Abstract
Polyclonal antimannan immunoglobulin G (IgG) activates the classical complement pathway and accelerates initiation of the alternative pathway by Canidida albicans. This dual role was assessed for two antimannan IgM monoclonal antibodies (MAbs). MAb B6.1 is specific for an epitope on the acid-labile portion of C. albicans phosphomannan; MAb B6 is specific for an epitope on the acid-stable region. Both MAbs were potent activators of the classical pathway but poor facilitators of alternative pathway initiation.
Candida albicans activates the human complement system via both the classical and the alternative pathways, leading to deposition of opsonic complement fragments on the yeast cell surface (8, 10, 18). In previous studies, we described a critical role for naturally occurring antimannan immunoglobulin G (IgG) in complement activation by C. albicans. Those studies used a kinetic assay for C3 deposition on the yeast and immunofluorescence evaluation of the sites of C3 binding (10, 17, 18). Deposition of C3 onto C. albicans cells incubated in normal human serum (NHS) occurs rapidly via the classical pathway and can be detected within the first 2 min of incubation. If the classical pathway is blocked by chelation of Ca2+ with EGTA, C3 deposition occurs via the alternative pathway, but C3 deposition is delayed and a 6-min incubation is required before bound C3 is readily detectable on the yeast surface. Removal of naturally occurring antimannan IgG from the serum by mannan absorption profoundly delays accumulation of C3 on the yeast cell surface, with 12 min or more of incubation being required before appreciable amounts of bound C3 are detected. However, this 12-min delay can be overcome by supplementation of the mannan-absorbed serum with affinity-purified human antimannan IgG in the absence of EGTA to mediate classical pathway initiation or shortened to 6 min in the presence of EGTA to allow antibody-facilitated activation of the alternative pathway. These observations demonstrate a dual role for antimannan IgG in serum from healthy adults in complement activation by C. albicans. Antimannan IgG mediates activation of the classical pathway and facilitates initiation of the alternative pathway (17, 18).
In studies described above, we used polyclonal antimannan IgG purified from pooled human plasma. Since C. albicans cells express a number of immunodominant mannan components recognized by rabbits (15, 16), the human polyclonal antimannan IgG likely contains a range of specificities for distinct mannan determinants. It has been shown that rabbit antibodies that are reactive with three different cell wall determinants of group A streptococci display differential abilities to activate the classical or alternative pathway (2). Although the antibodies specific for three different cell wall epitopes all activated the classical pathway, only antibody specific for the N-acetyl-d-glucosamine epitope activated the alternative pathway (2). In a separate study, capsular as well as noncapsular antibodies were found to direct classical-pathway-mediated killing of Haemophilus influenzae type b, whereas only the capsular antibodies promoted killing by the alternative pathway (12). These studies provide evidence that epitope specificity may influence the ability of an antibody to activate the alternative pathway and prompted us to examine whether antibodies that recognize different mannan determinants are able to mediate activation of the classical and alternative pathways by C. albicans.
Two IgM monoclonal antibodies (MAbs) that recognize distinct mannan determinants were compared for their abilities to activate the classical or alternative pathway. MAb B6.1 is specific for an acid-labile component of the Candida phosphomannan complex, and MAb B6 is specific for an acid-stable component (5). The MAbs were produced commercially (Montana ImmunoTech, Inc., Bozeman, Mont.).
C. albicans CA-1 was grown as yeast forms to stationary phase in glucose (2%)-yeast extract (0.3%)-peptone (1%) broth for 24 h at 37°C as described elsewhere (4, 6, 10). The mannan of CA-1 yeast was purified as described previously (7, 18) and coupled to CNBr-Sepahrose 4B (Pharmacia Biotech, Uppsala, Sweden) (18).
Pooled NHS was prepared from peripheral blood from at least 10 healthy adult donors and stored at −80°C. C3 was isolated from frozen human plasma (9, 13) and stored at −80°C until used. C3 was labeled with 125I as described previously (3) by use of IODO-GEN reagent (Pierce, Rockford, Ill.). NHS was absorbed with mannan-Sepharose 4B to remove antimannan antibodies (18).
Kinetics of C3 binding were assayed by the method of Kozel et al. (10). To determine whether MAb B6 or B6.1 activates the classical pathway, 2 × 106 yeast cells were incubated at 37°C in 1 ml of a complement binding medium that contained (i) 40% NHS, mannan-absorbed serum, or mannan-absorbed serum supplemented with MAb B6 or B6.1, (ii) sodium Veronal (5 mM)-buffered saline (142 mM, pH 7.3) containing 0.1% gelatin, 1.5 mM CaCl2, and 1 mM MgCl2, and (iii) 125I-labeled C3. To study whether MAb B6 or B6.1 plays a role in alternative pathway initiation, yeast cells were incubated in the manner described above except that the binding medium was not supplemented with Ca2+ and contained 5 mM EGTA and 5 mM MgCl2. At various time intervals from 2 to 16 min, 50-μl samples were withdrawn in duplicate and added to 200 μl of phosphate-buffered saline–0.1% sodium dodecyl sulfate–20 mM EDTA in Millipore MABX-N12 filter plates fitted with BV 1.2-μm-pore-size filter membranes (Millipore, Bedford, Mass.). The cells were washed with phosphate-buffered saline–0.1% sodium dodecyl sulfate, and filter-bound radioactivity was determined with a gamma counter. Nonspecific binding was estimated from cells incubated in NHS containing EDTA and was subtracted from the total counts.
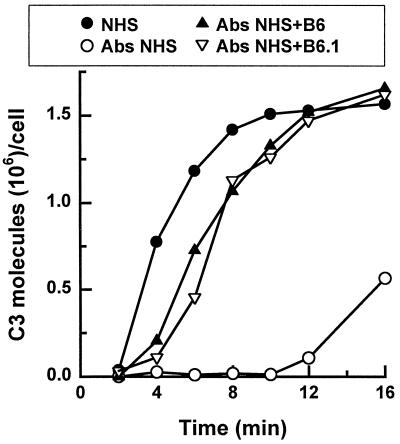
Mannan absorption of serum profoundly delayed C3 accumulation on yeast from 2 min to approximately 10 min (Fig. 1 and 2). However, addition of either MAb B6 or MAb B6.1 at 50 μg per ml of reaction mixture to the absorbed serum generated rapid activation kinetics characteristic of C3 deposition via the classical pathway (Fig. 1) (10, 17, 18). This observation was not unexpected, as polyvalent IgM is known to be a potent activator of the classical pathway.
FIG. 1.
Effect of MAb B6 or B6.1 on the kinetics of C3 deposition on C. albicans cells via the classical pathway. Yeast cells were incubated in a C3 binding medium containing (i) 40% NHS (•), (ii) 40% mannan-absorbed NHS (○), (iii) 40% mannan-absorbed NHS supplemented with MAb B6 (▴), or (iv) 40% mannan-absorbed NHS supplemented with MAb B6.1 (▿) at 50 μg per ml of reaction mixture. C3 deposition patterns from three independent assays were similar; results from one representative assay are shown.
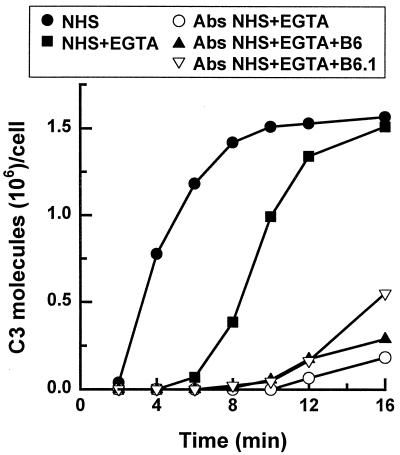
FIG. 2.
Effect of MAb B6 or B6.1 on the kinetics of C3 deposition on C. albicans cells via the alternative pathway. Yeast cells were incubated in a C3 binding medium containing (i) 40% NHS (•), (ii) 40% NHS–EGTA (■), (iii) 40% mannan-absorbed NHS containing EGTA (○), (vi) 40% mannan-absorbed NHS containing EGTA supplemented with MAb B6 (▴), or (iv) 40% mannan-absorbed NHS supplemented with MAb B6.1 (▿) at 50 μg per ml of reaction mixture. C3 deposition patterns from four independent assays were similar; results from one representative assay are shown.
The effects of MAbs B6 and B6.1 on activation of the alternative pathway were assessed by addition of the antibodies to mannan-absorbed serum in the presence of EGTA. The results (Fig. 2) showed that neither MAb B6 nor MAb B6.1 at 50 μg per ml of reaction mixture altered the alternative pathway activity of the mannan-absorbed serum. To determine whether the inability of MAb B6 or B6.1 to facilitate initiation of the alternative pathway was influenced by antibody concentration, the experiment represented in Fig. 2 was repeated with mannan-absorbed serum that was supplemented with 10 to 160 μg of MAb B6 or B6.1 per ml. These antibody concentrations were chosen because in our previous studies we found that affinity-purified human antimannan IgG activates both the classical and alternative pathways (17). However, at 10, 40, or 160 μg per ml of reaction mixture, both antibodies failed to enhance alternative pathway activity of mannan-absorbed serum but promoted classical pathway activity (data not shown).
The observation that both MAbs were unable to enhance alternative pathway activity was unexpected. Our previous studies showed that addition of polyclonal antimannan IgG to mannan-absorbed NHS containing EGTA produced C3 binding kinetics that were indistinguishable from the kinetics observed with nonabsorbed NHS containing EGTA (17). We further demonstrated IgG-dependent initiation of the alternative pathway by C. albicans using the six purified alternative pathway proteins (17).
There are at least three possible explanations for the failure of MAbs B6 and B6.1 to facilitate activation of the alternative pathway. First, it is possible that antimannan antibodies of the IgM class are unable to enhance C3 deposition via the alternative pathway. However, there is evidence that polyclonal IgM is able to enhance alternative pathway-mediated lysis of rabbit erythrocytes by NHS (11, 14). Second, the ability of an antibody to facilitate deposition of C3 via the alternative pathway could be epitope specific; MAbs B6 and B6.1 could have the wrong epitope specificity. As noted above, Eisenberg and Schwab (2) found that polyclonal antibodies specific for one antigen found on group A streptococcal cell walls were able to facilitate initiation of the alternative pathway, whereas antibodies specific for two other antigens were not. If antibody-facilitated activation of the alternative pathway is dependent on epitope specificity, such a finding might influence strategies for induction of protective immunity to Candida. Optimal immunization may require an immunogen that induces antibodies with epitope specificities needed to facilitate activation of the alternative pathway. Finally, we cannot exclude the possibility that human antimannan antibodies are able to facilitate activation of the alternative pathway, whereas mouse antibodies lack this capability.
In studies involving a murine model of disseminated candidiasis, MAb B6.1 was shown to be protective, whereas MAb B6 was not (4). However, the protection mechanisms remain to be elucidated. In an in vitro assay, MAb B6.1 but not MAb B6 was found to enhance candidacidal activity of polymorphonuclear leukocytes in the presence of fresh mouse serum, suggesting the involvement of mouse complement in the killing (1). Although assessing the role of complement in MAb B6.1-mediated protection was beyond the scope of this study, our observation that the two antibodies mediate similar kinetics of C3 deposition for C. albicans does not preclude the possibility that the composition and/or accessibility of opsonic complement fragments bound to the yeast cells might differ following complement activation by these two antibodies. Alternatively, the concerted action of several protective functions, including activation of the complement system, may be required for MAb B6.1-mediated protection.
Acknowledgments
This work was supported in part by National Institutes of Health grants AI 14209, AI 37194, and AI 24912.
We thank Kevin Wall for technical assistance.
REFERENCES
- 1.Caesar-TonThat T C, Cutler J E. A monoclonal antibody to Candida albicans enhances mouse neutrophil candidacidal activity. Infect Immun. 1997;65:5354–5357. doi: 10.1128/iai.65.12.5354-5357.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eisenberg R A, Schwab J H. Arthropathic group A streptococcal cell walls require specific antibody for activation of human complement by both the classical and alternative pathways. Infect Immun. 1986;53:324–330. doi: 10.1128/iai.53.2.324-330.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fraker P J, Speck J C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a, 6a-diphenylglycoluril. Biochem Biophys Res Commun. 1978;80:849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- 4.Han Y, Cutler J E. Antibody response that protects against disseminated candidiasis. Infect Immun. 1995;63:2714–2719. doi: 10.1128/iai.63.7.2714-2719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han Y, Kanbe T, Cherniak R, Cutler J E. Biochemical characterization of Candida albicans epitopes that can elicit protective and nonprotective antibodies. Infect Immun. 1997;65:4100–4107. doi: 10.1128/iai.65.10.4100-4107.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanbe T, Han Y, Redgrave B, Riesselman M H, Cutler J E. Evidence that mannans of Candida albicans are responsible for adherence of yeast forms to spleen and lymph node tissue. Infect Immun. 1993;61:2578–2584. doi: 10.1128/iai.61.6.2578-2584.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi H, Shibata N, Mitobe H, Ohkubo Y, Suzuki S. Structural study of phosphomannan of yeast-form cells of Candida albicans J-1012 strain with special reference to application of mild acetolysis. Arch Biochem Biophys. 1989;272:364–375. doi: 10.1016/0003-9861(89)90230-0. [DOI] [PubMed] [Google Scholar]
- 8.Kozel T R, Brown R R, Pfrommer G S T. Activation and binding of C3 by Candida albicans. Infect Immun. 1987;55:1890–1894. doi: 10.1128/iai.55.8.1890-1894.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozel T R, Pfrommer G S T. Activation of the complement system by Cryptococcus neoformans leads to binding of iC3b to the yeast. Infect Immun. 1986;52:1–5. doi: 10.1128/iai.52.1.1-5.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozel T R, Weinhold L C, Lupan D M. Distinct characteristics of initiation of the classical and alternative complement pathways by Candida albicans. Infect Immun. 1996;64:3360–3368. doi: 10.1128/iai.64.8.3360-3368.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polhill R B, Jr, Newman S L, Pruitt K M, Johnston R B., Jr Kinetic assessment of alternative complement pathway activity in a hemolytic system. II. Influence of antibody on alternative pathway activation. J Immunol. 1978;121:371–376. [PubMed] [Google Scholar]
- 12.Steele N P, Munson R S, Jr, Granoff D M, Cummins J E, Levine R P. Antibody-dependent alternative pathway killing of Haemophilus influenzae type b. Infect Immun. 1984;44:452–458. doi: 10.1128/iai.44.2.452-458.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tack B F, Janatova J, Thomas M L, Harrison R A, Hammer C H. The third, fourth, and fifth components of human complement: isolation and biochemical properties. Methods Enzymol. 1981;80:64–101. [Google Scholar]
- 14.Tomlinson S, Nussenzweig V. Human alternative complement pathway-mediated lysis of rabbit erythrocytes is enhanced by natural anti-galα1-3gal antibodies. J Immunol. 1997;159:5606–5609. [PubMed] [Google Scholar]
- 15.Tsuchiya T, Fukazawa Y, Kawakita S. Significance of serological studies on yeasts. Mycopathol Mycol Appl. 1965;26:1–15. doi: 10.1007/BF02098585. [DOI] [PubMed] [Google Scholar]
- 16.Tsuchiya T, Fukazawa Y, Taguchi M, Nakase T, Shinoda T. Serological aspects of yeast identification. Mycopathol Mycol Appl. 1974;53:77–92. doi: 10.1007/BF02127199. [DOI] [PubMed] [Google Scholar]
- 17.Zhang M X, Kozel T R. Mannan-specific immunoglobulin G antibodies in normal human serum accelerate binding of C3 to Candida albicans via the alternative complement pathway. Infect Immun. 1998;66:4845–4850. doi: 10.1128/iai.66.10.4845-4850.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang M X, Lupan D M, Kozel T R. Mannan-specific IgG antibodies in normal human serum mediate classical pathway initiation of C3 binding to Candida albicans. Infect Immun. 1997;65:3822–3827. doi: 10.1128/iai.65.9.3822-3827.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]