Abstract
In Portugal erythromycin resistance of 26.6% (n = 352) remained constant during 1998 to 2003, however in 1998 the MLSB phenotype dominated (85%), whereas in 2003 the M phenotype prevailed (77%). A decline in T12/emm22 MLSB isolates could partially explain the drop in this phenotype, but the rise of the M phenotype was not due to clonal expansion.
Although penicillin remains the antibiotic of choice in the treatment of Lancefield group A streptococci (GAS) infections, macrolides and lincosamides are recommended as suitable alternatives for patients allergic to penicillin (5). Newer macrolides, such as azithromycin, may be given once a day, making this an attractive option for the treatment of pharyngitis due to Streptococcus pyogenes. In Portugal, where penicillin V is not available, macrolides and lincosamides have the additional advantage of being a therapeutic option with an oral route of administration. High macrolide resistance in GAS was previously identified in Portugal (13) in line with other European countries (1, 4, 6) but in contrast to others (14, 16). The aims of this study were to determine the prevalence of macrolide resistance phenotypes and its temporal trends and to evaluate the correlation with T and emm-types.
A total of 1,321 GAS from clinical infections were collected from 30 laboratories, geographically distributed throughout Portugal, from January 1998 to December 2003. The isolates were distributed in the study period as follows: 153 in 1998, 240 in 1999, 213 in 2000, 216 in 2001, 270 in 2002, and 229 in 2003. The laboratories were asked to submit all nonduplicate GAS isolated from outpatients during the study period. Antimicrobial susceptibility testing, T-typing, and macrolide resistance phenotype and genotype were determined as previously described (7, 13). Strains were emm typed according to the recommendations of the Centers for Disease Control and Prevention (CDC) (http://www.cdc.gov/ncidod/biotech/strep/protocols.htm). The sequences of representatives of each restriction profile were searched against GenBank as well as the emm CDC database (http://www.cdc.gov/ncidod/biotech/strep/strepindex.htm). An isolate was considered to be of a given emm type if it had >95% identity over the 160 bases considered (3).
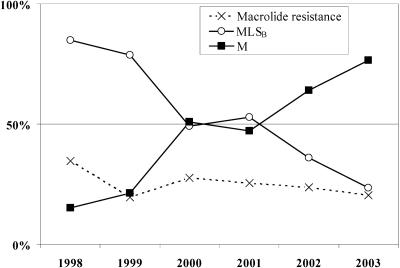
Among this collection, 352 isolates (26.6%) were erythromycin resistant. Although there was a higher prevalence of resistant isolates recovered in 1998 (34.6%), the variation of the overall prevalence in the following years (19.6% in 1999, 27.7% in 2000, 25.5% in 2001, 23.7% in 2002, and 20.5% in 2003) was not significant (χ2 test, P = 0.22) (Fig. 1). Only the 325 erythromycin-resistant isolates recovered from throat swabs associated with a diagnosis of pharyngitis were characterized further.
FIG. 1.
Erythromycin resistance and prevalence of macrolide resistance phenotypes in Portugal during 1998 to 2003.
Resistance to tetracycline was expressed by 38.7% (n = 126) of the isolates (MIC90 = 96 μg/ml, MIC range, 12 to 512 μg/ml). The distribution of tetracycline-resistant isolates among the study years was as follows: 84.9% of the isolates recovered in 1998, 70.2% in 1999, 28.8% in 2000, 30.9% in 2001, 12.5% in 2002, and 12.8% in 2003.
The cMLSB phenotype was expressed by 170 isolates (52.3%), 151 (46.4%) presented the M phenotype, and only 4 isolates (1.2%) the iMLSB phenotype. Erythromycin resistance determinants were detected in all isolates by multiplex PCR. All isolates presenting the M phenotype carried the mefA gene, including one which carried both mefA and ermB genes. Nine of the isolates presenting the MLSB phenotype (4.9%) carried both the mefA and ermB resistance determinants whereas all other isolates presenting this phenotype yielded a single PCR product consistent with the presence of the ermB gene. Three isolates presenting the iMLSB phenotype carried the erm(A) gene, and one carried the ermB gene.
A summary of the number of T/emm-type associations with the two macrolide resistance phenotypes and their distribution over the different years of the study period is presented in Table 1. The majority (90%) of the tetracycline-resistant isolates were included in the T12 emm22 group.
TABLE 1.
Distribution of T and emm types and macrolide resistance phenotypes among 325 pharyngitis-associated erythromycin-resistant GAS in Portugal during 1998 to 2003
| T(emm)a | Resultb for yr:
|
Totalc | |||||
|---|---|---|---|---|---|---|---|
| 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | ||
| 12(12) | 1/1 | 0/5 | 0/4 | 1/5 | 0/7 | 0/2 | 2 (1)/24 |
| 12(22) | 42/0 | 31/0 | 20/1 | 15/0 | 9/0 | 8/0 | 125 (113)/1 |
| 28(28) | 2/0 | 8/0 | 7/0 | 10/0 | 1/1 | 28 (2)/1 | |
| 1(1) | 0/3 | 0/11 | 0/17 | 0/4 | 0/35 | ||
| 25(75) | 0/3 | 0/5 | 0/3 | 0/11 | |||
| 4(4) | 0/5 | 0/1 | 0/6 | 0/2 | 0/6 | 0/12 | 0/32 |
| 12(11) | 2/0 | 2 (2)/0 | |||||
| B3264(4) | 0/2 | 0/2 | |||||
| 5/27/44(4) | 0/2 | 0/2 | |||||
| 1(12) | 0/2 | 0/2 | |||||
| NT(12)d | 0/2 | 0/4 | 0/1 | 0/7 | |||
| NT(28)d | 2/0 | 2/0 | |||||
| 13(22) | 3/0 | 3/0 | |||||
| 2(2) | 0/2 | 0/2 | |||||
| 2(4) | 0/2 | 0/2 | |||||
| 9(9) | 0/6 | 0/1 | 0/7 | ||||
| Othere | 2/0 | 1/2 | 1/4 | 5/4 | 2/5 | 1/8 | 12 (10)/23 |
| Total | 45/8 | 37/10 | 29/30 | 30/25 | 23/41 | 10/37 | 174/151 |
All T/emm type combinations presenting ≥2 isolates in any year of the study period are discriminated.
Number of isolates presenting each of the erythromycin resistance phenotypes (MLSB/M).
Numbers in parenthesis indicate tetracycline-resistant isolates among those presenting the MLSB phenotype. None of the isolates presenting the M phenotype was resistant to tetracycline.
NT, nontypeable by T serotyping.
Single isolates presenting unique T/emm-type combinations in each year. Most (16/24) of these T/emm combinations were represented by a single isolate during the entire study period. The remaining 8 were represented by more than one isolate each recovered in different years: T13 emm4, T13 emm77, and T12 emm28 were expressed by 3 isolates; T12 emm4, T(B3264) emm89, T1 emm4, T13 emm1, and T(nontypeable) emm1 were expressed by 2 isolates.
The situation found in 1998 and 1999, where strains expressing the MLSB phenotype accounted for approximately 80% of all macrolide-resistant isolates (Fig. 1), was similar to that found among a group of erythromycin-resistant isolates recovered in Italy in 2000 (6) and in France (2002 to 2003) (4). However, the dominance of the MLSB phenotype was in sharp contrast to the situation found in neighboring Spain where isolates collected around the same years (1996 to 1999) expressed overwhelmingly (95%) the M phenotype (1). The tendency for the strains expressing the MLSB phenotype to present a limited number of T/emm-type associations and for at most two associations (T12 emm22 and T28 emm28) to account for more than 80% of isolates recovered in each year was a constant during the study period. The only exception was 2001 when, not only was an unusually high number of associations identified (n = 9) but three different associations were necessary to account for 80% of the isolates (compare to 2000 when, for the same number of isolates, only three associations were identified) (Table 1). This indicates that other T/emm-type associations expressing the MLSB phenotype existed that could replace the T12 emm22 group; however none rose to significant numbers.
In the following years there was an increase in prevalence of isolates expressing the M phenotype, that accounted for 76.6% of erythromycin resistant isolates in 2003 (Fig. 1). In sharp contrast to the data presented here, the increases in occurrence of strains expressing the M phenotype documented elsewhere were also paralleled by an increase in the overall macrolide resistance rate (8, 9, 11). Contrary to isolates expressing the MLSB phenotype, those expressing the M phenotype were found among a diverse group of T/emm-type associations and usually at least one-half of the total number of associations found each year was necessary to account for 80% of the isolates. This increased diversity is better illustrated by the strains exhibiting the M phenotype recovered in 2003, when 17 different T/emm-type associations were found among the 36 isolates (Table 1). Unlike these findings studies from elsewhere in Europe have documented similar numbers of T/emm-type associations among isolates expressing the two phenotypes (4, 6). The contrast to the situation in Spain, where only four emm types accounted for 85% of the isolates expressing the M phenotype (1), indicates that, despite the geographic proximity, macrolide-resistant GAS isolated in the two countries constitute two different populations. Moreover, it suggests that the rise in isolates of the M phenotype was not due to invasion of the few emm groups prevalent in Spain.
The decrease observed in tetracycline resistance paralleled the decline of isolates presenting the MLSB phenotype, namely, those associated with the T12 emm22 types. This could be explained by the presence of erm and tetracycline resistance genes in the same transposon (12) in this group of isolates.
Although a situation where either phenotype dominates is not unusual, such a rapid shift in prevalence of the different phenotypes, while maintaining the same overall erythromycin resistance rate (Fig. 1), was not previously described in GAS to the best of our knowledge. This shift could not be attributed simply to the reduction in the number of isolates defined by T12 emm22 and the absence of other T/emm-type associations expressing the MLSB phenotype that could take its place, or by the emergence of a limited number of highly successful strains expressing the M phenotype and specific T/emm-type associations as documented in North America (8, 11). Since the data presented do not offer firm clues, we can only speculate as to the reasons behind this shift. Although Portugal is among the largest antibiotic consumers in Europe (www.ua.ac.be/ESAC), it is not remarkable for its macrolide consumption. Decreases in macrolide use were associated to a reduction of macrolide-resistant GAS (16). However, there was only a slight decrease in consumption for human use in the ambulatory setting during the study period in Portugal (www.ua.ac.be/ESAC). If, as suggested by Nielsen et al. (15), tetracycline use together with macrolide use could be correlated to macrolide resistance, especially the MLSB phenotype, then the slight reduction in tetracycline use (www.ua.ac.be/ESAC) could have an additive effect. These factors, as well as a possible immunity developed by the human population against the limited number of T antigens and M proteins associated with the MLSB phenotype, could explain the observed reduction in prevalence. The diversity among the strains expressing the M phenotype could be explained by a higher mobility of the genetic element containing the mefA gene (10). Interestingly, an unusual chimeric genetic element combining a transposon and a bacteriophage, that could mediate the transfer of the mefA gene by a process similar to transduction, was recently described (2).
The data presented documents a major shift in the prevalence of macrolide resistance phenotypes in an unusual short time period, not paralleled elsewhere in Europe and without a concomitant change in the frequency of macrolide resistance. Continued surveillance is needed to establish if this shift will stabilize and if the major groups of isolates sharing the same T/emm-types are also found elsewhere.
Acknowledgments
This work was partly supported by Fundação Calouste Gulbenkian, Portugal.
The technical support of Letícia Santos and Catarina Laborinho is gratefully acknowledged.
Members of the Portuguese Surveillance Group for the Study of Respiratory Pathogens: A. Fonseca, A. Coutinho, Centro Hospitalar de Cascais, Cascais; A. F. Alves, Centro Hospitalar de Coimbra, Coimbra; F. Fonseca, Centro Hospitalar da Póvoa do Varzim/Vila do Conde, Póvoa do Varzim e Vila do Conde; P. Lopes, I. Calheiros, Felício, C. Magalhães, L. Sobral, Centro Hospitalar de Vila Nova de Gaia, Vila Nova de Gaia; T. Vaz, M. Gião, Hospital do Barlavento Algarvio, Portimão; T. Afonso, Hospital Cruz do Carvalho, Funchal; M. J. Silvestre, H. Peres, T. Pina, Hospital Curry Cabral, Lisboa; C. Roldão, Hospital Distrital de Abrantes, Abrantes; J. Dantas, R. Farto, Hospital Distrital de Angra do Heroísmo, Angra do Heroísmo; E. Carvalho, Hospital do Divino Espírito Santo, Ponta Delgada; E. Ramalheira, A. Rodrigues, Hospital Infante D. Pedro, Aveiro; R. M. Barros, M. I. Peres, Hospital D. EstefÂnia, Lisboa; J. Diogo, A. Rodrigues, I. Nascimento, Hospital Garcia de Orta, Almada; M. P. Pinheiro, R. Semedo, Hospital Dr. José Maria Grande, Portalegre; V. Alves, A. Read, Hospital Pedro Hispano, Matosinhos; M. Abecassis, I. Alves, P. Cabral, Hospital de Pulido Valente, Lisboa; L. Cabral, O. Neto, F. Antunes, Hospital dos S.A.M.S., Lisboa; I. Fontes, Hosptial de Santa Luzia, Elvas; L. Lito, M. L. Fernandes, M. J. Salgado, Hospital de Santa Maria, Lisboa; M. Pinto, H. Choon, Hospital de Santa Marta, Lisboa; L. Ferreira, Hospital de Santo André, Leiria; A. P. Castro, M. H. Ramos, J. M. Amorim, Hospital de Santo António, Porto; F. Martins, M. A. Pessanha, E. Gonçalves, Hospital de São Francisco Xavier, Lisboa; F. Cotta, J. Correia da Fonseca, Hospital de São João, Porto; M. O. Spencer, J. Marques, Hospital de São José Lisboa; I. Marques, J. M. Ribeiro, Hospital de São Teotónio, Viseu; A. P. M. Vieira, F. B. Moniz, Hospital Senhora da Oliveira, Guimarães; R. Velho, R. Tomé, L. Boaventura, Hospitais da Universidade de Coimbra, Coimbra; A. P. Castro, Hospital de Vila Real, Vila Real; M. O. Basílio, M. G. Martins, Instituto Nacional de Saúde Dr. Ricardo Jorge, Porto.
REFERENCES
- 1.Albertí, S., C. Garcia-Rey, M. A. Dominguez, L. Aguilar, E. Cercenado, M. Gobernado, A. Garcia-Perea, and the Spanish Surveillance Group for Respiratory Pathogens. 2003. Survey of emm gene sequences from pharyngeal Streptococcus pyogenes isolates collected in Spain and their relationship with erythromycin susceptibility. J. Clin. Microbiol. 41:2385-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banks, D. J., S. F. Porcella, K. D. Barbian, J. M. Martin, and J. M. Musser. 2003. Structure and distribution of an unusual chimeric genetic element encoding macrolide resistance in phylogenetically diverse clones of group A Streptococcus. J. Infect. Dis. 188:1898-1908. [DOI] [PubMed] [Google Scholar]
- 3.Beall, B., R. R. Facklam, J. A. Elliott, A. R. Franklin, T. Hoenes, D. Jackson, L. Laclaire, T. Thompson, and R. Viswanathan. 1998. Streptococcal emm types associated with T-agglutination types and the use of conserved emm gene restriction fragment patterns for subtyping group A streptococci. J. Med. Microbiol. 47:893-898. [DOI] [PubMed] [Google Scholar]
- 4.Bingen, E., P. Bidet, L. Mihaila-Amrouche, C. Doit, S. Forcet, N. Brahimi, A. Bouvet, and R. Cohen. 2004. Emergence of macrolide-resistant Streptococcus pyogenes strains in French children. Antimicrob. Agents Chemother. 48:3559-3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bisno, A. L., M. A. Gerber, J. M. Gwaltney, Jr., E. L. Kaplan, and R. H. Schwartz. 2002. Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. Clin. Infect. Dis. 35:113-125. [DOI] [PubMed] [Google Scholar]
- 6.Dicuonzo, G., E. Fiscarelli, G. Gherardi, G. Lorino, F. Battistoni, S. Landi, M. De Cesaris, T. Petitti, and B. Beall. 2002. Erythromycin-resistant pharyngeal isolates of Streptococcus pyogenes recovered in Italy. Antimicrob. Agents Chemother. 46:3987-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Figueira-Coelho, J., M. Ramirez, M. J. Salgado, and J. Melo-Cristino. 2004. Streptococcus agalactiae in a large Portuguese teaching hospital: antimicrobial susceptibility, serotype distribution, and clonal analysis of macrolide-resistant isolates. Microb. Drug Resist. 10:31-36. [DOI] [PubMed] [Google Scholar]
- 8.Green, M., J. M. Martin, K. A. Barbadora, B. Beall, and E. R. Wald. 2004. Reemergence of macrolide resistance in pharyngeal isolates of group A streptococci in southwestern Pennsylvania. Antimicrob. Agents Chemother. 48:473-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsueh, P. R., L. J. Teng, L. N. Lee, P. C. Yang, S. W. Ho, H. C. Lue, and K. T. Luh. 2002. Increased prevalence of erythromycin resistance in streptococci: substantial upsurge in erythromycin-resistant M phenotype in Streptococcus pyogenes (1979-1998) but not in Streptococcus pneumoniae (1985-1999) in Taiwan. Microb. Drug Resist. 8:27-33. [DOI] [PubMed] [Google Scholar]
- 10.Kataja, J., P. Huovinen, M. Skurnik, the Finnish Study Group for Antimicrobial Resistance, and H. Seppälä. 1999. Erythromycin resistance genes in group A streptococci in Finland. Antimicrob. Agents Chemother. 43:48-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz, K. C., A. J. McGeer, C. L. Duncan, A. Ashi-Sulaiman, B. M. Willey, A. Sarabia, J. McCann, S. Pong-Porter, Y. Rzayev, J. S. de Azavedo, and D. E. Low. 2003. Emergence of macrolide resistance in throat culture isolates of group A streptococci in Ontario, Canada, in 2001. Antimicrob. Agents Chemother. 47:2370-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Bouguenec, C., G. de Cespedes, and T. Horaud. 1990. Presence of chromosomal elements resembling the composite structure Tn3701 in streptococci. J. Bacteriol. 172:727-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melo-Cristino, J., M. L. Fernandes, and the Portuguese Surveillance Group for the Study of Respiratory Pathogens. 1999. Streptococcus pyogenes isolated in Portugal: macrolide resistance phenotypes and correlation with T types. Microb. Drug Resist. 5:219-225. [DOI] [PubMed] [Google Scholar]
- 14.Nagai, K., P. C. Appelbaum, T. A. Davies, L. M. Kelly, D. B. Hoellman, A. T. Andrasevic, L. Drukalska, W. Hryniewicz, M. R. Jacobs, J. Kolman, J. Miciuleviciene, M. Pana, L. Setchanova, M. K. Thege, H. Hupkova, J. Trupl, and P. Urbaskova. 2002. Susceptibility to telithromycin in 1,011 Streptococcus pyogenes isolates from 10 central and eastern European countries. Antimicrob. Agents Chemother. 46:546-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nielsen, H. U., A. M. Hammerum, K. Ekelund, D. Bang, L. V. Pallesen, and N. Frimodt-Moller. 2004. Tetracycline and macrolide co-resistance in Streptococcus pyogenes: co-selection as a reason for increase in macrolide-resistant S. pyogenes? Microb. Drug Resist. 10:231-238. [DOI] [PubMed] [Google Scholar]
- 16.Seppälä, H., T. Klaukka, J. Vuopio-Varkila, A. Muotiala, H. Helenius, K. Lager, P. Huovinen, and the Finnish Study Group for Antimicrobial Resistance. 1997. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N. Engl. J. Med. 337:441-446. [DOI] [PubMed] [Google Scholar]