003). Deliberazione della Giunta Regionale n. 7/12764. Linee giuda relative alla costruzione e all'esercizio degli impianti di produzione di compost. Revoca della D.G.R. 16 luglio 1999 n. 44263 [Regional Decree n. 7/12764. Regional guidelines for the construction and management of composting plants. Revocation of the decree 16/07/1999 n. 44263]. Lombardy Region Official Gazette n. 20 of May 13, 2003. Available online: https://www.isprambiente.gov.it/it/progetti/cartella-progetti-in-corso/suolo-e-territorio-1/uso-dei-fanghi-di-depurazione-in-agricoltura-attivita-di-controllo-e-vigilanza-del-territorio/files/LOMBARDIA_DGR_12764_2003_Linee_guida_Compostaggio.pdf (accessed on 9 February 2023).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.