Abstract
Recent studies have implicated rodent mast cells in the innate immune response to infectious bacteria. We report that cord blood-derived human mast cells (CBHMC) obtained from culture of cord blood progenitors phagocytozed and killed various gram-negative and gram-positive bacteria and simultaneously released considerable amounts of tumor necrosis factor alpha. Overall, the extent of the endocytic and exocytic response of CBHMC correlated with the number of adherent bacteria. Thus, human mast cells are intrinsically capable of mediating microbial recognition and of actively contributing to the host defense against bacteria.
Mast cells are preferentially found in large numbers at the interface of the host and environment and are primarily known for their capacity to secrete significant amounts of pharmacologically active mediators (4–6, 8, 9, 12, 14). They are also an extremely heterogeneous collection of cells exhibiting striking differences in morphology and responsiveness to agonists (13, 17, 18, 32). These distinct traits are seen among mast cells from different species and even among mast cells from different sites in the same animal (17, 33). Recently, several in vitro and in vivo studies involving mucosal-type and connective tissue-type rodent mast cells have suggested that mast cells play a critical and hitherto unrecognized role in host defense against infectious agents (3, 7, 15, 23, 24). There exist two subpopulations of human mast cells, some containing measurable amounts of the neutral proteases tryptase and chymase, and others containing tryptase but no chymase (18, 34). In the present study, we sought to investigate if cultured CBHMC expressing tryptase but not chymase (2, 29, 31) were capable of recognizing and responding to different gram-negative and gram-positive bacteria.
Binding of CBHMC to gram-negative and gram-positive bacteria.
CBHMC were obtained following 10 weeks of culture of cord blood progenitor cells in the presence of stem cell factor and interleukin 6 (11). The cells were determined to be >97% CBHMC from their morphological appearance following May-Grünwald-Giemsa staining and by their positivity for tryptase (10, 28, 29). To examine the binding of CBHMC to various bacteria, we exposed monolayers of CBHMC grown on eight-chamber tissue culture slides (Nalge Nunc, Naperville, Ill.) to suspensions of various bacteria at a ratio of 100 bacteria to 1 mast cell (22). After 1 or 3 h of incubation, nonadherent bacteria were removed by gentle rinsing, and the CBHMC monolayers were fixed and stained. The number of adherent bacteria on at least 400 CBHMC was determined by light microscopy. Adherence to the following clinical bacterial isolates was investigated: Citrobacter freundii CI125, Streptococcus faecium CI126, Staphylococcus aureus CI127, and Klebsiella pneumoniae CI128. Also included in this study was the laboratory Escherichia coli strain ORN103(pSH2), which expresses recombinant type 1 fimbriae and its FimH-negative derivative, ORN103(pUT2002) (21, 22).
CBHMC recognized and bound all clinical strains as well as the laboratory E. coli strain expressing recombinant type 1 fimbriae, albeit to various degrees (Table 1). Among clinical strains, CBHMC bound S. faecium CI126 the most and K. pneumoniae CI128 the least. Also shown in Table 1 is CBHMC binding of bacteria after 3 h of incubation. It appears that with the exception of the FimH-negative mutant and K. pneumoniae CI128, mast cell binding of bacteria increased substantially with prolonged incubation. The limited binding of CBHMC to K. pneumoniae CI128 may be attributable to the large capsular material coating this bacterium. Although C. freundii CI125 appeared to be encapsulated, it was readily bound by CBHMC. Thus, the molecular basis for these adhesion reactions is, for the most part, unclear. Recent studies have shown that rodent mast cells bound E. coli and several other gram-negative enteric bacteria by recognizing the mannose-binding lectin FimH, which is expressed on their type 1 fimbrial organelles (22). We investigated whether FimH was the bacterial determinant recognized by CBHMC by comparing mast cell binding to a laboratory E. coli strain, ORN103(pSH2), which expresses recombinant type 1 fimbriae and to its FimH-negative mutant derivative, ORN103(pUT2002). Whereas binding of CBHMC to the FimH-negative bacterium was limited, the level of mast cell binding to the wild-type strain was remarkably high (Table 1). Also shown in Table 1 is the almost complete inhibition of binding of CBHMC to E. coli ORN103(pSH2) by d-mannose (Table 1). Since the binding interactions mediated by bacterial FimH are inhibited by d-mannose (21, 22), we assume that binding of CBHMC to E. coli ORN103(pSH2) involves bacterial FimH. We have recently determined that the putative receptor on rodent mast cells for bacterial FimH is the mannose-containing and glycosylphosphoinositol-anchored moiety CD48, which is a member of the immunoglobulin superfamily (23a). Although CD48 is also present on the surface of human mast cells (1), whether it serves as the receptor on CBHMC for bacterial FimH remains to be established. It must be emphasized that since the binding interactions between CBHMC and various bacteria were undertaken in the presence of serum, the adherence reactions observed here may be facilitated by any one of several humoral opsonins, including bacterium-specific antibodies, complement, collectins, and various extracellular matrix proteins (30).
TABLE 1.
In vitro adherence of various bacteria to CBHMC following incubation at different time intervals
| Bacterium | Mean no. of bacteria/CBHMC (± SEM) after incubation fora:
|
|
|---|---|---|
| 1 h | 3 h | |
| S. aureus CI127 | 4.1 ± 2 | 15.2 ± 4 |
| S. faecium CI126 | 7.6 ± 3 | 16.8 ± 3 |
| K. pneumoniae CI128 | 1.1 ± 0.4 | 1.7 ± 0.8 |
| C. freundii CI125 | 6.5 ± 2 | 11.2 ± 4 |
| E. coli ORN103(pSH2) | 6.6 ± 3 | 15.1 ± 3 |
| E. coli ORN103(pSH2) + d-mannose | 0.3 ± 0.2 | 0.3 ± 0.1 |
| E. coli ORN103(pUT2002) | 0.5 ± 0.1 | 0.6 ± 0.2 |
At least 400 CBHMC were examined for each time point in three different sets of experiments.
CBHMC-mediated phagocytosis and killing of bacteria.
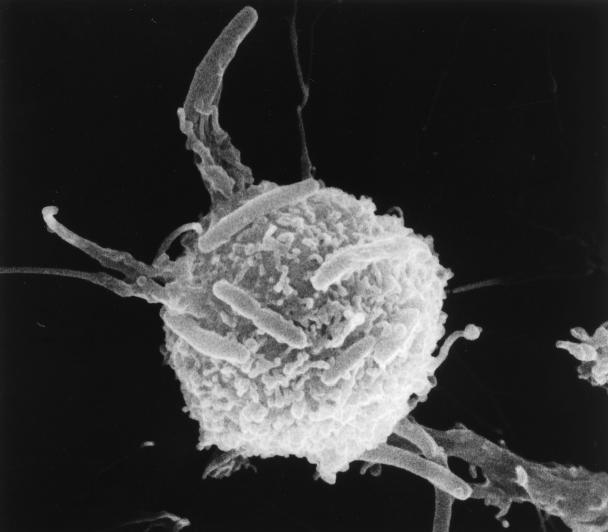
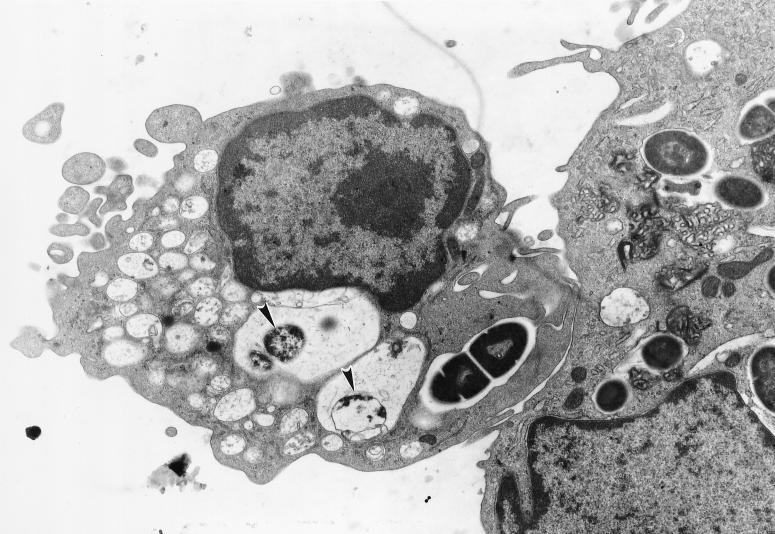
The morphological aspects of the interaction between CBHMC and selected bacteria were examined by scanning and transmission electron microscopy (SEM and TEM, respectively) (22). These techniques showed that mast cells employ protoplasmic protrusions on their surfaces to entrap bacteria. The formation of these mast cell protrusions around E. coli ORN103(pSH2) is shown in Fig. 1. Examination of cross-sections of mast cells after exposure to bacteria revealed a significant number of bacteria encased in vacuoles. A cross-section of mast cells containing several intracellular S. faecium CI126 cells is shown in Fig. 2. It is interesting that whereas most of the S. faecium CI126 associated with the CBHMC appeared to be intracellular, a significant number of the gram-negative bacteria still remained attached on the outside of the cell (data not shown), implying that the rate of internalization of the gram-positive bacteria was markedly higher than that of the gram-negative bacteria. Conceivably, the uptake of S. faecium CI126 is a rapid process triggered spontaneously upon contact with the mast cell membrane, whereas uptake of the gram-negative bacteria could be a more gradual process which is initiated only after the adherence of a critical number of bacteria.
FIG. 1.
SEM examination of CBHMC surface following exposure to E. coli ORN103(pSH2). Notice the formation of filopod-like structures employed by the mast cell to grip bacteria.
FIG. 2.
TEM examination of cross-sections of CBHMC showing internalized bacteria following exposure to S. faecium CI126. Notice the degraded bacteria (arrowheads) within vacuoles.
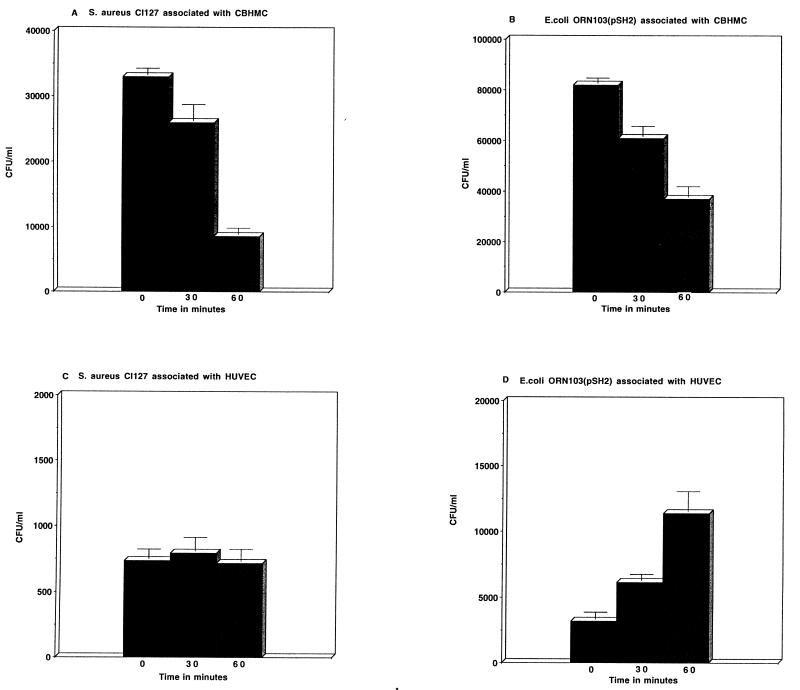
To investigate if adherent bacteria were indeed killed by CBHMC, we monitored the viability of S. aureus CI127 and E. coli ORN103(pSH2) bacteria that were adherent to monolayers of CBHMC as described previously (22). The cells were incubated in antibiotic-free medium containing serum. As a control, we monitored the viability of the same two bacteria that were adherent on human umbilical vein endothelial cells (HUVEC). We found that there was an appreciable and time-dependent decrease in viability of bacteria associated with CBHMC during the 1-h period of incubation (Fig. 3A and B). In contrast, the numbers of bacteria exposed to HUVEC either remained constant, as in the case of S. aureus CI127, or increased to reflect growth, as in the case of E. coli ORN103(pSH2) (Fig. 3C and D). Morphological evidence in support of intracellular bacterial killing by CBHMC could be obtained from the examination of cross-sections of CBHMC for the presence of degraded bacteria within vacuoles. For example, some of the S. faecium CI126 cells found within vacuoles in CBHMC shown in Fig. 2B appear to be partially degraded (see arrows). Thus, regardless of possible variation in the recognition mechanisms, rate of phagocytosis, and efficiency of intracellular killing of different bacteria, CBHMC possess the intrinsic capacity to kill adherent bacteria.
FIG. 3.
Viability of S. aureus CI127 (A and C) and E. coli ORN103(pSH2) (B and D) incubated with CBHMC or HUVEC. Bacteria were added to a monolayer of CBHMC or HUVEC in antibiotic-free and serum-containing medium in wells of a 96-well tray at a ratio of 100:1. The mixture was incubated for 30 min, after which all unbound bacteria were removed and fresh medium was added. At different time points thereafter, the viability of residual bacteria was assessed by solubilizing the cells in each well with Triton X-100, and the number of viable bacteria was assessed by plating onto agar.
CBHMC release of TNF-α.
Mast cells are renown for their capacity to release a large array of pharmacologically active mediators. Among mast cell mediators, tumor necrosis factor alpha (TNF-α) is of particular interest because mast cells are the only cell type in the body known to prestore TNF-α and spontaneously to release the cytokine upon activation (16). We examined the capacity of CBHMC to release TNF-α following exposure to various bacteria. CBHMC at a concentration of 106 per ml were incubated with 107 bacteria for 0.5, 1, 3, or 6 h at 37°C. After centrifugation (1,000 × g for 10 min at 4°C), cell-free supernatants were collected and TNF-α was measured by an enzyme-linked immunosorbent assay (ELISA) method (human TNF-α ELISA kit; Genzyme, Cambridge, Mass.). The results were expressed in picograms per milliliter, with the detection limit of the kit being 15 pg/ml. As shown in Table 2, CBHMC elaborated a substantial TNF-α response even after 0.5 h of incubation with various bacteria. However, it is not known at this time if this TNF-α is actually derived from presynthesized stores or from the rapid translation of untranslated TNF-α mRNA. Further incubation with bacteria (1, 3, and 6 h) revealed even greater amounts of TNF-α release, reflecting substantial de novo synthesis and release of TNF-α. The greatest amounts of TNF-α were elicited from CBHMC by S. faecium CI126 and S. aureus CI127, and the least amounts were elicited by K. pneumoniae CI128 and the FimH-negative mutant E. coli ORN103(pUT2002) (Table 2). It is interesting that at 6 h, the amounts of mast cell TNF-α elicited by S. faecium CI126 and S. aureus CI127 were comparable to the amount elicited by the combined action of the well-known mast cell agonists phorbol myristate acetate and calcium ionophore. Although on the whole there appeared to be a correlation between CBHMC binding of bacteria and TNF-α release (Tables 1 and 2), there were some notable exceptions. For example, the laboratory E. coli strain ORN103(pUT2002) exhibited a low level of binding but elicited appreciable TNF-α release from mast cells, especially after 6 h. It is conceivable that during the lengthy incubation period, bacteria could have released agents such as proteases and toxins which can potentially elicit a TNF-α response. Indeed, mast cell activation by such bacterial products as cholera toxin and lipopolysaccharide has been demonstrated (19, 20, 25, 26). Nevertheless, the capacity of CBHMC to release large amounts of TNF-α following contact with bacteria is relevant to host defense, considering the recent findings for mice showing that the early burst of mast cell-derived TNF-α following bacterial challenge was critical to the survival of mice (7, 23, 27).
TABLE 2.
Time-dependent release of TNF-α by CBHMC following exposure to various bacteria and mast cell agonists
| Bacterium/agonist | TNF-α release (pg/ml) after incubation for (h)a:
|
|||
|---|---|---|---|---|
| 0.5 h | 1 h | 3 h | 6 h | |
| None (medium alone) | 0 ± 0 | 2 ± 1 | 3 ± 5 | 14 ± 7 |
| S. aureus CI127 | 21 ± 6 | 161 ± 19 | 617 ± 96 | 1,944 ± 222 |
| S. faecium CI126 | 9 ± 9 | 201 ± 36 | 1,009 ± 264 | 3,209 ± 171 |
| K. pneumoniae CI128 | 29 ± 8 | 26 ± 2 | 48 ± 4 | 99 ± 17 |
| C. freundii CI125 | 44 ± 3 | 138 ± 7 | 379 ± 70 | 662 ± 64 |
| E. coli ORN103(pSH2) | 44 ± 8 | 131 ± 15 | 764 ± 2 | 1,393 ± 27 |
| E. coli ORN103(pSH2) | 37 ± 17 | 31 ± 7 | 104 ± 11 | 156 ± 21 |
| +d-Mannose | ||||
| E. coli ORN103 (pUT2002) | 33 ± 6 | 35 ± 7 | 83 ± 11 | 174 ± 12 |
| PMAb/Ca ionophore | 3 ± 2 | 164 ± 26 | 1,190 ± 32 | 1,461 ± 57 |
Values are the means ± standard errors of the means from three different sets of experiments.
PMA, phorbol myristate acetate.
Since their discovery more than 100 years ago, an unequivocal physiological role for mast cells in the body has been sought. We have shown here that CBHMC, which express tryptase but little or no chymase (29), recognize and mediate at least two potentially antimicrobial activities against several gram-negative and gram-positive bacteria. Thus, in spite of the noted heterogeneity in mast cell properties between species, their capacity to recognize different microorganisms is an intrinsic trait. This finding is consistent with the idea that an important function for mast cells in the body is to mobilize the immune defenses against infectious agents.
Acknowledgments
We express our appreciation to AMGEN Inc. (Thousand Oaks, Calif.) and to Novartis Biotechnology (Basel, Switzerland) for their continuous and generous supply of cytokines. We also thank Viviane Tricottet for expert advice concerning electron microscopy.
This work was supported in part by grants from the Fondation pour la Recherche Médicale and the National Institutes of Health (AI-35678 and DK-50814).
REFERENCES
- 1.Agis H, Fureder W, Bankl H C, Kundi M, Sperr W R, Willheim M, Boltz-Nitulescu G, Butterfield J H, Kishi K, Lechner K, Valent P. Comparative immunophenotypic analysis of human mast cells, blood basophils and monocytes. Immunology. 1996;87:535–543. doi: 10.1046/j.1365-2567.1996.493578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arock M, Hervatin F, Guillosson J J, Mencia-Huerta J M, Thierry D. Differentiation of human mast cells from bone-marrow and cord-blood progenitor cells by factors produced by a mouse stromal cell line. Ann N Y Acad Sci. 1994;725:59–68. doi: 10.1111/j.1749-6632.1994.tb39790.x. [DOI] [PubMed] [Google Scholar]
- 3.Bidri M, Vouldoukis I, Ktorza S, Issaly F, Mossalayi M D, Mazier D, Debré P, Guillosson J J, Arock M. Evidence for direct interaction between mast cell and leishmania parasites. Parasite Immunol. 1997;19:475–483. doi: 10.1046/j.1365-3024.1997.d01-153.x. [DOI] [PubMed] [Google Scholar]
- 4.Bradding P. Human mast cell cytokines. Clin Exp Allergy. 1996;26:13–19. doi: 10.1111/j.1365-2222.1996.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 5.Charlesworth E N. The role of basophils and mast cells in acute and late reactions in the skin. Allergy. 1997;52:31–43. doi: 10.1111/j.1398-9995.1997.tb04809.x. [DOI] [PubMed] [Google Scholar]
- 6.Church M K, Levi-Schaffer F. The human mast cell. J Allergy Clin Immunol. 1997;99:155–160. doi: 10.1016/s0091-6749(97)70089-7. [DOI] [PubMed] [Google Scholar]
- 7.Echtenacher B, Mannel D N, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 8.El-Lati S G, Dahinden C A, Church M K. Complement peptides C3a- and C5a-induced mediator release from dissociated human skin mast cells. J Invest Dermatol. 1994;102:803–806. doi: 10.1111/1523-1747.ep12378589. [DOI] [PubMed] [Google Scholar]
- 9.Erdei A, Kerekes K, Pecht I. Role of C3a and C5a in the activation of mast cells. Exp Clin Immunogenet. 1997;14:16–18. [PubMed] [Google Scholar]
- 10.Fodinger M, Fritsch G, Winkler K, Emminger W, Mitterbauer G, Gadner H, Valent P, Mannhalter C. Origin of human mast cells: development from transplanted hematopoietic stem cells after allogeneic bone marrow transplantation. Blood. 1994;84:2954–2959. [PubMed] [Google Scholar]
- 11.Fodinger M, Mannhalter C. Molecular genetics and development of mast cells: implications for molecular medicine. Mol Med Today. 1997;3:131–137. doi: 10.1016/S1357-4310(96)10061-7. [DOI] [PubMed] [Google Scholar]
- 12.Galli S J, Costa J J. Mast-cell-leukocyte cytokine cascades in allergic inflammation. Allergy. 1995;50:851–862. doi: 10.1111/j.1398-9995.1995.tb02490.x. [DOI] [PubMed] [Google Scholar]
- 13.Galli S J. New approaches for the analysis of mast cell maturation, heterogeneity, and function. Fed Proc. 1987;46:1906–1914. [PubMed] [Google Scholar]
- 14.Galli S J, Gordon J R, Wershil B K. Cytokine production by mast cells and basophils. Curr Opin Immunol. 1991;3:865–872. doi: 10.1016/s0952-7915(05)80005-6. [DOI] [PubMed] [Google Scholar]
- 15.Galli S J, Wershil B K. The two faces of the mast cell. Nature. 1996;381:21–22. doi: 10.1038/381021a0. [DOI] [PubMed] [Google Scholar]
- 16.Gordon J R, Galli S J. Release of both preformed and newly synthesized tumor necrosis factor alpha (TNF-alpha)/cachectin by mouse mast cells stimulated via the Fc epsilon R1. A mechanism for the sustained action of mast cell-derived TNF-alpha during IgE-dependent biological responses. J Exp Med. 1991;174:103–107. doi: 10.1084/jem.174.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He D, Esquenazi-Behar S, Soter N A, Lim H W. Mast-cell heterogeneity: functional comparison of purified mouse cutaneous and peritoneal mast cells. Invest Dermatol. 1990;95:178–185. doi: 10.1111/1523-1747.ep12477951. [DOI] [PubMed] [Google Scholar]
- 18.Irani A M, Schwartz L B. Human mast cell heterogeneity. Allergy Proc. 1994;15:303–308. doi: 10.2500/108854194778816472. [DOI] [PubMed] [Google Scholar]
- 19.Leal-Berumen I, Conlon P, Marshall J S. IL-6 production by rat peritoneal mast cells is not necessarily preceded by histamine release and can be induced by bacterial lipopolysaccharide. J Immunol. 1994;152:5468–5476. [PubMed] [Google Scholar]
- 20.Leal-Berumen I, Snider D P, Barajas-Lopez C, Marshall J S. Cholera toxin increases IL-6 synthesis and decreases TNF-alpha production by rat peritoneal mast cells. J Immunol. 1996;156:316–321. [PubMed] [Google Scholar]
- 21.Malaviya R, Ross E A, Jakschik B A, Abraham S N. Mast cell degranulation induced by type 1 fimbriated Escherichia coli in mice. J Clin Invest. 1994;93:1645–1653. doi: 10.1172/JCI117146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malaviya R, Ross E A, MacGregor J I, Ikeda T, Little J R, Jakschik B A, Abraham S N. Mast cell phagocytosis of FimH-expressing enterobacteria. J Immunol. 1994;152:1907–1914. [PubMed] [Google Scholar]
- 23.Malaviya R, Ikeda T, Ross E A, Abraham S N. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 23a.Malaviya, R., et al. Unpublished results.
- 24.Mécheri S, David B. Unravelling the mast cell dilemma: culprit or victim of its generosity. Immunol Today. 1997;18:212–215. doi: 10.1016/s0167-5699(97)01034-7. [DOI] [PubMed] [Google Scholar]
- 25.Norn S. Microorganisms and mediator release: new aspects in airway diseases. Agents Actions. 1992;36:57–77. [PubMed] [Google Scholar]
- 26.Norn S, Jensen C, Dahl B T, Stahl Skov P, Baek L, Permin H, Jarlov J O, Sorensen H. Endotoxins release histamine by complement activation and potentiate bacteria-induced histamine release. Agents Actions. 1986;18:149–152. doi: 10.1007/BF01988007. [DOI] [PubMed] [Google Scholar]
- 27.Prodeus A P, Zhou X, Maurer M, Galli S J, Carroll M C. Impaired mast cell-dependent natural immunity in complement C3-deficient mice. Nature. 1997;390:172–175. doi: 10.1038/36586. [DOI] [PubMed] [Google Scholar]
- 28.Rottem M, Okada T, Goff J P, Metcalfe D D. Mast cells cultured from the peripheral blood of normal donors and patients with mastocytosis originate from a CD34+/Fc epsilon RI-cell population. Blood. 1994;84:2489–2496. [PubMed] [Google Scholar]
- 29.Saito H, Ebisawa M, Tachimoto H, Shichijo M, Fukagawa K, Matsumoto K, Iikura Y, Awaji T, Tsujimoto G, Yanagida M, Uzumaki H, Takahashi G, Tsuji K, Nakahata T. Selective growth of human mast cells induced by Steel factor, IL-6, and prostaglandin E2 from cord blood mononuclear cells. J Immunol. 1996;157:343–350. [PubMed] [Google Scholar]
- 30.Sher A, Hein A, Moser G, Caulfield J P. Complement receptors promote the phagocytosis of bacteria by rat peritoneal mast cells. Lab Invest. 1979;41:490–499. [PubMed] [Google Scholar]
- 31.Toru H, Eguchima M, Tsumoto R, Yanagida M, Yata J, Nakahata T. Interleukin-4 promotes the development of tryptase and chymase double-positive human mast cells accompanied by cell maturation. Blood. 1998;91:187–195. [PubMed] [Google Scholar]
- 32.Valent P. Cytokines involved in growth and differentiation of human basophils and mast cells. Exp Dermatol. 1995;4:255–259. doi: 10.1111/j.1600-0625.1995.tb00254.x. [DOI] [PubMed] [Google Scholar]
- 33.Weidner N, Austen K F. Heterogeneity of mast cells at multiple body sites. Fluorescent determination of avidin binding and immunofluorescent determination of chymase, tryptase, and carboxypeptidase content. Pathol Res Pract. 1993;189:156–162. doi: 10.1016/S0344-0338(11)80086-5. [DOI] [PubMed] [Google Scholar]
- 34.Welle M. Development, significance, and heterogeneity of mast cells with particular regard to the mast cell-specific proteases chymase and tryptase. J Leukoc Biol. 1997;61:233–245. doi: 10.1002/jlb.61.3.233. [DOI] [PubMed] [Google Scholar]