Abstract
Goats which have recovered from acute Anaplasma ovis infection remain seropositive, although infected erythrocytes cannot be detected by microscopic examination. Persistence of A. ovis 17 to 21 months following experimental infection was demonstrated by PCR detection of the msp-5 gene. Quantitative analysis of persistent rickettsemia over time showed that all levels were below the limit of microscopic detection and ranged from a low of 102 organisms/ml to peaks of 106 organisms/ml. Two patterns of persistent rickettsemia were observed: the first was characterized by cyclic fluctuations at 6- to 9-week intervals, similar to the pattern described for A. marginale-infected cattle, while in the second pattern, repetitive cycles did not occur and the rickettsemia levels were relatively constant. The msp-2 and msp-3 multigene families, which provide the genetic capacity for outer membrane protein antigenic variation during persistent A. marginale rickettsemia, were identified in the A. ovis genome by Southern blot analysis, and expression of an MSP-2 homologue was confirmed by using immunoblots.
Natural transmission of pathogens of ehrlichial genogroups I and II requires infected, host-seeking ixodid ticks (14–17, 26, 34). Maintaining a sufficiently high tick infection rate for transmission is dependent on either continual presence of an infected animal reservoir or maintenance of infection within the tick population. The lack of transovarial transmission of ehrlichial pathogens indicates the importance of the infected host in providing a continual reservoir for tick infection (17, 28). In cattle infected with Anaplasma marginale, a member of ehrlichial genogroup II (5, 32, 34), low-level infection persists for the life of the animal and is characterized by repetitive, fluctuating cycles of rickettsemia (8, 9). A. marginale levels in these cycles progress from a low of 102 organisms per ml of blood to a peak of 106 to 107 organisms per ml and then precipitously decline (10, 13). Importantly, the levels of persistent A. marginale rickettsemia affect the infection rate of ixodid ticks feeding on these carrier animals (9). Consequently, changes in the rickettsial levels at the peak or trough of the cycles or changes in the frequency of cycles may dramatically alter the vector infection rate and, consequently, transmission.
Is low-level persistent infection a common feature of pathogens in the genus Anaplasma? Infection of goats with Anaplasma ovis results in acute rickettsemia (≥108 organisms per ml) and anemia (4, 27), but repeated microscopic examination of Giemsa-stained blood smears from 73 to 192 days post-experimental infection did not reveal any infected cells (20). However, these goats remained seropositive in the absence of additional exposure. This maintenance of high antibody titers in experimentally infected goats and the detection of seropositive goats in the field that give negative results upon microscopic examination of blood are consistent with persistence of A. ovis in immunocompetent hosts (20), as has been described for A. marginale (7, 8, 13). To detect persistent A. ovis infection and quantitate rickettsemia levels, we used a quantitative competitive PCR based on the genus-conserved msp-5 gene (10, 33). Four Saanen goats that had been infected with the Idaho strain 17 months (68 weeks) previously (20) and then housed in a tick-free facility were examined for A. ovis persistence. No A. ovis-infected erythrocytes had been detected, by microscopic examination of Giemsa-stained smears, in any of the goats for over 14 months (data not shown). Blood was collected in EDTA, and DNA was isolated from 100 μl of whole blood by using a Puregene (Gentra) DNA extraction kit. The quantitative competitive PCR was performed as previously described (10). Briefly, a constant amount of DNA extracted from the blood was amplified in the presence of 10-fold dilutions of an msp-5 mimic. The primers amplify a 457-bp msp-5 genomic fragment and a 202-bp msp-5 mimic fragment (10). PCR products were evaluated densitometrically following electrophoresis on a 2% agarose gel. The initial molar amount of target msp-5 DNA, N0t, was extrapolated from a logarithmic plot of At/As versus N0s, where At is the amount of amplified target, As is the amount of amplified mimic, and N0s is the initial molar amount of the added mimic. N0t is equal to N0s added to the reaction mixture when an equimolar ratio of the two products is produced (i.e., where log10 At/As = log10 1/1 = 0). Since the msp-5 gene is present as a single genomic copy, each molecule of msp-5 represents a single organism (33). Consequently, the number of organisms per milliliter of blood can be calculated from the initial msp-5 molar concentration. Variation in the assay, including the efficiency of DNA extraction, is <100.5 amol/μl, as previously determined by using five separate aliquots of the same blood sample, each separately extracted and amplified on different days (10).
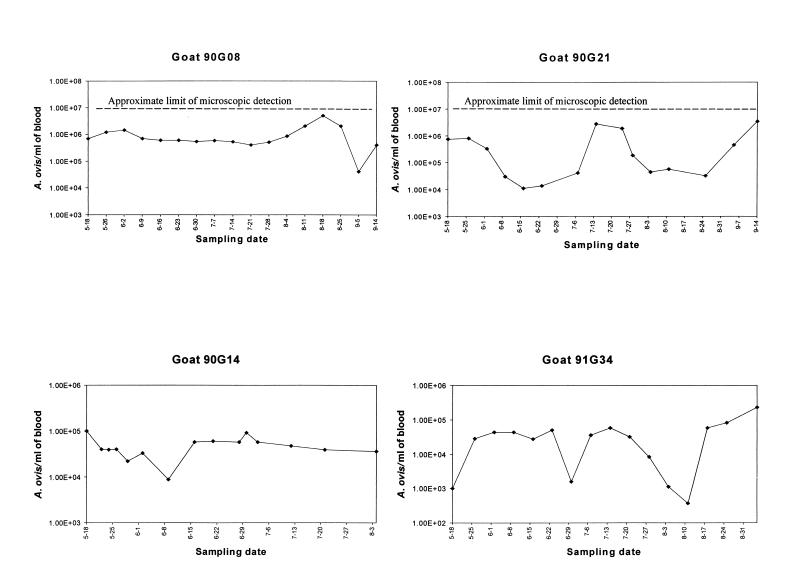
The PCR detected msp-5 in all blood samples from goats 90G14 (15 samples obtained over 11 weeks), 91G34 (16 weekly samples), 90G08 (17 weekly samples), and 90G21 (15 samples during a 17-week period) but consistently gave negative results when blood from a seronegative, uninfected goat was tested as a negative control. These data confirmed that infected goats remained persistently rickettsemic at organism levels below the limits of microscopic detection for a minimum of 21 months. The highest levels of persistent rickettsemia detected, 4.9 × 106 organisms/ml on 18 August 1995 in goat 90G08 and 3.4 × 106 organisms/ml on 14 September 1995 in goat 90G21, were below the 107 organisms/ml that can be reliably detected microscopically (Fig. 1). The lowest detected level was 4 × 102 A. ovis organisms/ml in goat 91G34, while minimal rickettsemias in the other three goats during the study period were in the range of 104 to 105 organisms/ml (Fig. 1). This logarithmic range of A. ovis rickettsemia during persistent infection is similar to the previously described levels of A. marginale in infected cattle, which fluctuate from lows of 102 organisms/ml to peaks of ≥106 organisms/ml (10, 13). Goats 91G34 and 90G21 had repetitive rickettsemic cycles, defined by a ≥2 log10 increase and subsequent decrease in the number of A. ovis organisms per milliliter, that occurred at 6- to 8-week intervals (Fig. 1). These cyclic fluctuations of ≥102 A. ovis organisms/ml are similar to those described for A. marginale, although the latter infection is typified by fluctuations of ≥103 organisms/ml (9, 10, 13). However, in contrast to the pattern for all infected cattle that have been examined, where cycles of A. marginale rickettsemia occur repetitively at 6- to 9-week intervals (8–10, 13), well-defined repeated cycles were not detected in goats 90G08 and 90G14, which showed only minor peaks of rickettsemia followed or preceded by relatively constant rickettsial levels (Fig. 1).
FIG. 1.
Quantitative detection of A. ovis rickettsemia by competitive PCR in four goats infected 17 months previously. The month and day for each sample are indicated on the x axis.
Persistent infections in the mammalian reservoir hosts have been reported for other ehrlichial genogroup I and II pathogens, including Ehrlichia canis (11), Cowdria ruminantium (3), the agent of human granulocytic ehrlichiosis (HGE) (12, 30), and Ehrlichia chaffeensis (6, 15). The duration of low-level rickettsemia has not been defined for most ehrlichial pathogens, but the detection of A. marginale in 100% of cattle examined 7 years after infection demonstrates that ehrlichial pathogens can persist for prolonged periods in immunocompetent hosts (8, 13). Thus, persistent infection of the reservoir host appears to be a common feature for ehrlichial genogroup I and II pathogens and would provide the ready source of infection for competent tick vectors during their seasonal emergence.
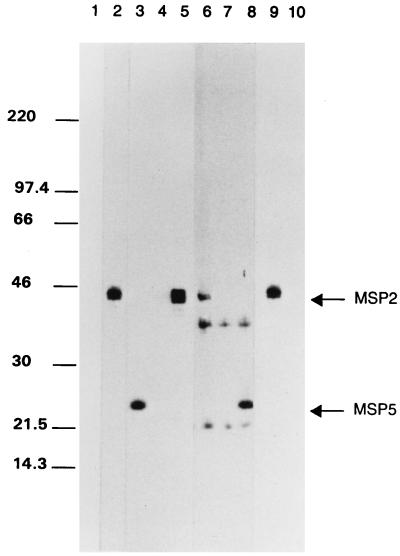
How ehrlichial pathogens persist in an immunocompetent host is unknown. A. marginale cyclic rickettsemia is associated with emergence of antigenic variants of an immunoprotective outer membrane protein, MSP-2 (23, 29). MSP-2 is encoded by a polymorphic multigene family, resulting in expression of structurally and antigenically distinct surface proteins during cycles of persistent A. marginale rickettsemia (7, 10, 22). Interestingly, MSP-2 homologues have been identified in the ehrlichial genogroup I pathogens, E. chaffeensis (21), E. canis (25), and C. ruminantium (31), and in the genogroup II agent of HGE (35). The presence in A. ovis of an immunodominant antigen of approximately 40 kDa, similar in size to A. marginale MSP-2, suggested the presence of a homologue (1, 19). To determine if A. ovis expressed MSP-2, lysates of the Idaho strain of A. ovis were analyzed by polyacrylamide gel electrophoresis and immunoblotting, performed as previously described (20). A. ovis MSP-2 was detected by using monospecific rabbit antibody against conserved regions of A. marginale MSP-2 (22) (Fig. 2, lane 9) and, following a longer exposure, by using anti-MSP-2 monoclonal antibody (MAb) ANAF19E2 (22, 23) (Fig. 2, lane 6). The approximate molecular size of A. ovis MSP-2 is similar to that of A. marginale (Fig. 2, lanes 2 and 5) and to that of the 44-kDa MSP-2 homologue recently identified in the closely related HGE agent (35). MAb ANAF16C1, which reacts with a genus-conserved MSP-5 epitope (18, 33), was the positive antibody control (Fig. 2, lanes 8 [A. ovis] and 3 [A. marginale]). Tryp1E1, which reacts with a Trypanosoma brucei variable surface glycoprotein, and normal rabbit serum were used as negative antibody controls and were unreactive with both A. ovis and A. marginale (Fig. 2).
FIG. 2.
Expression of MSP-2 by A. ovis. Lysates of A. ovis (lanes 6 to 10) were electrophoretically separated by using sodium dodecyl sulfate-containing polyacrylamide gels, transferred to nitrocellulose, and probed for MSP-2 expression. A. marginale lysates (lanes 1 to 5) were treated identically as positive antigen controls. MSP-2 expression was detected by using a 1:1,000 dilution of rabbit anti-MSP-2 antiserum (lanes 2 and 9) and 2 μg of MAb ANAF19E2/ml (lanes 5 and 6). Normal rabbit serum (lanes 1 and 10) and a MAb directed against T. brucei VSG (lanes 4 and 7) were used as negative antibody controls. MAb ANAF16C1, directed against a genus-conserved MSP-5 epitope, was used as a positive-control antibody (lanes 3 and 8). The molecular size markers are indicated on the left, and the positions of MSP-2 and MSP-5 are indicated on the right.
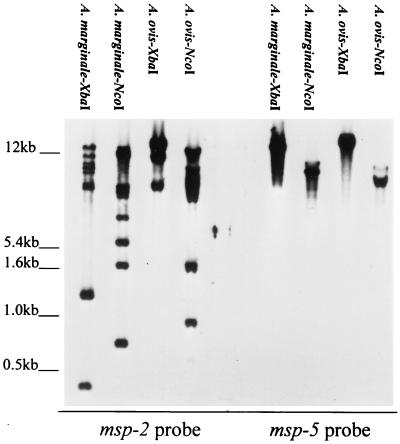
The msp-2 gene family is composed of multiple polymorphic copies widely dispersed within the A. marginale chromosome (7, 22). In contrast, the msp-2 homologue in C. ruminantium, map-1, is a single-copy gene which is polymorphic only between strains (24, 31). To determine whether A. ovis contained a single copy or multiple msp-2 genes, genomic DNA was extracted from the Idaho strain and digested with either XbaI or NcoI. Neither of these enzymes cut within any of the msp-2 genes identified in A. marginale (7, 10, 22). The electrophoretically separated and blotted fragments were hybridized with a digoxigenin-labeled msp-2 probe under stringent conditions as previously described (22). The probe was generated by PCR amplification of plasmid pCKR11.2 msp-2 by using a forward primer representing nucleotides 375 to 395 and a reverse primer representing nucleotides 710 to 729 (numbering based on pCKR11.2 [22]). Several restriction fragments derived from either XbaI or NcoI digestion hybridized with the msp-2-specific probe (Fig. 3). These multiple fragments are not attributable to partial digestion of genomic DNA, as the identical digests hybridized with only a single msp-5 fragment as predicted for this single gene copy (Fig. 3). The fact that multiple A. ovis restriction fragments hybridized with the region of msp-2 containing the open reading frame suggests that a mechanism for encoding MSP-2 antigenic variants, via expression of variant gene copies or recombination among partial gene copies, may exist. Determination of whether these fragments include multiple complete genes and the extent of polymorphism will require sequencing of genomic and expressed copies. However, the presence of a large repertoire of complete A. marginale msp-2 genes which express polymorphic full-length MSP-2 during persistent rickettsemia (7, 10) provides a precedent for the importance of the multiple msp-2 sequences in A. ovis.
FIG. 3.
Presence of multiple msp-2 copies in the A. ovis genome. A. ovis or A. marginale genomic DNA was digested with the restriction enzymes indicated above each lane and, following Southern blotting, hybridized with either msp-2 or msp-5. The molecular size markers are indicated on the left.
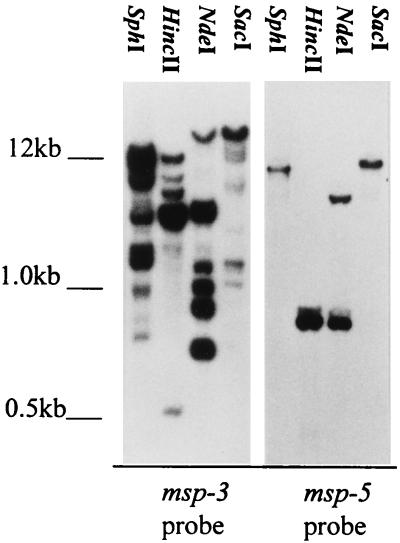
A. marginale contains a second multigene family, in addition to msp-2, that also encodes polymorphic outer membrane proteins. This gene family, designated msp-3, is estimated to comprise as much as 3% of the 1,250-kb A. marginale genome (2). To date, homologues of msp-3 have not been reported for any other ehrlichial pathogens, including A. ovis. The presence of msp-3 in A. ovis was determined by SphI, HincII, NdeI, or SacI digestion of genomic DNA followed by Southern blotting. None of these four enzymes cleaves within the defined A. marginale msp-3 genes (2), and only NdeI cuts within the single-copy msp-5 (2, 33). The blots were hybridized under stringent conditions by using a digoxigenin-labeled msp-3 probe generated by PCR amplification of plasmid msp3-12 with a forward primer derived from nucleotides 1309 to 1329 and a reverse primer derived from nucleotides 2313 to 2333 (numbering based on msp3-12 [2]). Multiple genomic fragments derived from each restriction enzyme digest hybridized with the msp-3-specific probe (Fig. 4). In contrast, the msp-5 probe bound only a single fragment in the SphI, HincII, and SacI digests and, as predicted by the single NdeI recognition site in msp-5, two fragments in the NdeI digest (Fig. 4). Similar to the results for msp-2, the hybridization of msp-3 with multiple A. ovis genomic fragments indicates that this multigene family, initially defined in A. marginale, is present among distinct species within the genus.
FIG. 4.
Presence of multiple msp-3 copies in the A. ovis genome. A. ovis or A. marginale genomic DNA was digested with the restriction enzymes indicated above each lane and, following Southern blotting, hybridized with either msp-3 or msp-5. The molecular size markers are indicated on the left.
Long-term persistent infection with A. ovis, similar to the situation with A. marginale, is postulated to be required for efficient natural transmission via ixodid ticks. Together the msp-2 and msp-3 gene families compose greater than 4% of the A. marginale genome and provide the genetic capacity for prolonged antigenic variation in the outer membrane proteins targeted by the immune response (2, 22, 29). The emergence of new A. marginale MSP-2 variants during each rickettsemic cycle suggests that the msp-2 multigene family is causally involved in persistence (10). Whether these multigene families have a similar role in persistent A. ovis infection, which differs from infection with A. marginale by the occurrence of long periods of relatively constant rickettsemia levels as well as cyclic fluctuations, is unknown.
Acknowledgments
This work was supported by U.S. Department of Agriculture National Research Initiative Competitive Grants Program grant 95-37204-2348, NIH grants 5T32 AI07367 and 5K08 AI01371, and the U.S.-Israel BARD Program.
We acknowledge Beverly Hunter, Kay Morris, and Carla Robertson for excellent technical assistance.
REFERENCES
- 1.Adams J H, Smith R D, Kuhlenschmidt M S. Identification of antigens of two isolates of Anaplasma marginale, using a western blot technique. Am J Vet Res. 1986;47:501–506. [PubMed] [Google Scholar]
- 2.Alleman A R, Palmer G H, McGuire T C, McElwain T F, Perryman L E, Barbet A F. Anaplasma marginale major surface protein-3 (MSP-3) is encoded by a polymorphic multigene family. Infect Immun. 1997;65:156–163. doi: 10.1128/iai.65.1.156-163.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrew H R, Norval R A. The carrier status of sheep, cattle, and African buffalo recovered from heartwater. Vet Parasitol. 1989;34:261–266. doi: 10.1016/0304-4017(89)90056-3. [DOI] [PubMed] [Google Scholar]
- 4.Bevan L E W. Anaplasmosis of sheep. Vet J. 1912;68:392–400. [Google Scholar]
- 5.Dame J B, Mahan S M, Yowell C A. Phylogenetic relationship of Cowdria ruminantium, agent of heartwater, to Anaplasma marginale and other members of the order Rickettsiales determined on the basis of 16S rRNA sequence. Int J Syst Bacteriol. 1992;42:270–274. doi: 10.1099/00207713-42-2-270. [DOI] [PubMed] [Google Scholar]
- 6.Dawson J E, Stallknecht D E, Howerth E W, Warner C, Biggie K, Davidson W R, Lockhart J M, Nettles V F, Olson J G, Childs J E. Susceptibility of white-tailed deer (Odocoileus virginianus) to infection with Ehrlichia chaffeensis, the etiologic agent of human ehrlichiosis. J Clin Microbiol. 1994;32:2725–2728. doi: 10.1128/jcm.32.11.2725-2728.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eid G, French D M, Lundgren A, Barbet A F, McElwain T F, Palmer G H. Expression of major surface protein 2 antigenic variants during acute Anaplasma marginale rickettsemia. Infect Immun. 1996;64:836–841. doi: 10.1128/iai.64.3.836-841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eriks I S, Palmer G H, McGuire T C, Allred D R, Barbet A F. Detection and quantitation of Anaplasma marginale in carrier cattle by using a nucleic acid probe. J Clin Microbiol. 1989;27:279–284. doi: 10.1128/jcm.27.2.279-284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eriks I S, Stiller D, Palmer G H. Impact of persistent Anaplasma marginale rickettsemia on tick infection and transmission. J Clin Microbiol. 1989;31:2091–2096. doi: 10.1128/jcm.31.8.2091-2096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.French D F, McElwain T F, McGuire T C, Palmer G H. Expression of Anaplasma marginale major surface protein 2 variants during persistent cyclic rickettsemia. Infect Immun. 1998;66:1200–1207. doi: 10.1128/iai.66.3.1200-1207.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrus S, Waner T, Aizenberg I, Foley J E, Poland A M, Bark H. Amplification of ehrlichial DNA from dogs 34 months after infection with Ehrlichia canis. J Clin Microbiol. 1998;36:73–76. doi: 10.1128/jcm.36.1.73-76.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodzic E, Ijdo J W, Feng S, Katavolos P, Sun W, Maretzki C H, Fish D, Fikrig E, Telford III S R, Barthold S W. Granulocytic ehrlichiosis in the laboratory mouse. J Infect Dis. 1998;177:737–745. doi: 10.1086/514236. [DOI] [PubMed] [Google Scholar]
- 13.Kieser S T, Eriks I S, Palmer G H. Cyclic rickettsemia during persistent Anaplasma marginale infection of cattle. Infect Immun. 1990;58:1117–1119. doi: 10.1128/iai.58.4.1117-1119.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kocan K M, Hair J A, Ewing S A, Stratton L G. Transmission of Anaplasma marginale Theiler in Dermacentor andersoni Stiles and Dermacentor variablis. Am J Vet Res. 1981;42:15–18. [PubMed] [Google Scholar]
- 15.Lockhart J M, Davidson W R, Stallknecht D E, Dawson J E, Howerth E W. Isolation of Ehrlichia chaffeensis from white-tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J Clin Microbiol. 1997;35:1681–1686. doi: 10.1128/jcm.35.7.1681-1686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lockhart J M, Davidson W R, Stallknecht D E, Dawson J E, Little S E. Natural history of Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) in the Piedmont physiographic province of Georgia. J Parasitol. 1997;83:887–894. [PubMed] [Google Scholar]
- 17.McLeod J, Gordon W S. Studies in tick-borne fever of sheep. I. Transmission by the tick, Ixodes ricinus, with a description of the disease produced. Parasitology. 1933;25:273–283. [Google Scholar]
- 18.Munodzana D, McElwain T F, Knowles D P, Palmer G H. Conformational dependence of Anaplasma marginale major surface protein 5 surface-exposed B-cell epitopes. Infect Immun. 1998;66:2619–2624. doi: 10.1128/iai.66.6.2619-2624.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura Y, Kawazu S I, Minami T. Antigen profiles of Anaplasma ovis and A. mesaeterum and cross infection trials with them and A. marginale. Vet Microbiol. 1993;37:19–30. doi: 10.1016/0378-1135(93)90179-b. [DOI] [PubMed] [Google Scholar]
- 20.Ndung’u L W, Aguirre C, Rurangirwa F R, McElwain T F, McGuire T C, Knowles D P, Palmer G H. Detection of Anaplasma ovis infection in goats by major surface protein 5 competitive inhibition enzyme-linked immunosorbent assay. J Clin Microbiol. 1995;33:675–679. doi: 10.1128/jcm.33.3.675-679.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohashi N, Zhi N, Zhang Y, Rikihisa Y. Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect Immun. 1998;66:132–139. doi: 10.1128/iai.66.1.132-139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer G H, Eid G, Barbet A F, McGuire T C, McElwain T F. The immunoprotective Anaplasma marginale major surface protein 2 is encoded by a polymorphic multigene family. Infect Immun. 1994;62:3808–3816. doi: 10.1128/iai.62.9.3808-3816.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer G H, Oberle S M, Barbet A F, Davis W C, Goff W L, McGuire T C. Immunization with a 36-kilodalton surface protein induces protection against homologous and heterologous Anaplasma marginale challenge. Infect Immun. 1988;56:1526–1531. doi: 10.1128/iai.56.6.1526-1531.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddy G R, Sulsona C R, Mahan S M, Burridge M J, Barbet A F. Sequence heterogeneity of the major antigenic protein 1 genes from Cowdria ruminantium isolates from different geographical areas. Clin Diagn Lab Immunol. 1996;4:417–422. doi: 10.1128/cdli.3.4.417-422.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy G R, Sulsona C R, Barbet A F, Mahan S M, Burridge M J, Alleman A R. Molecular characterization of a 28kDa surface antigen gene family of the tribe Ehrlichiae. Biochem Biophys Res Commun. 1998;247:636–643. doi: 10.1006/bbrc.1998.8844. [DOI] [PubMed] [Google Scholar]
- 26.Rikihisa Y. The tribe Ehrlichieae and ehrlicheal diseases. Clin Microbiol Rev. 1991;4:286–308. doi: 10.1128/cmr.4.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Splitter E J, Anthony H D, Twiehaus M J. Anaplasma ovis in the United States: experimental studies with sheep and goats. Am J Vet Res. 1956;17:487–491. [PubMed] [Google Scholar]
- 28.Stich R W, Kocan K M, Palmer G H, Ewing S A, Hair J A, Barron S J. Transstadial and attempted transovarial transmission of Anaplasma marginale by Dermacentor variablis. Am J Vet Res. 1989;50:1377–1380. [PubMed] [Google Scholar]
- 29.Tebele N, McGuire T C, Palmer G H. Induction of protective immunity using Anaplasma marginale initial body membranes. Infect Immun. 1991;59:3199–3204. doi: 10.1128/iai.59.9.3199-3204.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Telford S R, Dawson J E, Katavolos P, Warner C K, Kolbert C P, Persing D H. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci USA. 1996;93:6209–6214. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Vliet A H, Jongejan F, van Kleef M, van der Zeijst B A. Molecular cloning, sequence analysis, and expression of the gene encoding the immunodominant 32-kilodalton protein of Cowdria ruminantium. Infect Immun. 1994;62:1451–1456. doi: 10.1128/iai.62.4.1451-1456.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Vliet A H, Jongejan F, van der Zeijst B A. Phylogenetic position of Cowdria ruminantium (Rickettsiales) determined by analysis of amplified 16S rDNA sequences. Int J Syst Bacteriol. 1992;42:494–498. doi: 10.1099/00207713-42-3-494. [DOI] [PubMed] [Google Scholar]
- 33.Visser E S, McGuire T C, Palmer G H, Davis W C, Shkap V, Pipano E, Knowles D P. The Anaplasma marginale msp5 gene encodes a 19-kilodalton protein conserved in all recognized Anaplasma species. Infect Immun. 1992;60:5139–5144. doi: 10.1128/iai.60.12.5139-5144.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker D H, Dumler J S. Emergence of the ehrlichioses as human health problems. Emerg Infect Dis. 1996;2:18–29. doi: 10.3201/eid0201.960102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhi N, Ohashi N, Rikihisa Y, Horowitz H W, Wormser G P, Hechemy K. Cloning and expression of the 44-kilodalton major outer membrane protein gene of the human granulocytic ehrlichiosis agent and application of the recombinant protein to serodiagnosis. J Clin Microbiol. 1998;36:1666–1673. doi: 10.1128/jcm.36.6.1666-1673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]