Abstract
The use of post-transplantation cyclophosphamide (PTCy) for graft-versus-host disease (GVHD) prophylaxis is not established after reduced intensity conditioning (RIC) hematopoietic stem cell transplantation (HSCT) from fully matched donors. This was a randomized, open-label, multicenter, phase 2 trial. All patients received a RIC regimen with fludarabine, intravenous busulfan for 2 days (Flu-Bu2), and a peripheral blood stem cell (PBSC) graft from a matched related or 10/10 HLA-matched unrelated donor. Patients were randomly assigned to receive anti-thymocyte globulin (ATG) 5 mg/kg plus standard GVHD prophylaxis or PTCy 50 mg/kg/d at days +3 and +4 plus standard GVHD prophylaxis. The primary endpoint was the composite endpoint of GVHD- and relapse-free survival (GRFS) at 12 months after HSCT. Eighty-nine patients were randomly assigned to receive either PTCy or control prophylaxis with ATG. At 12 months, disease-free survival was 65.9% in the PTCy group and 67.6% in the ATG group (P = 0.99). Cumulative incidence of relapse, non-relapse mortality, and overall survival were also comparable in the two groups. GRFS at 12 months was 54.5% in the PTCy group versus 43.2% in the ATG group (P = 0.27). The median time to neutrophil and platelet count recovery was significantly longer in the PTCy group compared to the ATG group. Except for day +30, where EORTC QLQ-C30 scores were significantly lower in the PTCy compared to the ATG group, the evolution with time was not different between the two groups. Although the primary objective was not met, PTCy is effective for GVHD prophylaxis in patients receiving Flu-Bu2 conditioning with a PBSC graft from a fully matched donor and was well tolerated in term of adverse events and quality of life. This trial was registered at clinicaltrials.gov: NCT02876679.
Subject terms: Haematological diseases, Clinical trial design
Introduction
The use of allogeneic hematopoietic stem cell transplantation (HSCT) is still increasing worldwide, mainly due to the development of reduced intensity conditioning (RIC) regimens, increase in age of patients and increasing stem cell sources [1]. However, graft-versus-host disease (GVHD) still represents a major limitation of HSCT. The combination of fludarabine and 2 days of busulfan (Flu-Bu2) is a widely used RIC regimen in many centers in Europe [2–6]. However, the best GVHD prophylaxis combination in the Flu-Bu2 RIC regimen has not yet been established. While the combination of cyclosporine A (CsA), and a short course of methotrexate (MTX) after transplantation is considered as the gold standard for GVHD prophylaxis after conventional myeloablative HSCT from HLA-identical siblings [7], there is no consensus on the optimal preventive regimen for GVHD prophylaxis after RIC HSCT [8]. In a retrospective study, similar outcomes in the group of patients who received MTX or mycophenolate mofetil (MMF) and CsA without ATG were observed, but this group had a higher risk of chronic (c) GVHD leading to worse survival. In the context of ATG-containing regimens, the addition of MMF or MTX to CsA did not reduce the risk of acute (a) GVHD, but significantly increased that of relapse incidence (RI), possibly as a consequence of the relatively reduced risk of cGVHD, leading to worse disease-free survival (DFS) and overall survival (OS) [9]. Considering the source of donors, the updated recommendations, based on several high-level evidence publications [10], suggest that rabbit anti-thymocyte globulin (ATG) or anti-T-lymphocyte globulin should be used for GVHD prophylaxis in patients undergoing matched unrelated donor (MUD) HSCT [11]. However, ATG delays immune reconstitution and has been shown to be associated with more infections, especially viral infections [12, 13]. On the other hand, post-transplant cyclophosphamide (PTCy) is now well established, successful, and widely utilized for GVHD prophylaxis after bone marrow (BM) haploidentical HSCT [14, 15]. The mechanism of action of PTCy has been described as inducing preferential elimination and clonal deletion of alloreactive T-cells [16]. Moreover, there is evidence supporting the importance of regulatory T-cells in mediating long-term post-transplant tolerance and GVHD control with PTCy [17]. However, BM is not the preferred source of stem cells after RIC HSCT, and the potential efficacy of PTCy on preventing GVHD when using PBSCs is still under debate. This point is of major concern, as PBSCs represent the main stem cell source of allogeneic cells worldwide. Results from two non-randomized phase 2 studies suggest that PTCy alone may not be the preferred GVHD prophylaxis following a RIC transplant with PBSCs [18, 19]. The incidences of grade II-IV and grade III-IV a GVHD were 45% and 27%, respectively, with a non-relapse mortality (NRM) of 36% at 1 and 2 years, suggesting the benefit of adding another immunosuppressive treatment in the PBSC transplantation setting. The hitherto inconclusive data highlight the need for a controlled trial in a standardized setting. We launched a phase 2 randomized clinical trial comparing at 12 months, the efficacy of PTCy versus ATG for GVHD prophylaxis in the setting of Flu-Bu RIC as determined by a composite endpoint of GVHD-free, relapse-free survival (GRFS), allogeneic PBSC transplantation. In this report, we present the final analysis of this study.
Subjects and methods
Study design and patients
This is a randomized, multicenter, open-label phase 2 trial comparing PTCy with standard ATG as GVHD prophylaxis in patients receiving a RIC regimen before HSCT from matched sibling donors (MSD) or 10/10-HLA MUD in 11 French medical centers. The trial was approved by the Comité de Protection des Personnes d’Aulnay-sous-Bois (France). Eligible patients were aged 18–70 years, diagnosed with a hematological malignancy, including acute myeloid or lymphoblastic leukemia, myelodysplastic syndrome, myeloproliferative disorder, chronic lymphocyte leukemia, and lymphoma, for which a RIC HSCT was indicated. Eligibility criteria for RIC HSCT included at least one of the following parameters: (i) patient age older than 50 years; (ii) heavily pre-treated patients who had received an autologous HSCT (auto-HSCT) or with more than two lines of chemotherapy before HSCT; and (iii) patients with poor performance status because of significant medical comorbidities [20] i.e., a Karnofsky score of at least 70. Only HLA-matched family donors and 10/10-HLA MUD were selected for the purpose of this study, donor selection was performed on the basis of high resolution (4 digit) typing of HLA-A, B, C, DRB1, DQB1. The complete eligibility criteria are in the protocol (Supplementary appendix 1). Mobilized PBSCs was the only stem cell source accepted. All patients gave informed consent.
Randomization
Eligible patients were randomly assigned in a 1:1 ratio to receive either PTCy (experimental arm) or ATG (control arm). Allocation was done centrally. Patients were included and assigned to treatment up to 30 days before transplantation. Investigators had access to treatment assignments at their own centers. The statistician had access to data, in keeping with the need to report to the Data and Safety Monitoring Committee.
Procedures
Fludarabine was administered intravenously over 30 min at a total dose of 150 mg/m2 divided into five daily doses of 30 mg/m2/day, from day −6 to day −2. Busulfan was infused once daily intravenously over 3 h at a dose of 130 mg/m2/day. In the experimental arm, cyclophosphamide (50 mg/kg/day) was given on days +3 and +4 post-transplant. In the control arm, rabbit ATG (Thymoglobuline®, Sanofi-Genzyme, Lyon, France) was given at 2.5 mg/Kg/day × 2 days on days −2 and −1. CsA was administered at a dose of 3 mg/kg/day by continuous intravenous infusion starting from day +5 in the experimental group and from day −3 in the control group, with doses adjusted to maintain a trough level of 200–400 ng/mL. CsA was changed to twice daily oral dosing as soon as it was tolerated. CsA was tapered over 4 weeks from day +62, if clinically possible. Oral MMF was given at a fixed dose of 2 g/day starting from day +5 in the experimental group and from day −3 in the control group. No treatment adjustment was performed for MMF. MMF was tapered over 4 weeks starting from day +35 if clinically possible. All supportive care was given in keeping with the local institutional practice.
Safety assessments included reports of adverse events (AEs) graded according to the Common Terminology Criteria Adverse Events version 4.02. Grading of aGVHD was performed according to the revised Glucksberg criteria [21]. cGVHD was recorded (as well as the requirement for a systemic immunosuppressive therapy) and the maximum grade achieved according to the NIH Consensus Criteria [22]. All Epstein-Barr Virus (EBV) and cytomegalovirus (CMV) reactivations requiring treatment had to be reported, as well as all cardiac AEs.
To assess health-related quality of life, patients completed the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Core Questionnaire (QLQ-C30) [23] and the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) [24] at days −7 (baseline), +30, +90, +180 and +360.
Outcomes
The primary endpoint was the composite endpoint of GRFS at 12 months after HSCT. In fact, it is well established that such composite endpoints acknowledge that both survival and rates of other critical events are important when testing new interventions. The Blood and Marrow Transplant Clinical Trials Network (BMT CTN) recognized the potential utility of a composite endpoint in HSCT trials [25]. GRFS after HSCT, including grade III-IV aGVHD, cGVHD requiring systemic treatment, relapse, or death, is a clinically meaningful one because it represents ideal recovery from HSCT (at 1 year) and a measure of cure without ongoing morbidity. Secondary endpoints were aGVHD, cGVHD, NRM, RI, DFS, OS, chronic GVHD-free, relapse-free survival (CRFS) and quality of life (QoL). CRFS was defined as survival in the absence of cGVHD and relapse. NRM was defined as death without evidence of relapse or progression. DFS was defined as survival with no evidence of relapse or progression. OS was defined as the time from HSCT to death, regardless of the cause. RI and NRM rates were estimated using cumulative incidence (CI) functions and considered as competing risks. For aGVHD and cGVHD, death and relapse were considered as competing events. For CI of serious AEs, death was considered as a competing event. Engraftment was defined as achieving an absolute neutrophil count greater than or equal to 0.5 × 109/L for three consecutive days. Platelet engraftment was defined as independence from platelet transfusion for at least 7 days with a platelet count ≥20 × 109/L.
Statistical analysis
With 80 analyzable patients, the study design had 60% power to identify PTCy as superior to ATG when the GRFS at 1 year was 20% better than ATG at the one-sided significance level of 5% (two-sided level of 10%). The full analysis set included all patients who underwent HSCT for whom GRFS could be estimated. We also compared OS and DFS from time of randomization, in an intent-to-treat analysis. Probabilities of GRFS, DFS and OS were calculated using Kaplan–Meier estimates. Univariate analyses were performed using Gray’s test for CI and the log-rank test for GRFS, DFS and OS. The follow-up was censored at 1 year for 1-year comparisons. We also updated the follow-up of all patients in order to give the estimates at 3 years. Results of QoL after scoring were compared at each time point using the Mann-Whitney test and a generalized linear model was used to compare the evolution with time. Statistical analyses were performed with R version 4.0.1 (R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/). This study was registered with ClinicalTrials.gov, identifier number: NCT02876679.
Results
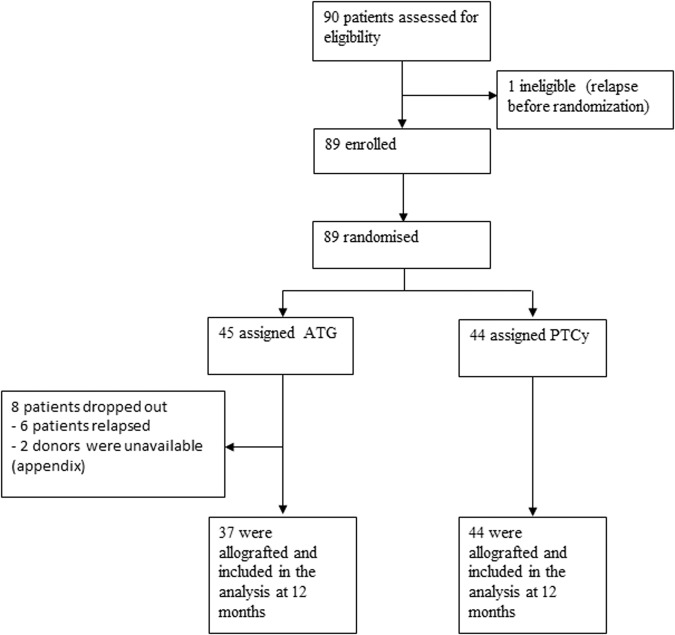
Between April 6, 2017, and October 10, 2019, a total of 90 patients from 11 centers were included. However, because one patient relapsed before randomization, only 89 patients were randomly assigned to receive either PTCy (44 patients) or control prophylaxis with ATG (45 patients) (Fig. 1). In the ATG-arm, only 37 patients underwent HSCT because six patients relapsed before transplant (4 cytologic relapses and two molecular relapses; 3 of these patients underwent HSCT with a sequential conditioning regimen chosen by the investigators) and two donors became unavailable (one unrelated donor became unavailable and one donor had an EBV replication) (Supplementary appendix 2-Table S1); in the PTCy group, 44 underwent HSCT. Overall, the median age was 64 years (range 21–71), and 69% patients were male. The baseline demographic and transplantation-related characteristics were well balanced between the two arms (Table 1). The median follow-up was 56 months in the PTCy group and 55 in the ATG group.
Fig. 1.
Study flowchart.
Table 1.
Patient and transplant related characteristics.
| PTCy (n = 44) | ATG (n = 37) | P value | ||
|---|---|---|---|---|
| Median follow-up (months) [95% CI] | 12 [11.97–12.16] | 12 [11.93–12.3] | 0.19 | |
| Patient age (years) | median (min-max) [IQR] | 64.4 (36–71.1) [57.5–66.5] | 64.3 (21.3–70.7) [59.4–67.6] | 0.73 |
| Patient gender | male | 30 (68.2%) | 26 (70.3%) | 0.84 |
| female | 14 (31.8%) | 11 (29.7%) | ||
| Recipient cytomegalovirus serostatus | Negative | 19 (43.2%) | 17 (47.2%) | 0.29 |
| Positive | 25 (56.8%) | 17 (47.2%) | ||
| missing | 0 | 3 | ||
| ECOG | 0 | 25 (61%) | 19 (57.6%) | 0.78 |
| 1 | 15 (36.6%) | 12 (36.4%) | ||
| 2 | 1 (2.4%) | 2 (6.1%) | ||
| missing | 3 | 4 | ||
| Diagnosis | acute myeloid leukemia | 21 (47.7%) | 17 (45.9%) | 0.66 |
| acute lymphoblastic leukemia | 2 (4.5%) | 2 (5.4%) | ||
| multiple myeloma | 1 (2.3%) | 3 (8.1%) | ||
| lymphoma | 8 (18.2%) | 5 (13.5%) | ||
| chronic lymphocytic leukemia | 1 (2.3) | 0 (0%) | ||
| myelodysplastic syndrome | 8 (18.2%) | 8 (21.6%) | ||
| myeloproliferative disorder | 1 (2.3%) | 2 (5.4%) | ||
| other | 2 (4.5%) | 0 | ||
| Status at transplantation | diagnosis | 1 (2.3%) | 2 (5.4%) | 0.15 |
| CR1 | 29 (65.9%) | 17 (45.9%) | ||
| CR2 | 2 (4.5%) | 7 (18.9%) | ||
| CR3 | 4 (9.1%) | 2 (5.4%) | ||
| PR | 4 (9.1%) | 7 (18.9%) | ||
| R/R | 4 (9.1%) | 2 (5.4%) | ||
| Time randomization-conditioning regimen (days) | median (min-max) [IQR] | 7 (1–21) [2–9.5] | 7 (1–23) [2–10] | 0.72 |
| Year of transplant | median (min-max) | 2018 (2017–2019) | 2018 (2017–2019) | 0.54 |
| Donor | matched related | 17 (38.6%) | 15 (40.5%) | 0.86 |
| 10/10 matched unrelated | 27 (61.4%) | 22 (59.5%) | ||
| Donor sex | male | 23 (52.3%) | 21 (58.3%) | 0.59 |
| female | 21 (47.7%) | 15 (41.7%) | ||
| missing | 0 | 1 | ||
| Donor age (years) | median (min-max) [IQR] | 38 (18–69) [27–54.2] | 31 (21–69) [25–56] | 0.66 |
| missing | 2 | 2 | ||
| Donor cytomegalovirus serostatus | negative | 25 (58.1%) | 20 (57.1%) | 0.93 |
| positive | 18 (41.9%) | 15 (42.9%) | ||
| missing | 1 | 2 | ||
| Graft cell content | ||||
| CD34 × 10.6/Kg | median (min-max) [IQR] | 6 (1.5–11.1) [4.7–7.6] | 6.6 (2.6–10.9) [4.7–8.2] | 0.42 |
| missing | 1 | 1 | ||
| Associated IS | CsA +MMF | 28 (63.6%) | 24 (64.9%) | 0.91 |
| CsA alone | 16 (36.4%) | 13 (35.1%) |
CR complete remission, IS immunosuppressive drug, IQR interquartile range, PR partial response, R/R relapsed or refractory, PTCy post-transplant cyclophosphamide, ATG anti-thymocyte globulin, CsA cyclosporine A, MMF mycophenolate mofetil, ECOG Eastern Cooperative Oncology Group.
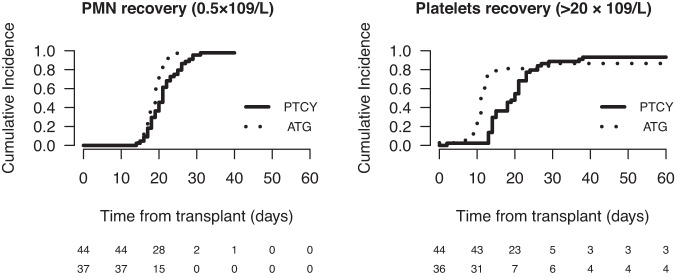
The median time to neutrophil recovery was significantly longer in the PTCy group at 21 days (range, 14–40) compared to 19 days in the ATG group (range, 14–27) (P = 0.01). Platelet count recovery was longer in the PTCy group, with a median time to achieve platelets >20 g/L of 20 days (range, 2–177) versus 11 days (range, 6–195) in the ATG group (P < 0.0001) (Fig. 2). Platelet numbers remained significantly lower at 1 year in the PTCy group compared to the ATG group. However, at 3 months and later, the number of platelet transfusions did not differ between the groups.
Fig. 2.
Polymorphonuclear neutrophil (PMN) and platelet engraftment after transplantation with post- cyclophosphamide (PTCy) or with anti-thymocyte globulin (ATG).
Table 2 shows the outcomes at 12 months. No statistically significant differences between the PTCy and ATG groups was observed.
Table 2.
Outcomes at 1, 3, and 5 years.
| Relapse | NRM | DFS | OS | GRFS | CRFS | chronic GVHD | chronic GVHD requiring systemic treatment | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 year | PTCy (n = 44) | 22.7% [11.6–36] | 11.4% [4.1–22.8] | 65.9% [50–77.8] | 79.5% [64.4–88.8] | 56.8% [41–69.9] | 56.8% [41–69.9] | 32.5% [18.5–47.2] | 13.6% [5.4–25.6] |
| ATG (n = 37) | 27% [13.9–42] | 8.1% [2–19.8] | 64.9% [47.3–77.9] | 81.1% [64.4–90.5] | 40.5% [24.9–55.7] | 40.5% [24.9–55.7] | 36.1% [20.7–51.8] | 24.3% [11.9–39.2] | |
| P value (censored at 1 year) | 0.61 | 0.66 | 0.82 | 0.91 | 0.12 | 0.11 | 0.72 | 0.58 | |
| 3 years | PTCy (n = 44) | 27.3% [15–41] | 11.4% [4.1–22.8] | 61.4% [45.4–73.9] | 72.7% [57–83.5] | 47.7% [32.5–61.5] | 47.7% [32.5–61.5] | 32.5% [18.5–47.2] | 18.2% [8.4–30.9] |
| ATG (n = 37) | 32.4% [18–47.7] | 10.8% [3.3–23.3] | 56.8% [39.4–70.8] | 64.9% [47.3–77.9] | 37.8% [22.6–53] | 37.8% [22.6–53] | 41.7% [25.2–57.4] | 27% [13.8–42.1] | |
| 5 years | PTCy (n = 44) | 27.3% [15–41] | 18.6% [7.3–33.8] | 54.2% [36.8–68.6] | 60.3% [42.5–74.2] | 43.2% [28.4–57.1] | 43.2% [28.4–57.1] | 32.5% [18.5–47.2] | 22.7% [11.6–36.1] |
| ATG (n = 37) | 37.6% [20.8–54.4] | 10.8% [3.3–23.3] | 51.6% [33.2–67.2] | 60.5% [42.1–74.7] | 37.8% [22.6–53] | 37.8% [22.6–53] | 41.7% [25.2–57.4] | 27% [13.8–42.1] | |
| p value (entire FU) | 0.52 | 0.57 | 0.77 | 0.94 | 0.39 | 0.37 | 0.44 | 0.58 |
NRM non-relapse mortality, DFS disease-free survival, OS overall survival, GRFS graft-versus-host disease- free, relapse-free survival, CRFS chronic graft-versus-host disease-free, relapse-free survival, GVHD graft-versus-host disease, PTCy post-transplant cyclophosphamide, ATG anti-thymocyte globulin, FU follow-up.
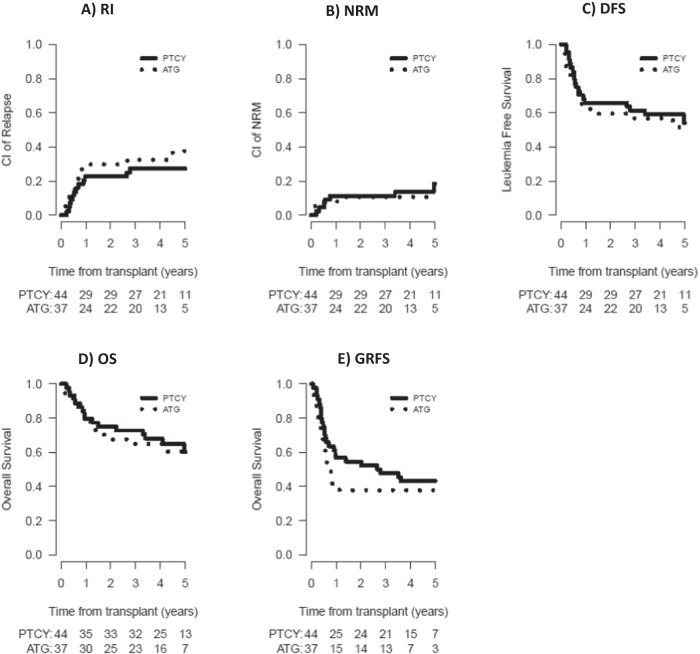
Likewise, at 5 years, there was no statistically significant difference in RI (27.3% vs. 37.6%, P = 0.52), CI of NRM (18.6% % vs. 10.8%, P = 0.57), DFS (54.2% vs. 51.6%, P = 0.77), OS (60.3% vs. 60.5%, P = 0.94), CRFS (43.2% vs. 37.8%, P = 0.37) or GRFS (43.2% vs. 37.8%, P = 0.39), between the PTCy and ATG groups, respectively (Fig. 3) (Table 2).
Fig. 3. Clinical outcomes according to the use of post-transplant cyclophosphamide (PTCy) or anti-thymocyte globulin (ATG).
Relapse incidence (RI) (A), non-relapse mortality (NRM) (B), disease-free survival (DFS) (C), overall survival (OS) (D), graft-versus-host disease-free, relapse-free survival (GRFS) (E) according to the use of post-transplant cyclophosphamide (PTCy) or anti-thymocyte globulin (ATG).
Finally, in an intent-to-treat analysis including all patients from randomization, 1-year DFS was 65.9% (95% CI, 50–77.8) vs. 62.2% (95% CI, 46.5–74.6) in the PTCy and ATG groups (P = 0.5), 1-year OS was 79.5% (95% CI, 64.4–88.8) vs. 73.3% (95% CI, 57.8–83.9), respectively (P = 0.41).
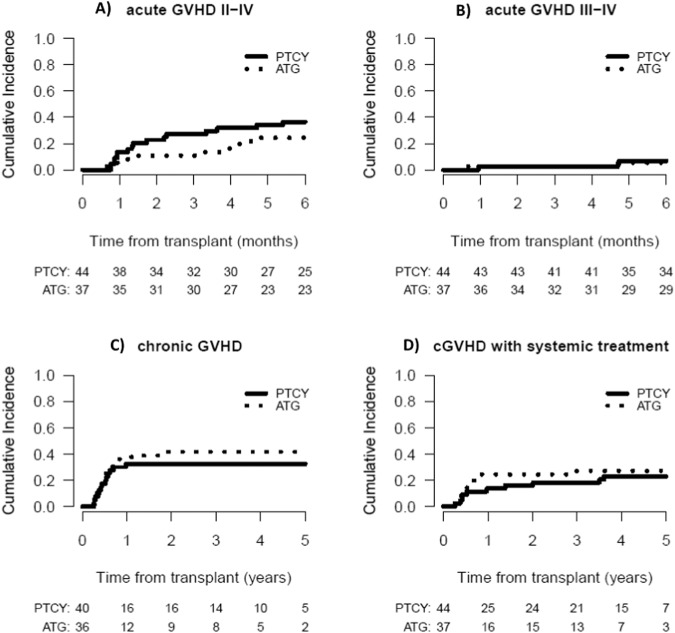
At 6 months, no significant difference was observed in the CI of aGVHD grade II-IV between the PTCy and ATG groups, with 36.4% and 24.3%, respectively (P = 0.35) (Fig. 4). In patients who received PTCy, 6.8% developed aGVHD grade III-IV in comparison to 5.4% of those who received ATG (P = 0.81). The incidence, organ involvement and severity of aGVHD were similar in the two groups (Supplementary appendix 2-Table S2). At 1 year, the CI of cGVHD was 32.5% in the PTCy group and 36.1% in the ATG group, with no significant difference observed between the two groups (Fig. 4), and the CI of cGVHD requiring systemic treatment was 13.6% in the PTCy group and 24.3% in the ATG group (P = 0.58) (Table 2).
Fig. 4. Cumulative incidence of GVHD.
aGVHD grade II-IV(A), aGVHD grade III-IV(B), cGVHD (C), cGVHD requiring systemic treatment (D) according to the use of post-transplant cyclophosphamide (PTCy) or anti-thymocyte globulin (ATG).
AEs numbered 37 in the PTCy group and 31 in the ATG group. Six and four patients developed a cardiac AE in the PTCy and ATG groups, respectively (Table 3). The CI of cardiac events at 1-year did not differ between the two groups with 11.4% (95%CI, 4.1–22.7) in the PTCy group and 8.1% (95%CI, 2–19.8) in the ATG group, P = 0.69. All cardiac complications occurred before 40 days except for two patients (1 in the PTCy group at day +323 and 1 in the ATG group at day +230) With respect to hemorrhagic cystitis, four events were observed in the PTCy group versus 1 in the ATG group (P = 0.37). Cystitis occurred within 20 days in the PTCy group and at day +201 in one patient who received ATG. EBV reactivation occurred in five patients in the PTCy group and in eight patients in the ATG group. The CI of EBV reactivation requiring treatment was 6.8% (95%CI, 1.7–16.9) and 21.6% (95%CI,10–36.1), P = 0.18. The CI for CMV reactivation requiring treatment did not differ between the groups: 29.5% (95%CI, 16.8–43.4) in PTCy and 32.4% (95%CI, 18–47.7) in the ATG group (P = 0.72). In the first month, 72.7% of patients experienced an infection in the PTCy group versus 59.5% in the ATG group (p = 0.21). Febrile neutropenia represented 78% of the infections in the PTCy group and 64% in the ATG group. Three cases of Clostridium difficile were reported in the PTCy group and two cases in the ATG group. Between the first and third month, 20 episodes of infection were reported in the PTCy group versus 19 in the ATG group (p = 0.73), it was mostly (70%) viral reactivation (in particular, CMV). In the PTCy group, the main cause of death was progression. Three patients died of GVHD, one of bacterial infection, one from hemorrhage. In the ATG group, progression was also the main cause of death with two patients dying of GVHD, one of pneumonia and one from hemorrhage (Supplementary appendix 2-Table S3).
Table 3.
Serious adverse events.
| PTCY (n = 44) | ATG (n = 37) | P | ||
|---|---|---|---|---|
| EBV reactivation (requiring treatment) | No | 39 (88.6%) | 29 (78.4%) | 0.21 |
| Yes | 5 (11.4%) | 8 (21.6%) | ||
| CMV reactivation (requiring treatment) | No | 31 (70.5%) | 25 (67.6%) | 0.78 |
| Yes | 13 (29.5%) | 12 (32.4%) | ||
| CARDIAC Events (Atrial Fibrillation/Arrhythmia/Unspecified Cardiac Disease) | No | 38 (86.4%) | 33 (89.2%) | 0.75 |
| Yes | 6 (13.6%) | 4 (10.8%) | ||
| Hemorrhagic CYSTITIS (BK Virus associated hemorrhagic cystitis/Non-BK Virus associated hemorrhagic cystitis) | No | 40 (90.9%) | 36 (97.3%) | 0.37 |
| Yes | 4 (9.1%) | 1 (2.7%) |
ATG anti-thymocyte globulin, CMV Cytomegalovirus EBV Epstein Barr Virus, PTCy post-transplant cyclophosphamide.
Except for day +30 where EORTC QLQ-C30 scores were significantly lower in the PTCy compared to the ATG group (P = 0.01), there were no significant differences between the two groups at days +90, +180, and +360. There was a suggestion of trend towards a lower FACT-BMT score at day +30 in the PTCy group (P = 0.051), but the scores were not significantly different at the other time points. The evolution with time was not different between the two groups (P = 0.24 and P = 0.70 for the EORTC functional score and FACT-BMT score, respectively).
Discussion
Despite progress in prophylaxis regimens, GVHD remains a major complication post-HSCT, leading to increased morbidity, mortality, and altered QoL. The differential impacts of PTCy and ATG are an important area of clinical and biological research. However, only retrospective studies with different aims have been conducted. This randomized phase 2 trial did not show significant differences between PTCy and ATG in the acute and cGVHD incidence in patients who underwent RIC HSCT with an MSD or a 10/10 HLA MUD. Even with over 4 years of follow-up, the absence of a statistically significant difference persists between the two groups. Considering donor type, the results are in accordance with a retrospective study from the EBMT on 174 and 1452 patients transplanted with a 10/10 HLA MUD, receiving PTCy and ATG, respectively, in which no significant difference between the PTCy and ATG groups was observed for incidence of grade II-IV aGVHD [26]. Bailen et al. have reported on 60 patients undergoing a matched or 9/10 mismatched unrelated donor (MMUD) HSCT with ATG-based prophylaxis combined with MTX-CsA, and 72 using a PTCy-based regimen [27]. PTCy showed a reduction in the rate of aGVHD but it is worth noting that 9/10 MMUDs were included. Recently, Bolanos-Meade et al. published the results of a phase 3 trial that randomized PTCy-tacrolimus-MMF or tacrolimus-MTX in patients who underwent HSCT from an HLA-matched related donor or a matched or 7/8 mismatched unrelated donor after a RIC regimen [28]. GRFS was significantly longer in the group who received PTCy, and the analysis showed a significantly lower hazard of grade III or IV aGVHD and of cGVHD with the PTCy-prophylaxis regimen than with the standard-prophylaxis regimen. In the phase 3 HOVON 96 trial, PTCy associated with CsA was compared to mycophenolic acid and CsA in patients with a RIC regimen [29]. This trial showed that PTCy was superior to CsA-MMF with respect to GFRS at 1 year. These important results emphasize the beneficial effect of PTCy in HSCT, more significantly than in the haploidentical setting. However, ATG was not included in these trials which is the standard GVHD prophylaxis in most of European countries for RIC regimens. In a retrospective study comparing the outcomes of adults with acute myeloid leukemia undergoing HSCT from HLA-MSD after the use of PTCy or ATG, no differences were observed at 1 year, in the transplantation outcomes, except for cGVHD which was significantly lower in the ATG group [30]. Although there was heterogeneity in terms of conditioning regimens and association of immunosuppressive drugs, the DFS and RI were comparable in both groups. These are important data especially considering that RIC HSCT mainly relies on the development of an immunological graft-versus-leukemia (GVL) effect, in contrast to a myeloablative conditioning regimen [31]. It is now established that the effects of ATG on RIC are dose-dependent, and that intermediate doses of ATG between 4 and 6 mg/kg seem to prevent GVHD optimally while sparing the GVL effect [32]. In our trial, using PTCy at the dose of 50 mg/kg/d on days +3 and +4 post-transplantation resulted in similar DFS and RI incidence, suggesting a retention of the GVL effect.
This trial did not find a significant difference in the safety profiles of PTCy and ATG. The cardiac toxicity profile of PTCy compared with non-PTCy GVHD prophylaxis is still under debate [33]. In our trial, 14% developed a cardiac AE in the PTCy group versus 8% in the ATG group, a statistically non-significant difference. Yeh et al. performed a retrospective analysis to evaluate cardiac toxicities after HLA-matched HSCT [34]. They found that baseline cardiac comorbidities were associated with a higher incidence of cardiac toxicities after HSCT, but PTCy-based GVHD prophylaxis did not appear to impact their development. Viral reactivation is an important issue, especially during the first few months post-transplant. ATG is a well-defined risk factor for EBV reactivation and EBV post-transplantation lymphoproliferative disorder [35]. Goldsmith et al. reported that PTCy, regardless of donor, was associated with a higher incidence of CMV infection [36]. However, we did not find significant differences for EBV and CMV reactivation between the two groups. This could reflect the low intensity of the Flu-Bu2 conditioning regimen with a faster immune reconstitution. Likewise, the rate of hemorrhagic cystitis was similar after PTCy and ATG.
Finally, the QoL study pointed to a significantly lower score for the PTCy group initially, at day +30; however, at months 3, 6, and 12, no difference was observed between the two groups.
Limitations of the study included heterogeneity of patients’ hematological diseases. RIC regimens typically have less antineoplastic activity but have limited toxicity and are thus better tolerated by patients who are not eligible for myeloablative conditioning. In our cohort, 20 patients were not in complete remission at transplant, of whom eight were treated for B-mature lymphoid malignancies or Hodgkin lymphoma and, therefore, had already received several lines of chemotherapy. It could explain why investigators decided to perform a Flu-Bu2 conditioning regimen, regarding the numerous previous lines of treatment and the median age of the cohort. However, the main objective of this study was to compare PTCy and ATG as GVHD prophylaxis and their impact on GRFS prevention regardless of the diagnosis. The distribution of diseases was well balanced between the two groups, however, the limited size of the cohort may mask subtle differences in patient characteristics such as disease status at transplant. Finally, the survival outcomes of DFS, RI, NRM, and OS were comparable between the two groups, but they should be confirmed in a larger study. We allowed 1 month between randomization and HSCT which led to the exclusion of eight patients, by chance, all in the ATG-arm, the main reason was relapse pre-transplant that modified the conditioning regimen. However, the characteristics of the population in each arm were comparable. Finally, as it was a phase 2 study, we were only able to include 40 patients per group therefore, the statistical power planned for in the protocol was obviously limited.
Although the primary objective was not met, the important results of this multicenter prospective study highlight some practical points. ATG has been extensively recognized as the ‘standard of care’ for GVHD prophylaxis in MSD and MUD HSCT [37]. However, it carries some limitations: it is not available everywhere, it is rather expensive, difficult to administer, and is often linked by investigators to the generation of opportunistic infections. The alternative drug, cyclophosphamide, is comparatively low-cost, widely available, and easy to use. Due to its significant success in the control of GVHD in the ‘difficult’ haploidentical HSCT setting, the question of whether it would be advantageous in MSD and MUD transplants is of paramount importance, particularly in centers where the routine use of ATG is precluded for the stated reasons. Despite some retrospective studies showing that PTCy is indeed effective in the latter setting, no conclusive data on its advantage or disadvantage over ATG was observed. Therefore, our randomized trial results demonstrate that the effectiveness and safety profile of the two drugs appear to be very similar, opening up a basis for an informed choice and paving the way for future studies.
Supplementary information
Acknowledgements
We thank the patients and their families. We acknowledge the crucial roles of the study coordinators in each center.
Author contributions
MM and EB were principal investigators, designed the research, and chaired the steering committee. ML was the statistician; she helped in the design of the study and did the statistical analyses. HL, GF, PC, TG, IY-A, MS, C-EB, AH, SC, A-LM, M-TR, PC, RD, SF, FM, and DB were transplant-center principal investigators, contributed patients, and approved the manuscript. EB, ML, and MM designed the study and wrote the manuscript. All authors commented on the manuscript and approved it for publication purposes.
Funding
PHRC-K 2015 (Ministry of Health).
Data availability
Data supporting the findings of this study including de-identified patient data are available after final completion of the trial report and are shared according to data sharing guidelines upon reasonable request to the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41408-024-00990-3.
References
- 1.Passweg JR, Baldomero H, Chabannon C, Basak GW, de la Camara R, Corbacioglu S, et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years. Bone Marrow Transpl. 2021;56:1651–64. doi: 10.1038/s41409-021-01227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohty M, Bay JO, Faucher C, Choufi B, Bilger K, Tournilhac O, et al. Graft-versus-host disease following allogeneic transplantation from HLA-identical sibling with antithymocyte globulin-based reduced-intensity preparative regimen. Blood. 2003;102:470–6. doi: 10.1182/blood-2002-12-3629. [DOI] [PubMed] [Google Scholar]
- 3.Yafour N, Serradj F, Osmani S, Brahimi M, Bouhass R, Arabi A, et al. Improving survival rates for patients with acute myeloid leukemia: Impacts of fludarabine/busulfan and antithymocyte globulin as reduced toxicity myeloablative conditioning for matched related donor allo-HCT. Curr Res Transl Med. 2020;68:145–8. doi: 10.1016/j.retram.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Le Bourgeois A, Lestang E, Guillaume T, Delaunay J, Ayari S, Blin N, et al. Prognostic impact of immune status and hematopoietic recovery before and after fludarabine, IV busulfan, and antithymocyte globulins (FB2 regimen) reduced-intensity conditioning regimen (RIC) allogeneic stem cell transplantation (allo-SCT) Eur J Haematol. 2013;90:177–86. doi: 10.1111/ejh.12049. [DOI] [PubMed] [Google Scholar]
- 5.Blaise D, Tabrizi R, Boher JM, Le Corroller-Soriano AG, Bay JO, Fegueux N, et al. Randomized study of 2 reduced-intensity conditioning strategies for human leukocyte antigen-matched, related allogeneic peripheral blood stem cell transplantation: prospective clinical and socioeconomic evaluation. Cancer. 2013;119:602–11. doi: 10.1002/cncr.27786. [DOI] [PubMed] [Google Scholar]
- 6.Lioure B, Bene MC, Pigneux A, Huynh A, Chevallier P, Fegueux N, et al. Early matched sibling hematopoietic cell transplantation for adult AML in first remission using an age-adapted strategy: long-term results of a prospective GOELAMS study. Blood. 2012;119:2943–8. doi: 10.1182/blood-2011-05-352989. [DOI] [PubMed] [Google Scholar]
- 7.Storb R, Deeg HJ, Whitehead J, Appelbaum F, Beatty P, Bensinger W, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N. Engl J Med. 1986;314:729–35. doi: 10.1056/NEJM198603203141201. [DOI] [PubMed] [Google Scholar]
- 8.Chhabra S, Liu Y, Hemmer MT, Costa L, Pidala JA, Couriel DR, et al. Comparative Analysis of Calcineurin Inhibitor-Based Methotrexate and Mycophenolate Mofetil-Containing Regimens for Prevention of Graft-versus-Host Disease after Reduced-Intensity Conditioning Allogeneic Transplantation. Biol Blood Marrow Transpl. 2019;25:73–85. doi: 10.1016/j.bbmt.2018.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubio MT, Labopin M, Blaise D, Socie G, Contreras RR, Chevallier P, et al. The impact of graft-versus-host disease prophylaxis in reduced-intensity conditioning allogeneic stem cell transplant in acute myeloid leukemia: a study from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2015;100:683–9. doi: 10.3324/haematol.2014.119339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker I, Panzarella T, Couban S, Couture F, Devins G, Elemary M, et al. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: a randomised, controlled, open-label, phase 3, multicentre trial. lancet Oncol. 2016;17:164–73. doi: 10.1016/S1470-2045(15)00462-3. [DOI] [PubMed] [Google Scholar]
- 11.Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020;7:e157–e67. doi: 10.1016/S2352-3026(19)30256-X. [DOI] [PubMed] [Google Scholar]
- 12.Soiffer RJ, Lerademacher J, Ho V, Kan F, Artz A, Champlin RE, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117:6963–70. doi: 10.1182/blood-2011-01-332007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Esser JW, van der Holt B, Meijer E, Niesters HG, Trenschel R, Thijsen SF, et al. Epstein-Barr virus (EBV) reactivation is a frequent event after allogeneic stem cell transplantation (SCT) and quantitatively predicts EBV-lymphoproliferative disease following T-cell—depleted SCT. Blood. 2001;98:972–8. doi: 10.1182/blood.V98.4.972. [DOI] [PubMed] [Google Scholar]
- 14.Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transpl. 2008;14:641–50. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bashey A, Zhang X, Sizemore CA, Manion K, Brown S, Holland HK, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013;31:1310–6. doi: 10.1200/JCO.2012.44.3523. [DOI] [PubMed] [Google Scholar]
- 16.Eto M, Mayumi H, Tomita Y, Yoshikai Y, Nishimura Y, Nomoto K. Sequential mechanisms of cyclophosphamide-induced skin allograft tolerance including the intrathymic clonal deletion followed by late breakdown of the clonal deletion. J Immunol. 1990;145:1303–10. doi: 10.4049/jimmunol.145.5.1303. [DOI] [PubMed] [Google Scholar]
- 17.Wachsmuth LP, Patterson MT, Eckhaus MA, Venzon DJ, Gress RE, Kanakry CG. Post-transplantation cyclophosphamide prevents graft-versus-host disease by inducing alloreactive T cell dysfunction and suppression. J Clin Invest. 2019;129:2357–73. doi: 10.1172/JCI124218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradstock KF, Bilmon I, Kwan J, Micklethwaite K, Blyth E, Deren S, et al. Single-agent high-dose cyclophosphamide for graft-versus-host disease prophylaxis in human leukocyte antigen-matched reduced-intensity peripheral blood stem cell transplantation results in an unacceptably high rate of severe acute graft-versus-host disease. Biol Blood Marrow Transpl. 2015;21:941–4. doi: 10.1016/j.bbmt.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Holtick U, Chemnitz JM, Shimabukuro-Vornhagen A, Theurich S, Chakupurakal G, Krause A, et al. OCTET-CY: a phase II study to investigate the efficacy of post-transplant cyclophosphamide as sole graft-versus-host prophylaxis after allogeneic peripheral blood stem cell transplantation. Eur J Haematol. 2016;96:27–35. doi: 10.1111/ejh.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transpl. 1995;15:825–8. [PubMed] [Google Scholar]
- 22.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol Blood Marrow Transpl. 2015;21:389–401.e1. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 24.McQuellon RP, Russell GB, Cella DF, Craven BL, Brady M, Bonomi A, et al. Quality of life measurement in bone marrow transplantation: development of the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) scale. Bone Marrow Transpl. 1997;19:357–68. doi: 10.1038/sj.bmt.1700672. [DOI] [PubMed] [Google Scholar]
- 25.Pasquini MC, Logan B, Jones RJ, Alousi AM, Appelbaum FR, Bolanos-Meade J, et al. Blood and marrow transplant clinical trials network report on the development of novel endpoints and selection of promising approaches for graft-versus-host disease prevention trials. Biol Blood Marrow Transpl. 2018;24:1274–80. doi: 10.1016/j.bbmt.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brissot E, Labopin M, Moiseev I, Cornelissen JJ, Meijer E, Van Gorkom G, et al. Post-transplant cyclophosphamide versus antithymocyte globulin in patients with acute myeloid leukemia in first complete remission undergoing allogeneic stem cell transplantation from 10/10 HLA-matched unrelated donors. J Hematol Oncol. 2020;13:87. doi: 10.1186/s13045-020-00923-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bailen R, Kwon M, Pascual-Cascon MJ, Ferra C, Sanz J, Gallardo-Morillo A, et al. Post-transplant cyclophosphamide for GVHD prophylaxis compared to ATG-based prophylaxis in unrelated donor transplantation. Ann Hematol. 2021;100:541–53. doi: 10.1007/s00277-020-04317-7. [DOI] [PubMed] [Google Scholar]
- 28.Bolanos-Meade J, Hamadani M, Wu J, Al Malki MM, Martens MJ, Runaas L, et al. Post-transplantation cyclophosphamide-based graft-versus-host disease prophylaxis. N. Engl J Med. 2023;388:2338–48. doi: 10.1056/NEJMoa2215943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broers AEC, de Jong CN, Bakunina K, Hazenberg MD, van Marwijk Kooy M, de Groot MR, et al. Posttransplant cyclophosphamide for prevention of graft-versus-host disease: results of the prospective randomized HOVON-96 trial. Blood Adv. 2022;6:3378–85. doi: 10.1182/bloodadvances.2021005847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Battipaglia G, Labopin M, Hamladji RM, Blaise D, Chevallier P, Brissot E, et al. Post-transplantation cyclophosphamide versus antithymocyte globulin in patients with acute myeloid leukemia undergoing allogeneic stem cell transplantation from HLA-identical sibling donors: A retrospective analysis from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Cancer. 2021;127:209–18. doi: 10.1002/cncr.33255. [DOI] [PubMed] [Google Scholar]
- 31.McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–400. doi: 10.1182/blood.V97.11.3390. [DOI] [PubMed] [Google Scholar]
- 32.Baron F, Labopin M, Blaise D, Lopez-Corral L, Vigouroux S, Craddock C, et al. Impact of in vivo T-cell depletion on outcome of AML patients in first CR given peripheral blood stem cells and reduced-intensity conditioning allo-SCT from a HLA-identical sibling donor: a report from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transpl. 2014;49:389–96. doi: 10.1038/bmt.2013.204. [DOI] [PubMed] [Google Scholar]
- 33.Dulery R, Mohty R, Labopin M, Sestili S, Malard F, Brissot E, et al. Early cardiac toxicity associated with post-transplant cyclophosphamide in allogeneic stem cell transplantation. JACC CardioOncol. 2021;3:250–9. doi: 10.1016/j.jaccao.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeh J, Whited L, Saliba RM, Rondon G, Banchs J, Shpall E, et al. Cardiac toxicity after matched allogeneic hematopoietic cell transplant in the posttransplant cyclophosphamide era. Blood Adv. 2021;5:5599–607. doi: 10.1182/bloodadvances.2021004846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peric Z, Cahu X, Chevallier P, Brissot E, Malard F, Guillaume T, et al. Features of Epstein-Barr Virus (EBV) reactivation after reduced intensity conditioning allogeneic hematopoietic stem cell transplantation. Leukemia. 2011;25:932–8. doi: 10.1038/leu.2011.26. [DOI] [PubMed] [Google Scholar]
- 36.Goldsmith SR, Abid MB, Auletta JJ, Bashey A, Beitinjaneh A, Castillo P, et al. Post-Transplant Cyclophosphamide (PTCy) is associated with increased cytomegalovirus infection: a CIBMTR analysis. Blood. 2021;137:3291–305. [DOI] [PMC free article] [PubMed]
- 37.Kroger N, Solano C, Wolschke C, Bandini G, Patriarca F, Pini M, et al. Antilymphocyte globulin for prevention of chronic graft-versus-host disease. N. Engl J Med. 2016;374:43–53. doi: 10.1056/NEJMoa1506002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this study including de-identified patient data are available after final completion of the trial report and are shared according to data sharing guidelines upon reasonable request to the corresponding author.