Abstract
Hippocampal hyperactivity is a novel pharmacological target in the treatment of schizophrenia. We hypothesized that levetiracetam (LEV), a drug binding to the synaptic vesicle glycoprotein 2 A, normalizes hippocampal activity in persons with schizophrenia and can be measured using neuroimaging methods. Thirty healthy control participants and 30 patients with schizophrenia (28 treated with antipsychotic drugs), were randomly assigned to a double-blind, cross-over trial to receive a single administration of 500 mg oral LEV or placebo during two study visits. At each visit, we assessed hippocampal function using resting state fractional amplitude of low frequency fluctuations (fALFF), cerebral blood flow (CBF) with arterial spin labeling, and hippocampal blood-oxygen-level-dependent (BOLD) signal during a scene processing task. After placebo treatment, we found significant elevations in hippocampal fALFF in patients with schizophrenia, consistent with hippocampal hyperactivity. Additionally, hippocampal fALFF in patients with schizophrenia after LEV treatment did not significantly differ from healthy control participants receiving placebo, suggesting that LEV may normalize hippocampal hyperactivity. In contrast to our fALFF findings, we did not detect significant group differences or an effect of LEV treatment on hippocampal CBF. In the context of no significant group difference in BOLD signal, we found that hippocampal recruitment during scene processing is enhanced by LEV more significantly in schizophrenia. We conclude that pharmacological modulation of hippocampal hyperactivity in schizophrenia can be studied with some neuroimaging methods, but not others. Additional studies in different cohorts, employing alternate neuroimaging methods and study designs, are needed to establish levetiracetam as a treatment for schizophrenia.
Subject terms: Biomarkers, Schizophrenia, Cognitive neuroscience
Introduction
Neuroimaging studies have revealed that the anterior hippocampus is hyperactive in individuals at clinical high-risk for developing psychosis [1, 2] and in patients with schizophrenia [3, 4]. In addition, human post-mortem studies and animal models of schizophrenia have demonstrated that disruptions in hippocampal micro-circuitry result in an excitation-inhibition imbalance [5–10]. Potential mechanisms include hypo-functioning NMDA receptors on fast-spiking hippocampal interneurons [11] as well as abnormal glutamatergic and GABAergic neurotransmission [12–14].
There is growing evidence that a hyperactive hippocampus disrupts hippocampal-cortical networks in schizophrenia, resulting in positive symptoms [15, 16], negative symptoms [17], and cognitive deficits [18–20]. Further, previous studies have demonstrated that patients with a hyperactive hippocampus are unable to effectively engage, or recruit, the hippocampus during cognitive task performance [21, 22]. Therefore, decreasing hippocampal hyperactivity has promise in the treatment of schizophrenia [23]. Animal and human studies have explored levetiracetam (LEV), which targets synaptic vesicle glycoprotein 2 A (SV2A) [24] and normalizes glutamatergic neurotransmission [25], for the treatment of hippocampal hyperactivity. In animal models of hippocampal hyperactivity, LEV improved cognitive function [26], reduced behavioral abnormalities, and reversed hippocampal hyperactivity [27]. Critically, LEV remained effective when administered concurrently with an antipsychotic drug, implicating that LEV could be used as an adjunctive therapy. Several clinical trials in patients with schizophrenia are currently assessing the impact of LEV on clinical symptoms (NCT04317807), hippocampal hyperactivity (NCT03129360), or both (NCT02647437, NCT03034356). A recent randomized, controlled trial indicated that 8 weeks of LEV treatment improved clinical symptoms, especially negative symptoms and cognitive deficits, in 40 chronic schizophrenia patients [28].
Here we report findings of the first neuroimaging study of LEV treating hippocampal hyperactivity in schizophrenia. We used a randomized, double-blind, cross-over, placebo-controlled design to test two hypotheses. First, there is a hippocampal excitation-inhibition imbalance in schizophrenia, resulting in increased hippocampal activity and decreased recruitment in schizophrenia under placebo conditions (group effect). Second, LEV normalizes hippocampal excitation-inhibition imbalance in schizophrenia, decreasing hippocampal hyperactivity and enhancing recruitment (treatment effect).
We employed three complementary functional magnetic resonance imaging (fMRI) methods to measure the effects of LEV on hippocampal function: amplitude of low frequency fluctuations (ALFF), cerebral blood flow (CBF), and recruitment during scene processing. This rigorous, multi-modal approach enabled us to examine different components of hippocampal physiology under placebo and treatment conditions and utilize the different strengths of each approach to test our hypotheses. ALFF is an ideal method to test interneuron dysfunction in psychosis [29]. Interneuron abnormalities in schizophrenia desynchronize oscillatory activity in the gamma frequency range [30], which is tightly linked to the resting state fMRI signal [31]. Numerous studies have reported elevations in hippocampal ALFF in schizophrenia [32–36]. Fractional ALFF (fALFF), a derivative of the ALFF signal that is less susceptible to motion and physiological artifacts [37], is elevated across the longitudinal axis of the hippocampus in early stage psychosis patients [29]. Arterial spin labeling (ASL) generates a reliable, absolute measure of CBF [38]. Since CBF is tightly coupled to glucose metabolism, ASL provides an indirect measure of neuronal activity [39]. Hippocampal regional cerebral blood flow (rCBF) has been studied in schizophrenia using ASL [40–45], but results are difficult to interpret due to differences in acquisition parameters (e.g., using continuous or pulsed sequences instead of the preferred pseudo-continuous sequences), low-resolution data, and increasing evidence suggesting that antipsychotic treatment may impact the hippocampal rCBF signal [46, 47]. In the current study, we sought to overcome previous limitations by following recommended sequence parameters [48] and scanning at higher resolution. Hippocampal recruitment during task performance was measured with the scene-processing task (SPT). In a recent study we demonstrated that hyperactivity of the anterior hippocampus in schizophrenia, as measured by cerebral blood volume mapping, correlates negatively with BOLD signal changes during the SPT [22].
Methods
Study design and participants
Study design
Participants completed 2 study visits in a randomized, double-blind, within-subject crossover design, separated by a washout period of at least one week (Fig. 1). Both healthy control participants and schizophrenia patients were given oral placebo during one treatment session and 500 mg oral LEV (Keppra, UCB Laboratories) during the other treatment session, with the order counter-balanced (randomized, double-blind). Independent pharmacists at the Vanderbilt University Medical Center Investigational Drug Services dispensed either placebo or LEV according to a randomization list generated by an independent staff member. Placebo and LEV were in capsule form and identical in appearance. Capsules were prepackaged in containers and consecutively numbered for each participant based on the randomization list. Each participant was assigned an order number and received the capsules in a corresponding prepacked container. Two hours after placebo or LEV treatment, participants completed a MRI scan and blood draw (the average serum drug level was 8.86 + /− 4.73 ug/mL). Data safety was monitored by an independent study monitor in collaboration with the Vanderbilt IRB.
Fig. 1. Study design.
Each participant was randomized, double-blind, in a within-crossover design, with the order of treatment on LEV and placebo counterbalanced within each cohort. HC Healthy controls, SZ Patients with schizophrenia.
Participants
Thirty patients with a schizophrenia spectrum disorder (7 schizoaffective disorder, 23 schizophrenia; all schizoaffective disorder patients met full criteria for schizophrenia [49] and therefore the entire patient sample is referred to here as schizophrenia patients) were matched with 30 healthy control participants on age, gender, race, and parental education (Table 1, Supplementary Fig. 1) and recruited between September 2020 and July 2021. All patients were in the chronic stage of illness (duration of illness > 2 years). All participants provided written informed consent for participation in this study. The Vanderbilt University Institutional Review Board approved the study, which was pre-registered at clinicaltrials.gov (NCT04559529). Participant inclusion and exclusion criteria and clinical characteristics are detailed in the Supplementary Information.
Table 1.
Participant demographics and clinical characteristics.
| Healthy control participants |
Schizophrenia patients |
Healthy control participants > Schizophrenia patients |
||||
|---|---|---|---|---|---|---|
| N = 30 | N = 30 | |||||
| Mean | SD | Mean | SD | Statistic (t) | p | |
| Age (yrs) | 34.1 | 10.2 | 35.2 | 12.2 | −0.37 | 0.71 |
| Parental Education (yrs) | 15.0 | 2.11 | 15.3 | 2.49 | −0.58 | 0.56 |
| WTAR | 111.0 | 9.53 | 105.0 | 13.3 | 1.94 | 0.06 |
| CPZ Equivalents | 350.09 | 252.55 | ||||
| Duration of Illness (yrs) | 13.78 | 11.92 | ||||
| N | % | N | % | Statistic (X2) | p | |
| Gender (Male) | 21 | 0.70 | 21 | 0.70 | 0.00 | 1.00 |
| Race (White) | 21 | 0.70 | 24 | 0.80 | 0.36 | 0.55 |
| Tobacco Use | ||||||
| No | 26 | 0.87 | 14 | 0.47 | 12.43 | 0.002 |
| Yes, continued | 1 | 0.03 | 11 | 0.37 | ||
| Yes, quit | 3 | 0.10 | 5 | 0.17 | ||
| Cannabis Use (last month) | 1 | 0.03 | 7 | 0.23 | 3.06 | 0.06 |
| Treated with APD | 28 | 0.93 | ||||
| Diagnosis | ||||||
| Schizophrenia | 23 | 0.77 | ||||
| Schizoaffective DO | 7 | 0.23 | ||||
| PANSS | ||||||
| Positive | 16.45 | 9.30 | ||||
| Negative | 15.52 | 8.45 | ||||
| General | 25.90 | 6.85 | ||||
yrs years, SD standard deviation, APD antipsychotic drugs, DO disorder.
Bolded values indicate significance at p < 0.05 level.
Structural imaging
Structural MRI acquisition was completed on a 3 T Philips Ingenia scanner at the Vanderbilt University Institute of Imaging Sciences. The 3D T1-weighted scan (voxel resolution: 1 mm3; field of view = 256 × 256 mm; number of slices = 170; TE = 3.7 ms; TR = 8.0 ms) was inspected and determined to be free from motion or other artifacts (no images were removed).
T1 structural images were processed using the FreeSurfer 6 [50, 51] hippocampal subfield module [52]. We used segmentation output from FreeSurfer to construct individual-specific masks of the left and right, and whole, anterior, and posterior hippocampal regions for pseudocontinuous ASL (pCASL) and fMRI region of interest analyses, detailed below. Segmentations were visually inspected for errors and manually corrected (see Supplementary Information) for inclusion in this study. The anterior and posterior regions of the hippocampus are divided using an anatomical landmark, the uncus [53].
Amplitude of low frequency fluctuations
fALFF data acquisition
Resting state functional images were acquired with an echo planar imaging sequence (38 ascending slices, oriented at −15° relative to the intercommissural plane; voxel size = 3.0 × 3.0 × 3.2 mm; TR = 2 s; TE = 28.0 ms; flip angle = 90°; 203 volumes). Participants were instructed to remain still with their eyes closed during the scan. Data from three scans were removed because participants reported falling asleep during the imaging session.
fALFF analysis
As in our previous work [33], fALFF was calculated using AFNI’s 3dRSFC tool [54]. Linear and quadratic trends were removed from the preprocessed and denoised fMRI time-series, which was then converted to a power spectrum using a Fourier transform. fALFF was calculated as the average of the power in the range 0.01–0.1 Hz relative to the entire frequency spectrum. fALFF for each hippocampal voxel was normalized for whole brain mean fALFF. Average normalized fALFF values were extracted from hippocampal ROIs separately in each hemisphere and entered into statistical analyses as described below.
Cerebral blood flow imaging
ASL data acquisition
Hippocampal pCASL was not preceded by a functional task and was acquired as follows: spatial resolution = 3 × 3 × 5 mm3, TE = 18 ms, TR = 3750 ms, SENSE-factor = 2.3, flip angle = 90 degrees, labeling duration = 1650 ms train using a train of 0.5 ms Hanning-windowed pulses, and post-labeling delay = 1600 ms. The echo-planar imaging sequence acquired 40 paired (labeled, unlabeled) measurements covering 10 slices, oriented at −15° relative to the intercommissural plane, and utilized two background suppression pulses at 1710 and 2860 ms. An additional M0 ASL scan was acquired for equilibrium magnetization and for coregistration to the structural image. A second, lower spatial resolution, whole-brain pCASL sequence was also collected (Supplementary Information).
ASL data processing and analysis
Maps were motion-corrected using standard parameters. Difference images (control-label) were averaged and fit to a single-compartment kinetic model [48, 55] to quantify CBF in absolute units of ml blood/100 g tissue/min. Maps were corrected for slice labeling delay, coregistered to the T1-weighted structural data, and the inverse transform was used to bring the hippocampal ROIs into the native space of the ASL images. We collected two hippocampal pCASL sequences and rCBF values were generated using the average of both sequences.
Task fMRI
Data acquisition
We collected 111 volumes of whole brain fMRI data (38 ascending slices, oriented at −15° relative to the intercommissural plane; voxel size = 3.0 × 3.0 × 3.2 mm; TR = 2 s; TE = 28.0 ms; flip angle = 90°) after pCASL sequence completion.
Functional MRI scene-processing task (SPT)
Participants completed a single run of a block-design 1-back task (Supplementary Fig. 2) during fMRI scanning. The task is described in detail in McHugo et al. 2021 [56] and in the Supplementary Information. The processing and analysis of the SPT data methodology is documented in McHugo et al. 2021 [56] and can be found in detail in the Supplementary Information.
Statistical analysis
We conducted all statistical analyses in R (R Core Team, 2019), unless otherwise specified. We tested all dependent variables (i.e., normalized fALFF, rCBF, and BOLD percent signal change) in linear models or linear mixed models with the packages lme4 [57], and emmeans [58]. For all models, we conducted significance tests on the fixed effects using analysis of variance (ANOVA) on the model output. We tested all main effects and interaction effects using type 2 sum of squares. Significant interactions were followed up with contrasts adjusted for multiple comparisons using Bonferroni correction. For each analysis that demonstrated significant group differences after placebo treatment, we conducted a follow-up intermediary analysis to test whether hippocampal activity is normalized in patients treated with LEV (i.e., activity of patients treated with placebo > patients treated with LEV > control participants treated with placebo). Details of the statistical tests are provided in the Supplementary Information.
Results
Hippocampal activity in schizophrenia
During the placebo condition, we tested for increased hippocampal activity in schizophrenia with three complementary measures: resting state fMRI fluctuation amplitude (fALFF), resting state rCBF (ASL), and recruitment during scene processing (BOLD).
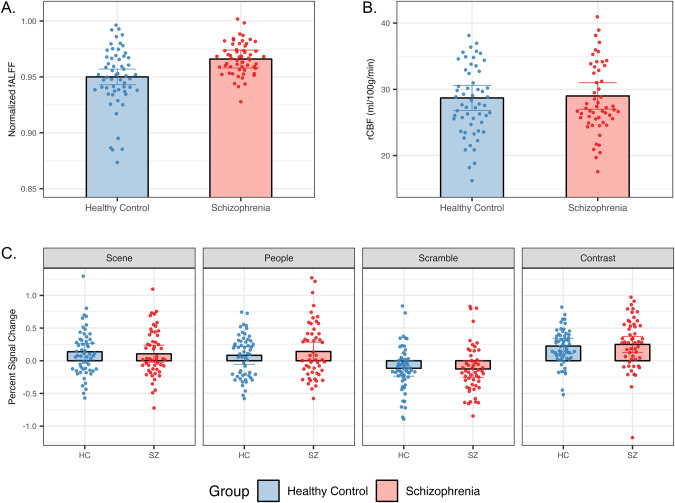
First, hippocampal fALFF was significantly elevated in patients with schizophrenia (F1,53 = 9.048, p = 0.004, Fig. 2A).
Fig. 2. Hippocampal fALFF, but not rCBF or BOLD PSC, is increased in schizophrenia.
A Linear mixed models indicate a significant group effect (p = 0.004) in hippocampal fALFF after placebo treatment (healthy control participants, n = 29; schizophrenia patients, n = 28). B Linear mixed models indicate no effect of group in hippocampal rCBF after placebo treatment using a high-resolution hippocampal ASL sequence (healthy control participants, n = 28; schizophrenia patients, n = 27). C Linear mixed models indicate no effect of group in the hippocampus after placebo treatment during the SPT (healthy control participants, n = 28; schizophrenia patients, n = 26). Both healthy control participants and schizophrenia patients demonstrate expected percent signal change patterns by each condition (increases in percent signal change for scene, people, and contrast condition; decrease in percent signal change for scramble condition). Error bars represent 95% confidence intervals. Individual points depict data from both hemispheres for each participant. HC Healthy controls, SZ Patients with schizophrenia.
Second, hippocampal resting state rCBF was not different in schizophrenia (Fig. 2B). Adjusting hippocampal rCBF by global gray matter rCBF did not change the results. There was no effect of group for global gray matter rCBF.
Third, both healthy individuals and schizophrenia patients significantly activated the hippocampus during the scene processing task (Fig. 2C, Supplementary Fig. 2b) and showed no between-group differences in anterior hippocampal BOLD signal change.
Effect of levetiracetam treatment on hippocampal activity
We tested for an effect of levetiracetam on all three measures of hippocampal activity.
First, we did not detect a statistically significant change in hippocampal fALFF, but we did find a trend-level decrease (F1,178 = 2.916, p = 0.089) in hippocampal fALFF for schizophrenia patients relative to healthy controls (0.11% reduction in controls versus 0.62% reduction in patients) after LEV treatment Fig. 3A).
Fig. 3. LEV enhances recruitment in scene processing in schizophrenia.
A Linear mixed models indicate a significant group effect (p = 0.005) and trend-level treatment effect (p = 0.089) in hippocampal fALFF after LEV treatment (healthy control participants, n = 29; schizophrenia patients, n = 29). B Linear mixed models indicate no effect of group or treatment in hippocampal rCBF after LEV treatment using a high-resolution hippocampal ASL sequence (healthy control participants, n = 30; schizophrenia patients, n = 29). C Linear mixed models demonstrate a group by treatment interaction (p = 0.005) in the scene condition of the SPT in healthy control participants (n = 29) and schizophrenia patients (n = 29). Error bars represent 95% confidence intervals. Individual points depict data from both hemispheres for each participant. HC Healthy controls, SZ Patients with schizophrenia, LEV levetiracetam.
Second, we did not find a significant effect of LEV treatment on hippocampal rCBF (Fig. 3B). There was no effect of treatment on global gray matter rCBF.
Third, we did not find a significant main effect of LEV treatment on BOLD signal change during scene processing (Fig. 3C). However, we observed a group by treatment interaction in the scene condition (F1,163 = 8.188, p = 0.005). Post-hoc tests revealed group differences after LEV treatment (t89 = 2.655, p = 0.019) but not after placebo treatment (t89 = −0.141, p = 1.000). When we limited our analysis to the participants’ first SPT session, we observed a main effect of group (F1,50 = 4.331, p = 0.043) and a trend-level group by treatment interaction (F1,50 = 3.436, p = 0.070) for the scene condition and a main effect of group (F1,50 = 7.747, p = 0.008) and a group by treatment interaction (F1,50 = 4.510, p = 0.039) for the scramble condition (Supplementary Fig. 3a). Post-hoc tests demonstrated a difference in group after LEV treatment (t50 = 3.47, p = 0.002) but no difference under placebo treatment (t50 = 0.49, p = 1.00). These effects were not found in the second SPT session (Supplementary Fig. 3b).
Intermediary analysis
We found a linear relationship of LEV treatment on hippocampal fALFF signal (F2,98 = 6.083, p = 0.003), in which hippocampal fALFF was highest in patients after placebo treatment, lowest in control participants after placebo treatment, and intermediary in patients after LEV treatment (Fig. 4). Post-hoc tests revealed differences between healthy control participants treated with placebo and patients with schizophrenia treated with placebo (t60 = 2.929, p = 0.014). In contrast, we found only a trend-level difference between patients treated with placebo and patients treated with LEV (t116 = 2.142, p = 0.052) and no difference between healthy control participants treated with placebo and patients with schizophrenia treated with LEV (t61 = 1.936, p = 0.173).
Fig. 4. Hippocampal activity does not differ between patients treated with LEV and controls treated with placebo.
There is a significant linear relationship (p = 0.003) between healthy control participants after placebo treatment, patients with schizophrenia after placebo treatment, and patients with schizophrenia after LEV treatment. Post-hoc tests reveal differences between healthy control participants treated with placebo and patients with schizophrenia treated with placebo (p = 0.014), but no difference between healthy controls treated with placebo and patients with schizophrenia treated with LEV (p = 0.173). There is a trend-level difference between patients treated with placebo and patients treatment with LEV (p = 0.052). Error bars represent 95% confidence intervals. Individual points depict data from both hemispheres for each participant. LEV levetiracetam.
Discussion
We investigated the impact of low-dose LEV on hippocampal hyperactivity, a potential treatment target for schizophrenia, using a randomized, double-blind, cross-over, placebo-controlled design and fMRI. We found evidence for hippocampal hyperactivity, suggestive of an excitation-inhibition imbalance, and an effect of LEV in schizophrenia with some neuroimaging measures of hippocampal function, but not others.
Notably, we replicated the finding of higher hippocampal fALFF in schizophrenia, consistent with previous studies demonstrating hippocampal hyperactivity in schizophrenia [29]. Although the effect was small and did not reach statistical significance, we found preliminary evidence that LEV may decrease hippocampal fALFF, more in schizophrenia than in healthy control participants. Crucially, we found that hippocampal recruitment during scene processing is enhanced more significantly in schizophrenia. To the best of our knowledge, this is the first published work detailing the effect of low-dose LEV on hippocampal hyperactivity in schizophrenia, but other trials are currently underway (e.g., NCT03129360, NCT02647437, NCT03034356).
Imaging hippocampal hyperactivity
Our findings suggest that fALFF is a promising neuroimaging measure to test the hypothesis of hippocampal hyperactivity in schizophrenia. After placebo treatment, only the fALFF analysis revealed a significant main effect of group. This finding adds to a growing literature demonstrating that hippocampal fALFF is elevated in psychosis [32–36]. The abnormal fALFF signal in schizophrenia may be linked to hippocampal interneurons [29] and therefore may be an ideal method to test the hypothesis of hippocampal interneuron dysfunction in psychosis [7].
A strength of this study is the multi-modal neuroimaging approach. A direct comparison of the differing results provides insight into potential hippocampal mechanisms of disease in schizophrenia. Resting state hippocampal rCBF was normal in our schizophrenia cohort. Previous hippocampal ASL studies had reported inconsistent findings [40–44]. Despite our methodological improvements in this study, we did not detect increased hippocampal resting rCBF in schizophrenia.
A growing literature highlights the limitations of using ASL to study hippocampal CBF in schizophrenia. Multiple ASL studies have demonstrated increased CBF in the striatum [41, 59] and decreased CBF in the frontal lobe [41, 59, 60], but few have reported altered CBF in hippocampal regions. Increased perfusion has only been reported in antipsychotic-naïve patients [40] and CHR-P individuals [61, 62] (10 and 13% taking antipsychotic medication, respectively). Antipsychotic drugs reduce hippocampal CBF, effectively normalizing CBF in schizophrenia patients [46, 47]. Similarly, antipsychotic drugs might explain the consistently reported increased striatal and decreased frontal CBF values in schizophrenia [63]. The mechanism by which antipsychotics impact CBF is unknown, although there is evidence suggesting that D2 receptor blockade drives striatal hyperperfusion [45]. Whether antipsychotics differentially impact neuroimaging signals (e.g., fALFF or the BOLD signal) is also unclear. Taken together, our finding of significant fALFF differences but normal CBF values in a cohort of chronic, treated schizophrenia patients is consistent with hippocampal interneuron dysfunction, but also highlights the potential confound of antipsychotic medication when testing the hypothesis.
When we restricted our analysis of the SPT to the first imaging session, we found hippocampal hyperactivity in schizophrenia, consistent with previous studies [22, 64]. The emergence of this group difference after LEV treatment indicates that LEV may have a subtle, differential impact on hippocampal activation. However, these effects were not seen when performing an analysis across both study sessions. This suggests a practice effect during the second performance of the SPT [65]. This limits the SPT as a neuroimaging measure of hippocampal activation in a repeated-measures study design.
Effect of levetiracetam treatment on hippocampal activity
LEV may normalize hippocampal hyperactivity in schizophrenia. The primary fALFF analysis demonstrated a trend-level main effect of treatment and an intermediary analysis revealed a significant linear relationship: patients who received LEV showed lower hippocampal fALFF values than patients after placebo treatment and did not significantly differ from healthy control participants after placebo treatment. Patients with schizophrenia demonstrate hippocampal hyperactivity and reductions in hippocampal activation during task performance, suggestive of a ceiling effect in which patients are unable to effectively recruit the hippocampus [21, 22]. Here, we report that patients increase recruitment of the hippocampus during scene processing after treatment with LEV. This provides preliminary evidence that LEV may impact hippocampal signal processing. Altogether, our results support a model in which hippocampal hyperactivity limits hippocampal recruitment in patients with schizophrenia. Targeting the excitation-inhibition imbalance in the hippocampus using pharmacologic modulation to reduce hippocampal hyperactivity may alleviate the ceiling effect and allow patients with schizophrenia to recruit the hippocampus more effectively, highlighting the therapeutic potential of hippocampal hyperactivity as a treatment target for schizophrenia.
Our study is an initial effort to measure the pharmacological modulation of hippocampal hyperactivity in schizophrenia. Future studies should study different patient cohorts (especially early stage psychosis) and explore different pharmacological regimens (e.g., different dosage, multiple versus single administration). Recent PET studies have demonstrated alterations in SV2A receptor expression in chronic schizophrenia patients [66–68] and mixed results in the early stages of psychosis [69, 70], suggesting that LEV may have distinct pharmacological properties in schizophrenia that differ by illness stage. Additionally, clinical trials using LEV to normalize hippocampal activity in patients with amnestic Mild Cognitive Impairment [71, 72] have demonstrated that LEV dosage differentially impacts hippocampal activation. LEV effectively modulated hippocampal activity and task performance at a dosage of 125 mg, but not at 250 mg. These studies also differed from the current study by administering LEV twice daily for two weeks before assessing changes in hippocampal activity. Here we tested the effect of LEV on hippocampal activity after a single administration of 500 mg LEV, which might have engaged the hippocampus differently.
Limitations and future directions
The confound of antipsychotic medication in the study of CBF and the fast habituation of hippocampal BOLD signal and/or practice effects limit our ability to make strong claims about the ability of LEV to normalize hippocampal hyperactivity and normalize recruitment during task performance. Despite these limitations, fALFF appears to be a promising proxy measure of hippocampal excitation-inhibition balance in schizophrenia, even in chronic, treated patients. In addition, other multimodal imaging methods, especially the quantification of glutamate and GABA levels, could clarify how LEV normalizes hippocampal hyperactivity [73]. Finally, pharmacologic agents other than LEV, that can reduce hippocampal hyperactivity in schizophrenia, should also be explored [74, 75].
In conclusion, this initial study suggests that LEV may normalize hippocampal excitation-inhibition balance in chronic schizophrenia.
Supplementary information
Author contributions
Study concept, design, and acquisition of funding: MJR and SH. Participant recruitment: MJR, KA, and SH. Acquisition and processing of data: MJR, MM, BR, MD, and SH. Statistical analyses and interpretation of data: MJR, MM, SA, MD, SH. Drafting of manuscript: MJR and SH. Critical revision of manuscript: MJR, MM, BR, KA, SA, MD, SH. Study supervision: SH. All authors read and approve the manuscript and vouch for the adherence of the trial to the protocol, the accuracy of the data and analyses, and reporting of adverse events.
Funding
Research reported in this publication was supported by the Charlotte and Donald Test Fund, the Vanderbilt Psychiatric Genotype/Phenotype Project, the National Institute of Mental Health (NIMH) grants R01-MH70560 (SH) and F30-MH125507 (MJR), the National Institute of General Medical Sciences (NIGMS) grant T32-GM007347, and the Vanderbilt Institute for Clinical and Translational Research (through grant UL1-TR000445 from the National Center for Research Resources/NIH).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-023-01730-0.
References
- 1.Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I, et al. Imaging Patients with Psychosis and a Mouse Model Establishes a Spreading Pattern of Hippocampal Dysfunction and Implicates Glutamate as a Driver. Neuron. 2013;78:81–93. doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Modinos G, Şimşek F, Azis M, Bossong M, Bonoldi I, Samson C, et al. Prefrontal GABA levels, hippocampal resting perfusion and the risk of psychosis. Neuropsychopharmacology. 2018;43:2652–9. doi: 10.1038/s41386-017-0004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, et al. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009;66:938–46. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talati P, Rane S, Kose S, Blackford JU, Gore J, Donahue MJ, et al. Increased hippocampal CA1 cerebral blood volume in schizophrenia. Neuroimage Clin. 2014;5:359–64. doi: 10.1016/j.nicl.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stan AD, Ghose S, Zhao C, Hulsey K, Mihalakos P, Yanagi M, et al. Magnetic resonance spectroscopy and tissue protein concentrations together suggest lower glutamate signaling in dentate gyrus in schizophrenia. Mol Psychiatry. 2015;20:433–9. doi: 10.1038/mp.2014.54. [DOI] [PubMed] [Google Scholar]
- 6.Benes FM. Evidence for altered trisynaptic circuitry in schizophrenic hippocampus. Biol Psychiatry. 1999;46:589–99. doi: 10.1016/S0006-3223(99)00136-5. [DOI] [PubMed] [Google Scholar]
- 7.Heckers S, Konradi C. GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. Schizophr Res. 2015;167:4–11. doi: 10.1016/j.schres.2014.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29:2344–54. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grace AA, Gomes FV. The Circuitry of Dopamine System Regulation and its Disruption in Schizophrenia: Insights Into Treatment and Prevention. Schizophr Bull. 2018. 10.1093/schbul/sbx199. [DOI] [PMC free article] [PubMed]
- 10.Kiemes A, Serrano Navacerrada ME, Kim E, Randall K, Simmons C, Rojo Gonzalez L, et al. Erbb4 Deletion From Inhibitory Interneurons Causes Psychosis-Relevant Neuroimaging Phenotypes. Schizophr Bull. 2023;49:569–80. doi: 10.1093/SCHBUL/SBAC192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–42. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Briend F, Nelson EA, Maximo O, Armstrong WP, Kraguljac NV, Lahti AC. Hippocampal glutamate and hippocampus subfield volumes in antipsychotic-naive first episode psychosis subjects and relationships to duration of untreated psychosis. Transl Psychiatry 2020;10. 10.1038/s41398-020-0812-z. [DOI] [PMC free article] [PubMed]
- 13.Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167:1178–93. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- 14.Lieberman JA, Girgis RR, Brucato G, Moore H, Provenzano F, Kegeles L, et al. Hippocampal dysfunction in the pathophysiology of schizophrenia: a selective review and hypothesis for early detection and intervention. Mol Psychiatry. 2018;23:1764–72. doi: 10.1038/mp.2017.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roiser JP, Howes OD, Chaddock CA, Joyce EM, McGuire P. Neural and behavioral correlates of aberrant salience in individuals at risk for psychosis. Schizophr Bull. 2013;39:1328–36. doi: 10.1093/schbul/sbs147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolthusen RPF, Coombs G, Boeke EA, Ehrlich S, DeCross SN, Nasr S, et al. Correlation Between Levels of Delusional Beliefs and Perfusion of the Hippocampus and an Associated Network in a Non–Help-Seeking Population. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:178–86. doi: 10.1016/j.bpsc.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makowski C, Bodnar M, Shenker JJ, Malla AK, Joober R, Chakravarty MM, et al. Linking persistent negative symptoms to amygdala–hippocampus structure in first-episode psychosis. Transl Psychiatry 2017;7. 10.1038/tp.2017.168. [DOI] [PMC free article] [PubMed]
- 18.Achim AM, Lepage M. Episodic memory-related activation in schizophrenia: Meta-analysis. Br J Psychiatry. 2005;187:500–9. doi: 10.1192/bjp.187.6.500. [DOI] [PubMed] [Google Scholar]
- 19.Ranganath C, Minzenberg MJ, Ragland JD. The Cognitive Neuroscience of Memory Function and Dysfunction in Schizophrenia. Biol Psychiatry. 2008;64:18–25. doi: 10.1016/j.biopsych.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo JY, Ragland JD, Carter CS. Memory and cognition in schizophrenia. Mol Psychiatry. 2019;24:633–42. doi: 10.1038/s41380-018-0231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ. et al. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1998. 10.1038/1137. [DOI] [PubMed]
- 22.McHugo M, Talati P, Armstrong K, Vandekar SN, Blackford JU, Woodward ND, et al. Hyperactivity and reduced activation of anterior hippocampus in early psychosis. Am J Psychiatry. 2019;176:1030–8. doi: 10.1176/appi.ajp.2019.19020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tregellas JR. Neuroimaging biomarkers for early drug development in schizophrenia. Biol Psychiatry. 2014;76:111–9. doi: 10.1016/j.biopsych.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Löscher W, Gillard M, Sands ZA, Kaminski RM, Klitgaard H. Synaptic Vesicle Glycoprotein 2A Ligands in the Treatment of Epilepsy and Beyond. CNS Drugs. 2016;30:1055–77. doi: 10.1007/S40263-016-0384-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjalieh SM, Matagne A, et al. The synaptic vesicle is the protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci USA. 2004;101:9861–6. doi: 10.1073/pnas.0308208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koh MT, Shao Y, Rosenzweig-Lipson S, Gallagher M. Treatment with levetiracetam improves cognition in a ketamine rat model of schizophrenia. Schizophr Res. 2018;193:119–25. doi: 10.1016/j.schres.2017.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavichioli AM, Santos-Silva T, Grace AA, Guimarães FS, Gomes FV. Levetiracetam Attenuates Adolescent Stress-induced Behavioral and Electrophysiological Changes Associated With Schizophrenia in Adult Rats. Schizophr Bull 2022. 10.1093/schbul/sbac106. [DOI] [PMC free article] [PubMed]
- 28.Behdani F, Hassanzadeh B, Eslamzadeh M, Moradi M, Hebrani P, Dadgarmoghaddam M, et al. Can levetiracetam improve clinical symptoms in schizophrenic patients? A randomized placebo-controlled clinical trial. Int Clin Psychopharmacol. 2022;37:159–65. doi: 10.1097/YIC.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 29.McHugo M, Rogers BP, Avery SN, Armstrong K, Blackford JU, Vandekar SN, et al. Increased amplitude of hippocampal low frequency fluctuations in early psychosis: A two-year follow-up study. Schizophr Res. 2022;241:260–6. doi: 10.1016/J.SCHRES.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathalon DH, Sohal VS. Neural Oscillations and Synchrony in Brain Dysfunction and Neuropsychiatric Disorders: It’s About Time. JAMA Psychiatry. 2015;72:840–4. doi: 10.1001/JAMAPSYCHIATRY.2015.0483. [DOI] [PubMed] [Google Scholar]
- 31.Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RAW. Neuroscience: Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309:948–51. doi: 10.1126/SCIENCE.1110948/SUPPL_FILE/NIESSING.SOM.PDF. [DOI] [PubMed] [Google Scholar]
- 32.Hare SM, Law AS, Ford JM, Mathalon DH, Ahmadi A, Damaraju E, et al. Disrupted Network Cross Talk, Hippocampal Dysfunction and Hallucinations in Schizophrenia. Schizophr Res. 2018;199:226. doi: 10.1016/J.SCHRES.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McHugo M, Rogers BP, Talati P, Woodward ND, Heckers S. Increased amplitude of low frequency fluctuations but normal hippocampal-default mode network connectivity in schizophrenia. Front Psychiatry. 2015;6:92. doi: 10.3389/FPSYT.2015.00092/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang Y, Zhou Q, Chang M, Chekroud A, Gueorguieva R, Jiang X, et al. Altered functional connectivity and low-frequency signal fluctuations in early psychosis and genetic high risk. Schizophr Res. 2019;210:172–9. doi: 10.1016/J.SCHRES.2018.12.041. [DOI] [PubMed] [Google Scholar]
- 35.Turner JA, Damaraju E, Van Erp TGM, Mathalon DH, Ford JM, Voyvodic J, et al. A multi-site resting state fMRI study on the amplitude of low frequency fluctuations in schizophrenia. Front Neurosci. 2013;7:47140. doi: 10.3389/FNINS.2013.00137/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoptman MJ, Zuo XN, Butler PD, Javitt DC, D’Angelo D, Mauro CJ, et al. Amplitude of low-frequency oscillations in schizophrenia: A resting state fMRI study. Schizophr Res. 2010;117:13–20. doi: 10.1016/J.SCHRES.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: Fractional ALFF. J Neurosci Methods. 2008;172:137–41. doi: 10.1016/J.JNEUMETH.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borogovac A, Asllani I. Arterial spin labeling (ASL) fMRI: Advantages, theoretical constrains and experimental challenges in neurosciences. Int J Biomed Imaging 2012;2012. 10.1155/2012/818456. [DOI] [PMC free article] [PubMed]
- 39.Petcharunpaisan S, Ramalho J, Castillo M. Arterial spin labeling in neuroimaging. World J Radiol. 2010;2:384. doi: 10.4329/WJR.V2.I10.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scheef L, Manka C, Daamen M, Kühn KU, Maier W, Schild HH, et al. Resting-state perfusion in nonmedicated schizophrenic patients: A continuous arterial spin-labeling 3.0-T MR study. Radiology. 2010;256:253–60. doi: 10.1148/radiol.10091224. [DOI] [PubMed] [Google Scholar]
- 41.Pinkham A, Loughead J, Ruparel K, Wu WC, Overton E, Gur R, et al. Resting quantitative cerebral blood flow in schizophrenia measured by pulsed arterial spin labeling perfusion MRI. Psychiatry Res Neuroimaging. 2011;194:64–72. doi: 10.1016/j.pscychresns.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walther S, Federspiel A, Horn H, Razavi N, Wiest R, Dierks T, et al. Resting state cerebral blood flow and objective motor activity reveal basal ganglia dysfunction in schizophrenia. Psychiatry Res Neuroimaging. 2011;192:117–24. doi: 10.1016/j.pscychresns.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Kindler J, Jann K, Homan P, Hauf M, Walther S, Strik W, et al. Static and dynamic characteristics of cerebral blood flow during the resting state in schizophrenia. Schizophr Bull. 2015;41:163–70. doi: 10.1093/schbul/sbt180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ota M, Ishikawa M, Sato N, Okazaki M, Maikusa N, Hori H, et al. Pseudo-continuous arterial spin labeling MRI study of schizophrenic patients. Schizophr Res. 2014;154:113–8. doi: 10.1016/j.schres.2014.01.035. [DOI] [PubMed] [Google Scholar]
- 45.Selvaggi P, Jauhar S, Kotoula V, Pepper F, Veronese M, Santangelo B, et al. Reduced cortical cerebral blood flow in antipsychotic-free first-episode psychosis and relationship to treatment response. Psychol Med. 2022:1–11. 10.1017/S0033291722002288. [DOI] [PMC free article] [PubMed]
- 46.Lahti AC, Holcomb HH, Weiler MA, Medoff DR, Tamminga CA. Functional effects of antipsychotic drugs: comparing clozapine with haloperidol. Biol Psychiatry. 2003;53:601–8. doi: 10.1016/S0006-3223(02)01602-5. [DOI] [PubMed] [Google Scholar]
- 47.Medoff DR, Holcomb HH, Lahti AC, Tamminga CA. Probing the human hippocampus using rCBF: Contrasts in schizophrenia. Hippocampus. 2001;11:543–50. doi: 10.1002/HIPO.1070. [DOI] [PubMed] [Google Scholar]
- 48.Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2015;73:102–16. doi: 10.1002/mrm.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seldin K, Armstrong K, Schiff ML, Heckers S. Reducing the Diagnostic Heterogeneity of Schizoaffective Disorder. Front Psychiatry 2017;8. 10.3389/FPSYT.2017.00018. [DOI] [PMC free article] [PubMed]
- 50.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 51.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/S0896-6273(02)00569-X. [DOI] [PubMed] [Google Scholar]
- 52.Iglesias JE, Augustinack JC, Nguyen K, Player CM, Player A, Wright M, et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. Neuroimage. 2015;115:117–37. doi: 10.1016/j.neuroimage.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woolard AA, Heckers S. Anatomical and functional correlates of human hippocampal volume asymmetry. Psychiatry Res Neuroimaging. 2012;201:48–53. doi: 10.1016/j.pscychresns.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taylor PA, Saad ZS. FATCAT: (an efficient) Functional and Tractographic Connectivity Analysis Toolbox. Brain Connect. 2013;3:523–35. doi: 10.1089/BRAIN.2013.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, Edelman RR. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med. 1998. 10.1002/mrm.1910400308. [DOI] [PubMed]
- 56.McHugo M, Avery S, Armstrong K, Rogers BP, Vandekar SN, Woodward ND, et al. Anterior hippocampal dysfunction in early psychosis: a 2-year follow-up study. Psychol Med. 2021:1–10. 10.1017/S0033291721001318. [DOI] [PMC free article] [PubMed]
- 57.Bates D, Machler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw. 2015;67:1–48.. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 58.Fox J, Weisberg S. Package ‘car’. Companion to Applied Regression, Second Edition. 2011.
- 59.Kindler J, Schultze-Lutter F, Hauf M, Dierks T, Federspiel A, Walther S, et al. Increased Striatal and Reduced Prefrontal Cerebral Blood Flow in Clinical High Risk for Psychosis. Schizophr Bull. 2018;44:182. doi: 10.1093/SCHBUL/SBX070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oliveira ÍAF, Guimarães TM, Souza RM, dos Santos AC, Machado-de-Sousa JP, Hallak JEC, et al. Brain functional and perfusional alterations in schizophrenia: an arterial spin labeling study. Psychiatry Res Neuroimaging. 2018;272:71–8. doi: 10.1016/J.PSCYCHRESNS.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 61.Allen P, Chaddock CA, Egerton A, Howes OD, Bonoldi I, Zelaya F, et al. Resting hyperperfusion of the hippocampus, midbrain, and basal ganglia in people at high risk for psychosis. Am J Psychiatry. 2016;173:392–9. doi: 10.1176/APPI.AJP.2015.15040485/ASSET/IMAGES/LARGE/APPI.AJP.2015.15040485F2.JPEG. [DOI] [PubMed] [Google Scholar]
- 62.Allen P, Azis M, Modinos G, Bossong MG, Bonoldi I, Samson C, et al. Increased Resting Hippocampal and Basal Ganglia Perfusion in People at Ultra High Risk for Psychosis: Replication in a Second Cohort. Schizophr Bull. 2018;44:1323.. doi: 10.1093/SCHBUL/SBX169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Handley R, Zelaya FO, Reinders AATS, Marques TR, Mehta MA, O’Gorman R. et al. Acute effects of single‐dose aripiprazole and haloperidol on resting cerebral blood flow (rCBF) in the human brain. Hum Brain Mapp. 2013;34:272.. doi: 10.1002/HBM.21436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ragland JD, Layher E, Hannula DE, Niendam TA, Lesh TA, Solomon M, et al. Impact of schizophrenia on anterior and posterior hippocampus during memory for complex scenes. Neuroimage Clin. 2017;13:82–8. doi: 10.1016/J.NICL.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kelly AMC, Garavan H. Human functional neuroimaging of brain changes associated with practice. Cereb Cortex. 2005;15:1089–102. doi: 10.1093/CERCOR/BHI005. [DOI] [PubMed] [Google Scholar]
- 66.Onwordi EC, Halff EF, Whitehurst T, Mansur A, Cotel MC, Wells L, et al. Synaptic density marker SV2A is reduced in schizophrenia patients and unaffected by antipsychotics in rats. Nat Commun. 2020. 10.1038/s41467-019-14122-0. [DOI] [PMC free article] [PubMed]
- 67.Radhakrishnan R, Skosnik PD, Ranganathan M, Naganawa M, Toyonaga T, Finnema S, et al. In vivo evidence of lower synaptic vesicle density in schizophrenia. Mol Psychiatry. 2021;26:7690–8. doi: 10.1038/S41380-021-01184-0. [DOI] [PubMed] [Google Scholar]
- 68.Howes OD, Cummings C, Chapman GE, Shatalina E. Neuroimaging in schizophrenia: an overview of findings and their implications for synaptic changes. Neuropsychopharmacology. 2022. 10.1038/s41386-022-01426-x. [DOI] [PMC free article] [PubMed]
- 69.Onwordi EC, Whitehurst T, Shatalina E, Mansur A, Arumuham A, Osugo M, et al. Synaptic Terminal Density Early in the Course of Schizophrenia: an in vivo UCB-J Positron Emission Tomographic Imaging Study of Synaptic Vesicle Glycoprotein 2A (SV2A). Biol Psychiatry. 2023. 10.1016/J.BIOPSYCH.2023.05.022. [DOI] [PubMed]
- 70.Yoon JH, Zhang Z, Mormino E, Davidzon G, Minzenberg MJ, Ballon J, et al. Reductions in synaptic marker SV2A in early-course Schizophrenia. J Psychiatr Res. 2023;161:213–7. doi: 10.1016/J.JPSYCHIRES.2023.02.026. [DOI] [PubMed] [Google Scholar]
- 71.Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, et al. Reduction of Hippocampal Hyperactivity Improves Cognition in Amnestic Mild Cognitive Impairment. Neuron. 2012;74:467–74. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bakker A, Albert MS, Krauss G, Speck CL, Gallagher M. Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance. Neuroimage Clin. 2015;7:688–98. doi: 10.1016/j.nicl.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lyseng-Williamson KA. Levetiracetam: A review of its use in epilepsy. Drugs. 2011;71:489–514. doi: 10.2165/11204490-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 74.Smucny J, Olincy A, Rojas DC, Tregellas JR. Neuronal effects of nicotine during auditory selective attention in schizophrenia. Hum Brain Mapp. 2016;37:410–21. doi: 10.1002/hbm.23040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kätzel D, Wolff AR, Bygrave AM, Bannerman DM. Hippocampal Hyperactivity as a Druggable Circuit-Level Origin of Aberrant Salience in Schizophrenia. Front Pharmacol 2020;11. 10.3389/FPHAR.2020.486811. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.