Abstract
Background
The inconformity (IC) between pathological and imaging remissions after neoadjuvant immunotherapy in patients with NSCLC can affect the evaluation of curative effect of neoadjuvant therapy and the decision regarding the chance of surgery.
Materials and methods
Patients who achieved disease control(CR/PR/SD) after neoadjuvant chemoimmunotherapy from a clinical trial (NCT04326153) and after neoadjuvant chemotherapy during the same period were enrolled in this study. All patients underwent radical resection and systematic mediastinal lymphadenectomy after neoadjuvant treatments. The pathological remission, immunohistochemistry (CD4, CD8, CD20, CD56, FoxP3, CD68, CD163, CD11b tumor-infiltrating lymphocytes, or macrophages), and single-source dual-energy computed tomography (ssDECT) scans were assessed. The IC between imaging remission by CT and pathological remission was investigated. The underlying cause of IC, the correlation between IC and DFS, and prognostic biomarkers were explored.
Results
After neoadjuvant immunotherapy, enhanced immune killing and reduced immunosuppressive performance were observed. 70 % of neoadjuvant chemoimmunotherapy patients were in high/medium IC level. Massive necrosis and repair around and inside the cancer nest were the main pathological changes observed 30–45 days post-treatment with PD1/PD-L1 antibody and were the main causes of IC between the pathology and imaging responses after neoadjuvant immunotherapy. High IC and preoperative CD8 expression (H score ≥ 3) indicate a high pathological response rate and prolonged DFS. Iodine material density ssDECT images showed that the iodine content in the lesion causes hyperattenuation in post-neoadjuvant lesion in PCR patient.
Conclusions
Compared to chemotherapy and targeted therapy, the efficacy of neoadjuvant immunotherapy was underestimated based on the RECIST criteria due to the unique antitumor therapeutic mechanism. Preoperative CD8+ expression and ssDECT predict this IC and evaluate the residual tumor cells. This is of great significance for screening immune beneficiaries and making more accurate judgments about the timing of surgery.
Keywords: Immunotherapy, Neoadjuvant therapy, Non-small cell lung cancer, Pathology, Tumor microenvironment, Single-source dual-energy computed tomography
Introduction
Lung tumor is the most common type and the leading cause of cancer-related deaths worldwide [1]. Surgical resection is the mainstay treatment for patients with localized non-small cell lung cancer (NSCLC). In recent years, immune checkpoint inhibitors against programmed cell death-1 (PD-1) and programmed cell death ligand-1 (PD-L1) have made significant progress in neoadjuvant therapy of resectable early-stage NSCLC [2], [3], [4], [5]. The improvement in major pathological ression (MPR) rates following neoadjuvant immunotherapy with or without chemotherapy (approximately 45–83 %) was more significant than that following chemotherapy and molecular-targeted therapy alone. The pathological complete response (PCR) rate following neoadjuvant chemotherapy was reported to be only 2.2 % [6]. Therefore, a renewed interest has been developed in studying these agents in patients with potentially resectable NSCLC at high risk of metastatic recurrence. Currently, chemoradiation is the perennial standard of care for IIIA/IIIB NSCLC, and the subsequent addition of durvalumab as consolidation therapy significantly improves the three-year overall survival (OS) compared to placebo (57.0 % vs. 43.5 %). Our phase II clinical trial (clinicaltrials.gov, identifier: NCT04326153) confirmed that advanced immunotherapy improves the MPR rate and two-year disease-free survival (DFS) of potential resectable IIIA/IIIB NSCLC. Moreover, an inconformity (IC) was detected between pathological and radiological remission after neoadjuvant therapy; despite a high pathological remission, radiological remission may not be detected in a timely manner. The IC seriously affected the timing, causing some patients to miss out on the opportunity for surgery. Therefore, exploring the causes of the IC, searching for biomarkers or examination methods, and accurately screening the beneficiaries of treatment are crucial for potentially resectable stage IIIA/IIIB NSCLC.
Tumor-infiltrating T lymphocytes (TILs) play a major role in antitumor immune responses within the tumor microenvironment (TME) [7]. Tumor-infiltrating B cells harbor both immunostimulatory [8] and immunosuppressive [9] tumor-associated macrophages (TAMs), an abundant cell type within the TME, and despite growing research, their role in cancer progression remains ambiguous. Tumor-infiltrating T regulatory cells (Tregs) and myeloid-derived suppressor cells (MDSCs) are key to sustained immunosuppression [10]. These biomarkers may be related to the prognosis of immunotherapy [11].
In a phase II preliminary study of 22 untreated and resectable patients with IB–IIIA NSCLC [2], the objective response is considered a solution to the problem of lesion property, which was easily misinterpreted in contrast-enhanced conventional computed tomography (CCT). The use of x-ray beams at two different kVp energy levels reduces or eliminates beam-hardening artifacts [12], while virtual monoenergetic images decrease the pseudo enhancement artifacts, accurately measuring the density [13,14]. Therefore, in the present study, a secondary analysis was performed on a previously conducted clinical trial that combined sintilimab with nab-paclitaxel+ carboplatin as the neoadjuvant therapy to treat patients with potentially resectable stage IIIA/IIIB NSCLC. The underlying causes for the IC between pathological and imaging remissions were examined. Also, the clinical implications of this IC, biomarkers, and imaging examination methods for predicting prognosis were explored.
Materials and methods
Patients selection
A secondary analysis was performed on data from patients who underwent neoadjuvant chemoimmunotherapy in a previous prospective, single-center, single-arm, phase II clinical trial of sintilimab combined with nab-paclitaxel + carboplatin as the neoadjuvant therapy in patients with potentially resectable stage IIIA/IIIB NSCLC (clinicaltrials.gov, identifier: NCT04326153). In addition, patients who received neoadjuvant chemotherapy within the same study period were also selected for analysis. The study protocol was approved by the ethics committee of the First Hospital of Jilin University, Changchun, China. Informed consent was waived due to the retrospective design of the study. The patients who achieved disease control (complete response(CR)/partial response(PR)/stable disease(SD)) after neoadjuvant chemoimmunotherapy or neoadjuvant chemotherapy within the same study period were selected for this study. Other criteria were as follows: age ≥18 years; histologically or cytologically documented, treatment-naive, stage IIIA/IIIB (American Joint Committee on Cancer, 8th edition), and potentially resectable NSCLC (direct surgery was not feasible; putative surgery after neoadjuvant treatment through MDT of Oncology, Thoracic Surgery, Radiotherapy, and Imaging Departments); normal organ function and adequate pulmonary function; an Eastern Cooperative Oncology Group Performance Status of 0-1. The exclusion criteria were as follows: presence of known epidermal growth factor receptor-sensitive mutations; active autoimmune or infectious diseases; ongoing systemic corticosteroid or other immunosuppressive therapy; history of symptomatic grade 3 or 4 interstitial lung disease; clinically significant concurrent cancer; previous allogeneic organ transplantation or hemopoietic stem cell transplantation; uncontrolled hypertension; any medical, mental, or psychological conditions that affect study completion according to the investigator's discretion; history of allergy to the study drugs. All patients underwent a radical resection of the pulmonary carcinoma and systematic mediastinal lymphadenectomy in 30–45 days after neoadjuvant treatment. The imaging remission rate was evaluated by CT based on RECIST1.1 criteria.
Assessment of pathological remission rate
The assessment of the pathological remission rate is based on previous literature [4]. PCR was defined as the absence of residual tumor cells. MPR was defined as residual tumor cell ratio ≤ 10 %. The same pathologists analyzed the cellular components of each pathological specimen and calculated the average values. All components were classified into three categories. The necrosis components included cholesterol crystal formation, foamy histiocytes, and multinucleated giant cell reactions. The reparative changes included angiogenesis and fibrosis. The infiltrative changes in immune cells included massive lymphocytic infiltration, plasma cell infiltration, tertiary lymphoid structure, and granuloma formation.
IC was defined as the differing values between the pathological and imaging response rates. (IC = Pathological remission−Radiological remission). The values < 40 %, 40 % ≤ IC < 70 %, and IC ≥ 70 % were classified as low, medium, and high IC, respectively.
Immunohistochemical (IHC) staining
IHC staining was performed using the UltraSensitiveTM SP IHC Kit (MXB Biotechnologies, Fuzhou, China). Tissue samples were sliced into 5-μm-thick sections. The primary antibodies used were as follows: CD4 (RMA-0620, MXB), CD8 (MAB-0021, MXB), CD20 (KIT-0001 MXB), CD56 (MAB-0743 MXB), FoxP3 (ab20034, Abcam), CD86 (ab269587 Abcam), CD163 (MAB-0869 MXB), and CD11b (FITC-65055, Proteintech).
Protocol: (1) Dewax paraffin sections for hydration. (2) Heat sections in Tris EDTA buffer (pH 9.0) at 121°C for 2 min for antigen retrieval, followed by washes with distilled water. (3) Block endogenous peroxidase activity with 3 % hydrogen peroxide at room temperature for 15 min. (4) Wash with 0.01 M phosphate-buffered saline (PBS) three times for 5 min each. (5) Block with 5 % normal goat serum at room temperature for 30 min. (6) Incubate with primary antibodies overnight at 4°C. (7) Wash with 0.01 M PBS three times for 5 min each. (8) Incubate with biotin-labeled secondary antibody at room temperature for 20 min. (9) Wash with 0.01M PBS thrice for 5 min each. (10) Incubate with streptavidin-labeled horseradish enzyme at room temperature for 20 min. (11) Wash with 0.01 M PBS three times for 5 min each. (12) Develop color using DAB chromogenic agent and observe microscopically. (13) Rinse with tap water, counterstain with hematoxylin, dehydrate with gradient alcohol, make sections transparent with xylene, and seal with neutral gum.
The slides were scanned using the Olympus microscope (Olympus, BX53, Japan). An experienced pathologist determined the scores based on cell staining intensity and the percentage of positive cells for each biomarker. According to the intensity of cell staining, it was rated as level 4: 0 point for no positive staining (negative), 1 point for light yellow (weakly positive), 2 points for brownish-yellow (positive), and 3 points for brown (strongly positive). According to the percentage of positive cells, it is rated as level 4: 1 point for ≤25 %, 2 points for 26–50 %, 3 points for 51–75 %, and 4 points for >75 %. The final histochemistry score (H-score) was obtained by multiplying the two scores.
Assessment of the radiological remission rate and spectral CT analysis
All patients were subjected to CT scanning at baseline, after two cycles of neoadjuvant therapy, before surgery, and around 12 weeks after the surgery (follow-up). The CT scans were obtained at the end of inspiration during one breath hold. The scanning range covered the entire area from the apex to the base of the lung, with the patient lying supine.
All CT examinations were performed using Revolution (GE Medical Systems, USA). The scanning parameters of non-enhanced CT scans were as follows: pitch, 1.0; matrix, 1,024 × 1,024; FOV, 300 mm; 120 kVp and 200 mA. After non-enhanced CT scanning, a double-cylinder high-pressure syringe pump was used to inject 2 mL/kg body weight of iodine contrast agent (Iophorol 370 mgI/mL) into the elbow vein, with an 18-gauge needle, followed by 20 mL of normal saline at a flow rate of 3 mL/s. Enhanced CT scans were acquired 25 s and 75 s after drug infusion, respectively. When a lesion was detected, an High Resolution Computed Tomography (HRCT) target scan was carried out between arterial and venous phases at the following parameters: pitch, 1.0; section thickness and interval, 1.0 and 1.0 mm; matrix, 1,024 × 1,024; FOV, 150 mm; 120 kVp and 200 mA. The images of the contrast-enhanced CT lesions (HRCT target scans) were stored as Dicom files for image texture feature extraction.
Exploratory spectral CT scan and basic material decomposition analysis (iodine and water) were performed. Regions of interest (ROI) were drawn on the arterial and venous phase iodine maps before and after treatment. Three ROIs were randomly selected at the level of the largest lesion to obtain the average iodine value. Then, the iodine value in the thoracic aorta at the same level was calculated. NIC = IClesion/ICaorta, where NIC stands for normalized iodine concentration, IClesion denotes iodine concentration in the lesion, and ICaorta represents iodine concentration in the thoracic aorta. Next, we preliminarily explored the efficacy of neoadjuvant therapy for lung cancer by comparing the changes in the average iodine value and NIC value within the lesions before and after treatment.
Statistical analysis
Measurement data were presented as mean, standard deviation, median, maximum, and minimum interquartile range values (IQR). Enumeration and ranked data were presented as number (composition ratio) and rate. Post-hoc comparisons were performed by categorizing the patients into groups based on IC. The categorical variables were analyzed by Pearson's χ2 test. The exact two-sided 95 % confidence intervals (CIs) were calculated using the Clopper–Pearson method. The Kaplan–Meier method was used to estimate the DFS. The reverse Kaplan–Meier method was used to calculate the median follow-up time and the corresponding IQR. Statistical analyses were performed with R software (ver. 4.3.0, Vienna, Austria) and SPSS (ver. 20.0; SPSS Inc, Chicago, IL, USA), and a P-value < 0.05 indicated a statistically significant difference.
Results
Pathological and radiological remission
A total of 20 patients receiving neoadjuvant chemoimmunotherapy and 12 receiving neoadjuvant chemotherapy were assessed. Their baseline and tumor characteristics are listed in Supplemental Table 1. All patients underwent R0 resection. Among the 20 patients treated with chemoimmunotherapy, 18 were diagnosed squamous cell carcinoma and 2 were diagnosed adenocarcinoma. Among the 12 patients treated with chemotherapy, 10 were diagnosed squamous cell carcinoma and 2 were diagnosed adenocarcinoma. PCR were observed in 8/20 chemoimmunotherapy-treated patients, while MPR(not PCR) were observed in 6 patients (Fig. 1A). Moreover, only 2 patients who received chemotherapy were observed in both PR and MPR(not PCR). PCR was observed in none of the patient receiving chemotherapy. In 8 patients who was observed in PCR after chemoimmunotherapy (Supplemental Table 2), PR was observed in 37.5 % (3/8) patients and stable disease (SD) was observed in 62.5 % (5/8) patients. In 5 patients who was observed in MPR, not PCR after chemoimmunotherapy, PR was observed in 80 % (4/5) patients and SD was observed in 20 % (1/5). Abnormal shadows were observed in the thoracic CT results of the chemoimmunotherapy-treated patients. The imaging remission rate was lower than the pathological remission rate, with IC13.46–92.28 % (Fig. 1B). Among the 20 chemoimmunotherapy-treated patients, 6 (30 %), 8 (40 %), and 6 (30 %) had high, medium, and low IC, respectively. However, the IC of patients treated with chemotherapy was low (Fig. 1B).
Fig. 1.
Pathological remission, RECIST1.1 criteria, and their inconformity(IC).
Pathological specimen analysis
The examination of the chemoimmunotherapy and chemotherapy postoperative specimens revealed a tertiary lymphatic structure, dense lymphocytes, plasma cell infiltration, lymphoid follicle formation, granuloma formation infiltration, fibrotic granuloma tissue, and features of substantial cell death, such as necrosis, cholesterol crystals, giant cells, and foam cells, indicating immune cell infiltration, tumor cell necrosis, and tissue cell repair, in the preoperative cancer nest of lung cancer tissue (Fig. 2). The calculation of the average cellular components of different classification revealed the major cellular components responsible for IC in necrosis and cell repair. The infiltration of inflammatory cells accounted for a small proportion of IC. Strikingly, the cellular component ratios of pathological specimens varied from person to person and treatment to treatment when compared between patients. The predominant type of infiltrated immune cells consisted of lymphocytes with a small amount of fibrous hyperplasia and repair. The predominant type of cell repair showed a small number of lymphocytes with substantial fibrous tissue hyperplasia that blocked the lymphocytes from moving further into the cancer nests. The predominant type of necrosis showed considerable cell death and a small amount of fibrous hyperplasia, repair, and immune cells. In 20 patients treated with chemoimmunotherapy, the patients with low IC had a similar rate of three components. Among eight patients with medium IC, 4, 2, and 2 had a predominant type of repair, a predominant type of necrosis, and a predominant type of immune cell infiltration, respectively. In 6 patients with high IC, 4 had a predominant type of repair and 2 had a predominant type of necrosis (Fig. 3, Supplemental Table 3). In contrast, among patients treated with chemotherapy, 5 had a predominant type of necrosis, 5 had a predominant type of repair, and the remaining 2 had a predominant type of immune cell infiltration.
Fig. 2.
Thoracic CT and postoperative specimens. (A) CT at baseline. (B) CT after neoadjuvant chemoimmunotherapy. Shadows in the thoracic CT results of a chemoimmunotherapy-treated PCR patient. (C) Overview of postoperative specimens. (D) Postoperative specimens revealed immune cell infiltration (yellow arrow), tumor cell necrosis (black arrow), and tissue cell repair (blue arrow).
Fig. 3.
Different cellular components with varied levels of IC in different chemoimmunotherapy patients. (Left to right for each patient) Necrosis, fibroproliferation and repair, and inflammatory infiltration of lymphocytes.
DFS and OS
By August 31, 2023, the median follow-up was 39.7 months. 70 % (14/20) of patients who underwent neoadjuvant chemoimmunotherapy did not experience recurrence, but the median DFS and OS were not attained. All patients who underwent chemotherapy relapsed. The 2-year DFS and OS for neoadjuvant chemoimmunotherapy was 75 % and 80 %, respectively (Fig. 4A-B). For subgroup analysis, the high IC group was significantly corelated to DFS (P = 0.0087). The higher the IC, the longer the DFS in patients (Fig. 4C).
Fig. 4.
IC and DFS. (A) DFS of neoadjuvant chemoimmunotherapy. (B) OS of neoadjuvant chemoimmunotherapy. (C) Correlation between IC and DFS.
Biomarkers and TME
A significant increase was observed in the number of infiltrating CD4+ (T cell), CD8+ (T cell), CD20+ (B cell) cells after neoadjuvant chemoimmunotherapy, and significant decrease was noted in the number of infiltrating FoxP3+ (Treg cells). An increased trend was visualized in the number of infiltrating CD86+ (M1 macrophages) and CD56+ (NK cells) and decreased trend was noted in CD163+ (M2 macrophages) and CD11b+ cells (MDSCs) (Fig. 5A-B). The analysis of the correlation between H scores and IC found a statistical difference in pretreatment H score of CD8 between the IC < 40 % group and IC ≥ 40 % group (P = 0.001). Pretreatment overexpression of CD8 (H score ≥ 3) indicated a high IC (Fig. 5C). Therefore, pretreatment of CD8 H score (≥ 3) predicted the efficacy and DFS of neoadjuvant chemoimmunotherapy effectively.
Fig. 5.
IHC analysis. (A) CD4+, CD8+, CD20+, FoxP3+, CD86+, CD56+, CD163+, CD11b+ at baseline and after neoadjuvant chemoimmunotherapy. (B) Radar map of IHC H scores. (C) Correlation between IC and CD8 H score.
ssDECT scan in remission evaluation
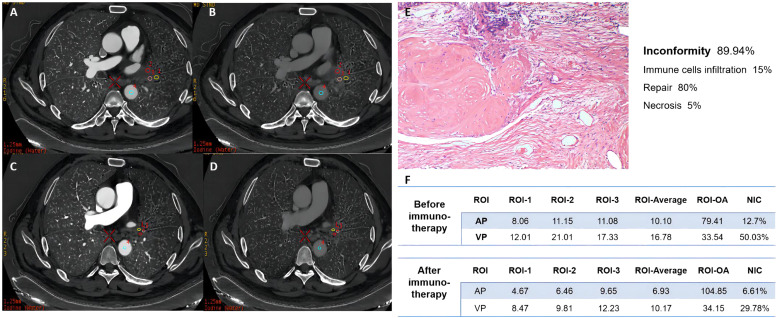
Exploratory spectral CT scan and basic material decomposition analysis (iodine and water) were performed. For example, a 64-year-old male patient with stage IIIA squamous cell carcinoma achieved 100 % pathological remission and had a predominant type of repair. The IC degree was high, and the tumor had a predominant type of repair. The average iodine value and normalized iodine concentration (NIC) of the lesions decreased after treatment, which is consistent with complete postoperative pathological remission (Fig. 6). The ssDECT images identified the properties of residual lesions compared to their standard presentation in ordinary thoracic CT.
Fig. 6.
ssDECT examination to evaluate radiological remission. (A-B) ssDECT examination before chemoimmunotherapy. (C-D) ssDECT examination after chemoimmunotherapy. (E) Postoperative specimens. (F) Average iodine value and NIC in ssDECT.
Discussion
The pathological response after immunotherapy is different from that after chemotherapy and targeted therapy, as characterized by high MRP and PCR rates. This difference is mainly reflected by varied levels of T cell infiltration. PD-1 receptor is expressed on the surface of activated T and B cells. When PD-L1 on tumor cells binds to targeted PD-1 on T cells, the migration and proliferation of T cells and the secretion of cytotoxic mediators can be inhibited [15]. Blocking this key tumor immune escape mechanism is the underlying rationale for designing PD-1/PD-L1 inhibitors for restoring the effective antitumor T cell responses. Moreover, T cells can be activated to generate CD4+ helper T cells (Th) and CD8+ cytotoxic T lymphocytes (CTLs) by blocking PD‐1/PD‐L1 interactions. Activated CTLs destroy tumors directly, and collapsed tumor cells release new tumor antigens to further amplify the secondary immune response [16]. New antigens are presented to specific effector CTLs of tumors at different sites. In addition, effector CTLs return to the residual and micrometastatic tumor lesions through systemic circulation [17], [18], [19]. These steps may lead to T cell infiltration and permanent control of residual and micrometastatic tumor lesions [20]. Thus, we speculated that the extensive tumor necrotic tissue cannot dissolve easily and be absorbed after a long-term repeated infiltration of inflammatory cells. This necrotic tissue could be disorganized and surrounded by new connective tissue. The changes in the TME mentioned above led to an IC between imaging and pathological remission, ultimately affecting the selection of surgical timing. The reasons for the above phenomena and finding biomarkers that reflect pathological remission are critical for predicting the timing of surgery in clinical practice.
We also explored the potential changes caused by this specific pathological response. In the present study, imaging remission was lower than pathologic remission since the shadows in the images were specific components rather than tumor cells. Thus, it can be speculated that necrosis and increased repair responses occur at the tumor site with the former treatment after a longer inflammatory reaction and more T lymphocyte infiltration in tumor specimens treated with PD-1/PD-L1 immune checkpoint inhibitors compared to chemotherapy and molecular-targeted therapy. However, different responses to immunotherapy were shown across patients. Tumor necrosis could be reduced by immunotherapy in patients with extensive lymphocyte infiltration, finally achieving PCR. In the postoperative pathology examination, some patients showed obvious fibrous tissue proliferation, which might block the lymphocytes from moving further into the cancer nests. As shown in Fig. 2, these patients would not have significantly increased lymphocyte infiltration despite prolonged immunotherapy and should receive early surgical resection because the benefit of immunotherapy is reduced. Moreover, the three states, including lymphocyte infiltration, necrosis, and repair after immunotherapy, change dynamically. Lymphocyte infiltration occurs shortly after neoadjuvant chemoimmunotherapy. Hence, we could not conduct pathological dynamic monitoring and observe the time window of each reaction. In this study, postoperative pathological specimens were obtained 30–45 days after immunotherapy, due to which the majority of patients were characterized by necrosis and repair. The current study showed changes in increased immune killing and reduced immune suppression after immune therapy, which was consistent with previous findings [21]. These pathological changes suggested that based on the modified TME, we can search for early biomarkers to predict the true efficacy and accurately screen the population that would benefit from the treatment.
As described previously, neoadjuvant immunotherapy significantly increases pathological remission [2], [3], [4], which has a noticeable effect on OS [22]. MPR reflects treatment-specific antitumor activity and indicates the magnitude of the treatment effects on survival, which might apply to all stages of NSCLC. A CD8+T cell infiltration before surgery indicates that the patient's basic immune function is activated, leading to stronger antitumor effects and pronounced reactions to necrosis and repair after immunotherapy, resulting in a significant difference between the actual imaging manifestations and the number of remaining tumor cells. Preoperative CD8+T cell infiltration indicates IC between radiological and pathological remission, which are closely related to the patient's pathological response and DFS. Therefore, preoperative CD8+ high expression (H score ≥ 3) may be an essential biomarker that needs to be evaluated simultaneously in imaging when RESIST criteria are not appropriate for judging the efficacy of immunotherapy. Some clinical studies have also screened CD8+T cells as indicators related to the efficacy of immunotherapy. However, this study focused on the potential resectable NSCLC in stage IIIA/IIIB, explored the direction of imaging and pathological remission differences, and applied simple and feasible immunohistochemical methods to clarify the threshold of CD8+T cells related to prognosis.
Furthermore, the efficacy of the neoadjuvant immunotherapy was underestimated using the RECIST criteria due to the unique tumor treatment method and the limitations of ordinary CT. Also, FDG is not a tumor-specific tracer and may accumulate in benign inflammatory nodes [23]. Hence, selecting the methods of treatment assessment closely related to the pathological response rate is crucial to determine the presence of active tumor cells accurately. In the present study, we used ssDECT to assess remission by measuring electron density (ED) in tissues [24]. The ED reflects the probability that an electron is present at a specific location, which in turn, is influenced by the molecular structure of the tissue. Increased iodine enhancement of lung nodules has been described as an indicator of both vascularity and malignancy [25,26]. A phantom study [27] showed a linear correlation between tissue ED (as measured by DECT) and density. This information might provide valuable insights regarding the changes in the elemental composition of the tissue in the presence of pathology. ssDECT can characterize different materials based on elemental composition. Ren et al. [12] indicated the putative role of iodine-related quantification in ssDECT while evaluating the lung cancer response to treatment as a feasible substitute for the 18FDG PET-CT examination. Herein, we proposed that PET-CT can predict tissue composition rather scientifically. ssDECT was convenient and economical for patients as it reduces unnecessary imaging examinations and minimizes the wastage of medical resources. In this study, ssDECT was used to evaluate the radiological remission; the results showed that the iodine content reduced in the region with hyperattenuation in the post-neoadjuvant lesion reduced. Nonetheless, in-depth studies are required to evaluate the role of special CT techniques, such as ssDECT, with respect to the treatment response of immunotherapy.
Conclusions
Compared to chemotherapy and targeted therapy, the efficacy of neoadjuvant immunotherapy was underestimated based on the RECIST criteria due to the unique antitumor therapeutic mechanism. Massive necrosis and repair around and inside the cancer nest were the main pathological changes observed 30–45 days post-treatment with PD-1/PD-L1 antibody and were the main causes of IC between the pathology and imaging responses after neoadjuvant immunotherapy. High IC and preoperative CD8 expression (H score ≥ 3) indicate a high pathological response rate and prolonged DFS. ssDECT may have potential applications in the preoperative evaluation of neoadjuvant immunotherapy of lung cancer.
Ethics approval
All included patients provided oral and written informed consent. The study was approved by the Ethics Committee (Regional Ethics Committee of Jilin University First Hospital; reference number 19K112-001).
Data availability statement
All data generated or analysed during this study are included in this published article.
Funding
This study received no funding.
CRediT authorship contribution statement
Chao Sun: Methodology, Writing – original draft. Xiaobo Ma: Data curation. Fanyang Meng: Data curation. Xi Chen: Validation. Xu Wang: Resources. Wenyu Sun: Resources. Yinghui Xu: Resources. Hua He: Software. Huimao Zhang: Supervision, Methodology. Kewei Ma: Project administration, Writing – review & editing.
Declaration of competing interest
All authors declare no financial or non-financial competing interests.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neo.2024.100977.
Contributor Information
Huimao Zhang, Email: huimao@jlu.edu.cn.
Kewei Ma, Email: makw@jlu.edu.cn.
Appendix. Supplementary materials
References
- 1.Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021 doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Forde P.M., et al. Neoadjuvant PD-1 bockade in resectable lung cancer. N. Engl. J. Med. 2018;378:1976–1986. doi: 10.1056/NEJMoa1716078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwiatkowski D.J., et al. Neoadjuvant atezolizumab in resectable non-small cell lung cancer (NSCLC): Interim analysis and biomarker data from a multicenter study (LCMC3) J. Clin. Oncol. 2019;37:8503. Suppl 15. [Google Scholar]
- 4.Provencio M., et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21:1413–1422. doi: 10.1016/S1470-2045(20)30453-8. [DOI] [PubMed] [Google Scholar]
- 5.Gao S.G., et al. Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. J. Thorac. Oncol. 2020;15(5):816–826. doi: 10.1016/j.jtho.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Forde P.M., Spicer J., Lu S., et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N. Engl. J. Med. 2022;386(21):1973–1985. doi: 10.1056/NEJMoa2202170. May 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galon J., Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019;18(3):197–218. doi: 10.1038/s41573-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 8.DeFalco J., Harbell M., Manning-Bog A., Baia G., Scholz A., Millare B., et al. Non-progressing cancer patients have persistent B cell responses expressing shared antibody paratopes that target public tumor antigens. Clin. Immunol. 2018;187:37–45. doi: 10.1016/j.clim.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Shalapour S., Font-Burgada J., Di Caro G., Zhong Z., Sanchez-Lopez E., Dhar D., et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature. 2015;521(7550):94–98. doi: 10.1038/nature14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabrilovich D.I., Ostrand-Rosenberg S., Bronte V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012;12(4):253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horvath L., Thienpont B., Zhao L., Wolf D., Pircher A. Overcoming immunotherapy resistance in non-small cell lung cancer (NSCLC) - novel approaches and future outlook. Mol. Cancer. 2020;19(1):141. doi: 10.1186/s12943-020-01260-z. 1221 3. Sep 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren Y.P., et al. Dual-energy computed tomography-based iodine quantitation for response evaluation of lung cancers to chemoradiotherapy/radiotherapy: a comparison with fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography-based positron emission tomography/computed tomography response evaluation criterion in solid tumors. J. Comput. Assist. Tomogr. 2018;42:614–622. doi: 10.1097/RCT.0000000000000734. [DOI] [PubMed] [Google Scholar]
- 13.Tkaczyk J.E., et al. Quantization of liver tissue in dual kVp computed tomography using linear discriminant analysis. Proceedings of the SPIE 7258, Medical Imaging 2009: Physics of Medical Imaging: Physics of Medical Imaging; Orlando, FL, USA; 2009. 26 March. [Google Scholar]
- 14.Dilmanian F.A. Computed tomography with monochromatic X rays. Am. J. Physiol. Imaging. 1992;7:175–193. [PubMed] [Google Scholar]
- 15.Palucka K., Ueno H., Fay J., Banchereau J. Dendritic cells and immunity against cancer. J. Intern. Med. 2011;269:64–73. doi: 10.1111/j.1365-2796.2010.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casares N., et al. Caspase-dependentimmunogenicity of doxorubicin-induced tumor cell death. J. Exp. Med. 2005;202:1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou W.P., Wolchok J.D., Chen L.P. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 2016;2(8):328. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khalil D.N., Smith E.L., Brentjens R.J., Wolchok J.D. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat. Rev. Clin. Oncol. 2016;13:273–290. doi: 10.1038/nrclinonc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Genova C., et al. Therapeutic implications of tumor microenvironment in lung cancer: focus on immune checkpoint blockade. Front. Immunol. 2023;12 doi: 10.3389/fimmu.2021.799455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cottrell T.R., et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC) Ann. Oncol. 2018;29(8):1853–1860. doi: 10.1093/annonc/mdy218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hui Z., et al. Single-cell profiling of immune cells after neoadjuvant pembrolizumab and chemotherapy in IIIA non-small cell lung cancer (NSCLC) Cell Death Dis. 2022;13:607. doi: 10.1038/s41419-022-05057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang F., et al. Three-year follow-up of neoadjuvant programmed cell death protein-1 inhibitor (Sintilimab) in NSCLC. J. Thorac. Oncol. 2022;17(7):909–920. doi: 10.1016/j.jtho.2022.04.012. [DOI] [PubMed] [Google Scholar]
- 23.Shreve P.D., Anzai Y., Wahl R.L. Pitfalls in oncologic diagnosis with FDG PET imaging: physiologic and benign variants. Radiographics. 1999;19:61–77. doi: 10.1148/radiographics.19.1.g99ja0761. [DOI] [PubMed] [Google Scholar]
- 24.Hua C.H., Shapira N., Merchant T.E., Klahr P., Yagil Y. Accuracy of electron density, effective atomic number, and iodine concentration determination with a dual-layer dual-energy computed tomography system. Med. Phys. 2018;45:2486–2497. doi: 10.1002/mp.12903. [DOI] [PubMed] [Google Scholar]
- 25.Swensen S.J., Silverstein M.D., Ilstrup D.M., Schleck C.D., Edell E.S. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch. Intern. Med. 1997;157:849–855. [PubMed] [Google Scholar]
- 26.Swensen S.J., Brown L.R., Colby T.V., Weaver A.L. Pulmonary nodules: CT evaluation of enhancement with iodinated contrast material. Radiology. 1995;194:393–398. doi: 10.1148/radiology.194.2.7824716. [DOI] [PubMed] [Google Scholar]
- 27.Tatsugami F., et al. Measurement of electron density and effective atomic number by dual-energy scan using a 320-detector computed tomography scanner with raw data-based analysis: a phantom study. J. Comput. Assist. Tomogr. 2014;38:824–827. doi: 10.1097/RCT.0000000000000129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article.