Key summary points
Aim
To investigate the correlations of sarcopenia and its components with peak expiratory flow (PEF) among Chinese community-dwelling elderly people.
Findings
The baseline sarcopenia status was related to PEF and PEF decline in Chinese community-dwelling elderly population by cross-sectional and longitudinal analysis. Also, the changes in physical performance were associated with changes in PEF during a 4-year follow-up.
Message
Improving sarcopenia, especially physical performance may contribute to the increase of PEF.
Supplementary Information
The online version contains supplementary material available at 10.1007/s41999-023-00838-2.
Keywords: Sarcopenia, Skeletal muscle mass, Hand grip strength, Physical performance, Peak expiratory flow, Community-dwelling elderly
Abstract
Purpose
To cross-sectionally and longitudinally investigate the correlations of sarcopenia and its components with peak expiratory flow (PEF) among Chinese community-dwelling elderly people.
Methods
The data were extracted from the China Health and Retirement Longitudinal Study (CHARLS). A total of 4053 participants aged ≥ 60 years were enrolled from CHARLS 2011, and 2810 were followed up until 2015. Participants were classified into no-sarcopenia, non-severe sarcopenia, and severe sarcopenia groups based on skeletal muscle mass index (SMI), hand grip strength (HGS), and physical performance [gait speed, five-repetition chair stand test (5CST) and short physical performance battery (SPPB)]. Multivariate linear and logistic regression analyses were used to evaluate the associations of sarcopenia and its components with PEF cross-sectionally and longitudinally.
Results
In the cross-sectional analysis, the prevalence of non-severe sarcopenia was 14.6% and severe sarcopenia was 4.9%. The results of linear regression analysis revealed that sarcopenia and its components were all correlated with PEF and PEF%pred. In the longitudinal analysis, compared with non-sarcopenia, subjects with severe sarcopenia were associated with a higher risk of PEF (OR = 2.05, 95%CI = 1.30–3.26) and PEF%pred (OR = 1.83, 95%CI = 1.17–2.86) decline. The changes in physical performance were correlated with changes in PEF and PEF%pred. No associations were observed between changes in SMI and PEF as well as PEF%pred.
Conclusions
We demonstrated the associations of baseline sarcopenia status with PEF and longitudinal PEF decline. Also, the changes in physical performance were associated with changes in PEF during a 4-year follow-up. It indicates that improving sarcopenia, especially physical performance may increase PEF.
Supplementary Information
The online version contains supplementary material available at 10.1007/s41999-023-00838-2.
Introduction
Sarcopenia is a geriatric syndrome characterized by loss of skeletal muscle mass plus loss of muscle strength and/or reduced physical performance [1]. According to the 2019 consensus of the Asian Working Group for Sarcopenia (AWGS), the prevalence of sarcopenia in Asia ranged from 5.5 to 25.7% [2]. In China, a recent meta revealed that the prevalence of sarcopenia in community-dwelling older adults was 12.9% in men and 11.2% in women [3]. What is more, the prevalence of sarcopenia increases with age. Sarcopenia in the elderly was usually occult in onset but could result in physical dysfunction. Previous studies have demonstrated that sarcopenia is related to various adverse events, including cardiovascular diseases, falls, disability, mortality, and so on [4–6].
Similarly to systemic skeletal muscle, aging could cause loss of respiratory muscle mass and respiratory muscle strength, and/or decline of pulmonary function, which is known as respiratory sarcopenia [7]. It is reported that low skeletal muscle mass and sarcopenia were related to poor pulmonary function in chronic obstructive pulmonary disease (COPD) [8]. A systematic review including twenty-three studies involving 9637 participants with COPD revealed that people with sarcopenia had lower predicted forced expiratory volume in the first second (FEV1) and poorer exercise tolerance and quality of life compared with those who did not [9]. A few cross-sectional studies also investigated the relationship between sarcopenia and pulmonary function in general adults without clinically apparent lung diseases, and had come to a similar conclusion [10–12]. Moreover, peripheral muscle strength such as hand grip strength may affect respiratory muscle strength [13].
The peak expiratory flow (PEF), a simple screening tool of pulmonary function, refers to the instantaneous velocity of expiratory flow when it is the fastest in the process of forced spirometry, and reflects the strength of the respiratory muscle [14]. Recently, some scholars proposed that among community-dwelling older people, the definition of respiratory sarcopenia based on peak expiratory flow was useful and correlated with conventional sarcopenia and long-term care insurance certification [15]. However, to our knowledge, few studies have investigated the correlation of sarcopenia and its components with PEF based on longitudinal studies.
Therefore, in this study, using data from the China Health and Retirement Longitudinal Study (CHARLS), we conducted a cross-sectional analysis to evaluate the association of sarcopenia with pulmonary function, as assessed via PEF. We also longitudinally examined the associations of the change trajectories between sarcopenic components with PEF over a 4-year follow-up among Chinese community-dwelling elderly people.
Methods
Study design and population
The data of this study were extracted from the CHARLS, an ongoing nationally representative longitudinal cohort survey targeting the Chinese population aged 45 years and older. Detailed information about the CHARLS design and methods has been previously described in detail [16, 17]. In brief, the CHARLS project conducted a national baseline survey in 2011–2012 by multistage probability-proportionate-to-size sampling technology and was followed up consecutively through face-to-face computer-assisted personal interviews. The baseline survey recruited 17,708 participants from 450 villages/residents and 150 counties/districts within 28 provinces in China. The follow-up surveys were conducted in 2013, 2015, and 2018. The CHARLS was approved by the Institutional Review Board of Peking University (IRB00001052-11015) and informed consent was obtained from all subjects.
In this study, we obtained the baseline data from CHARLS 2011 and the followed-up information from 2015. The exclusion criteria at baseline were as follows: (1) no information of sarcopenia; (2) aged less than 60 years old; (3) individuals with a self-reported history of chronic lung diseases or asthma; (4) lack of PEF or not trying hard enough; and (5) missing data of height or weight or the type of cooking fuel. Finally, 4053 subjects were included in the baseline survey. During the follow-up wave, 1243 individuals lacked information of sarcopenia or PEF, or lost in 2015, leaving 2810 eligible participants for the final longitudinal analysis. The detailed flowchart of this current study is shown in Fig. 1.
Fig. 1.
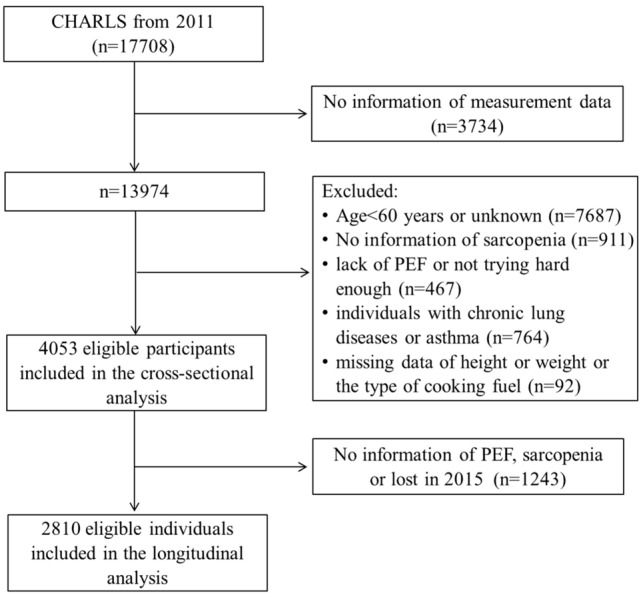
The detailed flowchart of this study
Covariates
The socio-demographic and health-related information on age, sex, educational level, smoking and drinking status, height, body weight (BW), marital status (married or widowed/divorced/single), residential area (urban or rural), type of cooking fuels, and self-reported comorbid diseases including hypertension, diabetes, heart problems, stroke, and kidney disease of all participants was collected by well-trained investigators through face-to-face interviews. The body mass index (BMI) was calculated by dividing the BW (in kilograms) by the height (in meters) squared. The education level was divided into elementary school or below, secondary school, and college or above. The smoking status was divided into never smokers, former smokers, and current smokers. The drinking was defined as drinking in the last year. Clean fuel for household cooking was defined as natural gas, marsh gas, liquefied petroleum gas, and electric, while solid cooking fuel referred to coal and crop residue/wood burning.
Assessment of sarcopenia
The details about the definitions for sarcopenia components in the CHARLS have been described previously [18, 19]. The skeletal muscle mass was estimated by the appendicular skeletal muscle mass (ASM), which was calculated with a previously validated anthropometric equation in Chinese adults [20].
Of this equation, the BW, height, and age were measured in kilograms, centimeters, and years, respectively. For sex, males were assigned the value of 1, and females were assigned 2. The skeletal muscle mass index (SMI) was calculated by ASM/height2. The cut-off value for defining low muscle mass was based on the sex-specific lowest 20% of the SMI among the study population [18, 21], with < 6.85 kg/m2 in men and < 4.98 kg/m2 in women.
Hand grip strength (HGS) was measured using the right or left hand with a dynamometer (Yuejian™ WL-1000, Nantong, China) in kilograms at a right angle (90˚) for a few seconds as hard as possible. Each hand was tested twice and the maximum value of the four attempts was extracted for the next analysis [22]. The cut-off points for low grip strength were defined as < 28.0 kg for men and < 18.0 kg for women.
Gait speed was calculated by the time to complete a 2.5-m course at a normal pace. The average of available values twice was used for statistical analysis. The five-repetition chair stand test (5CST) was assessed by the time taken to stand from a chair five times in seconds as quickly as possible [19]. Besides gait speed and 5CST, the assessment of short physical performance battery (SPPB) also included the balance tests including semi-tandem stands, full-tandem stands, and side-by-side stands. The summary score of SPPB was 12 points with 4 points for each physical performance measurement [23]. AWGS 2019 recommends defining low physical performance based on either SPPB ≤ 9, gait speed < 1 m/s, or 5CST ≥ 12 s.
Sarcopenia is defined as low muscle mass plus low muscle strength or low physical performance. Subjects with low muscle mass, low muscle strength, and low physical performance are considered severe sarcopenia.
Ascertainment of PEF
PEF was measured using a peak flow meter (Everpure ™, Shanghai, China). The participants were asked to take a deep breath and place their lips around the mouthpiece, and then blow as hard and fast as possible. The process was repeated three times at 30-s intervals and the highest value was used in the next analysis.
The predicted PEF was calculated with previously validated equations by Zhong Nanshan in Chinese adults: 75.6 + 20.4 × age−0.41 × age2 + 0.002 × age3 + 1.19 × height for males and 282.0 + 1.79 × age−0.046 × age2 + 0.68 × height for females. Of the equations, age and height were measured in years and centimeters, respectively. The percentage of actual value to predicted PEF was expressed as PEF%pred. PEF%pred < 80% was defined as airflow limitation [24].
Statistical analysis
All continuous variables of the study were shown as mean ± standard deviation. Statistical analysis of continuous variables was performed using an unpaired Student t-test between two groups and one-way analysis of variance followed by a least-squares difference test for multiple comparisons. Categorical variables were expressed as frequency and percentages, and performed by chi-square test. Multivariate linear regression analysis with enter method was performed to evaluate the cross-sectional associations of sarcopenia and its components with PEF, as well as the associations of the change trajectories between sarcopenic components with PEF from 2011 to 2015, respectively. Multivariate logistic regression analysis with enter method was used to investigate the associations between airflow limitation and sarcopenia, including its defining components. The same method was used to explore the incidence of PEF or PEF%pred decline according to baseline sarcopenia status in the longitudinal analysis. Three adjusted models were used in the multivariate linear and logistic regression analysis: model 1 unadjusted; model 2 adjusted for age and sex; model 3 adjusted for model 2 plus education level, smoking status, drinking status, BMI, marital status, residential area, type of cooking fuels, and comorbid diseases. In the longitudinal analysis, baseline PEF or PEF%pred was also added to model 3. The regression coefficients (β) and 95% confidence intervals (CI) were expressed by linear regression analysis, while odds ratios (OR) and 95% CI were computed by logistic regression analysis.
All tests were carried out using SPSS version 22.0 statistical software. Differences were considered statistically significant at a two-tailed P value < 0.05.
Results
Baseline characteristics of subjects
The baseline characteristics of enrolled subjects are listed in Table 1. A total of 4053 participants were included in the cross-sectional analysis. The mean age was 67.39 years old, and 50.4% of participants were men. Among these 4053 elderly, the prevalence of sarcopenia was 19.5%, of which, non-severe sarcopenia was 14.6% and severe sarcopenia was 4.9%. Compared to non-sarcopenia, the participants with sarcopenia were more likely to be older and single, live in rural, had a lower level of education, smoke, and use solid fuels for household cooking (all P < 0.01). The values of PEF and PEF%pred were significantly lower, while the proportion of airflow limitation was higher in sarcopenic subjects than in non-sarcopenia, especially in the severe sarcopenia group (all P < 0.01).
Table 1.
Baseline characteristics of enrolled subjects
| Total (n = 4053) | Non-sarcopenia (n = 3263) | Non-severe sarcopenia (n = 592) | Severe sarcopenia (n = 198) | P value | |
|---|---|---|---|---|---|
| Age (years) | 67.39 ± 6.25 | 66.44 ± 5.63 | 70.53 ± 6.98 | 73.72 ± 6.89 | < 0.001 |
| Gender, n (%) | 0.077 | ||||
| Male | 2042 (50.4) | 1646 (50.4) | 283 (47.8) | 113 (57.1) | |
| Female | 2011 (49.6) | 1617 (49.6) | 309 (52.2) | 85 (42.9) | |
| Marital status, n (%) | < 0.001 | ||||
| Married | 3184 (78.6) | 2639 (80.9) | 426 (72.0) | 119 (60.1) | |
| Widowed/divorced/single | 869 (21.4) | 624 (19.1) | 166 (28.0) | 79 (39.9) | |
| Residential area, n (%) | < 0.001 | ||||
| Urban | 790 (19.5) | 725 (22.2) | 51 (8.6) | 14 (7.1) | |
| Rural | 3263 (80.5) | 2538 (77.8) | 541 (91.4) | 184 (92.9) | |
| Education level, n (%) | < 0.001 | ||||
| Elementary school or below | 3308 (81.6) | 2597 (79.6) | 524 (88.5) | 187 (94.4) | |
| Secondary school | 674 (16.6) | 600 (18.4) | 65 (11.0) | 9 (4.6) | |
| College and above | 71 (1.8) | 66 (2.0) | 3 (0.5) | 2 (1.0) | |
| Smoking status | < 0.001 | ||||
| Never | 2375 (58.6) | 1955 (59.9) | 319 (53.9) | 101 (51.0) | |
| Former | 404 (10.0) | 339 (10.4) | 44 (7.4) | 21 (10.6) | |
| Current | 1274 (31.4) | 969 (29.7) | 229 (38.7) | 76 (38.4) | |
| Current drinking | 0.879 | ||||
| No | 2750 (67.9) | 2209 (67.7) | 407 (68.7) | 134 (67.7) | |
| Yes | 1303 (32.1) | 1054 (32.3) | 185 (31.3) | 64 (32.9) | |
| Type of cooking fuels | < 0.001 | ||||
| Clean | 1589 (39.2) | 1369 (42.0) | 167 (28.2) | 53 (26.8) | |
| Solid | 2464 (60.8) | 1894 (58.0) | 425 (71.8) | 145 (73.2) | |
| Height (cm) | 156.74 ± 8.73 | 157.48 ± 8.33 | 154.16 ± 9.69 | 152.19 ± 9.34 | < 0.001 |
| Weight (kg) | 56.60 ± 10.96 | 59.67 ± 9.62 | 44.20 ± 5.91 | 43.03 ± 5.58 | < 0.001 |
| BMI (kg/m2) | 22.96 ± 3.61 | 24.02 ± 3.13 | 18.56 ± 1.50 | 18.57 ± 1.70 | < 0.001 |
| Hypertension, n (%) | 1238 (30.5) | 1098 (33.7) | 101 (17.1) | 39 (19.7) | < 0.001 |
| Diabetes or high blood sugar, n (%) | 265 (6.5) | 245 (7.5) | 13 (2.2) | 7 (3.5) | < 0.001 |
| Heart problems, n (%) | 546 (13.5) | 471 (14.4) | 53 (9.0) | 22 (11.1) | 0.001 |
| Stroke, n (%) | 109 (2.7) | 95 (2.9) | 8 (1.4) | 6 (3.0) | 0.095 |
| Kidney disease, n (%) | 228 (5.6) | 191 (5.9) | 26 (4.4) | 11 (5.6) | 0.389 |
| HGS (kg) | 30.18 ± 9.43 | 31.02 ± 9.50 | 29.35 ± 7.26 | 18.85 ± 5.80 | < 0.001 |
| Gait speed (m/s) | 0.64 ± 0.23 | 0.66 ± 0.23 | 0.59 ± 0.21 | 0.52 ± 0.19 | < 0.001 |
| 5CST (s) | 11.66 ± 4.76 | 11.52 ± 4.84 | 11.64 ± 3.60 | 14.04 ± 5.87 | < 0.001 |
| SPPB | 9.60 ± 1.97 | 9.73 ± 1.94 | 9.37 ± 1.85 | 8.17 ± 2.23 | < 0.001 |
| ASM (kg) | 16.35 ± 4.16 | 17.06 ± 3.92 | 13.46 ± 3.91 | 13.29 ± 3.66 | < 0.001 |
| SMI (kg/m2) | 6.55 ± 1.14 | 6.79 ± 1.03 | 5.54 ± 1.04 | 5.63 ± 1.05 | < 0.001 |
| PEF (L/min) | 273.29 ± 117.03 | 283.27 ± 117.29 | 241.76 ± 106.61 | 203.05 ± 101.01 | < 0.001 |
| PEF%pred | 79.11 ± 30.00 | 80.54 ± 29.66 | 75.60 ± 30.45 | 65.99 ± 30.22 | < 0.001 |
| Airflow limitation, n (%) | 2089 (51.5) | 1608 (49.3) | 350 (59.1) | 131 (66.2) | < 0.001 |
BMI body mass index, HGS hand grip strength, 5CST five-repetition chair stand test, SPPB short physical performance battery, ASM appendicular skeletal muscle mass, SMI skeletal muscle mass index, PEF peak expiratory flow
Cross-sectional associations of sarcopenia with PEF
Table 2 shows the associations of sarcopenia and its components with PEF and PEF%pred by linear regression analysis. After adjusting for covariates in model 3, we found that SMI, HGS, gait speed, and SPPB were positively correlated with PEF and PEF%pred, while 5CST and sarcopenia were negatively correlated with PEF and PEF%pred, respectively (all P < 0.01).
Table 2.
Associations of sarcopenia and its components with PEF and PEF%pred in the cross-sectional analysis
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| β (95%CI) | P value | β (95%CI) | P value | β (95%CI) | P value | |
| PEF | ||||||
| HGS | 5.68 (5.34, 6.02) | < 0.001 | 3.58 (3.15, 4.01) | < 0.001 | 3.13 (2.68, 3.57) | < 0.001 |
| Gait speed | 116.40 (101.10, 131.70) | < 0.001 | 68.51 (54.22, 82.80) | < 0.001 | 61.52 (47.25, 75.80) | < 0.001 |
| 5CST | − 5.85 (− 6.59, − 5.10) | < 0.001 | − 3.30 (− 4.00, − 2.59) | < 0.001 | − 3.07 (− 3.77, − 2.37) | < 0.001 |
| SPPB | 19.06 (17.33, 20.79) | < 0.001 | 11.53 (9.81, 13.25) | < 0.001 | 10.66 (8.91, 12.40) | < 0.001 |
| SMI | 42.27 (39.39, 45.16) | < 0.001 | 18.26 (13.77, 22.74) | < 0.001 | 69.65 (47.21, 92.08) | < 0.001 |
| Sarcopenia | − 40.66 (− 47.33, − 33.99) | < 0.001 | − 26.74 (− 33.08, − 20.40) | < 0.001 | − 19.15 (− 26.69, − 11.62) | < 0.001 |
| PEF%pred | ||||||
| HGS | 0.65 (0.55, 0.74) | < 0.001 | 0.96 (0.84, 1.09) | < 0.001 | 0.84 (0.71, 0.97) | < 0.001 |
| Gait speed | 18.29 (14.30, 22.28) | < 0.001 | 19.37 (15.27, 23.48) | < 0.001 | 17.51 (13.40, 21.62) | < 0.001 |
| 5CST | − 0.93 (− 1.12,− 0.73) | < 0.001 | − 1.00 (− 1.21, − 0.80) | < 0.001 | − 0.95 (− 1.15, − 0.75) | < 0.001 |
| SPPB | 2.98 (2.52, 3.44) | < 0.001 | 3.46 (2.97, 3.96) | < 0.001 | 3.24 (2.74, 3.74) | < 0.001 |
| SMI | 3.03 (2.22, 3.83) | < 0.001 | 5.24 (3.96, 6.53) | < 0.001 | 22.95 (16.50, 29.39) | < 0.001 |
| Sarcopenia | − 6.36 (− 8.09, − 4.63) | < 0.001 | − 7.82 (− 9.64, − 6.00) | < 0.001 | − 5.82 (− 7.98, − 3.65) | < 0.001 |
HGS hand grip strength, 5CST five-repetition chair stand test, SPPB short physical performance battery, SMI skeletal muscle mass index, PEF peak expiratory flow
Model 1, unadjust
Model 2, adjust for age and sex
Model 3, adjust for age, sex, education level, smoking status, drinking status, BMI, marital status, residential area, type of cooking fuels, hypertension, diabetes, heart problems, stroke, and kidney disease
We also explored the associations of sarcopenia and its components with airflow limitation by logistic regression analysis (Table S1). After adjusting for covariates in model 3, we found that compared with the non-sarcopenia group, subjects in the severe sarcopenia group were associated with a higher risk of airflow limitation (OR = 1.66, 95% CI = 1.18–2.33, P = 0.004). Also, higher SMI, HGS, gait speed, and SPPB were significantly associated with lower odds of airflow limitation, while the opposite pattern was found between 5CST and airflow limitation (all P < 0.01).
Associations between baseline sarcopenia status and 4-year PEF decline
Figure 2 shows the changes in PEF and PEF%pred according to baseline sarcopenia status from 2011 to 2015. We found that the changes in PEF and PEF%pred were both significantly different in the non-sarcopenia and sarcopenia groups (P < 0.05).
Fig. 2.
Changes in PEF (A) and PEF%pred (B) according to sarcopenia status from 2011 to 2015. Data are shown as mean ± standard deviation
Table S2 shows the associations of the baseline sarcopenia status and its components with PEF and PEF%pred changes by linear regression analysis. After adjusting for covariates, we found that the baseline SMI, HGS, gait speed, and SPPB were positively correlated with PEF and PEF%pred changes, while sarcopenia was negatively correlated with PEF and PEF%pred changes (P < 0.05).
Table 3 presents the incidence of PEF or PEF%pred decline according to baseline sarcopenia status from 2011 to 2015. After adjusting for covariates in model 3, the results revealed that compared with the non-sarcopenia group, subjects in the severe sarcopenia group were both associated with a higher risk of PEF decline (OR = 2.05, 95%CI = 1.30–3.26, P = 0.002) and PEF%pred decline (OR = 1.83, 95%CI = 1.17–2.86, P = 0.008).
Table 3.
Incidence of PEF or PEFpred% decline according to baseline sarcopenia status from 2011 to 2015
| No. of cases/total | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | ||||
| PEF decline | |||||||||
| No sarcopenia | 1053/2299 | Ref | Ref | Ref | |||||
| Non-severe sarcopenia | 193/406 | 1.06 (0.86, 1.31) | 0.568 | 0.98 (0.79, 1.22) | 0.846 | 1.04 (0.78, 1.39) | 0.800 | ||
| Severe sarcopenia | 65/115 | 1.53 (1.05, 2.23) | 0.028 | 1.32 (0.89, 1.95) | 0.165 | 2.05 (1.30, 3.26) | 0.002 | ||
| PEF%pred decline | |||||||||
| No sarcopenia | 883/2289 | Ref | Ref | Ref | |||||
| Non-severe sarcopenia | 167/406 | 1.11 (0.90, 1.38) | 0.330 | 1.03 (0.83, 1.29) | 0.790 | 0.96 (0.72, 1.28) | 0.759 | ||
| Severe sarcopenia | 58/115 | 1.62 (1.11, 2.36) | 0.012 | 1.43 (0.97, 2.11) | 0.070 | 1.83 (1.17, 2.86) | 0.008 | ||
Model 1, unadjust
Model 2, adjust for age and sex
Model 3, adjust for age, sex, education level, smoking status, drinking status, BMI, marital status, residential area, type of cooking fuels, baseline PEF or PEF%pred, hypertension, diabetes, heart problems, stroke, and kidney disease
Associations of the change trajectories between sarcopenic components and PEF from 2011 to 2015
Table 4 shows the associations of the change trajectories between sarcopenic components with PEF or PEF%pred from 2011 to 2015. After adjusting for covariates in model 3, we found that the change trajectories of physical performance including gait speed, 5CST, and SPPB were correlated with PEF and PEF%pred (all P < 0.01). The change in HGS was only significantly correlated with PEF%pred (P = 0.030), not with PEF (P = 0.056). No associations were observed between changes in SMI and PEF as well as PEF%pred.
Table 4.
Associations of the change trajectories between sarcopenic components with PEF or PEFpred% from 2011 to 2015
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| β (95%CI) | P value | β (95%CI) | P value | β (95%CI) | P value | |
| PEF change | ||||||
| HGS change | 0.42 (− 0.10, 0.93) | 0.112 | 0.36 (− 0.16, 0.87) | 0.176 | 0.41 (− 0.01, 0.82) | 0.056 |
| Gait speed change | 17.80 (3.19, 32.40) | 0.017 | 13.01 (− 1.75, 27.76) | 0.084 | 24.96 (12.98, 36.94) | < 0.001 |
| 5CST change | − 2.32 (− 3.22, − 1.41) | < 0.001 | − 2.16 (− 3.07, − 1.25) | < 0.001 | − 1.92 (− 2.66, − 1.19) | < 0.001 |
| SPPB change | 4.78 (2.90, 6.65) | < 0.001 | 4.19 (2.30, 6.08) | < 0.001 | 3.66 (2.13, 5.19) | < 0.001 |
| SMI change | − 9.12 (− 20.59, 2.35) | 0.119 | − 10.19 (− 21.64, 1.27) | 0.081 | 2.22 (− 7.29, 11.74) | 0.647 |
| PEFpred% change | ||||||
| HGS change | 0.14 (− 0.01, 0.30) | 0.070 | 0.13 (− 0.02, 0.29) | 0.093 | 0.15 (0.02, 0.28) | 0.030 |
| Gait speed change | 5.35 (0.96, 9.74) | 0.017 | 4.27 (− 0.17, 8.71) | 0.060 | 7.62 (3.85, 11.39) | < 0.001 |
| 5CST change | − 0.71 (− 0.98, − 0.44) | < 0.001 | − 0.68 (− 0.95,− 0.41) | < 0.001 | − 0.61 (− 0.84, − 0.38) | < 0.001 |
| SPPB score change | 1.42 (0.86, 1.98) | < 0.001 | 1.30 (0.74, 1.87) | < 0.001 | 1.16 (0.68, 1.65) | < 0.001 |
| SMI change | − 2.35 (− 5.80, 1.10) | 0.182 | − 2.67 (− 6.12, 0.78) | 0.130 | 1.07 (− 1.92, 4.07) | 0.482 |
HGS hand grip strength, 5CST five-repetition chair stand test, SPPB short physical performance battery, SMI skeletal muscle mass index, PEF peak expiratory flow
Model 1, unadjust
Model 2, adjust for age and sex
Model 3, adjust for age, sex, education level, smoking status, drinking status, BMI, marital status, residential area, type of cooking fuels, baseline PEF or PEF%pred, hypertension, diabetes, heart problems, stroke, and kidney disease
Discussion
In this study, we evaluated the cross-sectional and longitudinal associations of sarcopenia and its components with pulmonary function, as assessed via PEF and PEF%pred among Chinese community-dwelling elderly people. We demonstrated that sarcopenia, skeletal muscle mass, grip strength, and physical performance were all correlated with PEF, PEF%pred, and risk of airflow limitation in the cross-sectional analysis. We also found that the individuals with severe sarcopenia were at a higher risk of incident PEF or PEF%pred decline during a 4-year follow-up. In addition, the changes in physical performance were associated with changes in PEF and PEF%pred in the longitudinal analysis. To the best of our knowledge, this is the first longitudinal study to investigate the associations of the change trajectories between sarcopenic components and PEF among Chinese community-dwelling elderly people.
Previous research on associations between sarcopenia and pulmonary function was mainly based on forced vital capacity (FVC) and FEV1 in COPD patients [8, 25, 26]. PEF is a cheap and simple screening tool for pulmonary function. According to the 2010 consensus by the European Working Group on Sarcopenia in older people, PEF is determined by the strength of respiratory muscles in people without lung diseases [1]. It can be used as an indicator of respiratory sarcopenia [15]. Recently, an increasing number of studies explored the associations between PEF and sarcopenia [27] as well as its defining components. A previous cross-sectional study including 240,562 Korean adults without clinical lung diseases demonstrated the association between PEF and skeletal muscle mass [11]. Another study focused on community-dwelling older adults reported that HGS and gait speed were more strongly associated with PEF than skeletal muscle mass [28]. In this present cross-sectional study, we added new evidence that SMI, HGS, and gait speed were associated with PEF, PEF%pred, and risk of airflow limitation.
The 5CST and SPPB are important parts of sarcopenia. It was reported that 5CTS and SPPB were responsive to pulmonary rehabilitation in patients with COPD [29]. Some previous cross-sectional studies found that abnormal SPPB was associated with abnormal pulmonary function, as assessed via FEV1 or FVC, in both COPD [30] and the aging population [31]. It is also reported by Charles et al. that compared to elderly subjects with normal PEF, subjects with reduced PEF had a lower SPPB [32]. However, the relationship between 5CST and pulmonary function remains controversial. Choi et al. found that 5CST did not exhibit a linear relationship with FEV1 and FVC [31], while Landi et al. proved that FVC, FEV1, and PEF were all linearly correlated with 5CST [33]. In our study, we provided more evidence about the relationship between SPPB and PEF, as well as 5CST and PEF.
To our knowledge, few studies have investigated the correlation of sarcopenia and its components with PEF based on longitudinal studies. Recently, a report showed that the baseline paraspinal muscle density predicted FEV1%pred decline among women with asthma [34]. In this study, we longitudinally investigate the relationship of baseline sarcopenia status with the decline of PEF during a 4-year follow-up. We found that the changes of PEF and PEF%pred were both significantly different in the non-sarcopenia and sarcopenia groups. However, just as mentioned in a previous study [35], the increase of PEF and PEF%pred during a 4-year follow-up seemed irrational in the elderly population even in the non-sarcopenia group. The reason for this increase may be associated with improved air quality and a healthy community lifestyle. The results also revealed the baseline sarcopenia status and its components including SMI, HGS, gait speed, and SPPB were all associated with PEF and PEF%pred changes, respectively. Compared with the non-sarcopenia group, subjects in the severe sarcopenia group were associated with a 2.05-times risk of PEF decline and a 1.83-times risk of PEF%pred decline.
In addition, skeletal muscle mass, grip strength, and physical performance can be changed over time. Therefore, it is necessary to examine the associations of the change trajectories between sarcopenic components with PEF. A previous longitudinal study found that an increase in skeletal muscle mass was associated with attenuated FEV1 decline in healthy men by the Pearson’s methods. Furthermore, it is proved that even prominent loss of muscle mass over time is associated with attenuated FEV1 decline in men unless it is combined with prominent gain of fat mass. The results weakened the association between muscle mass changes over time and pulmonary function change [36]. In our study, we found that the change trajectories of physical performance including gait speed, 5CST, and SPPB were associated with changes in PEF and PEF%pred over a 4-year follow-up. However, SMI did not exhibit this relationship. These differences may exist due to different populations, pulmonary function parameters, and evaluation methods for muscle mass. Our results indicated that improvement in physical performance is more important than muscle mass. As far as we know, PEF reflects respiratory muscle strength rather than respiratory muscle mass [37]. Additional studies for the relationship between pulmonary function and muscle mass changes over time are warranted.
The strengths of our study are as follows: we focused on a large elderly population from Chinese community-dwelling, and we performed both cross-sectional and longitudinal analyses to evaluate the associations of sarcopenia with PEF. However, some limitations still should be noted in our study. First, according to the 2019 consensus of AWGS, dual-energy X-ray absorptiometry (DXA) and bioelectrical impedance analysis were recommended to measure skeletal muscle mass. However, in our study, skeletal muscle mass was estimated by a previously validated anthropometric equation which may introduce some bias. According to reports in the literature, skeletal muscle mass calculated by this formula was in good agreement with DXA and widely used as a method to quantify sarcopenia [20, 38, 39]. Second, gait speed was calculated by the time to complete a 2.5-m course at a normal pace rather than a standard distance of 6-m. A systematic review including 48 studies showed that among the elderly, the walkway lengths did not produce differences in the recorded gait speed [40]. Finally, even though we have adjusted as many relevant covariates as possible in the multivariate regression analysis, there are still confounding factors that could not be completely ruled out which may contribute to a different outcome.
Conclusions
In conclusion, we demonstrated the associations of sarcopenia with PEF as well as PEF decline in Chinese community-dwelling elderly population by cross-sectional and longitudinal analysis. We also found that changes in physical performance were associated with changes in PEF during a 4-year follow-up. Our findings suggest that improving sarcopenia, especially physical performance may contribute to the increase of PEF.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
This study is supported by the National Key R & D Program of China (2020YFC2006205, 2020YFC2006202, 2020YFC2004803) and Capital General Practice Research Project (12100102).
Data availability
The datasets generated and/or analyzed during the current study are available in the China Health and Retirement Longitudinal Study (CHARLS) repository, http://charls.pku.edu.cn/.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
The CHARLS study was approved by the Ethical Review Committee of Peking University (IRB00001052-11015). All methods were carried out in accordance with relevant guidelines and regulations.
Informed consent
Informed consent was obtained from all subjects.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in older people. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen LK, Woo J, Assantachai P et al (2020) Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 21(3):300–7.e2. 10.1016/j.jamda.2019.12.012 [DOI] [PubMed]
- 3.Chen Z, Li WY, Ho M, et al. The prevalence of sarcopenia in Chinese older adults: meta-analysis and meta-regression. Nutrients. 2021;13(5):1441. doi: 10.3390/nu13051441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damluji AA, Alfaraidhy M, AlHajri N, et al. Sarcopenia and cardiovascular diseases. Circulation. 2023;147(20):1534–1553. doi: 10.1161/circulationaha.123.064071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitamura A, Seino S, Abe T, et al. Sarcopenia: prevalence, associated factors, and the risk of mortality and disability in Japanese older adults. J Cachexia Sarcopenia Muscle. 2021;12(1):30–38. doi: 10.1002/jcsm.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Huang P, Dou Q, et al. Falls among older adults with sarcopenia dwelling in nursing home or community: a meta-analysis. Clin Nutr. 2020;39(1):33–39. doi: 10.1016/j.clnu.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Nagano A, Wakabayashi H, Maeda K, et al. Respiratory sarcopenia and sarcopenic respiratory disability: concepts, diagnosis, and treatment. J Nutr Health Aging. 2021;25(4):507–515. doi: 10.1007/s12603-021-1587-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martínez-Luna N, Orea-Tejeda A, González-Islas D, et al. Association between body composition, sarcopenia and pulmonary function in chronic obstructive pulmonary disease. BMC Pul Med. 2022;22(1):106. doi: 10.1186/s12890-022-01907-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sepúlveda-Loyola W, Osadnik C, Phu S, et al. Diagnosis, prevalence, and clinical impact of sarcopenia in COPD: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2020;11(5):1164–1176. doi: 10.1002/jcsm.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeon YK, Shin MJ, Kim MH, et al. Low pulmonary function is related with a high risk of sarcopenia in community-dwelling older adults: the Korea National Health and Nutrition Examination Survey (KNHANES) 2008–2011. Osteoporos Int. 2015;26(10):2423–2429. doi: 10.1007/s00198-015-3152-8. [DOI] [PubMed] [Google Scholar]
- 11.Park CH, Yi Y, Do JG, et al. Relationship between skeletal muscle mass and lung function in Korean adults without clinically apparent lung disease. Medicine (Baltimore) 2018;97(37):e12281. doi: 10.1097/md.0000000000012281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Komici K, D’Amico F, Verderosa S, et al. Impact of body composition parameters on lung function in athletes. Nutrients. 2022 doi: 10.3390/nu14183844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bahat G, Tufan A, Ozkaya H, et al. Relation between hand grip strength, respiratory muscle strength and spirometric measures in male nursing home residents. Aging Male. 2014;17(3):136–140. doi: 10.3109/13685538.2014.936001. [DOI] [PubMed] [Google Scholar]
- 14.Fragoso CAV, Gill TM. Respiratory impairment and the aging lung: a novel paradigm for assessing pulmonary function. J Gerontol A Biol Sci Med Sci. 2012;67(3):264–275. doi: 10.1093/gerona/glr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kera T, Kawai H, Hirano H, et al. Definition of respiratory sarcopenia with peak expiratory flow rate. J Am Med Dir Assoc. 2019;20(8):1021–1025. doi: 10.1016/j.jamda.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Hu Y, Smith JP, et al. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS) Int J Epidemiol. 2014;43(1):61–68. doi: 10.1093/ije/dys203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu S, Cai X, Xie B, et al. Normalized creatinine-to-cystatin C ratio and risk of diabetes in middle-aged and older adults: the China Health and Retirement Longitudinal Study. Diabetes Metab J. 2022;46(3):476–485. doi: 10.4093/dmj.2021.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao K, Cao LF, Ma WZ, et al. Association between sarcopenia and cardiovascular disease among middle-aged and older adults: findings from the China health and retirement longitudinal study. EClinicalMedicine. 2022;44:101264. doi: 10.1016/j.eclinm.2021.101264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu X, Li X, Xu M, et al. Sarcopenia prevalence and associated factors among older Chinese population: findings from the China Health and Retirement Longitudinal Study. PLoS ONE. 2021;16(3):e0247617. doi: 10.1371/journal.pone.0247617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wen X, Wang M, Jiang C-M, et al. Anthropometric equation for estimation of appendicular skeletal muscle mass in Chinese adults. Asia Pac J Clin Nutr. 2011;20(4):551–556. [PubMed] [Google Scholar]
- 21.Alexandre TdS, Duarte YAdO, Santos JLF, et al. Sarcopenia according to the European Working Group on Sarcopenia in Older People (EWGSOP) versus dynapenia as a risk factor for mortality in the elderly. J Nutr Health Aging. 2014;18(8):751–756. doi: 10.1007/s12603-014-0540-2. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Liu Y, Hu J, et al. Association of handgrip strength with all-cause mortality: a nationally longitudinal cohort study in China. J Sci Med Sport. 2022;25(11):878–883. doi: 10.1016/j.jsams.2022.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Zhong BX, Zhong HL, Zhou GQ, et al. Physical performance and risk of hip fracture in community-dwelling elderly people in China: a 4-year longitudinal cohort study. Maturitas. 2021;146:26–33. doi: 10.1016/j.maturitas.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Pulmonary function professional group of respiratory branch of Chinese medical association Guideline of pulmonary function testing—peak expiratory flow and its variability. Chin J Tubere Respir Dis. 2017;40(6):426–430. [Google Scholar]
- 25.Mansour KMK, Goulart CdL, Carvalho-Junior LCSd, et al. Pulmonary function and functional capacity cut-off point to establish sarcopenia and dynapenia in patients with COPD. J Bras Pneumol. 2019;45(6):e20180252. doi: 10.1590/1806-3713/e20180252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cesari M, Pedone C, Chiurco D, et al. Physical performance, sarcopenia and respiratory function in older patients with chronic obstructive pulmonary disease. Age Ageing. 2012;41(2):237–241. doi: 10.1093/ageing/afr167. [DOI] [PubMed] [Google Scholar]
- 27.Ridwan ES, Wiratama BS, Lin M-Y, et al. Peak expiratory flow rate and sarcopenia risk in older Indonesian people: a nationwide survey. PLoS ONE. 2021;16(2):e0246179. doi: 10.1371/journal.pone.0246179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kera T, Kawai H, Hirano H, et al. Relationships among peak expiratory flow rate, body composition, physical function, and sarcopenia in community-dwelling older adults. Aging Clin Exp Res. 2018;30(4):331–340. doi: 10.1007/s40520-017-0777-9. [DOI] [PubMed] [Google Scholar]
- 29.Stoffels AA, Brandt JD, Meys R et al (2021) Short physical performance battery: response to pulmonary rehabilitation and minimal important difference estimates in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil 102(12):2377–84.e5. 10.1016/j.apmr.2021.05.011 [DOI] [PubMed]
- 30.Medina-Mirapeix F, Bernabeu-Mora R, Sánchez-Martínez MP, et al. Mobility limitations related to reduced pulmonary function among aging people with chronic obstructive pulmonary disease. PLoS ONE. 2018;13(5):e0196152. doi: 10.1371/journal.pone.0196152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi HC, Son KY, Cho B, et al. An implication of the short physical performance battery (SPPB) as a predictor of abnormal pulmonary function in aging people. Arch Gerontol Geriatr. 2012;54(3):448–452. doi: 10.1016/j.archger.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 32.Charles A, Buckinx F, Cataldo D, et al. Relationship between peak expiratory flow and incidence of frailty, deaths and falls among nursing home residents: results of the SENIOR cohort. Arch Gerontol Geriatr. 2019;85:103913. doi: 10.1016/j.archger.2019.103913. [DOI] [PubMed] [Google Scholar]
- 33.Landi F, Salini S, Zazzara MB, et al. Relationship between pulmonary function and physical performance among community-living people: results from Look-up 7+ study. J Cachexia Sarcopenia Muscle. 2020;11(1):38–45. doi: 10.1002/jcsm.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tattersall MC, Lee KE, Tsuchiya N, et al. Skeletal muscle adiposity and lung function trajectory in the severe asthma research program. Am J Respir Crit Care Med. 2023;207(4):475–484. doi: 10.1164/rccm.202203-0597OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo L, Yang L, Rao L, et al. Too depressed to breathe: The longitudinal association between depressive symptoms and lung function among general middle-aged and older adults. Arch Gerontol Geriatr. 2022;103:104797. doi: 10.1016/j.archger.2022.104797. [DOI] [PubMed] [Google Scholar]
- 36.Park HK, Lee SH, Lee SY, et al. Relationships between lung function decline and skeletal muscle and fat mass changes: a longitudinal study in healthy individuals. J Cachexia Sarcopenia Muscle. 2021;12(6):2145–2153. doi: 10.1002/jcsm.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato S, Miyazaki S, Tamaki A, et al. Respiratory sarcopenia: a position paper by four professional organizations. Geriatr Gerontol Int. 2023;23(1):5–15. doi: 10.1111/ggi.14519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang M, Hu X, Wang H, et al. Sarcopenia predicts readmission and mortality in elderly patients in acute care wards: a prospective study. J Cachexia Sarcopenia Muscle. 2017;8(2):251–258. doi: 10.1002/jcsm.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu Y, Peng W, Ren R, et al. Sarcopenia and mild cognitive impairment among elderly adults: the first longitudinal evidence from CHARLS. J Cachexia Sarcopenia Muscle. 2022;13(6):2944–2952. doi: 10.1002/jcsm.13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peel NM, Kuys SS, Klein K. Gait speed as a measure in geriatric assessment in clinical settings: a systematic review. J Gerontol A Biol Sci Med Sci. 2013;68(1):39–46. doi: 10.1093/gerona/gls174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the China Health and Retirement Longitudinal Study (CHARLS) repository, http://charls.pku.edu.cn/.



