Abstract
Despite the improved efficacy of highly active antiretroviral therapy (HAART) in viral suppression, emerging evidence indicates an increased burden of noncommunicable diseases in people living with HIV (PLWH). Immune activation and persistently elevated levels of inflammation have been associated with endothelial dysfunction in PLWH, likely contributing to the development of cardiovascular diseases (CVDs). Here, electronic search databases including PubMed, Google Scholar, Cochrane Library, and Science Direct were used to retrieve scientific evidence reporting on any association between markers of endothelial function and CVD-related outcomes in PLWH on HAART. Extracted data was subjected to quality assessment using the Downs and Black checklist. Most (60 %) of the results indicated the presence of endothelial dysfunction in PLWH on HAART, and this was mainly through reduced flow mediated dilation and elevated serum makers of adhesion molecules like ICAM-1, VCAM-1, and P-selectin. The summarized evidence indicates an association between persistently elevated markers of endothelial dysfunction and a pro-inflammatory state in PLWH on HAART. Only a few studies reported on improved endothelial function markers in PLWH on HAART, while limited evidence is available to prove that endothelial dysfunction is associated with CVD-risk, which could be attributed to therapeutic effects of HAART. Limited studies with relatively high quality of evidence were included in this systematic review. In conclusion, results from this review lay an important foundation for future research, even a meta-analysis, that will improve the understanding of the contributing factors to the burden of CVDs in PLWH on HAART.
Keywords: Endothelial dysfunction, Markers of oxidative stress, Inflammatory markers, Cardiovascular disease risk, Highly active antiretroviral therapy, Human immunodeficiency virus, Inflammation, Endothelial makers, Endothelial function tests
Graphical abstract
Highlights
-
•
HAART may have negative effects endothelial function which may result in CVDs.
-
•
Inflammation may be associated with endothelial dysfunction in PLWH on HAART.
-
•
Duration of HAART remains a critical factor for CVD-related adverse effects.
1. Introduction
It has been more than four decades since the human immunodeficiency virus (HIV) was first discovered [1]. However, this condition continues to be one of the leading causes of death in low- and middle-income countries, especially those in the sub-Saharan Africa [2]. With the World Health Organization (WHO) documenting about 38.4 million active cases of HIV globally at the end of 2021 [3]. The Joint United Nations Programme on HIV/Acquired Immune Deficiency Syndrome (UNAIDS) indicates approximately 7.5 million active cases in South Africa alone [2]. HIV describes a compromised immune response that hinders the body's ability to fight-off or recover from infection. However, the discovery of nucleoside reverse transcriptase inhibitors (NRTIs) proved important for the early treatment of HIV [4,5]. The early availability of this class of drugs was fundamental for advancements in new generations of antiretroviral drugs, especially the discovery of highly active antiretroviral therapy (HAART) [5]. The latter describes a combination of drugs from at least two different classes, including protease inhibitors (PIs) and currently remains the leading regimen for the management of HIV [6].
The benefits of HAART in prolonging the lives of people living with HIV (PLWH) are well-known [7,8], however emerging evidence indicates that continued use of this combination therapy may render the endothelium susceptible to damage that may lead to endothelial dysfunction [[9], [10], [11]]. This consequence describes diminished vasodilation and vasoconstriction that is likely to be accompanied by coagulation and a prothrombotic state [12,13]. Available evidence already indicates the importance of flow mediated dilation (FMD) as a non-invasive measure of endothelial dysfunction to predict cardiovascular disease (CVD)-risk in PLWH on HAART [14]. People living with HIV (PLWH) present with an increased CVD risk when compared to individuals without the virus, especially in the presence of other comorbidities such as obesity, diabetes, hypertension, and kidney diseases [[15], [16], [17]]. Plasma levels of oxidative products and pro-inflammatory markers, including high sensitivity C-reactive protein (hs-CRP), tumor necrosis factor (TNF)-α, interleukin (IL)-6, as well as reduced nitric oxide (NO) bioavailability are all considered viable predictors of endothelial dysfunction [[18], [19], [20]]. Cellular adhesion molecules (CAMs), including E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) can also serve as biomarkers to predict endothelial dysfunction [21,22]. Due to ongoing research indicating a rising toll of cardiovascular-related complications in PLWH [23,24], it has become essential to determine whether prominent biomarkers of endothelial dysfunction can be used to predict CVD-risk in PLWH, especially those on HAART.
This systematic review contributes to already available evidence that is meant to underscore the importance of factors that lead to increased risk of CVD in PLWH on HAART [25,26]. It systematically evaluates literature of scientific studies reporting on any association between markers of endothelial function and CVD-risk in PLWH on HAART. Specific emphases are placed on understanding the potential correlation between endothelial dysfunction and CVD-risk in PLWH on HAART.
2. Methodology
This systematic review was compiled following the Cochrane Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA) (Supplementary file 1). The updated Cochrane Handbook for Systematic Reviews of Interventions guidelines were followed [27]. The systematic review was not registered with the International Prospective Register for Systematic Reviews (PROSPERO), but this database and other online registries were searched to make sure this review is not replicated.
2.1. Search strategy
Electronic databases including PubMed, Google Scholar, Cochrane Library, and Science Direct were searched to retrieve relevant literature. This included scientific studies, from 1996 until June 2023, reporting on any association between markers of endothelial function and CVD-related outcomes in PLWH on HAART. The primary search was conducted through PubMed, using advanced search and clinical queries adjusting for keywords and clinical subject headings (Supplementary file 2). Keywords and subject headings (including relevant synonyms) used during the search included ‘endothelial function’, ‘human immune deficiency virus’, ‘inflammation’, and ‘highly active antiretroviral therapy’.
2.2. Eligibility criteria
The review included primary publications reporting on endothelial function among PLWH on HAART. We specifically included clinical studies, randomized controlled trials, cross-sectional, cohort, retrospective, case-control, and longitudinal studies on PLWH on HAART (≥18 years old). Studies reporting on PLWH who were HAART-naïve as their main study population with HIV-negative individuals as their control group were excluded from our pool of interest. Moreover, studies reporting on PLWH on HAART with cancer, neuropathies, and those with existing CVD-risk including hypertension, diabetes mellitus, coagulopathies, and lipodystrophy with its associated symptoms were also excluded. We further excluded PLWH on HAART who were pregnant, lactating, those making use of children, and those reporting on individuals taking other drugs such as statins and aspirins to recover endothelial function. The focus of the current review was on clinical applications; hence animal and in-vitro studies were excluded. Publications not in the English language were excluded, provided they could not be translated. Studies were deemed relevant if they reported on indicators or measurements of endothelial function, including FMD, as well as biomarkers like E/P-selectin, ICAM-1, and VCAM-1. Since both oxidative stress and inflammation are major pathological features of endothelial dysfunction [28,29], levels of malondialdehyde (MDA), hs-CRP, TNF-α, IL-6, as well as NO bioavailability were also assessed as potential contributors to endothelial dysfunction and CVD-risk. The current study was conducted to address the following: Patient/population, Exposure, Comparison, and Outcomes (PECO).
2.2.1. Population
Study population included PLWH (≥18 years of age).
2.2.2. Exposure
PLWH on any type of HAART regimen, including drug combinations of atazanavir (ATV), ritonavir (RTV), lopinavir (LPV), efavirenz (EFV), tenofovir disoproxil fumarate (TDF) or emtricitabine (FTC).
2.2.3. Comparison
Studies reporting on PLWH on HAART and HAART-naïve or HIV-negative individuals.
2.2.4. Outcome/s
Primary outcomes were indicators and markers of endothelial function. Secondary outcome was CVD-related outcomes, including markers of oxidative stress and inflammation.
2.3. Data extraction and quality assessment
The main extracted data items from eligible studies included the type of HAART regimen used and exposure period, as well as study outcomes/key findings on the status of endothelial function in PLWH on HAART. Other extracted items were sample size, country, as well as CD4 count and viral load of study participants (Supplementary file 3). Importantly, data on risk factors of endothelial dysfunction and CVD-risk including the age, sex, and ethnicity of participants were extracted from all the eligible studies.
2.4. Quality assessment
The quality of evidence for all the included studies was independently assessed using the Downs and Black Checklist (Supplementary file 4) [30]. The adopted checklist consisted of twenty-seven (27) domains, which are efficient at informing on the quality of evidence. For all the included studies, quality assessment was based only on the data available in the published full-text article. Two authors independently assessed the quality of evidence of each article, while disagreements were addressed by consulting a third author. Rater agreement expressed as a Cohen's Kappa (K) value was determined using IBM Statistical Package for the Social Sciences (SPSS) version 29.0, to verify the validity and reliability of the rater outcomes.
3. Results
3.1. Characteristic features of included studies
The search strategy and selection process for eligible studies is demonstrated in Fig. 1. The search strategy of online databases initially identified 393 related records. However, after removing nonqualifying studies, only ten clinical studies were deemed suitable and included in this systematic review. All included studies reported on endothelial function and relevant CVD-related parameters in PLWH on HAART. In terms of regional distribution, most studies were from the United States (n = 4) and Switzerland (n = 2), while other countries presented with one study, including South Africa (n = 1), Denmark (n = 1), France (n = 1), and Botswana (n = 1). In terms of sex and age, 70 % of the participants were male, with an average age of 40 years (Table 1). With included evidence further showing that NRTI base regimen was the most prescribed HAART (n = 7 studies), which was followed by PI-base regimen (n = 6 studies) (see Fig. 2).
Fig. 1.
Flow diagram depicting study selection and inclusion process. Briefly, the search strategy of online databases initially identified 393 related records on PubMed, Google Scholar, Cochrane Library, and Science Direct. However, after removing duplicates and irrelevant studies, only ten clinical studies were deemed suitable and included in this systematic review. All incorporated studies reported on the modulation of endothelial function and relevant cardiovascular disease-related parameters in PLWH on HAART.
Table 1.
A synopsis of studies reporting on endothelial dysfunction and cardiovascular disease (CVD)-risk in people living with human immunodeficiency virus (HIV) on highly active antiretroviral therapy (HAART).
| Author, year | Country | Study design | Study population | Type of HAART regimen | Key findings |
|---|---|---|---|---|---|
| [32] | United States | Cross-sectional study | PLWH (n = 37) on HAART, with an average age of 42 years (81 % male), without chronic lifestyle diseases. No reported ethnicity | 1 participant took amprenavir (APV), 11 indinavir (IDV), 6 nelfinavir (NFV), 2 RTV, 6 saquinavir (SQV), 2 abacavir (ABC), 3 didanosine (ddI), 18 lamivudine (3 TC), 8 stavudine (d4T), 6 zidovudine (ZDV), 2 delaviridine (DLV), and 3 nevirapine (NVP). Participants were monitored for ≥6 months | Participants presented with increased total cholesterol and triglyceride levels, characterized by high concentrations of very low-density lipoprotein (vLDL). This was followed by endothelial dysfunction marked by impaired flow mediated dilation (FMD) |
| [33] | United States | Randomized controlled trial | PLWH (n = 82) on HAART, with an average age of 35 years (91 % male). Mixed ethnic groups, White (54 %), Black/Asian (32 %), and Hispanic (15 %) | NRTIs plus EFV, NRTIs plus lopinavir/ritonavir (LPV/r), or a NRTI-sparing regimen of EFV plus LPV/r. Participants were monitored for up to 6 months | Participants presented with improved endothelial function demonstrated increased FMD |
| [34] | Switzerland | Randomized controlled trial | PLWH (n = 145) on HAART, with an average age of 34 years (62 % females). Thai (100 %) ethnicity | RTV boosted SQV/d4T, TDF/3 TC, TDF/FTC. Participants were monitored for up to 6 months | Participants presented with marked decrease in markers of endothelial dysfunction, soluble- vascular cell adhesion molecule 1 (VCAM-1), P-selectin, leptin, and D-dimer, whereas mediators of anti-inflammation like adiponectin and interleukin (IL)-10 were increased after initiation of HAART. However, this effect was diminished at 12 weeks after randomization, and even associated with increase in plasma HIV-RNA |
| [31] | Switzerland | Randomized controlled trial | PLWH (n = 39) on HAART, with an age between 18 and 65 years (77 % male). No reported ethnicity | 20 participants taking ATV and 19 were on ATV-free PIs. Participants were monitored for up to 3 months | Participants on ATV presented with more pronounced effect in improved lipid profiles, including total cholesterol, high-density lipoprotein (HDL), and triglycerides. This included oxidised LDL, accompanied by endothelial dysfunction as shown by reduced FMD |
| [35] | Denmark | Longitudinal study | PLWH (n = 12) on HAART, with an age between 18 and 60 years (100 % male). White (100 %) ethnicity | 9 participants were on TDF/FTC/EFV, 1 took 3TC/ZDV/EFV, 1 took 3TC/ABC/EFV, and 1 took TDF/FTC/raltegravir (RAL). Participants were monitored before and 5 weeks (24–67 days) after initiation of ART | Participants presented with reduced maximal myocardial perfusion and decreased myocardial perfusion reserve soon after initiating HAART. This was accompanied by endothelial dysfunction as seen with reduced FMD |
| [36] | United States | Randomized controlled trial | PLWH (n = 50) on HAART, with an average age of 43 years (84 % males). White (66 %) ethnicity | 26 participants switched to ATV (all continued RTV); 24 remained on their PIs. Participants were monitored for up to 6 months | Participants switched to zidovudine presented with improved total cholesterol, triglycerides, and HDL levels. However, endothelial function as measure by FMD was not affected, including inflammatory and metabolic markers |
| [37] | United States | Randomized controlled trial | PLWH (n = 101) on HAART, with an average age of 34 years (68 % male). Mixed ethnicity, African American (60 %), Hispanic (38 %), and Asian (2 %) | 51 participants took fosamprenavir (FPV)/RTV; 50 took EFV/ABC/3 TC. Participants were monitored after 24 months | Participants presented with improved cardiovascular biomarkers. This included favourable decline in thrombotic activity (marked by D-dimer) and endothelial activation (marked by VCAM-1). However, inflammation increased as reflected by high sensitivity C-reactive protein (hs-CRP) |
| [38] | France | Randomized controlled trial | PLWH (n = 44) on HAART, with an average age of 41 years (93 % male). No reported ethnicity | ZDV/RTV (300/100 mg), plus a fixed combination of either ABC/3 TC (600/300 mg) or FTC/TDF (200/245 mg) for 24 months | Participants presented with stable markers of inflammation (hs-CRP, IL-6) and endothelial activation (VCAM-1) during the treatment period |
| [39] | Botswana | Randomized controlled trial | PLWH (n = 112) on HAART, with an average age of 40 years (51 % females). Black African (100 %) ethnicity | In addition to a nonnucleoside reverse transcriptase inhibitor (NNRTIs) (73 %) or PI (26 %), the HAART regimen backbone consisted of either TDF/3 TC (49 %), ZDV/3 TC (47 %), ABC/3 TC (2 %) or 3 TC (2 %) for 14 months | Participants presented with endothelial dysfunction, marked by elevated intercellular adhesion molecule 1 (ICAM-1) and VCAM-1. Monocyte activation marker (sCD163) was also associated with increased ICAM-1. However, inflammatory marker, IL-6 was not affected |
| [40] | South Africa | Case-control | PLWH (n = 100) on HAART, with an average age of 43 years (75 % female). Black African (100 %) ethnicity | TDF/FTC/EFV or TDF/3TC/EFV Participants were monitored over 18 months | Participants presented with comparable markers of endothelial dysfunction and cardiometabolic profiles to HIV-free individuals. The %FMD was reduced in some participants, based on socio-economic differences |
Fig. 2.
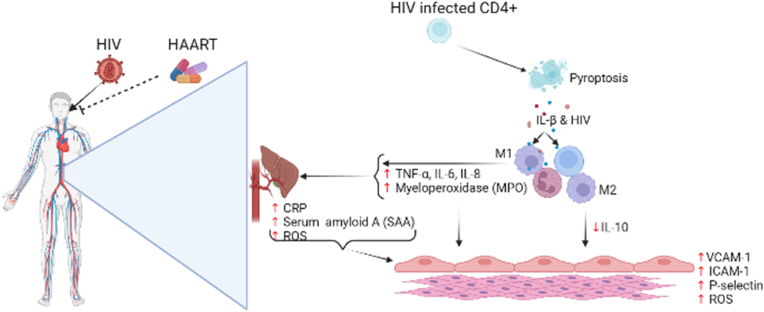
Upon infection with HIV, the virus infiltrates cluster differentiation positive (CD4+) T-lymphocytes and induce pro-inflammatory intracellular micro-environment marked by increased IL-β. The CD4+ T-lymphocytes undergo pyroptosis showering adjacent uninfected immune cell (including CD4+ T-lymphocytes) with IL-1β and new viral cells. Interleukin-1β activates M1 macrophages to secrete pro-inflammatory cytokines (IL-6, IL-8, and TNF-α) and neutrophils to release myeloperoxidase (MPO). The increased release of pro-inflammatory cytokines stimulates hepatocytes to release the acute phase response proteins serum amyloid A and C-reactive proteins, while elevated levels of MPO promotes the formation of reactive oxygen species (ROS). pro-inflammatory cytokines (IL-6, IL-8, and TNF-α), MPO, and the cute phase response proteins induces endothelial activation marked by elevated serum levels of VCAM-1, ICAM-1, P-selectin, and endothelial ROS. Sustained secretion of pro-inflammatory cytokines (IL-6, IL-8, and TNF-α), MPO, acute phase response proteins, and ROS overpowers the anti-inflammatory response pathway by M2 macrophages preceding chronic inflammation and endothelial dysfunction (Created with BioRender.com).
3.2. Quality of included studies
The quality of included studies (n = 10) was assessed using the modified Downs and the Black checklist. Majority of the studies had good quality of evidence, with one study [31] classified as having excellent quality after rating 25 out of 27 possible scores. Two studies had the lowest rating (17 and 18 scores out of 27 possible scores) but were classified as having fair quality of evidence. Based on the 5 evaluated domains, all studies had excellent reporting bias, with a median of 10 (9–11) out of 10 possible scores and a good rater agreement (Cohen's kappa) value of (K = 0.58) between two independent reviewers, and external validity with a median of 2 (0–2) out of the three possible scores and a slight rater agreement value (K = 0.14). The included studies had good internal validity, demonstrated by a median score of 5 (4–6) out of 7 possible scores (K = 0.41) and a median of 4 (2–6) out of the 6 possible scores (K = 0.25). All studies had great power of ≥90 % indicating that these studies had no errors of omissions (type 2 error) to their findings, as supported by a median score range of 1 (0–1) and a (K = 1.0) (Supplementary file 5).
3.3. Conflicting evidence on the status of endothelial dysfunction in PLWH on HAART
There is a considerable interest in understanding the status of CVD risk in PLWH, especially the role of endothelial function as a major predictor of cardiac abnormality [26,41,42]. A PubMed search shows a rapid growth in studies that have been published reporting on markers of endothelial function in PLWH on HAART [[42], [43], [44]]. With summarized evidence showing a potential role of endothelial dysfunction in PLWH on HAART (Table 1). However, the presented evidence indicated some conflicting results, with many factors potentially contributing to this outcome. For example, earlier evidence by Stein et al. [32] indicated that PLWH present with endothelial dysfunction, marked by reduced FMD after taking HIV PIs or NRTIs for a period of ≥6 months. Flammer et al. [31] also did not show any improvement in endothelial function as measured using FMD after taking zidovudine for up to 6 months. Murphy et al. [36] did not see any effect on endothelial function in PLWH measured by FMD after switching from RTV to ATV for 6 months. Kristoffersen et al. [35] affirmed these results, showing that PLWH present with endothelial dysfunction as seen with reduced FMD after taking a combination therapy of TDF/FTC/EFV, 3TC/ZDV/EFV, 3TC/ABC/EFV or TDF/FTC/RAL, in which the study participants were monitored for 24–67 days. Mosepele et al. [39] also showed that PLWH presented with endothelial dysfunction, marked by elevated ICAM-1 and VCAM-1 after taking NNRTIs or a combination of TDF/3 TC, ZDV/3 TC, or ABC/3 TC for 14 months. Although these findings suggest the presence of endothelial dysfunction in PLWH on HAART, there were fewer participants (12–145) enrolled in these studies.
Other included studies within the current review do support improved outcomes in markers of endothelial function in PLWH on HAART (Table 1). For example, Torriani et al. [33] showed that PLWH on HAART presented with improved endothelial function demonstrated by increased FMD after taking NRTIs plus EFV or LPV/r for up to 6 months. A follow up study by Calmy et al. [34] showed that PLWH presented with a marked decrease in markers of endothelial dysfunction, including VCAM-1, P-selectin, leptin, and D-dimer after taking RTV boosted SQV/d4T, TDF/3 TC, and TDF/FTC for up to 3 months. Kumar et al. [37] also showed a favourable decline in thrombotic activity (marked by D-dimer) and endothelial activation (marked by VCAM-1) in PLWH after taking FPV/RTV or EFV/ABC/3 TC for up to 24 months. Such positive effects are also confirmed in PLWH by Porith et al. [38], after taking ZDV/RTV plus a fixed combination of either ABC/3 TC or FTC/TDF for 24 months. Interestingly, recent studies by different groups [39,40] indicate that NNRTI or PIs can improve endothelial function by reducing markers such as FMD, ICAM-1 and VCAM-1, when used for a period of 14–18 months. Notably, the duration of these studies spanned from as short as 24 days, up to 24 months, however with most studies reporting a duration of 6 months. And study duration was evenly distributed between those indicating the negative or positive effects of HAART on endothelial function in PLWH.
3.4. HAART may potentially lower CVD-risk in PLWH on HAART
Since endothelial dysfunction may potentially serve as a predictor of cardiovascular abnormality (Hadi et al., 2005), it was important to determine whether elevated markers of endothelial dysfunction were linked to CVD-related outcomes in PLWH on HAART. Noteworthy, fewer of the included studies (n = 4) support the notion that endothelial dysfunction is not linked with CVD-risk in PLWH on HAART [31,[35], [36], [37]]. The introduction of HAART for viral suppression, contributed to enhanced endothelial health, blocking the development of atherosclerosis. Atherogenic factors as well as diminished maximal myocardial perfusion were predominant markers to indicate CVD-risk in PLWH on HAART (Table 1). Whereas the predominantly used HAART regimen consisted of a varied combination of ATV-free PIs, TDF/FTC or (3 TC)/EFV, 3TC/ZDV/ABC, TDF/FTC/RAL, and ATV, which was for a period of 6–24 months [31,[35], [36], [37]]. Notably, a study by Stein et al. [32] provided the only evidence indicating that increased CVD-risk was consistent with endothelial dysfunction in PLWH on HAART, as shown by increased levels of total cholesterol, triglyceride, and very low-density lipoprotein (vLDL). Here, the type of HAART regimen (because this evidence is about two decades ago), or even the study design being a non-randomized control trial, could be a contributing factor in such negative results. Nonetheless, well-designed additional studies are required to verify these findings.
3.5. Implications of oxidative stress and inflammation during endothelial dysfunction in PLWH on HAART
Oxidative stress and inflammation are the leading pathological complications that are increasingly linked with the development of endothelial dysfunction [45,46], well beyond its role in causing other CVD-related complications [46]. Thus, it remained essential to establish whether there is a link between elevated markers of oxidative stress and inflammation with endothelial dysfunction in PLWH on HAART. Notably, only one study reported on oxidative stress, through oxidised LDL, which was in PLWH on HAART [31]. Oxidised LDL characterizes a variety of alterations for both lipid and apolipoprotein B components, which can promote atherosclerosis through the formation of macrophage foam cells [26,47]. Alternations in both inflammatory and immunologic mechanisms is fundamental during this process [48]. The summarized evidence showed that anti-inflammatory factors including adiponectin and IL-10 were reduced after initiation of HAART (a combination of RTV boosted SQV/d4T, TDF/3 TC, and TDF/FTC) in PLWH [34]. This is in agreement with evidence by Porith et al. [38], which showed that inflammatory (hs-CRP, IL-6) and endothelial activation (VCAM-1) markers were stable in PLWH after taking ZDV/RTV, plus a fixed combination of either ABC/3 TC, or FTC/TDF for 24 months. These findings are however inconsistent because others have reported the opposite effects. With two studies [36,39] not showing any changes in inflammatory markers incorporating IL-6, IL-8, and TNF-α, while monocyte activation marker (sCD163) was linked with increased ICAM-1 in PLWH on HAART [39]. Further showing that different factors, especially socio-economic status in some populations, may negatively influence the effect of HAART in PLWH (Table 1). For example, Swart et al. [40] observed no significant differences in endothelial function between PLWH on HAART and HIV-free black South Africans with an average age of 43-years, while Mosepele et al. [39] observed that black Africans from Botswana with an average age of 40-years on HAART present with endothelial dysfunction. Swart et al. [40] further motivated that some of the study participants presented with endothelial dysfunction due to socio-economic differences. There is a need for additional studies to affirm the beneficial effects of HAART on reducing inflammation to further protect against endothelial dysfunction in PLWH.
4. Discussion
This review systematically discusses the close association in clinical evidence reporting on endothelial dysfunction, or its markers thereof, and CVD-risk in PLWH on HAART. Through the search of electronic databases, ten studies were identified to match the inclusion criteria, showing conflicting evidence on the modulation of markers of endothelial function in PLWH on HAART. Importantly, it was evident that most studies showed elevated makers of endothelial dysfunction in PLWH on HAART, including reduced FMD, which might be concurrent with increased serum makers incorporating ICAM-1, VCAM-1, and P-selectin (Table 1). In terms of HAART regimen, it was evident that administering treatment containing TDF, 3 TC, or EFV is associated with either lipodystrophy, inflammation, and/or endothelial dysfunction [32,34,35,39]. Recent evidence indicates persistent immune activation and inflammation in PLWH on HAART, occurring independent of viral suppression [49,50]. Despite these outcomes, it remains of interest to understand whether ethnic differences and other confounding factors hold a pivotal role in the pathogenesis of endothelial dysfunction and associated CVD-risk factors.
To date, there is still ongoing investigations to understand the link between HAART and endothelial dysfunction, both in a cellular and molecular level [15,51,52]. However, it is currently acknowledged that some HAART drug components may be beneficial on the endothelium, whilst some combinations may potentially exacerbate lipodystrophy and inflammation preceding endothelial dysfunction (Mar et al., 2013). For example, Torriani et al. [33] argued that administering HAART containing PIs, especially LPV/r increases blood vessel FMD as a measure of improved endothelial function. Contrary to these findings, Murphy et al. [36] indicated that PI-based regimen containing RTV improved lipid profile but has no apparent effect on endothelial function and inflammation as its associated risk factor. Endothelial dysfunction may be a feature in PLWH on HAART and may explain the increased prevalence of CVDs observed in this population group. However, more studies, looking at a wider pool of CVD-related outcomes are required to confirm this.
A variety of antiretroviral (ARV) drugs independently suppress HIV replication, however, their effectiveness is enhanced with adherence and improved lifestyle modifications [2]. Majority of the identified studies which reported endothelial dysfunction recruited PLWH who were exposed to at least one or more of the following HAART drugs: ATV, EFV, TDF, TFC, and RTV-boosted protease inhibitors. Highly active antiretroviral therapy containing two NRTIs (e.g., ABC/FTC/TDF) and an NNRTIs or a PI could potentially improve endothelial function [33,36,37,53]. However, contradictory findings remain regarding these drug combinations. Also noting that HAART-associated adverse health effects may be intensified by confounding risk factors including ethnicity, sex, and socio-economic status.
5. Study limitations and strengths
The major limitation of the present systematic review was extracting limited studies meeting the inclusion criteria. While another limitation was the diversity of the extracted study designs that could not support the execution of a meta-analysis. This partially implies that a mixture of study designs, including cross-sectional, case-control, and longitudinal studies limited the quality of evidence. Although the outcomes of the included studies were comparable and could be pooled purposefully, the interventions (specific HAART drugs) and comparators across the studies differed which further limited the execution of a meta-analysis. We could not fully address the differences in FMD and inflammation in our study population due to differences reporting across the included studies. Our pool of studies consisted of an uneven gender distribution with male being predominant, which could potentially influence the quality of their conclusions. An even balance gender is important for any analysis, especially to understand the effect of HAART on the vascular endothelium in PLWH. However, the reliability of evidence from the included studies assessed using the Downs and Black checklist indicated that these studies had good reporting bias and reporting power, because some were randomized controlled trials. The qualitative approach in analysing the current data brings strength by taking into consideration the many factors presented by each study before conclusions are drawn. Moreover, the included studies reported on the effects of HAART on the vascular endothelium and the potential manifestation of CVDs over deviating HIV treatment duration, which elevates the reliability of pooled data. These could also potentially explain the contrasting existing evidence on whether HAART promotes endothelial recovery, or it perpetuates endothelial dysfunction.
6. Conclusion
This systematic review acknowledges conflicting evidence informing on the status of endothelial function in PLWH on HAART. Although some evidence shows that elevated markers of inflammation, and those of endothelial dysfunction persists in PLWH, the introduction of HAART may hinder this and protect individuals against CVD-related complications. However, these findings need validation since they are based on very limited evidence, while more research is required to get a better understanding on the pathogenesis of endothelial function in PLWH on HAART at increased risk of developing CVDs.
Funding statement
Phiwayinkosi V. Dludla is supported in part by the National Research Foundation (NRF) (Grant numbers: 117829 and 141929). Sidney Hanser is also funded by the NRF (Grant number: TTK2204082828). The content hereof is the sole responsibility of the authors and do not necessarily represent the official views of the NRF.
Author contribution
Authors, Haskly Mokoena, Sihle E. Mabhida, Joel Choshi, Phiwayinkosi V. Dludla, and Sidney Hanser conceived and contributed to drafting the original manuscript. All other authors, including, Bongani B. Nkambule, Zandile J.R. Mchiza, Duduzile E. Ndwandwe, and André P. Kengne reviewed the manuscript. All authors approved the final version of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The work by Sihle E. Mabhida, reported herein was made possible through partial funding by the South African Medical Research Council through its Division of Research Capacity Development under the Researcher Development Award Programme. The content hereof is the sole responsibility of the authors and do not necessarily represent the official views of the funders.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.athplu.2024.01.003.
Abbreviations
- 3 TC
Lamivudine
- ABC
Abacavir
- APV
Amprenavir
- ATV
Atazanavir
- CAMs
Cell Adhesion Molecules
- CD4 count
Cluster Differentiation 4 Count
- CVDs
Cardiovascular diseases
- D4T
Stavudine
- ddI
Didanosin
- EFV
Efavirenz
- E-selectin
Endothelial Selectin
- FMD
Flow Mediated Dilation
- FPV
Fosamprenavir
- FTC
Emtricitabine
- HAART
Highly Active Antiretroviral therapy
- HDL
High density lipoprotein
- HIV
Human immunodeficiency virus
- Hs-CRP
Highly sensitive C-reactive protein
- ICAM-1
Intercellular adhesion molecule-1
- IDV
Indinavir
- IL-10
Interleukin-10
- IL-6
Interleukin-6
- K
Cohen's Kappa
- LDL
Low-density lipoprotein
- LPV/r
Lopinavir boosted ritonavir
- LPV
Lopinavir
- MDA
Malondialdehyde
- NFV
Nelfinavir
- NIH
National Institute of Health
- NNRTIs
Non-nucleoside reverse transcriptase inhibitors
- NO
Nitric oxide
- NVP
Nevirapine
- PECO
Population, Exposure, Comparison, Outcome(s)
- PIs
Protease inhibitors
- PLWH
People living with human immunodeficiency virus
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROSPERO
International Prospective Register for Systematic Reviews
- P-selectin
Platelet selectin
- RAL
Raltegravir
- RTV
Ritonavir
- sCD163
Soluble cluster differentiation 163
- SPSS
Statistical package for the social sciences
- SQV
Saquinavir
- TDF
Tenofovir disoproxil fumarate
- TNF-α
Tumor necrosis factor-alpha
- UNAIDS
United Nations on Acquired Immunodeficiency Syndrome
- VCAM-1
Vascular adhesion molecule-1
- vLDL
Very low-density lipoprotein
- WHO
World Health Organization
- DLV
Delavirdine
- ZDV
Zidovudine.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Barré-Sinoussi F., Chermann J.C., Rey F., Nugeyre M.T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C., Rozenbaum W. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 2.UNAIDS . United Nations AIDS; 2021. HIV and AIDS estimates.https://WWW.UNAIDS.ORG/EN/REGIONSCOUNTRIES/COUNTRIES/SOUTHAFRICA Available from: [Google Scholar]
- 3.WHO . World Health Organization; 2022. HIV data and statistics.https://WWW.WHO.INT/TEAMS/GLOBAL-HIV-HEPATITIS-AND-STIS-PROGRAMMES/HIV/STRATEGIC-INFORMATION/HIV-DATA-AND-STATISTICS [Google Scholar]
- 4.Patel P.H., Zulfiqar H. StatPearls; 2022. Reverse transcriptase inhibitors.https://WWW.NCBI.NLM.NIH.GOV/BOOKS/NBK551504/ Available from: [PubMed] [Google Scholar]
- 5.NIH . National Institute of Allergy and Infectious Diseases; 2020. HIV/AIDS treatment: factsheet.https://WWW.NIAID.NIH.GOV/DISEASES-CONDITIONS/HIV-TREATMENT Available from: [Google Scholar]
- 6.Eggleton J.S., Nagalli S. Highly active antiretroviral therapy (HAART) StatPearls. 2022 https://WWW.NCBI.NLM.NIH.GOV/BOOKS/NBK554533/ Available from: [PubMed] [Google Scholar]
- 7.Bigna J.J., Ndoadoumgue A.L., Nansseu J.R., Tochie J.N., Nyaga U.F., Nkeck J.R., Foka A.J., Kaze A.D., Noubiap J.J. Global burden of hypertension among people living with HIV in the era of increased life expectancy: a systematic review and meta-analysis. J Hypertens. 2020;38:1659–1668. doi: 10.1097/HJH.0000000000002446. [DOI] [PubMed] [Google Scholar]
- 8.Martinez J.D.A., Tania B.G.S., Alejandro A.M.D.O.D., Sacramento V.S.A., Jose C.F.A. Risk of fractures in older adults living with HIV. Journal ISSN. 2022;2766:2276. [Google Scholar]
- 9.Kamau F., Strijdom H., Mwangi P., Blackhurst D., Imperial E., Salie R. Antiretroviral drug-induced endothelial dysfunction is improved by dual PPARα/γ stimulation in obesity. Vasc Pharmacol. 2019;121 doi: 10.1016/j.vph.2019.106577. https://WWW.DOI.ORG.10.1016/J.VPH.2019.106577 [DOI] [PubMed] [Google Scholar]
- 10.Ramteke A.P., Kumar V.R. Study of cardiac abnormalities in HIV patients and their correlation with Cd4 count. European Journal of Molecular & Clinical Medicine. 2022;9:1946–1959. [Google Scholar]
- 11.Hurwitz B.E., McIntosh R.C., Greeson J.M. In: Handbook of cardiovascular behavioral medicine. Waldstein S.R., Kop W.J., Suarez E.C., Lovallo W.R., Katzel L.I., editors. Springer; New York: 2022. HIV-1 spectrum disease, psychological distress, and cardiometabolic risk.https://WWW.DOI.ORG/10.1007/978-0-387-85960-6_59 [Google Scholar]
- 12.Biswas I., Khan G.A. In: Basic and clinical understanding of microcirculation. Shad K.F., Saravi S.S.S., Bilgrami N.L., editors. 2019. Endothelial dysfunction in cardiovascular diseases.https://WWW.DOI.ORG/10.5772/INTECHOPEN.89365 [Google Scholar]
- 13.Nkambule B.B., Mxinwa V., Mkandla Z., Mutize T., Mokgalaboni K., Nyambuya T.M., Dludla P.V. Platelet activation in adult HIV-infected patients on antiretroviral therapy: a systematic review and meta-analysis. BMC Med. 2020;18:357. doi: 10.1186/S12916-020-01801-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nkeh-Chungag B.N., Goswami N., Engwa G.A., Sewani-Rusike C.R., Mbombela V., Webster I., De Boever P., Kessler H.H., Stelzl E., Strijdom H. Relationship between endothelial function, antiretroviral treatment and cardiovascular risk factors in HIV patients of African descent in South Africa: a cross-sectional study. J Clin Med. 2021;10:392. doi: 10.3390/jcm10030392. https://WWW.DOI.ORG/10.3390/JCM10030392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marincowitz C., Genis A., Goswami N., De Boever P., Nawrot T.S., Strijdom H. Vascular endothelial dysfunction in the wake of HIV and ART. FEBS J. 2019;286:1256–1270. doi: 10.1111/febs.14657. [DOI] [PubMed] [Google Scholar]
- 16.Anand A.R., Rachel G., Parthasarathy D. HIV proteins and endothelial dysfunction: implications in cardiovascular disease. Frontiers in Cardiovascular Medicine. 2018;5:185. doi: 10.3389/fcvm.2018.00185. https://WWW.DOI.ORG/10.3389/FCVM.2018.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Röling J., Schmid H., Fischereder M., Draenert R., Goebel F.D. HIV-associated renal diseases and highly active antiretroviral therapy—induced nephropathy. Clin Infect Dis. 2006;42:1488–1495. doi: 10.1086/503566. [DOI] [PubMed] [Google Scholar]
- 18.Sun H.J., Wu Z.Y., Nie X.W., Bian J.S. Role of endothelial dysfunction in cardiovascular diseases: the link between inflammation and hydrogen sulfide. Front Pharmacol. 2019;10:1568. doi: 10.3389/fphar.2019.01568. https://WWW.DOI.ORG/10.3389/FPHAR.2019.01568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solages A., Vita J.A., Thornton D.J., Murray J., Heeren T., Craven D.E., Horsburgh C.R. Endothelial function in HIV-infected persons. Clin Infect Dis. 2006;42:1325–1332. doi: 10.1086/503261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nkambule B.B., Mxinwa V., Nyambuya T.M., Dludla P.V. The mean platelet volume and atherosclerotic cardiovascular-risk factors in adults with obesity: a systematic review and meta-analysis of observational studies. BMC nutrition. 2022;8:47. doi: 10.1186/s40795-022-00541-8. https://WWW.DOI.ORG/10.1186/S40795-022-00541-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang S.J., Ballantyne C.M., Sharrett A.R., Smith L.C., Davis C.E., Gotto A.M., Boerwinkle E. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 1997;96:4219–4225. doi: 10.1161/01.cir.96.12.4219. [DOI] [PubMed] [Google Scholar]
- 22.Milošević N., Rütter M., David A. Endothelial cell adhesion molecules- (un)Attainable targets for nanomedicines. Frontiers in medical technology. 2022;4 doi: 10.3389/fmedt.2022.846065. https://WWW.DOI.ORG/10.3389/FMEDT.2022.846065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullis C., Swartz T.H. NLRP3 Inflammasome signaling as a link between HIV-1 infection and atherosclerotic cardiovascular disease. Frontiers in Cardiovascular Medicine. 2020;7:95. doi: 10.3389/fcvm.2020.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perkins M.V., Joseph S.B., Dittmer D.P., Mackman N. Cardiovascular disease and thrombosis in HIV infection. Arterioscler Thromb Vasc Biol. 2023;43:175–191. doi: 10.1161/ATVBAHA.122.318232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fogacci F., Borghi C., Grassi D., Cicero A.F.G. People living with human immunodeficiency virus: cardiovascular risk screening for an early and effective risk management. Atherosclerosis. 2022;353:28–29. doi: 10.1016/j.atherosclerosis.2022.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Avagimyan A., Pogosova N., Kakturskiy L., Sheibani M., Urazova O., Trofimenko A., Navarsdyan G., Jndoyan Z., Abgaryan K., Fogacci F., Galli M., Agati L., Kobalava Z., Shafie D., Marzilli M., Gogiashvili L., Sarrafzadegan N. HIV-related atherosclerosis: state-of-the-art-review. Curr Probl Cardiol. 2023;48 doi: 10.1016/j.cpcardiol.2023.101783. [DOI] [PubMed] [Google Scholar]
- 27.Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A. Cochrane Handbook for systematic reviews of interventions version 6.3. Cochrane. 2022 WWW.TRAINING.COCHRANE.ORG/HANDBOOK [Google Scholar]
- 28.Steven S., Frenis K., Oelze M., Kalinovic S., Kuntic M., Bayo Jimenez M.T., Vujacic-Mirski K., Helmstädter J., Kröller-Schön S., Münzel T., Daiber A. Vascular inflammation and oxidative stress: major triggers for cardiovascular disease. Oxid Med Cell Longev. 2019 doi: 10.1155/2019/7092151. https://WWW.DOI.ORG/10.1155/2019/7092151 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ngcobo S.R., Nkambule B.B., Nyambuya T.M., Mokgalaboni K., Ntsethe A., Mxinwa V., Ziqubu K., Ntamo Y., Nyawo T.A., Dludla P.V. Activated monocytes as a therapeutic target to attenuate vascular inflammation and lower cardiovascular disease-risk in patients with type 2 diabetes: a systematic review of preclinical and clinical studies. Biomedicine & pharmacotherapy, Biomedecine & pharmacotherapie. 2022;146 doi: 10.1016/j.biopha.2021.112579. https://WWW.DOI.ORG/10.1016/J.BIOPHA.2021.112579 [DOI] [PubMed] [Google Scholar]
- 30.O'Connor S.R., Tully M.A., Ryan B., Bradley J.M., Baxter G.D., McDonough S.M. Failure of a numerical quality assessment scale to identify potential risk of bias in a systematic review: a comparison study. BMC Res Notes. 2015;8:224. doi: 10.1186/s13104-015-1181-1. https://WWW.DOI.ORG/10.1186/S13104-015-1181-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flammer A.J., Vo N.T., Ledergerber B., Hermann F., Gämperli A., Huttner A., Evison J., Baumgartner I., Cavassini M., Hayoz D., Quitzau K., Hersberger M., Sudano I., Ruschitzka F., Lüscher T.F., Noll G., Weber R. Effect of atazanavir versus other protease inhibitor-containing antiretroviral therapy on endothelial function in HIV-infected persons: randomised controlled trial. Heart. 2009;95:385–390. doi: 10.1136/hrt.2007.137646. [DOI] [PubMed] [Google Scholar]
- 32.Stein J.H., Klein M.A., Bellehumeur J.L., McBride P.E., Wiebe D.A., Otvos J.D., Sosman J.M. Use of human immunodeficiency virus-1 protease inhibitors is associated with atherogenic lipoprotein changes and endothelial dysfunction. Circulation. 2001;104:257–262. doi: 10.1161/01.cir.104.3.257. [DOI] [PubMed] [Google Scholar]
- 33.Torriani F.J., Komarow L., Parker R.A., Cotter B.R., Currier J.S., Dubé M.P., Fichtenbaum C.J., Gerschenson M., Mitchell C.K., Murphy R.L., Squires K., Stein J.H. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: the ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol. 2008;52:569–576. doi: 10.1016/j.jacc.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calmy A., Gayet-Ageron A., Montecucco F., Nguyen A., Mach F., Burger F., Ubolyam S., Carr A., Ruxungtham K., Hirschel B., Ananworanich J. HIV increases markers of cardiovascular risk: results from a randomized, treatment interruption trial. AIDS. 2009;23:929–939. doi: 10.1097/qad.0b013e32832995fa. [DOI] [PubMed] [Google Scholar]
- 35.Kristoffersen U.S., Wiinberg N., Petersen C.L., Gerstoft J., Gutte H., Lebech A.M., Kjaer A. Reduction in coronary and peripheral vasomotor function in patients with HIV after initiation of antiretroviral therapy: a longitudinal study with positron emission tomography and flow-mediated dilation. Nucl Med Commun. 2010;31:874–880. doi: 10.1097/MNM.0b013e32833d82e6. [DOI] [PubMed] [Google Scholar]
- 36.Murphy R.L., Berzins B., Zala C., Fichtenbaum C., Dube M.P., Guaraldi G., Torriani F., Belsey E., Mitchell C., Stein J.H. Change to atazanavir/ritonavir treatment improves lipids but not endothelial function in patients on stable antiretroviral therapy. AIDS. 2010;24:885–890. doi: 10.1097/QAD.0b013e3283352ed5. [DOI] [PubMed] [Google Scholar]
- 37.Kumar P., DeJesus E., Huhn G., Sloan L., Small C.B., Edelstein H., Felizarta F., Hao R., Ross L., Stancil B., Pappa K., Ha B. Evaluation of cardiovascular biomarkers in a randomized trial of fosamprenavir/ritonavir vs. efavirenz with abacavir/lamivudine in underrepresented, antiretroviral-naïve, HIV-infected patients (SUPPORT): 96-week results. BMC Infect Dis. 2013;13:269. doi: 10.1186/1471-2334-13-269. https://WWW.DOI.ORG/10.1186/1471-2334-13-269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piroth L., Moinot L., Yeni P., Avettand-Fénoel V., Reynes J., Girard P.M., Marchou B., Georget A., Rouzioux C., Autran B., Duvillard L., Chêne G., Fagard C. Immunity, inflammationinflammation, and reservoir in patients at an early stage of HIV infection on intermittent ART (ANRS 141 TIPI Trial) J Antimicrob Chemother. 2015;71:490–496. doi: 10.1093/jac/dkv369. [DOI] [PubMed] [Google Scholar]
- 39.Mosepele M., Mohammed T., Mupfumi L., Moyo S., Bennett K., Lockman S., Hemphill L.C., Triant V.A. HIV disease is associated with increased biomarkers of endothelial dysfunction despite viral suppression on long-term antiretroviral therapy in Botswana. Cardiovascular Journal of Africa. 2018;29:155–161. doi: 10.5830/CVJA-2018-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swart C., Fourie C., De Boever P., Goswami N., Lammertyn L., Strijdom H., Kamau F., Botha-Le Roux S. Comparison of endothelial function and cardiometabolic profiles of people living with HIV in two South African regions: the EndoAfrica study. Cardiovascular Journal of Africa. 2022;33:15–20. doi: 10.5830/CVJA-2021-026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zifodya J.S., Duncan M.S., So-Armah K.A., Attia E.F., Akgün K.M., Rodriguez-Barradas M.C., Marconi V.C., Budoff M.J., Bedimo R.J., Alcorn C.W., Soo Hoo G.W., Butt A.A., Kim J.W., Sico J.J., Tindle H.A., Huang L., Tate J.P., Justice A.C., Freiberg M.S., Crothers K. Community-Acquired pneumonia and risk of cardiovascular events in people living with HIV. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.017645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goerlich E., Schär M., Bagchi S., Soleimani-Fard A., Brown T.T., Sarkar S., Bonanno G., Streeb V., Gerstenblith G., Barditch-Crovo P., Weiss R.G., Hays A.G. Coronary endothelial dysfunction in people living with HIV is related to body fat distribution. J Acquir Immune Defic Syndr. 2022;90:201–207. doi: 10.1097/QAI.0000000000002932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mouchati C., Durieux J.C., Zisis S.N., McComsey G.A. HIV and race are independently associated with endothelial dysfunction. AIDS. 2023;37:271–277. doi: 10.1097/QAD.0000000000003421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shrestha J., Santerre M., Allen C.N., Arjona S.P., Hooper R., Mukerjee R., Kaul M., Shcherbik N., Soboloff J., Sawaya B.E. HIV-1 gp120 protein promotes HAND through the calcineurin pathway activation. Mitochondrion. 2023;70:31–40. doi: 10.1016/j.mito.2023.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L., Cheng C.K., Yi M., Lui K.O., Huang Y. Targeting endothelial dysfunction and inflammation. Journal of molecular and cellular cardiology. 2022;168:58–67. doi: 10.1016/j.yjmcc.2022.04.011. [DOI] [PubMed] [Google Scholar]
- 46.Babcock M.C., DuBose L.E., Witten T.L., Stauffer B.L., Hildreth K.L., Schwartz R.S., Kohrt W.M., Moreau K.L. Oxidative stress and inflammation are associated with age-related endothelial dysfunction in men with low testosterone. The Journal of Clinical Endocrinology and Metabolism. 2022;107:500–514. doi: 10.1210/clinem/dgab715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsuura E., Hughes G.R., Khamashta M.A. Oxidation of LDL and its clinical implication. Autoimmun Rev. 2008;7:558–566. doi: 10.1016/j.autrev.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 48.Lv T., Cao W., Li T. HIV-related immune activation and inflammation: current understanding and strategies. Journal of immunology research. 2021;2021 doi: 10.1155/2021/7316456. https://WWW.DOI.ORG/10.1155/2021/7316456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zicari S., Sessa L., Cotugno N., Ruggiero A., Morrocchi E., Concato C., Rocca S., Zangari P., Manno E.C., Palma P. Immune activation, inflammation, and non-AIDS co-morbidities in HIV-infected patients under long-term ART. Viruses. 2019;11:200. doi: 10.3390/v11030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nyambuya T.M., Dludla P.V., Mxinwa V., Nkambule B.B. The effect of successful antiretroviral therapy on immune activation and reconstitution in HIV-infected adults: a systematic review and meta-analysis. AIDS Rev. 2021;23 doi: 10.24875/AIDSREV.20000039. [DOI] [PubMed] [Google Scholar]
- 51.Strijdom H., De Boever P., Walzl G., Essop M.F., Nawrot T.S., Webster I., Westcott C., Mashele N., Everson F., Malherbe S.T., Stanley K. Cardiovascular risk and endothelial function in people living with HIV/AIDS: design of the multi-site, longitudinal EndoAfrica study in the Western Cape Province of South Africa. BMC Infect Dis. 2017;17:1–9. doi: 10.1186/s12879-016-2158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kovacs L., Kress T.C., Belin de Chantemèle E.J. HIV, combination antiretroviral therapy, and vascular diseases in men and women. Basic to Translational Science. 2022;7:410–421. doi: 10.1016/j.jacbts.2021.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piroth et al., 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.