HIGHLIGHTS
-
•
Wildlife-to-human pathogen spillovers are increasing in frequency.
-
•
Western countries lead wildlife trade demand, exacerbating a public health threat.
-
•
Veterinarians and physicians have untapped potential to mitigate disease outbreaks.
-
•
One Health education and patient interactions are opportunities to expand roles.
Keywords: COVID-19, disease spillovers, wildlife trade, zoonotic disease, clinical care, One Health
Abstract
Introduction
The transmission of pathogens from wildlife to humans is a major global health threat that has been highlighted by the proposed origins of the COVID-19 pandemic. Numerous barriers impede pathogen spillover events from ensuing widespread human transmission, but human activity has accelerated the frequency of spillovers and subsequent disease outbreaks, in part through a booming wildlife trade whose impacts on health are not well understood.
Methods
A literature review was conducted to examine the risk that the wildlife trade poses to public health and the degree to which these risks are recognized and addressed in clinical practice and medical and veterinary education.
Results
The illicit aspects of the wildlife trade challenge efforts to understand its impacts on health. The U.S. and Europe play a leading role in the global wildlife trade that often goes unacknowledged. In particular, the consumption of wild meat and ownership of exotic pets poses public health risks. The potential role of clinicians is underutilized, both in the clinical setting and in clinical education.
Discussion
Physicians and veterinarians have the unique opportunity to utilize their clinical roles to address these knowledge gaps and mitigate future outbreaks. We outline a multifaceted approach that includes increasing clinical knowledge about the ecology of zoonotic diseases, leveraging opportunities for mitigation during patient/client–clinician interactions, and incorporating One Health core competencies into medical and veterinary school curricula.
Graphical Abstract

INTRODUCTION
The identification and early spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the etiologic virus behind the coronavirus disease 2019 (COVID-19) pandemic, offers crucial insights for future disease mitigation. On December 30, 2019, ophthalmologist Dr. Li Wenliang was credited as the first to alert the world of a potential outbreak after 7 patients developed a pattern of severe acute respiratory syndrome (SARS)–like illness at his hospital.1 All 7 patients were linked to the Huanan Seafood Wholesale Market, where vendors sold an array of animals and animal products, including live wildlife, and to which the earliest cases in the pandemic had been associated or were in close proximity.2 These events underscore 2 important points: (1) there are ecologic underpinnings to this pandemic and recent 21st-century outbreaks and (2) clinicians have an important role to play in detecting emerging infectious diseases.
In this study, we argue that clinicians have the potential to proactively mitigate disease outbreaks if given greater knowledge of the ecologic underpinnings of many infectious disease outbreaks, specifically the transmission of disease from animals to humans. We use the term clinician to refer to those charged with the medical management of diseases in either humans or animals. Clinical medicine's focus on disease pathology and treatment often precludes consideration of disease ecology during educational training and practice. Despite this traditional view of treating disease at the individual level, clinicians have the unique ability to be among the first to detect disease outbreaks in populations, as illustrated by the late Dr. Li with COVID-19. Subsequent discussion will focus on anthropogenic drivers of pathogen transmission from animals to humans and how clinicians are uniquely poised to mitigate the risk of disease spillover. Although much of the initial pandemic news coverage focused on factors promoting spillover risk in Asia, these threats are also present and need to be addressed in the U.S. and Europe.
METHODS
This narrative review was inspired by the authors’ multidisciplinary training in both clinical veterinary and human medicine and research expertise in disease ecology, epidemiology, and One Health. The literature review was conducted with Google Scholar and PubMed. Well-referenced disease ecology literature was synthesized to briefly identify the ecologic factors that impact the transmission of zoonotic pathogens to humans. The anthropological factors that have favored the emergence of infectious diseases were described with examples from recent disease outbreaks. Research was supplemented with a review of publications and policy guidelines from professional organizations and both international and domestic regulation agencies to assess the extent to which wildlife trade is recognized as a source of pathogen transmission. Recommendations for clinical practice were guided by the authors’ clinical training and a broad search of examples of how recognition of the disease ecology cycle impacted patient care. Finally, a literature search was conducted to identify ways that One Health curricula can be integrated into medical and veterinary education.
RESULTS/DISCUSSION
When Spillover Sparks a Pandemic: The Ecologic Underpinnings of SARS-CoV-2
The majority of emerging infectious diseases are zoonotic (transmitted from an animal source), and over 70% of zoonotic pathogens originate in wildlife.3 Novel zoonotic diseases in humans often stem from spillover events, where a pathogen in a reservoir host (a natural population in which a pathogen can reproduce and be transmitted) successfully infects a new host species.4 SARS-CoV-2 likely first entered the human population in November–December 2019 through spillover events at the Huanan Market, where a number of wildlife species susceptible to SARS-CoV-2 (e.g., raccoon dogs) were sold.5,6 Wildlife at the market has been implicated in playing the role of bridge host, acquiring SARS-CoV-2 from its natural animal reservoir (believed to be a bat species7) and continuing the interspecies transmission chain by subsequently transmitting it to humans.5,8
The emergence of transmissible human diseases by pathogens initially confined to animals is arguably an uncommon and stochastic process because there are many biological impediments to a successful spillover (Figure 1).4 Pathogens have a natural host species range that is determined in part by genetic adaptations they undergo during coevolution with their natural hosts, which create an impediment to infection of new species.4 If a pathogen is able to infect a host of a new species, it often fails to establish disease because the pathogen must then overcome the host's immune system to successfully disseminate into a population by shedding and transmitting to susceptible hosts.4 However, pathogen predilection for conserved host targets and anthropogenic drivers can overcome these barriers. For example, the SARS-CoV-2 virus has the potential to infect a variety of mammalian hosts by binding the angiotensin-converting enzyme 2 protein, a receptor that is highly conserved across mammalian species.9
Figure 1.
Simplified diagram depicting the ecology of emerging infectious diseases.
Natural ecologic barriers impede zoonotic pathogens from infecting humans and causing outbreaks. Anthropogenic activity can alter conditions to favor pathogen spillover and evolution for greater pathogenicity in humans, allowing for eventual dissemination in human populations. Increased travel and global connectivity further increase a pathogen's epidemic potential.
Human activities are accelerating the frequency of spillover events (Figure 1).10 Expansion of human settlements, agricultural lands, and road networks into wildlife habitats has brought people in closer proximity to wildlife, increasing opportunities for disease transmission at the human–wildlife interface. Furthermore, increased global connectivity and the wildlife trade (introduced in the following section) have resulted in the translocation of animals and pathogens to non-native locations.10 For example, severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1)—the etiologic agent of SARS—has been traced back to Chinese wildlife markets where the virus' proposed animal host (i.e., palm civets) was thrust in close proximity to humans.11,12 SARS-CoV-1 and SARS-CoV-2 are just 2 of many 21st-century outbreaks that are attributable to pathogen spillovers facilitated by human activity; others include Middle East respiratory syndrome coronavirus (domesticated camels, close contact),13 2013–2015 Ebola virus (bats, forest fragmentation from human activity),14 Nipah virus (bats and livestock pigs, agricultural intensification),15 and 2009 H1N1 influenza virus (livestock pigs, close contact) (Table 1).16
Table 1.
Examples of 21st-Century Outbreaks With Links to Wildlife or the Wildlife Trade
| Pathogen | Year(s) active | Role played by wildlife and/or the wildlife trade in the origin or amplification of the outbreak |
|---|---|---|
| Ebola virusa | 2000–2001, 2003–2005, 2007–2008, 2011–2012, 2014, 2017–2018, 2020–2022 | Bats are the suspected reservoir host, and nonhuman primates and duikers have been implicated as bridge hosts in spillover events to humans.17 Several outbreaks have been associated with consumption of chimpanzees that were found dead.18 |
| Hanta virus | 2000–2023 | Transmitted from rodents to humans through inhalation of rodent droppings and saliva. Transmission is hypothesized to stem from increased human exposure due to climate factors and land development.19 Sporadic cases/outbreaks have been linked to exposure to rodents with no evidence, to date, of human-to-human transmission.20 |
| Mpox virusa | 2003, 2022–2023 | 2003 U.S. outbreak: Mpox virus entered the U.S. through a pet trade shipment from Ghana that included infected giant pouched rats, rope squirrels, and dormice.21 The virus spread from imported animals to American native prairie dogs housed in the same facility, who then spread the disease to humans after they were adopted as pets.22,21 2022–2023 global epidemic: Initial cluster of cases were confirmed in the United Kingdom in May 2022, and human-to-human transmission has been rapid and widespread, affecting over 100 countries. The animal origins of the epidemic are unknown.23 |
| Nipah virus | 2001, 2003–2004, 2007–2012, 2015, 2018, 2023 | Fruit bats are the reservoir host and transmit the virus to domestic pigs (bridge host), who get infected by eating fruit contaminated by infected bats.24 Pigs transmit the virus to humans through direct contact, and human-to-human transmission can occur through direct contact, aerosols, or fomites.24 |
| Salmonella agbenia | 2016–2017 | 2017 U.S. outbreak with 76 confirmed cases linked to domestically farmed pet turtles.25 Traceback investigation did not identify a single common source farm, but genetic analysis indicated that the offending strain was the same as one isolated from a turtle in 2015 and one that caused a smaller 2016 outbreak (63% of cases linked to pet turtles).25 Small outbreaks of Salmonella species with links to exotic pets occur almost annually in the U.S. (e.g., S. typhimurium outbreak linked to pet turtles in 202026 and S. typhimurium outbreak linked to pet hedgehogs in 201927) |
| SARS-CoV-1a | 2002–2004 | Originated in bats and transmitted to farmed palm civets (bridge host) that were eventually transported to live animal markets in Guangdong where the virus spread among market civets before spillover to humans.28 |
| SARS-CoV-2a | 2019–ongoing | Originated in bats and transmitted to 1 or more wild mammalian species sold at the Huanan live animal market, where initial spillover to humans likely occurred.5,7 |
Note: Wildlife species and aspects of wildlife trade that played a role in outbreaks are indicated by bold and bold/underlined text, respectively.
Pathogen name denotes outbreaks mediated by the wildlife trade.
SARS-CoV-1, severe acute respiratory syndrome coronavirus 1; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Given the significant role of human activity in recent epidemics, we seek to explore some of the societal factors that have brought humans into close contact with nondomesticated animals. More specifically, we aim to understand the aspects of the wildlife trade that pose a risk to public health. Early responses to the pandemic included closure of Chinese live animal markets and calls to regulate the country's wildlife trade. However, these demands do not address the substantial role that Western countries play in the wildlife trade, which leaves open opportunities for future pathogen spillover. Throughout the following sections, we argue that clinicians through their clinical duties may uniquely address the drivers of the wildlife trade to mitigate spillovers (Figure 2).
Figure 2.
Expanding the clinician's role.
Recommendations for expanding the role of clinicians in the mitigation of zoonotic disease are provided.
Drivers of Wildlife Trade and Consumerism
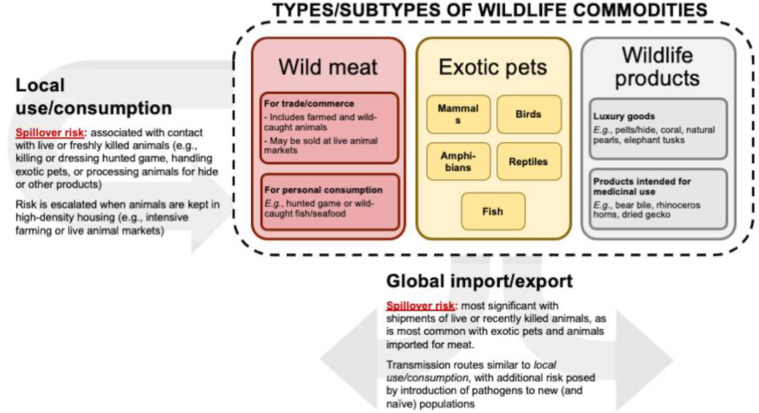
A brief overview of the global wildlife trade. Wildlife trade—the commerce of living or dead wild animals and their derived products—is a large legal and illegal global financial operation that transports thousands of diverse species worldwide for a variety of uses (Figure 3). The extent of wildlife trade and consumption is unknown because illegal wildlife trade also consists of products derived from protected animals, which may be reported under false names or not at all.29 The wildlife trade is estimated to include thousands of animal species, including mammals, birds, amphibians, reptiles, and fish.30 The U.S. and the European Union are among the top importers of legal and illegal wildlife shipments,31, 32, 33 and this global translocation of wild animals can result in the unintentional movement of the pathogens they harbor and introduce infectious diseases to new and potentially immunologically naïve populations.10
Figure 3.
Types of wildlife commodities and the spillover risks associated with their use in a local or global context.
Wildlife commodities can be roughly divided into wild meat, exotic pets, and wildlife products, which can be further subdivided into luxury goods and products intended for medicinal use. The spillover risks associated with both locally acquired and globally imported wildlife commodities are highest when contact is with live or freshly killed animals (e.g., handling exotic pets or freshly killed meat). Global importation of wildlife heightens the risk of disease outbreaks by increasing the likelihood of introducing pathogens to an immunologically naive population.
There are major regulatory gaps with regard to the wildlife trade, despite the significant risks posed to public health. There are currently no international or national conventions on pathogen surveillance for wildlife and wildlife products,34 and the U.S. does not screen for pathogens when importing wildlife shipments, so the majority of imports are untested.35 Furthermore, knowledge of both potential disease risk and safe handling practices is lacking, and guidance is limited. The potential function of physicians and veterinarians in addressing emerging infectious diseases is increasingly acknowledged, but their roles in mitigating potential disease outbreaks in the clinical setting remain poorly defined.36,37 In this study, we consider the function that clinicians can play in addressing zoonotic disease risk from wild animals because this is a neglected topic, especially when contrasted with the widespread awareness of zoonotic risk posed by domestic animals in the human and animal health professions.
In addition to recognizing sentinel events of emerging zoonotic disease, physicians may also serve as an important source of information while gathering patient data to fill a knowledge gap. The confidential visits between physicians and patients offer an unrealized opportunity to use screening questions to determine the extent to which wildlife products are being used at the individual and community levels. By learning about their patients’ usage of wildlife products, physicians would be better equipped to recognize the potential risk for disease outbreaks by broadening their differential diagnoses and counseling safer behavioral practices. Similarly, veterinarians who care for companion animals can educate clients about the zoonotic disease risks posed by exotic pets, and wildlife veterinarians can detect diseases in wildlife that may pose a risk to human health. We focus our discussion in the following section on 2 of the 3 categories of wildlife commodities that are depicted in Figure 3: wild meat and exotic pets. Although the trade in wildlife products is a massive industry, we reason that its health risks are minor compared with those of the other categories because these products (e.g., furs or horns) are processed before import to the U.S. and Europe and are significantly less likely to harbor pathogens than live or recently killed animals.
Wild meat. Wildlife is essential for food security because it is a crucial protein source in many regions around the world.38 Most wild meat is sourced and consumed locally, but international demand has resulted in both legal and illegal trading overseas.38 Although wild meat does not form a major part of the standard Western diet, imported bushmeat is considered a luxury good in some high-income countries and can drive demand for illegal imports.39 For example, an estimated 5 metric tons (about 11,000 pounds) of wild meat is smuggled in personal luggage from Africa into Paris, France, through Charles de Gaulle airport each week.39 The wild meat trade involves hunters, middle markets, and consumers, resulting in an extensive network of billions of direct and indirect contacts between humans and animals,40 and this global translocation of animals can subsequently facilitate the transmission of pathogens into new, densely populated regions.
In both the SARS-CoV-1 and SARS-CoV-2 outbreaks, significant attention was paid to the role of wet markets, a broad term that is used to describe markets where fresh foods—including fresh produce, meat, and fish—are sold. In this section, we focus specifically on the subset of wet markets that include the sale of live animals or slaughter of live animals upon purchase. These live animal markets pose a threat to human health because transport and market environments are characterized by close, dense quarters, which create stressful conditions that increase potential host animals’ susceptibility to disease.41,42 These conditions are conversely optimal for pathogen development and transmission to other hosts.43 For example, stress in animals has been suggested to lead to increased microbial invasion of the gastrointestinal tract, more severe and frequent infections, and increased shedding of microbes in excretions.44 Congregating diverse animals, especially those that are rare and are normally isolated, into close quarters increases opportunities for pathogens to jump from natural to novel hosts outside their native habitat. Successful pathogen spillovers can then be disseminated in human populations by heavy human traffic through the markets.
A survey of a live animal market after the first SARS outbreak demonstrated that along with palm civets (the proposed bridge host), raccoon dogs, ferret badgers, and humans also tested positive for the virus.45 Similarly, the Huanan Seafood Wholesale Market likely included infected animals and those that were SARS-CoV-2 susceptible at the start of the pandemic.5 In addition, almost two thirds of the over 36,000 birds observed in Thai markets were non-native species to the country,40 and a recent survey of wild meat commerce in Malaysia identified 51 zoonotic pathogens that could be hosted in the wild species for sale.46
Although the examples in this section are from overseas, this discussion is certainly pertinent to the West.47 Live animal markets in Minnesota with close swine–human contact have resulted in multiple human infections of swine-origin influenza A viruses,48 and the COVID-19 pandemic has fueled demands to not only shut down live animal markets overseas but in the U.S. as well. Furthermore, tens of thousands of pounds of meat from wild animals are smuggled into the U.S. for human consumption each year and pose an additional risk of pathogen transmission,49,50 such as simian foamy virus and herpesviruses identified in meat originating from nonhuman primates that were confiscated at U.S. international airports.51
In addition to wild animals imported for food consumption, the hunting and trapping of wild animals on domestic soil poses additional spillover risks. Human pathogen exposure can occur directly from wild game, through companion animals (e.g., hunting dogs) that may act as bridge hosts, or through insect vectors encountered in the outdoors. Americans who hunt wild game (estimated at 11.5 million in 2016) are at risk of zoonotic infection, such as tuberculosis and toxoplasmosis, which can be acquired from exposure to wild deer.52, 53, 54 In addition, SARS-CoV-2 has been circulating widely in white-tailed deer populations in many regions of the U.S. and has even resulted in a handful of suspected deer-to-human transmission events.55,56 Believed to stem from multiple reverse zoonotic (i.e., human-to-deer) transmission events followed by efficient deer-to-deer transmission,57,58 widespread SARS-CoV-2 transmission in white-tailed deer raises concern for their emergence as a stable reservoir species for SARS-CoV-2 and a conduit for the evolution of novel viral variants; this could complicate the control of transmission in humans in addition to endangering those with close deer contact.56, 57, 58, 59
What is the physician's role in mitigating the risks associated with wild meat and hunting? A survey of medical students, a cohort notable for taking extensive social history during patient visits, found that the majority did not believe that primary care physicians would ask about wildlife exposure when considering a disease of unknown etiology, highlighting a potential gap in medical education.60 During the routine social history component of clinical encounters, identifying patients who have frequent contact with wild animals would provide an opportunity for an open dialogue on safe, preventive practices. Recognizing the connection between wildlife and disease can be powerful in endorsing safe behavior. For example, there was a reduction in the consumption of bushmeat, a suggested source of initial pathogen spillover, during the Ebola virus outbreak.61 Furthermore, an interventional study with wet markets in Malaysia demonstrated changes in attitude and avoidance practices with leptospirosis in market workers.62
Patient education can similarly lead to beneficial behavioral changes, and clinical counseling has demonstrated efficacy in mitigating infectious disease risks, such as increasing vaccination rates before travel63,64 and guiding reproductive behavior during the 2015–2016 Zika epidemic.65 In addition to behavior counseling, clinical encounters also offer the opportunity to prophylactically decrease the risk of zoonotic disease transmission by offering vaccinations to those with significant animal contact or who are at increased risk owing to age, pregnancy, or immunocompromised status. Vaccination guidelines for groups at increased risk of animal-transmitted diseases have been published in other countries and can be adapted for use in the U.S.66,67 Additional resources for patient education on visiting food markets, handling meats, and safe hunting practices can be found in guidelines provided by the WHO, Codex Alimentarius, the U.S. Centers for Disease Control and Prevention, and the American Veterinary Medical Association.68, 69, 70, 71, 72
Veterinarians have long been integral in safeguarding the U.S. food supply from zoonotic and foodborne pathogens because they oversee health and preventative care for food animals, conduct federal inspections of animal products in slaughter and processing establishments, and provide food safety oversight for corporations. The role of wildlife veterinarians in monitoring and mitigating zoonotic disease risk in wild animals that could end up in the food supply is less often discussed but arguably equally crucial for the millions of Americans who hunt and consume wild game each year. Veterinarians who surveil and treat diseases in wildlife should alert state officials and researchers about concerning trends so that appropriate measures can be taken to safeguard public health.
Exotic pets. Exotic pets are wild animals that are kept within a domestic setting, either for companionship, entertainment, or other personal interests.73 The multibillion-dollar exotic pet trade consists of both legal and illegal trafficking of wild animals that are acquired through a variety of means, including those captured in the wild or taken from the wild as eggs as well as those born or bred in captivity.73,74 The American Veterinary Medical Association estimates that about 13% of American households owned specialty or exotic pets in 2016, representing a 25% increase from 2011,75 and a recent report that included 10 of 27 European Union member states estimated that there were 62 million exotic pets kept in private households in 2021.76 In addition to threatening local biodiversity,72 exotic pets also threaten human health because they can serve as a source of human infections, such as severe salmonellosis in children acquired from pet reptiles and ringworm acquired from African pygmy hedgehogs and chinchillas.47,77,25
The first community-acquired human cases of mpox in the U.S., which occurred nearly 2 decades before the current global outbreak, resulted from contact with pet prairie dogs (Table 1).22 The 2022 mpox outbreak, which has resulted in over 50,000 cases in over 100 countries, has focused less on animal-to-human spillover transmission than the potential for human-to-animal spillback transmission, which could introduce the virus to new reservoir species outside of endemic regions in Africa.78, 79 These fears have already been partially realized by the first documented case of human-to-animal mpox transmission, which occurred in a pet dog that was infected by its owners in France.80
Human and animal health clinicians have multifaceted roles in addressing the role of exotic pets and the potential for pathogen spillover. For physicians, asking about household pets is routine during the environmental/social examination but should explicitly encompass questions about exotic and specialty pets. Patient confidentiality needs to be reiterated, especially because there may be hesitation to report if animals were obtained illicitly. For those who handle exotic animals, such as through occupational exposure, additional counseling on disease risk and preventative behavior may be warranted.81 In addition, physicians providing care for patients with zoonotic diseases with potential for human-to-animal spillback transmission should counsel patients about preventing transmission to their pets. These protective measures may include avoiding close contact with their pets and isolating exposed pets from contact with people or other animals.
Veterinarians have unique capabilities in disease surveillance because they are among the first to recognize the potential emergence of infectious diseases in animals. Similar to human clinicians, veterinarians may educate owners, be familiar with current outbreaks, and report concerns appropriately. Veterinarians should also counsel clients about their choice of exotic pet and perhaps even broach discussions with potential owners about whether they should get an exotic pet at all. Emphasizing the substantial health risks that exotic pets can pose to potential owners and their families may be particularly persuasive because research shows that people are less interested in obtaining exotic pets when presented with facts about their associated zoonotic disease risks.82,83
One Health in Medical and Veterinary Education
Properly addressing spillover risks associated with human–wildlife interactions requires a One Health approach characterized by collaboration between the medical, veterinary, and environmental health sectors. One Health curricula in medical and veterinary schools are important for exposing the next generation of human and animal health clinicians to the tenets of One Health (i.e., that the health of humans and animals are interconnected and bound to the health of the environment); however, although One Health concepts are frequently introduced in veterinary schools, they seldom feature in medical school curricula.60 An interprofessional longitudinal health curriculum, such as case-based scenarios with students from pharmacy or dental schools, has been implemented in some medical schools to emphasize the importance of multidisciplinary care.84 Establishing similar partnerships between veterinary schools, medical schools, and environmental health programs could offer students invaluable insight into how these different disciplines can act synergistically to address many of society's most pressing issues, including climate change, antimicrobial resistance, global food security, and emerging zoonotic diseases.85,86
Effective prevention of zoonotic outbreaks of epidemic/pandemic potential will require identification of pathogens in the natural host before spillover occurs, regular surveillance of wildlife, and risk analysis at the human–wildlife interface.87,88 Although these activities will likely be led by scientists rather than clinicians, they will undoubtedly require the participation of physicians and veterinarians, who should be prepared to rise to the challenge posed by these types of interdisciplinary collaborations.89 Moreover, as we have argued in this paper, clinicians are critical in detecting sentinel cases of emerging diseases, and this role necessitates that they be able to communicate their frontline detection appropriately for further action by public health and environmental health practitioners.85 To this end, medical and veterinary program administrators can consider incorporating One Health core competencies into their curricula.90,91 These competencies span domains that include leadership, ability to work in and across teams, and systems thinking; these curricula are aimed to equip future clinicians and future leaders from other fields with the ability to communicate, coordinate, and collaborate with those from other sectors.90,91
CONCLUSIONS
The current COVID-19 pandemic is the worst public health crisis that most of the world's population has experienced in their lifetime, and it is unclear what perils lie ahead. Pathogen spillovers leading to significant disease outbreaks are accelerating in frequency, with outbreaks of Middle East respiratory syndrome coronavirus, Ebola, Zika, and SARS-CoV-2 occurring in just the last decade alone. Human activities contribute to increased contact with wildlife, but there is a lack of knowledge about the extent or demand for wild animals and wildlife products. Clinicians, in both human and veterinary medicine, have the unique opportunity not only to educate those they serve but to fill this knowledge gap. Rather than trying to adapt to a zoonotic outbreak in real time, preventing the emergence of another zoonotic spillover by addressing relevant societal drivers could reduce the cost in lives.
CRediT authorship contribution statement
Tam Tran: Conceptualization, Data curation, Investigation, Visualization, Writing – original draft, Writing – review & editing. Sherrie Xie: Data curation, Investigation, Visualization, Writing – original draft, Writing – review & editing.
ACKNOWLEDGMENTS
The authors would like to express their gratitude to Kayleigh O'Keeffe, Zach Oppler, and Dustin Brisson for their contributions to the intellectual development and writing of the manuscript.
The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Centers for Disease Control and Prevention.
This work was supported by the NIH (National Institute of Allergy and Infectious Diseases of the NIH, F31AI133871 and National Heart, Lung, and Blood Institute of the NIH, F31-HL142153).
Declaration of interest: none.
REFERENCES
- 1.Green A, Wenliang L. Li Wenliang. Lancet. 2020;395(10225):682. doi: 10.1016/S0140-6736(20)30382-2. [DOI] [Google Scholar]
- 2.Worobey M. Dissecting the early COVID-19 cases in Wuhan. Science. 2021;374(6572):1202–1204. doi: 10.1126/science.abm4454. [DOI] [PubMed] [Google Scholar]
- 3.Taylor LH, Latham SM, Woolhouse ME. Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci. 2001;356(1411):983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plowright RK, Parrish CR, McCallum H, et al. Pathways to zoonotic spillover. Nat Rev Microbiol. 2017;15(8):502–510. doi: 10.1038/nrmicro.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Worobey M, Levy JI, Malpica Serrano L, et al. The Huanan Seafood Wholesale Market in Wuhan was the early epicenter of the COVID-19 pandemic. Science. 2022;377(6609):951–959. doi: 10.1126/science.abp8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freuling CM, Breithaupt A, Müller T, et al. Susceptibility of raccoon dogs for experimental SARS-CoV-2 infection. Emerg Infect Dis. 2020;26(12):2982–2985. doi: 10.3201/eid2612.203733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. WHO-convened Global Study of the Origins of SARS-CoV-2 (including annexes). Geneva, Switzerland: WHO. https://www.who.int/health-topics/coronavirus/origins-of-the-virus. Published March 30, 2021. Accessed August 14, 2020.
- 8.Borremans B, Faust C, Manlove KR, Sokolow SH, JO Lloyd-Smith. Cross-species pathogen spillover across ecosystem boundaries: mechanisms and theory. Philos Trans R Soc Lond B Biol Sci. 2019;374(1782) doi: 10.1098/rstb.2018.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun K, Gu L, Ma L, Duan Y. Atlas of ACE2 gene expression reveals novel insights into transmission of SARS-CoV-2. Heliyon. 2021;7(1):e05850. doi: 10.1016/j.heliyon.2020.e05850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker RE, Mahmud AS, Miller IF, et al. Infectious disease in an era of global change. Nat Rev Microbiol. 2022;20(4):193–205. doi: 10.1038/s41579-021-00639-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosen GE, Smith KF. Summarizing the evidence on the international trade in illegal wildlife. EcoHealth. 2010;7(1):24–32. doi: 10.1007/s10393-010-0317-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.da Silva PG, Mesquita JR, de M São José, Nascimento, Ferreira VAM. Viral, host and environmental factors that favor anthropozoonotic spillover of coronaviruses: an opinionated review, focusing on SARS-CoV, MERS-CoV and SARS-CoV-2 [published correction appears in Sci Total Environ. 2020;745:142123] Sci Total Environ. 2021;750 doi: 10.1016/j.scitotenv.2020.141483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dudas G, Carvalho LM, Rambaut A, Bedford T. MERS-CoV spillover at the camel-human interface. Elife. 2018;7:e31257. doi: 10.7554/eLife.31257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rulli MC, Santini M, Hayman DT, D'Odorico P. The nexus between forest fragmentation in Africa and Ebola virus disease outbreaks. Sci Rep. 2017;7:41613. doi: 10.1038/srep41613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pulliam JR, Epstein JH, Dushoff J, et al. Agricultural intensification, priming for persistence and the emergence of Nipah virus: a lethal bat-borne zoonosis. J R Soc Interface. 2012;9(66):89–101. doi: 10.1098/rsif.2011.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neumann G, Noda T, Kawaoka Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature. 2009;459(7249):931–939. doi: 10.1038/nature08157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kock RA, Begovoeva M, Ansumana R, Suluku R. Searching for the source of Ebola: the elusive factors driving its spillover into humans during the West African outbreak of 2013–2016. Rev Sci Tech. 2019;38(1):113–122. doi: 10.20506/rst.38.1.2946. [DOI] [PubMed] [Google Scholar]
- 18.Leroy EM, Rouquet P, Formenty P, et al. Multiple Ebola virus transmission events and rapid decline of Central African wildlife [published correction appears in Science. 2004;303(5658):628] Science. 2004;303(5656):387–390. doi: 10.1126/science.1092528. [DOI] [PubMed] [Google Scholar]
- 19.Jonsson CB, Figueiredo LT, Vapalahti O. A global perspective on hantavirus ecology, epidemiology, and disease. Clin Microbiol Rev. 2010;23(2):412–441. doi: 10.1128/CMR.00062-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toledo J, Haby MM, Reveiz L, Sosa Leon L, Angerami R, Aldighieri S. Evidence for human-to-human transmission of hantavirus: a systematic review. J Infect Dis. 2022;226(8):1362–1371. doi: 10.1093/infdis/jiab461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown K, Leggat PA. Human monkeypox: current state of knowledge and implications for the future. Trop Med Infect Dis. 2016;1(1):8. doi: 10.3390/tropicalmed1010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.News From the Centers for Disease Control and Prevention Update: multistate outbreak of monkeypox—Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. JAMA. 2003;290(4):454–455. doi: 10.1001/jama.290.4.454. [DOI] [Google Scholar]
- 23.Laurenson-Schafer H, Sklenovská N, Hoxha A, et al. Description of the first global outbreak of mpox: an analysis of global surveillance data. Lancet Glob Health. 2023;11(7):e1012–e1023. doi: 10.1016/S2214-109X(23)00198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soman Pillai V, Krishna G, Valiya Veettil M. Nipah virus: past outbreaks and future containment. Viruses. 2020;12(4):465. doi: 10.3390/v12040465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koski L, Stevenson L, Huffman J, et al. Notes from the field: an outbreak of Salmonella Agbeni infections linked to turtle exposure-United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67(48):1350. doi: 10.15585/mmwr.mm6748a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Outbreak of salmonella infections linked to small pet turtles. U.S. Centers for Disease Control and Prevention. https://www.cdc.gov/salmonella/typhimurium-1-20/index.html Updated March 13, 2020. Accessed August 4, 2023.
- 27.Outbreak of salmonella infections linked to pet hedgehogs. U.S. Centers for Disease Control and Prevention. https://www.cdc.gov/salmonella/typhimurium-01-19/index.html. Updated October 2, 2019. Accessed August 4, 2023.
- 28.Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ditrich H. Illegal trading in endangered animal and plant species from an Austrian perspective. Eur Law Rev. 2020;19:71–93. https://www.researchgate.net/publication/343151262_Illegal_Trading_in_Endangered_Animal_and_Plant_Species_-an_Austrian_perspective Accessed September 20, 2020. [Google Scholar]
- 30.Scheffers BR, Oliveira BF, Lamb I, Edwards DP. Global wildlife trade across the tree of life. Science. 2019;366(6461):71–76. doi: 10.1126/science.aav5327. [DOI] [PubMed] [Google Scholar]
- 31.Bager Olsen MT, Geldmann J, Harfoot M, et al. Thirty-six years of legal and illegal wildlife trade entering the USA. Oryx. 2021;55(3):432–441. doi: 10.1017/S0030605319000541. [DOI] [Google Scholar]
- 32.Halbwax M. Addressing the illegal wildlife trade in the European Union as a public health issue to draw decision makers attention. Biol Conserv. 2020;251 doi: 10.1016/j.biocon.2020.108798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liew JH, Kho ZY, Lim RBH, et al. International socioeconomic inequality drives trade patterns in the global wildlife market. Sci Adv. 2021;7(19):eabf7679. doi: 10.1126/sciadv.abf7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watsa M, Wildlife Disease Surveillance Focus Group Rigorous wildlife disease surveillance. Science. 2020;369(6500):145–147. doi: 10.1126/science.abc0017. [DOI] [PubMed] [Google Scholar]
- 35.Smith KM, Zambrana-Torrelio C, White A, et al. Summarizing US wildlife trade with an eye toward assessing the risk of infectious disease introduction. Ecohealth. 2017;14(1):29–39. doi: 10.1007/s10393-017-1211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibbs EP. The evolution of One Health: a decade of progress and challenges for the future. Vet Rec. 2014;174(4):85–91. doi: 10.1136/vr.g143. [DOI] [PubMed] [Google Scholar]
- 37.Rabinowitz P, Conti L. One Health and emerging infectious diseases: clinical perspectives. Curr Top Microbiol Immunol. 2013;365:17–29. doi: 10.1007/82_2012_263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coad L, Fa JE, Abernethy K, et al., Towards A Sustainable, Participatory and Inclusive Wild Meat Sector. Bogor, Indonesia Center for International Forestry Research (CIFOR); https://www.cifor.org/publications/pdf_files/Books/BCoad1901.pdf. Published 2019. Accessed November 1, 2021.
- 39.Chaber A, Allebone-Webb S, Lignereux Y, Cunningham AA, Marcus Rowcliffe J. The scale of illegal meat importation from Africa to Europe via Paris. Conserv Lett. 2010;3(5):317–321. doi: 10.1111/j.1755-263X.2010.00121.x. [DOI] [Google Scholar]
- 40.Karesh WB, Cook RA, Bennett EL, Newcomb J. Wildlife trade and global disease emergence. Emerg Infect Dis. 2005;11(7):1000–1002. doi: 10.3201/eid1107.050194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seeber PA, Morrison T, Ortega A, East ML, Greenwood AD, Czirják GÁ. Immune differences in captive and free-ranging zebras (Equus zebra and E. quagga) Mamm Biol. 2020;100(2):155–164. doi: 10.1007/s42991-020-00006-0. [DOI] [Google Scholar]
- 42.Fischer CP, Romero LM. Chronic captivity stress in wild animals is highly species-specific. Conserv Physiol. 2019;7(1):coz093. doi: 10.1093/conphys/coz093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panayotova-Pencheva MS. Parasites in captive animals: a review of studies in some European zoos. Der Zoologische Garten. 2013;82(s1–2):60–71. doi: 10.1016/j.zoolgart.2013.04.005. [DOI] [Google Scholar]
- 44.Rostagno MH. Can stress in farm animals increase food safety risk? Foodborne Pathog Dis. 2009;6(7):767–776. doi: 10.1089/fpd.2009.0315. [DOI] [PubMed] [Google Scholar]
- 45.Guan Y, Zheng BJ, He YQ, et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302(5643):276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- 46.Cantlay JC, Ingram DJ, Meredith AL. A review of zoonotic infection risks associated with the wild meat trade in Malaysia. Ecohealth. 2017;14(2):361–388. doi: 10.1007/s10393-017-1229-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chomel BB, Belotto A, Meslin FX. Wildlife, exotic pets, and emerging zoonoses. Emerg Infect Dis. 2007;13(1):6–11. doi: 10.3201/eid1301.060480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi MJ, Torremorell M, Bender JB, et al. Live animal markets in Minnesota: a potential source for emergence of novel influenza A viruses and interspecies transmission. Clin Infect Dis. 2015;61(9):1355–1362. doi: 10.1093/cid/civ618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goyenechea A, Indenbaum RA. Combating wildlife trafficking from Latin America to the United States. Washington, DC: Defenders of Wildlife.https://defenders.org/sites/default/files/publications/combating-wildlife-trafficking-from-latin-america-to-the-united-states-and-what-we-can-do-to-address-it.pdf. Published 2015. Accessed July 20, 2020.
- 50.Devaux CA, Mediannikov O, Medkour H, Raoult D. Infectious disease risk across the growing human-non human primate interface: a review of the evidence. Front Public Health. 2019;7:305. doi: 10.3389/fpubh.2019.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith KM, Anthony SJ, Switzer WM, et al. Zoonotic viruses associated with illegally imported wildlife products. PLoS One. 2012;7(1):e29505. doi: 10.1371/journal.pone.0029505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.U.S. Fish and Wildlife Service, U.S. Census Bureau, National Survey of Fishing, Hunting and Wildlife-Associated Recreation, Washington, DC: U.S. Fish and Wildlife Service, U.S. Census; Published 2016. Accessed September 20, 2021.
- 53.Kuehn B. Tuberculosis in deer hunters. JAMA. 2019;322(18):1757. doi: 10.1001/jama.2019.18029. [DOI] [PubMed] [Google Scholar]
- 54.Gaulin C, Ramsay D, Thivierge K, et al. Acute toxoplasmosis among Canadian deer hunters associated with consumption of undercooked deer meat hunted in the United States. Emerg Infect Dis. 2020;26(2):199–205. doi: 10.3201/eid2602.191218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feng A, Bevins S, Chandler J, et al. Transmission of SARS-CoV-2 in free-ranging white-tailed deer in the United States. Nat Commun. 2023;14(1):4078. doi: 10.1038/s41467-023-39782-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pickering B, Lung O, Maguire F, et al. Divergent SARS-CoV-2 variant emerges in white-tailed deer with deer-to-human transmission. Nat Microbiol. 2022;7(12):2011–2024. doi: 10.1038/s41564-022-01268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuchipudi SV, Surendran-Nair M, Ruden RM, et al. Multiple spillovers from humans and onward transmission of SARS-CoV-2 in white-tailed deer. Proc Natl Acad Sci U S A. 2022;119(6) doi: 10.1073/pnas.2121644119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hale VL, Dennis PM, McBride DS, et al. SARS-CoV-2 infection in free-ranging white-tailed deer. Nature. 2022;602(7897):481–486. doi: 10.1038/s41586-021-04353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.AD Marques, S Sherrill-Mix, JK Everett, et al., Multiple introductions of SARS-CoV-2 Alpha and Delta variants into white-tailed deer in Pennsylvania, mBio, 13 (5), 2022, e02101-22, 10.1128/mbio.02101-22. [DOI] [PMC free article] [PubMed]
- 60.Docherty L, Foley PL. Survey of One Health programs in U.S. medical schools and development of a novel one health elective for medical students. One Health. 2021;12 doi: 10.1016/j.onehlt.2021.100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ordaz-Németh I, Arandjelovic M, Boesch L, et al. The socio-economic drivers of bushmeat consumption during the West African Ebola crisis. PLoS Negl Trop Dis. 2017;11(3) doi: 10.1371/journal.pntd.0005450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rahman MHAA, Hairon SM, Hamat RA, et al. Leptospirosis health intervention module effect on knowledge, attitude, belief, and practice among wet market workers in Northeastern Malaysia: an intervention study. Int J Environ Res Public Health. 2018;15(7):1396. doi: 10.3390/ijerph15071396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang M, Zhang J, Hao Y, et al. Vaccination knowledge, attitude and practice among Chinese travelers who visit travel clinics in Preparation for international travel. J Travel Med. 2016;23(6):taw051. doi: 10.1093/jtm/taw051. [DOI] [PubMed] [Google Scholar]
- 64.Tafuri S, Guerra R, Gallone MS, et al. Effectiveness of pre-travel consultation in the prevention of travel-related diseases: a retrospective cohort study. Travel Med Infect Dis. 2014;12(6, pt B):745–749. doi: 10.1016/j.tmaid.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 65.Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure - United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(5):122–127. doi: 10.15585/mmwr.mm6505e2. [DOI] [PubMed] [Google Scholar]
- 66.Monath TP. Vaccines against diseases transmitted from animals to humans: a one health paradigm. Vaccine. 2013;31(46):5321–5338. doi: 10.1016/j.vaccine.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vaccination for people who have regular contact with animals. Australian Government, Department of Health and Aged Care.https://immunisationhandbook.health.gov.au/resources/publications/infographic-vaccination-for-people-who-have-regular-contact-with-animals. Updated June 9, 2018. Accessed April 1, 2021.
- 68.Codex alimentarius: international food standards. Food and Agriculture Organization of the United states, WHO. http://www.fao.org/fao-who-codexalimentarius/en/. Updated 2024. Accessed October 5, 2020.
- 69.WHO. A guide to healthy food markets. Geneva, Switzerland: WHO. https://iris.who.int/bitstream/handle/10665/43393/9241593938_eng.pdf?sequence=1. Published 2006. Accessed January 31, 2021.
- 70.WHO. WHO recommendations to reduce risk of transmission of emerging pathogens from animals to humans in live animal markets or animal product markets. Geneva, Switzerland: WHO.https://apps.who.int/iris/bitstream/handle/10665/332217/WHO-2019-nCoV-Human_animal_risk-2020.2-eng.pdf?sequence=1&isAllowed=y. Published March 26, 2020. Accessed September 24, 2020.
- 71.Guidance to reduce the risk of SARS-CoV-2 spreading between people and wildlife. U.S. Centers for Disease Control and Prevention.https://www.cdc.gov/healthypets/covid-19/wildlife.html. Accessed February 11, 2022.
- 72.Disease precautions for hunters. American Veterinary Medical Association.https://www.avma.org/resources/public-health/disease-precautions-hunters. Accessed February 11, 2022.
- 73.Bush ER, Baker SE, Macdonald DW. Global trade in exotic pets 2006–2012. Conserv Biol. 2014;28(3):663–676. doi: 10.1111/cobi.12240. [DOI] [PubMed] [Google Scholar]
- 74.Lockwood JL, Welbourne DJ, Romagosa CM, et al. When pets become pests: the role of the exotic pet trade in producing invasive vertebrate animals. Front Ecol Environ. 2019;17(6):323–330. doi: 10.1002/fee.2059. [DOI] [Google Scholar]
- 75.American Veterinary Medical Association. AVMA releases latest stats on pet ownership and veterinary care. Schaumburg, IL: American Veterinary Medical Association.https://www.avma.org/news/press-releases/avma-releases-latest-stats-pet-ownership-and-veterinary-care. Published November 18, 2018. Accessed May 1, 2021.
- 76.Eurogroup for Animals. The current pet trade in the EU and its variation between Member States. Brussels, Belgium: Eurogroup for Animals.https://www.eurogroupforanimals.org/library/current-pet-trade-eu-and-its-variation-between-member-states. Published January 31, 2023. Accessed July 31, 2023.
- 77.Whiley H, Gardner MG, Ross K. A review of Salmonella and squamates (lizards, snakes and amphisbians): implications for public health. Pathogens. 2017;6(3):38. doi: 10.3390/pathogens6030038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.2022 Monkeypox outbreak global map. Centers for Disease Control and Prevention. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html. Updated September 1, 2022. Accessed September 2, 2022.
- 79.Alakunle EF, Okeke MI. Monkeypox virus: a neglected zoonotic pathogen spreads globally. Nat Rev Microbiol. 2022;20(9):507–508. doi: 10.1038/s41579-022-00776-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Seang S, Burrel S, Todesco E, et al. Evidence of human-to-dog transmission of monkeypox virus. Lancet. 2022;400(10353):658–659. doi: 10.1016/S0140-6736(22)01487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garland-Lewis G, Whittier C, Murray S, Trufan S, Rabinowitz PM. Occupational risks and exposures among wildlife health professionals. Ecohealth. 2017;14(1):20–28. doi: 10.1007/s10393-017-1208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moorhouse TP, D'Cruze NC, Macdonald DW. Information about zoonotic disease risks reduces desire to own exotic pets among global consumers. Front Ecol Evol. 2021;9 doi: 10.3389/fevo.2021.609547. [DOI] [Google Scholar]
- 83.Moorhouse TP, Balaskas M, D'Cruze NC, Macdonald DW. Information could reduce consumer demand for exotic pets. Conserv Lett. 2017;10(3):337–345. doi: 10.1111/conl.12270. [DOI] [Google Scholar]
- 84.Dyess AL, Brown JS, Brown ND, Flautt KM, Barnes LJ. Impact of interprofessional education on students of the health professions: a systematic review. J Educ Eval Health Prof. 2019;16 doi: 10.3352/jeehp.2019.16.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Machalaba C, Raufman J, Anyamba A, et al. Applying a one health approach in global health and medicine: enhancing involvement of medical schools and global health centers. Ann Glob Health. 2021;87(1):30. doi: 10.5334/aogh.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rabinowitz PM, Natterson-Horowitz BJ, Kahn LH, Kock R, Pappaioanou M. Incorporating one health into medical education. BMC Med Educ. 2017;17(1):45. doi: 10.1186/s12909-017-0883-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carroll D, Morzaria S, Briand S, et al. Preventing the next pandemic: the power of a global viral surveillance network. BMJ. 2021;372:n485. doi: 10.1136/bmj.n485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Plowright RK, Reaser JK, Locke H, et al. Land use-induced spillover: a call to action to safeguard environmental, animal, and human health. Lancet Planet Health. 2021;5(4):e237–e245. doi: 10.1016/S2542-5196(21)00031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dykstra MP, Baitchman EJ. A call for one health in medical education: how the COVID-19 pandemic underscores the need to integrate human, animal, and environmental health. Acad Med. 2021;96(7):951–953. doi: 10.1097/ACM.0000000000004072. [DOI] [PubMed] [Google Scholar]
- 90.Togami E, Gardy JL, Hansen GR, et al. Core Competencies in One Health Education: What are We Missing? National Academy of Medicine; Washington, DC: 2018. [Google Scholar]
- 91.Frankson R, Hueston W, Christian K, et al. One health core competency domains. Front Public Health. 2016;4:192. doi: 10.3389/fpubh.2016.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]