Abstract
This cross-sectional study aimed to develop and validate population-based machine learning models for examining the association between breastfeeding and metabolic syndrome in women. The artificial neural network, the decision tree, logistic regression, the Naïve Bayes, the random forest and the support vector machine were developed and validated to predict metabolic syndrome in women. Data came from 30,204 women, who aged 20 years or more and participated in the Korean National Health and Nutrition Examination Surveys 2010–2019. The dependent variable was metabolic syndrome. The 86 independent variables included demographic/socioeconomic determinants, cardiovascular disease, breastfeeding duration and other medical/obstetric information. The random forest had the best performance in terms of the area under the receiver-operating-characteristic curve, e.g., 90.7%. According to random forest variable importance, the top predictors of metabolic syndrome included body mass index (0.1032), medication for hypertension (0.0552), hypertension (0.0499), cardiovascular disease (0.0453), age (0.0437) and breastfeeding duration (0.0191). Breastfeeding duration is a major predictor of metabolic syndrome for women together with body mass index, diagnosis and medication for hypertension, cardiovascular disease and age.
Subject terms: Cardiovascular diseases, Metabolic disorders
Introduction
The occurrences of metabolic syndrome and its associated risk factors, like hypertension, dyslipidemia, insulin resistance, and central obesity, have increased over the past few decades1,2. The clinical importance of metabolic syndrome has been acknowledged for long time owing to its increased risk for type 2 diabetes and cardiovascular disease (CVD)3. There have been considerable research to find the factors reducing the risk of metabolic syndrome. As a series of events following pregnancy, such as delivery and breastfeeding are known to have long-term impacts on women’s health, multiple studies evaluated the association between the pregnancy-related factors and metabolic syndrome4–6. Especially, the protective role of breastfeeding received attentions in terms of resetting metabolic change caused by pregnancy which includes insulin resistance and accumulation of lipid6. Several studies reported that the breastfeeding was associated with reducing the risk of metabolic syndromes7,8. While some studies found no association between breastfeeding and metabolic syndrome8–10. In addition, various mediating factors should be considered to determine the association between breastfeeding and metabolic syndrome.
CVD and metabolic syndrome are closely related owing to shared predisposing risk factors11,12. The proportion of pregnant women with CVD has increased over the decades13–15. Additionally, the number of pregnant women with pregestational comorbidities, like diabetes and obesity, is also on the rise13,15–17. These changes are presumably associated with maternal metabolic syndrome, but the validated data is limited18–20.
Understanding the association between metabolic syndrome and breastfeeding is important in terms of suggesting another possible prevention of metabolic syndrome. Therefore, we aimed to investigate the association between obstetric characteristics like breastfeeding and metabolic syndrome and the presence of CVD in a large-scale Asian population-based cross-sectional study of women, using artificial intelligence. We developed a prediction model for metabolic syndrome using artificial intelligence, which assessed 86 variables, including general obstetric characteristics (e.g., parity, gravidity), medical information, demographics, dietary preferences, lifestyles, and socioeconomic factors.
Results
General obstetric characteristics and metabolic syndrome
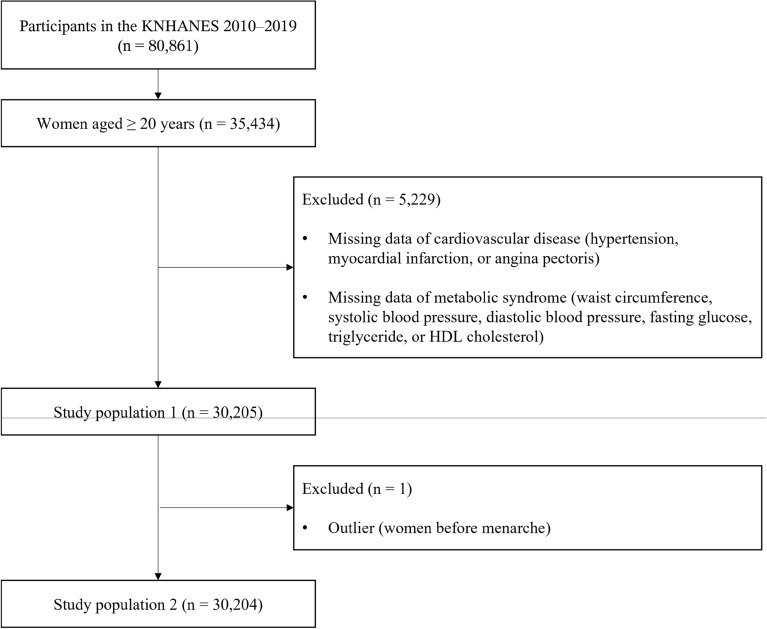
Among the 80,861 participants in the KNHANES 2010–2019, only women older than 20 years of age were included (n = 35,434). Patients with missing CVD or metabolic syndrome data were excluded (n = 5229). After excluding the outliers (n = 1), the data of 30,204 participants were analyzed (Fig. 1). The mean age of the participants was 50.93 years, and the prevalence of metabolic syndrome was 28.38% (8571/30,204) (Table 1). Among the study population, 21,865 (72.85%) had a history of breastfeeding. The prevalence of CVD was 23.50% (7097/30,204).
Figure 1.
A flow chart summarizing the experimental approach of the study. KNHANES, Korean National Health and Nutrition Examination Survey; HDL, high-density lipoprotein.
Table 1.
The baseline characteristics evaluated for the prediction of metabolic syndrome.
| Variables | Study population (n = 30,204) |
|---|---|
| Age at enrollment (years) | 50.93 ± 16.14 |
| Body mass index (kg/m2) | 23.48 ± 3.56 |
| Waist circumference (cm) | 79.09 ± 9.90 |
| Systolic blood pressure (mmHg) | 117.30 ± 17.84 |
| Diastolic blood pressure (mmHg) | 73.78 ± 9.72 |
| Fasting glucose (mg/dL) | 97.81 ± 21.70 |
| Triglycerides (mg/dL) | 115.90 ± 79.55 |
| HDL cholesterol (mg/dL) | 53.67 ± 12.53 |
| Age at menarche (years) | 14.37 ± 2.14 |
| Menstrual status, n (%) | |
| Menstruation | 13,976 (46.51%) |
| Pregnant | 211 (0.70%) |
| Breast-feeding | 318 (1.06%) |
| Menopause | 15,203 (50.59%) |
| Others | 344 (1.14%) |
| Married, n (%) | 26,448 (87.58%) |
| Nulligravida, n (%) | 4290 (14.20%) |
| Gravidity | 3.34 ± 2.38 (3.00) |
| Parous women, n (%) | 25,320 (84.29%) |
| Breastfeeding experience, n (%) | 21,865 (72.85%) |
| Number of breastfed children | 1.80 ± 1.57 (2.00) |
| Breastfeeding duration (months) | 25.11 ± 34.49 (13.00) |
| Use of oral contraceptive, n (%) | 5035 (16.75%) |
| Cardiovascular disease, n (%) | 7097 (23.50%) |
| Hypertension, n (%) | 6855 (22.70%) |
| Myocardial infarction, n (%) | 175 (0.58%) |
| Angina, n (%) | 563 (1.86%) |
| Major depressive disorder, n (%) | 3282 (10.87%) |
| Stroke, n (%) | 547 (1.81%) |
| Kidney failure, n (%) | 102 (0.34%) |
| Antihypertensive drug | 6429 (21.29%) |
| Drug treatment for glucose control | 2199 (7.28%) |
| Insulin | 203 (0.67%) |
| Oral hypoglycemic agents | 2143 (7.10%) |
| Lipid-lowering agent | 3443 (11.40%) |
| LDL cholesterol (mg/dL) | 115.51 ± 32.97 |
| Total cholesterol (mg/dL) | 192.36 ± 36.84 |
| White blood cell counts (Thous/µL) | 5.87 ± 1.66 |
| Red blood cell counts (Mil/µL) | 4.34 ± 0.35 |
| Hematocrit (%) | 39.78 ± 3.12 |
| Hemoglobin (g/dL) | 13.10 ± 1.15 |
| Serum creatinine (mg/dL) | 0.72 ± 0.20 |
| Blood urea nitrogen (mg/dL) | 14.05 ± 4.51 |
| Daily intake of calories (kcal) | 1687.99 ± 687.09 |
| Daily intake of fat (g) | 35.51 ± 27.51 |
| Daily intake of water (g) | 929.09 ± 566.30 |
| Daily intake of vitamin C (mg) | 89.43 ± 105.50 |
| Daily intake of sodium (mg) | 3373.46 ± 2378.88 |
| Daily intake of calcium (mg) | 459.05 ± 304.05 |
| Daily intake of carbohydrate (g) | 277.05 ± 113.80 |
| Daily intake of iron (mg) | 12.97 ± 10.53 |
| Daily intake of potassium (mg) | 2716.56 ± 1498.10 |
| Daily intake of protein (g) | 59.46 ± 31.15 |
| Daily intake of phosphorus (mg) | 962.27 ± 446.42 |
| Education level, n (%) | |
| Elementary school and below | 8204 (27.26%) |
| Middle school | 3078 (10.23%) |
| High school | 9350 (31.07%) |
| College and above | 9461 (31.44%) |
| Household income, n (%) | |
| Low | 5994 (19.96%) |
| Medium–low | 7609 (25.34%) |
| Medium–high | 8058 (26.84%) |
| High | 8365 (27.86%) |
| Economic activity, n (%) | 15,216 (50.53%) |
| Residential areas, n (%) | |
| Urban | 24,559 (81.31%) |
| Rural | 5645 (18.69%) |
| Frequency of drinking per year, n (%) | |
| Never | 5279 (17.58%) |
| Have not drunk in the last 1 year | 5529 (18.41%) |
| Less than once a month | 7300 (24.31%) |
| Once a month | 3275 (10.91%) |
| 2–4 times a month | 5533 (18.43%) |
| 2–3 times a week | 2447 (8.15%) |
| ≥ 4 times a week | 662 (2.20%) |
| Smoking status, n (%) | |
| Non-smoker | 26,793 (89.14%) |
| Smoker | 1575 (5.24%) |
| Ex-smoker | 1688 (5.62%) |
| Subjective body image, n (%) | |
| Very skinny | 1070 (3.56%) |
| A bit skinny | 2916 (9.70%) |
| Normal | 12,325 (40.98%) |
| A bit fat | 10,654 (35.42%) |
| Very fat | 3110 (10.34%) |
| Weight change in the last 1 year, n (%) | |
| Maintained | 18,872 (62.82%) |
| Lost | 3817 (12.71%) |
| Gained | 7353 (24.48%) |
| The days of weight training per week, n (%) | |
| 0 day | 24,997 (83.05%) |
| 1 day | 1037 (3.45%) |
| 2 days | 1265 (4.20%) |
| 3 days | 1178 (3.91%) |
| 4 days | 507 (1.68%) |
| ≥ 5 days | 1116 (3.71%) |
| EQ-5D index | 0.93 ± 0.13 (1.00) |
| Stress awareness, n (%) | |
| Feel a great deal of stress | 1522 (5.07%) |
| Feel much stress | 6845 (22.78%) |
| Feel some stress | 17,026 (56.66%) |
| Feel almost no stress | 4654 (15.49%) |
| Feeling depression in the last 1 year, n (%) | 3192 (10.60%) |
| Medical checkup in the last 2 years, n (%) | 18,958 (62.86%) |
Values are mean ± standard deviation (median) or n (%).
LDL, low-density lipoprotein; HDL, high-density lipoprotein; EQ-5D, European Quality of Life-5 Dimensions.
Prediction model for metabolic syndrome
The performance measures for the six prediction models for metabolic syndrome are summarized in Table 2. Among the six prediction models for metabolic syndrome, the random forest performed the best in terms of the area under the receiver operating characteristic curve (AUC); 90.7% (all participants), 87.7% (diagnosed with CVD), and 82.6% (no CVD diagnosis). The values and ranks of the random forest variable importance are summarized in Table 3. A predictor with the ranking of 26th or higher can be considered to be a major predictor in this study, given that it is a top 30% among 86 predictors here. According to the random forest variable importance in Table 3, the major predictors of metabolic syndrome were body mass index (BMI) (0.1032), use of antihypertensive drugs (0.0552), hypertension (0.0499), CVD (0.0453), age at enrollment (0.0437), white blood cell count (0.0297), low-density lipoprotein (LDL), cholesterol levels (0.0263), menstrual status (0.0247), use of lipid-lowering agents (0.0237), red blood cell count (0.0231), total cholesterol levels (0.0229), subjective body image (0.0221), education level (0.0214), daily fat intake (0.0198), hematocrit levels (0.0197), and breastfeeding duration (0.0191). Breastfeeding duration was a major predictor of metabolic syndrome. Let us take an example in which the random forest variable importance of BMI, CVD, or breastfeeding duration is 0.1032, 0.0453, or 0.0191, respectively. Here, the accuracy of the model will decrease by 10.32%, 4.53%, or 1.91% if the values of BMI, CVD, or breastfeeding duration are randomly permutated (or shuffled). The importance rankings of some major predictors showed dramatic changes in the subgroup analysis, i.e., between the participants with and without CVD. For example, the predictors of medication and diagnosis for hypertension ranked second and third for all participants, respectively, but these predictors went out of the top-30 ranking for both subgroups in Table 3. Likewise, the respective rankings of menstrual status and education were eighth and 13th for all the participants, but their rankings dropped to 23rd or lower for both the subgroups in the same table. Breastfeeding duration ranked 16th as a predictor for all the participants. However, it was ranked slightly higher at 14th for those without CVD and much lower at 26th for those with the condition.
Table 2.
Model performance: the average was measured for 50 runs.
| Model | All Participants | CVD undiagnosed | CVD diagnosed | |||
|---|---|---|---|---|---|---|
| Accuracy | AUC | Accuracy | AUC | Accuracy | AUC | |
| LR | 0.7727 | 0.8176 | 0.8712 | 0.8878 | 0.6922 | 0.8254 |
| DT | 0.7825 | 0.7347 | 0.8083 | 0.6553 | 0.6794 | 0.6469 |
| NB | 0.7954 | 0.8405 | 0.7995 | 0.7885 | 0.7682 | 0.7535 |
| RF | 0.8442 | 0.9065 | 0.8663 | 0.8765 | 0.6817 | 0.8260 |
| SVM | 0.7163 | 0.7964 | 0.8392 | 0.5283 | 0.6800 | 0.4989 |
| ANN | 0.6684 | 0.5319 | 0.7590 | 0.5135 | 0.8254 | 0.4986 |
CVD: cardiovascular disease; AUC: area under the receiver operating characteristic curve; LR: logistic regression; DT: decision tree; NB: naïve Bayes; RF: random forest; SVM: support vector machine; ANN: artificial neural network.
Table 3.
The variable importance from the Random Forest in predicting metabolic syndrome.
| All participants | Value | Rank | CVD undiagnosed | Value | Rank | CVD diagnosed | Value | Rank | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| v030 | BMI | 0.1032 | 1 | v030 | BMI | 0.1266 | 1 | v030 | BMI | 0.1177 | 1 |
| v090 | Antihypertensive drug | 0.0552 | 2 | v005 | Age | 0.0453 | 2 | v036 | WBC counts (Thous/µL) | 0.0459 | 2 |
| v053 | Hypertension | 0.0499 | 3 | v036 | WBC counts (Thous/µL) | 0.0353 | 3 | v075 | LDL | 0.0379 | 3 |
| v049 | CVD | 0.0453 | 4 | v075 | LDL | 0.0330 | 4 | v031 | Total cholesterol (mg/dL) | 0.0299 | 4 |
| v005 | Age | 0.0437 | 5 | v014 | Subjective body image | 0.0316 | 5 | v091 | Lipid-lowering agent | 0.0274 | 5 |
| v036 | WBC counts (Thous/µL) | 0.0297 | 6 | v031 | Total cholesterol (mg/dL) | 0.0303 | 6 | v037 | RBC counts (Mil/µL) | 0.0260 | 6 |
| v075 | LDL | 0.0263 | 7 | v037 | RBC counts (Mil/µL) | 0.0302 | 7 | v041 | Daily intake of fat (g) | 0.0256 | 7 |
| v076 | Menstrual status | 0.0247 | 8 | v033 | Hematocrit (%) | 0.0263 | 8 | v014 | Subjective body image | 0.0252 | 8 |
| v091 | Lipid-lowering agent | 0.0237 | 9 | v041 | Daily intake of fat (g) | 0.0255 | 9 | v046 | Daily intake of sodium (mg) | 0.0250 | 9 |
| v037 | RBC counts (Mil/µL) | 0.0231 | 10 | v032 | Hemoglobin (g/dL) | 0.0251 | 10 | v005 | Age | 0.0250 | 10 |
| v031 | Total cholesterol (mg/dL) | 0.0229 | 11 | v039 | Daily intake of water (g) | 0.0242 | 11 | v043 | Daily intake of calcium (mg) | 0.0249 | 11 |
| v014 | Subjective body image | 0.0221 | 12 | v046 | Daily intake of sodium (mg) | 0.0239 | 12 | v048 | Daily intake of vitamin C (mg) | 0.0246 | 12 |
| v007 | Education level | 0.0214 | 13 | v048 | Daily intake of vitamin C (mg) | 0.0237 | 13 | v039 | Daily intake of water (g) | 0.0244 | 13 |
| v041 | Daily intake of fat (g) | 0.0198 | 14 | v082 | BF durations (month) | 0.0235 | 14 | v042 | Daily intake of carbohydrate (g) | 0.0240 | 14 |
| v033 | Hematocrit (%) | 0.0197 | 15 | v035 | Serum creatinine (mg/dL) | 0.0233 | 15 | v033 | Hematocrit (%) | 0.0236 | 15 |
| v082 | BF duration (month) | 0.0191 | 16 | v043 | Daily intake of calcium (mg) | 0.0231 | 16 | v038 | Daily intake of calories (kcal) | 0.0234 | 16 |
| v092 | Drug treatment for glucose control | 0.0190 | 17 | v042 | Daily intake of carbohydrate (g) | 0.0230 | 17 | v035 | Serum creatinine (mg/dL) | 0.0234 | 17 |
| v032 | Hemoglobin (g/dL) | 0.0186 | 18 | v045 | Daily intake of iron (mg) | 0.0227 | 18 | v045 | Daily intake of iron (mg) | 0.0233 | 18 |
| v039 | Daily intake of water (g) | 0.0186 | 19 | v040 | Daily intake of protein (g) | 0.0223 | 19 | v040 | Daily intake of protein (g) | 0.0231 | 19 |
| v048 | Daily intake of vitamin C (mg) | 0.0184 | 20 | v038 | Daily intake of calories (kcal) | 0.0222 | 20 | v047 | Daily intake of potassium (mg) | 0.0231 | 20 |
| v046 | Daily intake of sodium (mg) | 0.0183 | 21 | v047 | Daily intake of potassium (mg) | 0.0222 | 21 | v044 | Daily intake of phosphorus (mg) | 0.0228 | 21 |
| v043 | Daily intake of calcium (mg) | 0.0181 | 22 | v044 | Daily intake of phosphorus (mg) | 0.0218 | 22 | v092 | Drug treatment for glucose control | 0.0225 | 22 |
| v035 | Serum creatinine (mg/dL) | 0.0180 | 23 | v007 | Education level | 0.0196 | 23 | v032 | Hemoglobin (g/dL) | 0.0218 | 23 |
| v042 | Daily intake of carbohydrate (g) | 0.0177 | 24 | v034 | Blood urea nitrogen (mg/dL) | 0.0188 | 24 | v034 | Blood urea nitrogen (mg/dL) | 0.0204 | 24 |
| v045 | Daily intake of iron (mg) | 0.0173 | 25 | v077 | Age at menarche (years) | 0.0170 | 25 | v094 | Oral hypoglycemic agents | 0.0204 | 25 |
| v047 | Daily intake of potassium (mg) | 0.0172 | 26 | v083 | Gravidity | 0.0159 | 26 | v082 | BF durations (month) | 0.0196 | 26 |
| v040 | Daily intake of protein (g) | 0.0172 | 27 | v076 | Menstrual status | 0.0158 | 27 | v093 | Insulin | 0.0193 | 27 |
| v038 | Daily intake of calories (kcal) | 0.0172 | 28 | v092 | Drug treatment for glucose control | 0.0153 | 28 | v002 | Age at enrollment (years) | 0.0158 | 28 |
| v094 | Oral hypoglycemic agents | 0.0171 | 29 | v094 | Oral hypoglycemic agents | 0.0152 | 29 | v077 | Age at menarche (years) | 0.0153 | 29 |
| v044 | Daily intake of phosphorus (mg) | 0.0171 | 30 | v002 | Age at enrollment (years) | 0.0148 | 30 | v011 | EQ-5D | 0.0146 | 30 |
| v093 | Insulin | 0.0171 | 31 | v093 | Insulin | 0.0139 | 31 | v083 | Gravidity | 0.0144 | 31 |
| v034 | Blood urea nitrogen (mg/dL) | 0.0154 | 32 | v081 | Number of breastfed children | 0.0137 | 32 | v090 | Antihypertensive drug | 0.0125 | 32 |
| v077 | Age at menarche (years) | 0.0136 | 33 | v091 | Lipid-lowering agent | 0.0130 | 33 | v081 | Number of breastfed children | 0.0123 | 33 |
| v083 | Gravidity | 0.0135 | 34 | v052 | Frequency of drinking per year | 0.0127 | 34 | v052 | Frequency of drinking per year | 0.0119 | 34 |
| v081 | Number of breastfed children | 0.0128 | 35 | v011 | EQ-5D | 0.0124 | 35 | v007 | Education level | 0.0100 | 35 |
| v011 | EQ-5D | 0.0119 | 36 | v006 | Household income | 0.0107 | 36 | v006 | Household income | 0.0093 | 36 |
| v002 | Age at enrollment (years) | 0.0116 | 37 | v013 | Occupation | 0.0098 | 37 | v016 | Weight control in the last 1 year | 0.0087 | 37 |
| v052 | Frequency of drinking per year | 0.0100 | 38 | v016 | Weight control in the last 1 year | 0.0080 | 38 | v017 | Stress awareness | 0.0084 | 38 |
| v006 | Household income | 0.0092 | 39 | v017 | Stress awareness | 0.0078 | 39 | v013 | Occupation | 0.0080 | 39 |
| v013 | Occupation | 0.0072 | 40 | v015 | Weight change in the last 1 year | 0.0061 | 40 | v015 | Weight change in the last 1 year | 0.0056 | 40 |
| v016 | Weight control in the last 1 year | 0.0067 | 41 | v018 | The days of weight training per week | 0.0054 | 41 | v018 | The days of weight training per week | 0.0048 | 41 |
| v017 | Stress awareness | 0.0061 | 42 | v057 | Osteoarthritis | 0.0040 | 42 | v009 | Participation in health examination | 0.0040 | 42 |
| v057 | Osteoarthritis | 0.0049 | 43 | v010 | Cancer screening for the last 2 years | 0.0039 | 43 | v021 | Diagnosis of HTN in mother | 0.0039 | 43 |
| v015 | Weight change in the last 1 year | 0.0044 | 44 | v012 | Economic activity | 0.0038 | 44 | v057 | Osteoarthritis | 0.0038 | 44 |
| v018 | The days of weight training per week | 0.0041 | 45 | v009 | Participation in health examination | 0.0037 | 45 | v010 | Cancer screening for the last 2 years | 0.0037 | 45 |
| v010 | Cancer screening for the last 2 years | 0.0030 | 46 | v051 | Smoking | 0.0035 | 46 | v053 | Hypertension | 0.0037 | 46 |
| v009 | Participation in health examination | 0.0029 | 47 | v021 | Diagnosis of HTN in mother | 0.0035 | 47 | v012 | Economic activity | 0.0036 | 47 |
| v012 | Economic activity | 0.0028 | 48 | v003 | Residental area (urban/rural) | 0.0034 | 48 | v019 | Use of oral contraceptive | 0.0036 | 48 |
| v003 | Residental area (urban/rural) | 0.0027 | 49 | v029 | Diagnosis of DM in mother | 0.0032 | 49 | v003 | Residental area (urban/rural) | 0.0036 | 49 |
| v019 | Use of oral contraceptive | 0.0026 | 50 | v019 | Use of oral contraceptive | 0.0032 | 50 | v076 | Menstrual status | 0.0035 | 50 |
| v021 | Diagnosis of HTN in mother | 0.0026 | 51 | v020 | Diagnosis of HTN in father | 0.0027 | 51 | v062 | Depression | 0.0032 | 51 |
| v051 | Smoking | 0.0023 | 52 | v074 | Melancholy in the last 1 year | 0.0027 | 52 | v020 | Diagnosis of HTN in father | 0.0030 | 52 |
| v020 | Diagnosis of HTN in father | 0.0022 | 53 | v062 | Depression | 0.0026 | 53 | v074 | Melancholy in the last 1 year | 0.0029 | 53 |
| v074 | Melancholy in the last 1 year | 0.0021 | 54 | v028 | Diagnosis of DM in father | 0.0024 | 54 | v027 | Diagnosis of stroke in mother | 0.0026 | 54 |
| v080 | History of breastfeeding | 0.0021 | 55 | v080 | History of breastfeeding | 0.0023 | 55 | v026 | Diagnosis of stroke in father | 0.0025 | 55 |
| v062 | Depression | 0.0020 | 56 | v027 | Diagnosis of stroke in mother | 0.0021 | 56 | v051 | Smoking | 0.0025 | 56 |
| v029 | Diagnosis of DM in mother | 0.0020 | 57 | v026 | Diagnosis of stroke in father | 0.0020 | 57 | v056 | Stroke | 0.0024 | 57 |
| v027 | Diagnosis of stroke in mother | 0.0017 | 58 | v061 | Thyroid disease | 0.0020 | 58 | v055 | angina | 0.0023 | 58 |
| v079 | Childbirth experience | 0.0017 | 59 | v079 | Childbirth experience | 0.0018 | 59 | v061 | Thyroid disease | 0.0022 | 59 |
| v026 | Diagnosis of stroke in father | 0.0016 | 60 | v008 | Marriage | 0.0017 | 60 | v058 | Rheumatic arthritis | 0.0020 | 60 |
| v028 | Diagnosis of DM in father | 0.0016 | 61 | v060 | Asthma | 0.0017 | 61 | v029 | Diagnosis of DM in mother | 0.0020 | 61 |
| v061 | Thyroid disease | 0.0015 | 62 | v078 | Pregnancy experience | 0.0015 | 62 | v080 | History of breastfeeding | 0.0019 | 62 |
| v078 | Pregnancy experience | 0.0013 | 63 | v059 | Tuberculosis | 0.0014 | 63 | v059 | Tuberculosis | 0.0019 | 63 |
| v008 | Marriage | 0.0012 | 64 | v064 | Atopic dermatitis | 0.0012 | 64 | v060 | Asthma | 0.0015 | 64 |
| v060 | Asthma | 0.0012 | 65 | v024 | Diagnosis of IHD in father | 0.0012 | 65 | v028 | Diagnosis of DM in father | 0.0013 | 65 |
| v059 | Tuberculosis | 0.0011 | 66 | v058 | Rheumatic arthritis | 0.0012 | 66 | v025 | Diagnosis of IHD in mother | 0.0011 | 66 |
| v058 | Rheumatic arthritis | 0.0011 | 67 | v023 | Diagnosis of hyperlipidemia in mother | 0.0012 | 67 | v023 | Diagnosis of hyperlipidemia in mother | 0.0010 | 67 |
| v056 | Stroke | 0.0011 | 68 | v025 | Diagnosis of IHD in mother | 0.0011 | 68 | v079 | Childbirth experience | 0.0009 | 68 |
| v023 | Diagnosis of hyperlipidemia in mother | 0.0009 | 69 | v056 | Stroke | 0.0010 | 69 | v064 | Atopic dermatitis | 0.0008 | 69 |
| v024 | Diagnosis of IHD in father | 0.0009 | 70 | v068 | Breast cancer | 0.0006 | 70 | v054 | Myocardial infarction | 0.0008 | 70 |
| v055 | Angina | 0.0009 | 71 | v022 | Diagnosis of hyperlipidemia in father | 0.0006 | 71 | v065 | Gastric cancer | 0.0007 | 71 |
| v025 | Diagnosis of IHD in mother | 0.0008 | 72 | v069 | Cervical cancer | 0.0005 | 72 | v024 | Diagnosis of IHD in father | 0.0006 | 72 |
| v064 | Atopic dermatitis | 0.0007 | 73 | v071 | Hepatitis B | 0.0004 | 73 | v078 | Pregnancy experience | 0.0006 | 73 |
| v068 | Breast cancer | 0.0005 | 74 | v067 | Colon cancer | 0.0004 | 74 | v069 | Cervical cancer | 0.0006 | 74 |
| v069 | Cervical cancer | 0.0004 | 75 | v072 | Hepatitis C | 0.0002 | 75 | v068 | Breast cancer | 0.0006 | 75 |
| v022 | Diagnosis of hyperlipidemia in father | 0.0004 | 76 | v073 | Liver cirrhosis | 0.0001 | 76 | v073 | Liver cirrhosis | 0.0005 | 76 |
| v071 | Hepatitis B | 0.0004 | 77 | v065 | Gastric cancer | 0.0001 | 77 | v008 | Marriage | 0.0005 | 77 |
| v067 | Colon cancer | 0.0003 | 78 | v063 | Chronic kidney disease | 0.0001 | 78 | v071 | Hepatitis B | 0.0005 | 78 |
| v065 | Gastric cancer | 0.0003 | 79 | v070 | Lung cancer | 0.0001 | 79 | v063 | Chronic kidney disease | 0.0005 | 79 |
| v054 | Myocardial infarction | 0.0003 | 80 | v066 | Liver cancer | 0.0000 | 80 | v067 | Colon cancer | 0.0003 | 80 |
| v073 | Liver cirrhosis | 0.0002 | 81 | v004 | Sex | 0.0000 | 81 | v022 | Diagnosis of hyperlipidemia in father | 0.0003 | 81 |
| v063 | Chronic kidney disease | 0.0002 | 82 | v049 | CVD | 0.0000 | 81 | v072 | Hepatitis C | 0.0002 | 82 |
| v072 | Hepatitis C | 0.0002 | 83 | v053 | Hypertension | 0.0000 | 81 | v070 | Lung cancer | 0.0001 | 83 |
| v070 | Lung cancer | 0.0001 | 84 | v054 | Myocardial infarction | 0.0000 | 81 | v066 | Liver cancer | 0.0000 | 84 |
| v066 | Liver cancer | 0.0000 | 85 | v055 | Angina | 0.0000 | 81 | v004 | Sex | 0.0000 | 85 |
| v004 | Sex | 0.0000 | 86 | v090 | Antihypertensive drug | 0.0000 | 81 | v049 | CVD | 0.0000 | 85 |
TG, triglyceride; LDL, low-density lipoprotein; HDL, high-density lipoprotein; BMI, body mass index; IHD, ischemic heart disease; DM, diabetes mellitus; HTN, hypertension; WBC, white blood cell; CVD, cardiovascular disease; RBC, red blood cell; EQ-5D, European Quality of Life-5 Dimension.
The logistic analysis results for each important variable, including obstetric characteristics, are presented in Supplementary Material 2. The breastfeeding duration was associated with a decreased risk of metabolic syndrome (adjusted odds ratio [aOR] 0.998; confidence interval [CI] [0.996–1.000]). The odds of metabolic syndrome will decrease by 0.2% if breastfeeding duration increases by 1 month. In other words, the odds of metabolic syndrome will decrease by 2.4% (or 4.8%) if breastfeeding duration increases by 1 year, i.e., 12 months (or 2 years, i.e., 24 months). The effect of breastfeeding duration on metabolic syndrome looks small on 1 month but it is big on 1 year or two. The odds ratio is not statistical significant at 5% level but it is still useful information in machine learning, given that variable importance is primary and statistical significance is supplementary in machine learning. Logistic regression requires adopting the unrealistic assumption of ceteris paribus, i.e., “all the other variables remain constant”. In this context, the results of the logistic regression would serve as supplementary information to the random forest variable importance.
Discussion
In summary, among the obstetric characteristics, one of the most significant factors associated with metabolic syndrome was the duration of breastfeeding. Among the six prediction models for metabolic syndrome, the random forest had the best performance in terms of the AUC, i.e., 90.7% (all participants). In the subgroup analysis, among the women without CVD, the importance of breastfeeding duration as a predictor of metabolic syndrome was ranked 14th (0.0235), which is as important as the daily intake of sodium (12th, 0.0239).
This study presents the most comprehensive analysis of the determinants of metabolic syndrome in women using a large-scale Asian population-based cross-sectional study of 30,204 participants. While there is one paper that has addressed the association between breastfeeding and metabolic syndrome in postmenopausal women using KHANES data, our study differs in that it targeted all adult women, included more recent data (2010 to 2018), and distinguished itself by constructing a predictive model for metabolic syndrome using machine learning9. This study investigated whether there were differences in metabolic syndrome-related factors between the women with and without CVD. In a recent meta-analysis, the authors assumed that breastfeeding may have a preventive effect on metabolic syndrome and that it was related to breastfeeding duration8. However, the pooled effect of breastfeeding on metabolic syndrome was not conclusive because of the study population heterogeneity, the criteria for breastfeeding, and confounding factors for metabolic syndrome8. In this large-scale population-based study, we evaluated the precise impact of breastfeeding on metabolic syndrome and compared its clinical importance to the other known risk factors known to predispose women to metabolic syndrome.
During pregnancy, the mother undergoes metabolic changes that increase insulin resistance and serum lipid levels (particularly triglyceride [TG])21,22. Breastfeeding reportedly restores the overall maternal postpartum metabolic changes faster back to the prenatal baselines23. It also has a long-term positive effect on maternal glucose levels, lipid metabolism, and adiposity23–25. The relationship between gravidity, parity, and metabolic syndrome is still debated, necessitating further research.
In this study, we investigated the importance of specific variables in the development of metabolic syndrome in women with and without CVD. The relative importance of different variables between the participants with and without CVD can have important clinical implications. First, in women without CVD, age (second vs. tenth), breastfeeding duration (14th vs. 26th), and gravidity (26th vs. 31st) were ranked higher as compared to women with CVD. These variables appeared to have a higher association with metabolic syndrome in the women without CVD and were less important in women with CVD. Second, in women with CVD, the importance of lipid-lowering agents or diabetes drugs was relatively higher. A previous meta-analysis reported that among the five factors of metabolic syndrome, the prognosis of CVD was especially poor in patients with dyslipidemia or impaired glucose tolerance26. In this study, it can also be hypothesized that dyslipidemia or impaired glucose tolerance has a stronger mediating effect on metabolic syndrome in women with CVD. Third, in the three models of this study (Table 3), the nutrient intake (especially fat intake) was highly correlated with metabolic syndrome, and the importance of nutrient intake was higher in women with CVD than in women without CVD. Previous studies have reported the significance of healthy diets for metabolic syndrome, which was further emphasized in this study27. Moreover, the importance of diet in metabolic syndrome was reported to be greater in women with CVD than in women without CVD. Additionally, white blood cell count ranked sixth or higher as a predictor of metabolic syndrome in women. Levels of C-reactive protein, plasma, and low-grade inflammation have been reported to be positively associated with metabolic syndrome28,29. It is reasonable to speculate that the white blood cell count also has a positive relationship with metabolic syndrome.
This study has limitations. First, a cross-sectional design was used. However, using data with a longitudinal design is expected to improve the validity of this study. Second, the duration of breastfeeding in this study is reliant on information that has been self-reported several years after the actual breastfeeding took place, which may introduce limitations to the accuracy of the data. Furthermore, although the medical history was presumed based on a physician's diagnosis, it may be subject to limitations in accuracy as it relied on self-report surveys by the participants. Similarly, an investigation into dietary intake involved a nutritionist conducting direct interviews during visits. However, there may be limitations to the objectivity of respondents' responses. Third, expanding this study to other diseases and predictors such as health utility usage might significantly contribute to this line of research. Fourth, we excluded the diagnostic criteria for the metabolic syndrome from the independent variables. However, to examine the influence of CVD and the use of cardiovascular medications on the metabolic syndrome, we included the presence of hypertension diagnosed by a physician and the use of cardiovascular medications as independent variables. Fifth, this study used random forest variable importance as primary results and logistic regression odds ratios as supplementary findings. That is, the former result was considered to be the strength of the association between metabolic syndrome and its major predictor, while the latter finding was considered to be the direction of the association. There would be other ways to examine the direction of the association, and this would make a great contribution for research in this direction. Finally, this study did not consider the possible mediating effects among the variables.
In the prediction model with a random forest of AUC 90.7%, the top predictors of metabolic syndrome included body mass index (0.1032), medication for hypertension (0.0552), hypertension (0.0499), cardiovascular disease (0.0453), age (0.0437) and breastfeeding duration (0.0191). Breastfeeding duration was one of the most important predictors of metabolic syndrome among the various obstetric characteristics.
Methods
Study population
This study was based on the fifth (2010–2012), sixth (2013–2015), seventh (2016–2018), and eighth (2019) Korean National Health and Nutrition Examination Survey (KNHANES) surveys. The KNHANES is a nationwide representative survey that obtains samples annually using a stratified multistage cluster sampling design. The KHANSE is conducted by a dedicated research team, visiting four regions each week (for a total of 192 regions annually). The survey is conducted over a period of 3 days in each region, with mobile examination vehicles visiting the area to perform health screenings, health surveys, and nutritional assessments. Health surveys and medical examinations are conducted in mobile examination vehicles, while nutritional assessments are performed by a specialized team of nutritionists who visit households directly. This data is used to assess the health status, prevalence of chronic diseases, and nutritional intake status of the population in South Korea. In the KNHANES 2010–2019, men and participants under the age of 20 years were excluded from the current analyses. The cases with missing data on the chronic occurrence or diagnosis of hypertension, myocardial infarction, angina, all the factors associated with the diagnosis of metabolic syndrome, and an outlier (the woman over 80 years old before menarche) were excluded.
The data were publicly available and de-identified. The requirement for ethical approval was waived by the institutional review board of Korea University Anam Hospital. All methods were conducted in accordance with relevant institutional/ethical committee guidelines and regulations. The requirement for informed consent was waived because all participant information was deidentified and encrypted to protect privacy.
Variables
The variables included in this study are summarized in Supplementary Materials 1. The sociodemographic characteristics, including the age at enrollment, sex, body mass index (BMI), household income (represented as quartiles), marital status, the level of education (elementary school and below, middle school, high school, and college and above), areas of residence, economic activities, and occupations, were assessed using questionnaires.
Information regarding the general obstetric characteristics, including gravidity, parity, breastfeeding (history of breasting, the number of children breastfed, and lifetime total breastfeeding duration), history of abortions, the age at menarche, and the menstrual status (menstruation, pregnancy, breastfeeding, menopause, and others), were also obtained from the questionnaires. The presence of the following diseases was defined based on an interview: (1) hypertension, (2) myocardial infarction, (3) angina, (4) stroke, (5) osteoarthritis, (6) rheumatoid arthritis, (7) pulmonary tuberculosis, (8) asthma, (9) thyroid-related disease, (10) major depressive disorder, (11) kidney failure, (12) hepatitis B, (13) hepatitis C, (14) liver cirrhosis, (14) cancers (gastric cancer, hepatic cancer, colorectal cancer, breast cancer, cervical cancer, and lung cancer), and (15) atopic dermatitis. Data on family histories of hypertension, hyperlipidemia, ischemic heart disease, stroke, and diabetes mellitus were also obtained from the questionnaires. Additionally, the questionnaires also provided the data on the use of (1) antihypertensive drugs, (2) lipid-lowering agents, (3) oral hypoglycemic agents, and (4) insulin.
The blood pressures, waist circumferences and body mass index (BMI) of the participants were measured. Levels of total cholesterol, TG, LDL, high-density lipoprotein (HDL), hemoglobin, hematocrit, blood urea nitrogen, blood creatinine, white blood cell, and red blood cell were also measured at the time of survey.
The participants answered questions about their insights and habits associated with their health. They were asked about their subjective body image, their goals associated with controlling their body weights, history of medical checkups for the past 2 years, history of smoking, frequency of alcohol consumption (per year), and weekly weight training routines. Data on mental health, including stress awareness and feelings of depression within a year, were also collected. The quality of life, based on health indicators, was assessed using the European Quality of Life-5 Dimensions (EQ-5D) scale30. The daily intake of energy (kcal), carbohydrates (g), protein (g), fat (g), sodium (mg), water (g), calcium (mg), phosphorus (mg), iron (mg), potassium (mg), and vitamin C (mg) was ascertained from the nutrition survey.
A diagnosis for CVD required the presence of at least one of the following: (1) hypertension, (2) myocardial infarction, or (3) angina. Based on the modified National Cholesterol Rationale Education Program Adult Treatment Program III criteria and the appropriate cutoff for central obesity in Korean adult women (suggested by the Korean Endocrine Society), metabolic syndrome was defined as having three or more of the following1,31: (1) central obesity (waist circumference ≥ 85 cm); (2) elevated TGs (serum TG concentration ≥ 150 mg/dL); (3) low HDL cholesterol (serum HDL cholesterol concentration < 50 mg/dL); (4) elevated blood pressure (systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg) or the prescription of antihypertensive drugs; (5) elevated fasting glucose (fasting serum glucose ≥ 100 mg/dL) or the prescription of diabetes drugs. And we excluded the variables corresponding to the diagnostic criteria of metabolic syndrome among the independent variables, including waist circumference, TG, HDL cholesterol, blood pressure measurements, and fasting glucose.
Statistical analysis
An artificial neural network, decision tree, logistic regression, naïve Bayes, random forest, and support vector machine were used to predict metabolic syndrome. Data on 30,204 observations with full information were divided into training and validation sets in a 70:30 ratio (21,143:9061). The AUC curve and accuracy (the ratio of correct predictions among the 9061 observations in the validation set) were employed as the standard for model validation. The random forest variable importance, the contribution of a certain variable to the random forest performance (accuracy), was used to examine the major predictors of metabolic syndrome. Let us assume that the importance of the random forest variable of CVD is 0.0453. Here, the accuracy of the model drops by 4.53% if the values of a predictor of CVD are randomly permutated (or shuffled). The random split and analysis were repeated 50 times and averaged for external validation32–34. R-Studio 1.3.959 (R-Studio Inc.: Boston, United States) and Python 3.52 (CreateSpace: Scotts Valley, United States) were employed for the analysis between February 1, 2022–March 31, 2022.
Supplementary Information
Acknowledgements
The data for this study were obtained from the Korean National Health and Nutrition Examination Survey (KNHANES).
Author contributions
Study concept and design: K.H.A. Statistical analyses: K.S.L., H.L., S.S. Manuscript writing: J.S.L., E.S.C., H.L., S.S., K.S.L. Critical revision of the manuscript for content/interpretation: J.S.L., E.S.C., K.S.L. K.H.A. K.H.A. accepts full responsibility for the conduct of the study, had access to the data. All authors controlled the decision to publish.
Funding
This work was supported by (1) the Korea University Medical Center grant (No. K1925051; Author Ki Hoon Ahn; https://www.kumc.or.kr/en/index.do), (2) the Korea Health Industry Development Institute grant (Korea Health Technology R&D Project) funded by the Ministry of Health & Welfare of South Korea (No. HI22C1463; Author Ki Hoon Ahn; https://www.khidi.or.kr/eps), and technically supported by 4P Lab, Co., Ltd for data analysis (Authors Ki Hoon Ahn & Kwang-Sig Lee), and (3) the Korea Health Industry Development Institute grant (Korea Health Technology R&D Project) funded by the Ministry of Health & Welfare of South Korea (No. HI22C1302; Author Kwang-Sig Lee; https://www.khidi.or.kr/eps). There was no additional external funding received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability
The data utilized in this study is available from the Korean National Health and Nutrition Examination Survey (KNHANES) (https://knhanes.kdca.go.kr/knhanes). The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: The Funding section in the original version of this Article was omitted. Full information regarding the correction made can be found in the correction for this Article.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jue Seong Lee and Eun-Saem Choi.
Change history
3/26/2024
A Correction to this paper has been published: 10.1038/s41598-024-57571-4
Contributor Information
Kwang-Sig Lee, Email: ecophy@hanmail.net.
Ki Hoon Ahn, Email: akh1220@hanmail.net.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-53137-6.
References
- 1.Grundy SM, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 2.Moore JX, Chaudhary N, Akinyemiju T. Metabolic syndrome prevalence by race/ethnicity and sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev. Chronic Dis. 2017;14:E24. doi: 10.5888/pcd14.160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alberti KG, et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 4.Akter S, et al. Higher gravidity and parity are associated with increased prevalence of metabolic syndrome among rural Bangladeshi women. PLoS ONE. 2013;8:e68319. doi: 10.1371/journal.pone.0068319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsunaga T, et al. Associations of breastfeeding history with metabolic syndrome and cardiovascular risk factors in community-dwelling parous women: The Japan Multi-Institutional Collaborative Cohort Study. PLoS ONE. 2022;17:e0262252. doi: 10.1371/journal.pone.0262252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stuebe AM, Rich-Edwards JW. The reset hypothesis: Lactation and maternal metabolism. Am. J. Perinatol. 2009;26:81–88. doi: 10.1055/s-0028-1103034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunderson EP, et al. Duration of lactation and incidence of the metabolic syndrome in women of reproductive age according to gestational diabetes mellitus status: A 20-year prospective study in CARDIA (Coronary Artery Risk Development in Young Adults) Diabetes. 2010;59:495–504. doi: 10.2337/db09-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torris C, Bjornnes AK. Duration of lactation and maternal risk of metabolic syndrome: A systematic review and meta-analysis. Nutrients. 2020;12:2718. doi: 10.3390/nu12092718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J, Kim T. Association of breastfeeding and risk of metabolic syndrome and its components in postmenopausal parous women: Korea national health and nutrition examination survey (2010–2016) Arch. Public Health. 2021;79:82. doi: 10.1186/s13690-021-00607-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho GJ, et al. The relationship between reproductive factors and metabolic syndrome in Korean postmenopausal women: Korea National Health and Nutrition Survey 2005. Menopause. 2009;16:998–1003. doi: 10.1097/gme.0b013e3181a03807. [DOI] [PubMed] [Google Scholar]
- 11.Guembe MJ, et al. Risk for cardiovascular disease associated with metabolic syndrome and its components: A 13-year prospective study in the RIVANA cohort. Cardiovasc. Diabetol. 2020;19:195. doi: 10.1186/s12933-020-01166-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson PW, D'Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham FG, Leveno KJ. Childbearing among older women—the message is cautiously optimistic. N. Engl. J. Med. 1995;333:1002–1004. doi: 10.1056/NEJM199510123331511. [DOI] [PubMed] [Google Scholar]
- 14.Roos-Hesselink JW, Stein JI. Pregnancy and cardiac disease. Rev. Esp. Cardiol. 2017;70:78–80. doi: 10.1016/j.recesp.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 15.Regitz-Zagrosek V, et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur. Heart J. 2018;39:3165–3241. doi: 10.1093/eurheartj/ehy340. [DOI] [PubMed] [Google Scholar]
- 16.Adam K. Pregnancy in women with cardiovascular diseases. Methodist Debakey Cardiovasc. J. 2017;13:209. doi: 10.14797/mdcj-13-4-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ananth CV, et al. Changes in the prevalence of chronic hypertension in pregnancy, United States, 1970 to 2010. Hypertension. 2019;74:1089–1095. doi: 10.1161/HYPERTENSIONAHA.119.12968. [DOI] [PubMed] [Google Scholar]
- 18.Pouta A, et al. Manifestations of metabolic syndrome after hypertensive pregnancy. Hypertension. 2004;43:825–831. doi: 10.1161/01.HYP.0000120122.39231.88. [DOI] [PubMed] [Google Scholar]
- 19.Lei Q, et al. Prehypertension during normotensive pregnancy and postpartum clustering of cardiometabolic risk factors: A prospective cohort study. Hypertension. 2016;68:455–463. doi: 10.1161/HYPERTENSIONAHA.116.07261. [DOI] [PubMed] [Google Scholar]
- 20.Puhkala J, et al. Metabolic syndrome in Finnish women 7 years after a gestational diabetes prevention trial. BMJ Open. 2017;7:e014565. doi: 10.1136/bmjopen-2016-014565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hadden DR, McLaughlin C. Normal and abnormal maternal metabolism during pregnancy. Semin. Fetal Neonatal Med. 2009;14:66–71. doi: 10.1016/j.siny.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Vrijkotte TG, et al. Maternal lipid profile during early pregnancy and pregnancy complications and outcomes: The ABCD study. J. Clin. Endocrinol. Metab. 2012;97:3917–3925. doi: 10.1210/jc.2012-1295. [DOI] [PubMed] [Google Scholar]
- 23.Stuebe AM. Does breastfeeding prevent the metabolic syndrome, or does the metabolic syndrome prevent breastfeeding? Semin. Perinatol. 2015;39:290–295. doi: 10.1053/j.semperi.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dewey KG, Cohen RJ, Brown KH, Rivera LL. Effects of exclusive breastfeeding for four versus six months on maternal nutritional status and infant motor development: Results of two randomized trials in Honduras. J. Nutr. 2001;131:262–267. doi: 10.1093/jn/131.2.262. [DOI] [PubMed] [Google Scholar]
- 25.McClure CK, et al. Breastfeeding and subsequent maternal visceral adiposity. Obesity. 2011;19:2205–2213. doi: 10.1038/oby.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, et al. Impact of metabolic syndrome and it's components on prognosis in patients with cardiovascular diseases: A meta-analysis. Front. Cardiovasc. Med. 2021;8:704145. doi: 10.3389/fcvm.2021.704145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castro-Barquero S, Ruiz-Leon AM, Sierra-Perez M, Estruch R, Casas R. Dietary strategies for metabolic syndrome: A comprehensive review. Nutrients. 2020;12:2983. doi: 10.3390/nu12102983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taube A, Schlich R, Sell H, Eckardt K, Eckel J. Inflammation and metabolic dysfunction: Links to cardiovascular diseases. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H2148–2165. doi: 10.1152/ajpheart.00907.2011. [DOI] [PubMed] [Google Scholar]
- 29.Reddy P, Lent-Schochet D, Ramakrishnan N, McLaughlin M, Jialal I. Metabolic syndrome is an inflammatory disorder: A conspiracy between adipose tissue and phagocytes. Clin. Chim. Acta. 2019;496:35–44. doi: 10.1016/j.cca.2019.06.019. [DOI] [PubMed] [Google Scholar]
- 30.Lee YK, et al. South Korean time trade-off values for EQ-5D health states: Modeling with observed values for 101 health states. Value Health. 2009;12:1187–1193. doi: 10.1111/j.1524-4733.2009.00579.x. [DOI] [PubMed] [Google Scholar]
- 31.Yoon YS, Oh SW. Optimal waist circumference cutoff values for the diagnosis of abdominal obesity in korean adults. Endocrinol. Metab. 2014;29:418–426. doi: 10.3803/EnM.2014.29.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JY, et al. Radiomic machine learning for predicting prognostic biomarkers and molecular subtypes of breast cancer using tumor heterogeneity and angiogenesis properties on MRI. Eur. Radiol. 2022;32:650–660. doi: 10.1007/s00330-021-08146-8. [DOI] [PubMed] [Google Scholar]
- 33.Park EK, et al. Machine learning approaches to radiogenomics of breast cancer using low-dose perfusion computed tomography: Predicting prognostic biomarkers and molecular subtypes. Sci. Rep. 2019;9:17847. doi: 10.1038/s41598-019-54371-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park HS, et al. Machine learning models that integrate tumor texture and perfusion characteristics using low-dose breast computed tomography are promising for predicting histological biomarkers and treatment failure in breast cancer patients. Cancers. 2021;13:6013. doi: 10.3390/cancers13236013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data utilized in this study is available from the Korean National Health and Nutrition Examination Survey (KNHANES) (https://knhanes.kdca.go.kr/knhanes). The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.