Abstract
Aims
We examined the cardiovascular effects of celiac disease (CeD) in a humanized mouse model, with a focus on vascular inflammation, endothelial dysfunction, and oxidative stress.
Methods and results
NOD.DQ8 mice genetically predisposed to CeD were subjected to a diet regime and oral gavage to induce the disease (gluten group vs. control). We tested vascular function, confirmed disease indicators, and evaluated inflammation and oxidative stress in various tissues. Plasma proteome profiling was also performed.
CeD markers were confirmed in the gluten group, indicating increased blood pressure and impaired vascular relaxation. Pro-inflammatory genes were upregulated in this group, with increased CD11b+ myeloid cell infiltration and oxidative stress parameters observed in aortic and heart tissue. However, heart function remained unaffected. Plasma proteomics suggested the cytokine interleukin-17A (IL-17A) as a link between gut and vascular inflammation. Cardiovascular complications were reversed by adopting a gluten-free diet.
Conclusion
Our study sheds light in the heightened cardiovascular risk associated with active CeD, revealing a gut-to-cardiovascular inflammatory axis potentially mediated by immune cell infiltration and IL-17A. These findings augment our understanding of the link between CeD and cardiovascular disease providing clinically relevant insight into the underlying mechanism. Furthermore, our discovery that cardiovascular complications can be reversed by a gluten-free diet underscores a critical role for dietary interventions in mitigating cardiovascular risks associated with CeD.
Keywords: Celiac disease, Arterial hypertension, Endothelial dysfunction, Oxidative stress, Vascular inflammation, Interleukin-17A
Graphical abstract
The central scheme illustrates the pathophysiological process initiated by celiac disease (CeD). Gluten comprises indigestible gliadin, which is taken up by the epithelium of the duodenum, the first part of the small intestine. Within the lamina propria, gliadin is deamidated and becomes capable of binding DQ2 or DQ8 antigens on antigen-presenting cells, thereby activating an immune response cascade involving T and B cells. This reaction can lead to intestinal changes such as villous atrophy, crypt hyperplasia, and intraepithelial lymphocytosis. We observed signs of intestinal inflammation, a decreased villus/crypt ratio, and upregulation of pro-inflammatory cytokines. Despite no immediate impairment in cardiac function, we detected elevated systolic and diastolic blood pressure, increased lipid peroxidation, and a heightened state of oxidative stress. Gliadin consumption also resulted in adverse effects on vascular function and an increase in vascular oxidative stress. Furthermore, we observed elevated oxidative burst, increased nitrate, and nitrite levels, and increased interleukin-17A plasma levels in the whole blood samples. These results underline the systemic impact of active CeD on the body's oxidative stress and inflammatory status.
Non-standard abbreviations and acronyms
- 3-NT
3-nitrotyrosine
- 4-HNE
4-hydroxy-2-nonenal
- BW
bodyweight
- CeD
celiac disease
- CI
confidence interval
- CTX
cholera toxin
- DD
distal duodenum
- DHE
dihydroethidium
- EWAT
epididymal white adipose tissue
- GCD
gluten-containing diet
- GFD
gluten-free diet
- HR
hazard ratio
- IL
interleukin
- iNOS
inducible •NO synthase (type 2)
- L-012
8-amino-5-chloro-7-phenylpyrido[3,4-d]pyridazine-1,4-(2H,3H)dione sodium salt
- Nox2
NADPH oxidase isoform 2
- PT
pepsin trypsin
- PVAT
perivascular adipose tissue
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- ROS
reactive oxygen species
- TG2
tissue transglutaminase, transglutaminase 2
- TTE
transthoracic echocardiography
1. Introduction
Cardiovascular diseases are a major cause of mortality worldwide [1]. Besides classical cardiovascular risk factors such as arterial hypertension, tobacco smoking, adiposity, diabetes, or dyslipidemia, there is increasing evidence for formerly unrecognized, non-classical risk factors such as noise, air pollution, and social loneliness, and also specific dietary components [2]. Moreover, autoinflammatory diseases such as systemic lupus erythematosus [3], psoriasis [4] or rheumatoid arthritis [5] are associated with increased cardiovascular morbidity and mortality.
Celiac disease (CeD) is a gluten-induced autoinflammatory enteropathy with a 0.5–2 % prevalence in most countries. The clinical picture of CeD is highly heterogeneous and symptoms include intestinal (diarrhea, abdominal pain, bloating, etc.) and extraintestinal (several associated autoimmune diseases, anemia, osteoporosis) manifestations [6,7]. CeD is grossly underdiagnosed since many patients only show mild or no overt disease manifestations [8]. Notably, even clinically mild CeD can lead to severe consequences with ongoing long-term gluten ingestion, such as the promotion of extraintestinal autoimmunity, hematological malignancies, diet refractory CeD, and intestinal T cell lymphoma [9]. Apart from extraintestinal autoimmunity, an enhanced risk of renal- and cardiovascular disease has been described in patients with untreated or insufficiently treated CeD [10,11], which could not be linked to vitamin or nutrient deficiencies that may go along with untreated CeD [[12], [13], [14], [15]].
In a Swedish cohort study with over 28,000 participants, CeD was strongly associated with death from ischemic heart disease (IHD; HR, 1.22; [95 % CI, 1.06–1.40]) [11]. In this line, a US cohort study of 49,829 patients with a follow-up of 12.5 years showed that overall mortality was significantly increased in CeD patients (HR, 1.21; [95 % CI, 1.17–1.25]) as was the risk for cardiovascular death (HR, 1.08; [95 % CI, 1.02–1.13]). Interestingly, the increased risk persisted beyond ten years after diagnosis, i.e., with the vast majority of patients being on a strict gluten-free diet since diagnosis, and was highest in the first five years (HR, 2.34; [95 % CI, 2.14–2.55]) [12]. However, the underlying pathophysiology linking the intestinal inflammation in CeD to cardiovascular disease remains elusive.
Therefore, we aimed to investigate the mechanisms underlying the adverse health outcome of a gluten-containing diet and small intestinal inflammation in CeD. To this aim we established and thoroughly studied both the gut and cardiovascular system of a humanized mouse model that replicates all key immunological and pathophysiological features of human CeD.
2. Methods
2.1. Pepsin-trypsin digest of gliadin/zein and deamidation with TG2
Gliadins are the major fraction of gluten proteins and contain the most immunogenic epitopes that trigger CeD in genetically predisposed individuals carrying HLA-DQ2 or -DQ8. Certain gliadin peptides that we generate by gliadin digestion with the gastrointestinal proteases pepsin and trypsin (PT) are further potentiated to elicit a gluten-specific T cell response after deamidation and crosslinking by TG2, the CeD autoantigen in the intestinal lamina propria. PT-digested and TG2-reacted gliadin (PT/TG2-gliadin) and the negative control PT/TG2-zein were prepared by enzymatic digestion as described previously with minor modifications [16]. Gliadin (G3375) or zein, the prolamin of maize (Z3625) [7] were digested with pepsin and trypsin, followed by TG2-mediated deamidation. Gliadin peptides thus generated were evaluated for their immunogenic potential. For a detailed description, see Online Supplement.
2.2. Animals and housing
All animals were treated in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the US National Institutes of Health and approval was granted by the Federal IACUC (Landesuntersuchungsamt) Rhineland Palatinate, Koblenz, Germany; permit number: 23 177-07/G 20-1-061. The studies were performed with 8–12 weeks old male transgenic NOD.DQ8 mice (provided by E. Verdu, McMaster University, Canada) that express human HLA-DQ8 (HLA-DQA1*0301; DQB1*0302) in the absence of endogenous mouse class II genes (Aβ0/0) [17]. Animals were housed under a 12-h light/dark cycle in the institutional animal facility in ventilated animal cabinets with free access to the gluten-free diet (GFD) and water.
2.3. Treatment of NOD.DQ8 mice with PT/TG2-gliadin (gluten group) or PT/TG2-zein (ctr)
NOD.DQ8 mice were randomly divided into a control group (with zein from maize) and a gluten group (with gluten and gliadin from wheat).
NOD.DQ8 mice from both groups were administered 3 oral gavages one week apart of 0.5 mg PT/TG2-gliadin or PT/TG2-zein together with 25 μg of cholera toxin (CTX, C8052) in 0.2 ml PBS. With the first gavage, mice were switched to the gluten-containing diet (GCD, gluten group). The zein-sensitized control group continued the gluten-free diet (GFD, control group).
In addition to the already described treatments of the control and gluten group, the OFF-study treatment scheme involved moving the now 10–14-week-old males to fresh cages with fresh water sources to eliminate potential gluten contamination and received GFD (for treatment schemes, see Fig. 1A and Fig. 6A; for diet specifications, see Suppl. Fig. S1).
Fig. 1.
Gluten feeding of gliadin sensitized NOD.DQ8 mice induces celiac disease and leads to hypertension and hypercholesterolemia.
(A) Treatment scheme; NOD.DQ8 mice, with a genetic predisposition for celiac disease (CeD), were raised on a corn-based, gluten-free diet (GFD). During the experiment, 8–12-week-old male mice received three gavages with a deamidated peptic-tryptic digest of zein or gliadin together with cholera toxin (PT-TG2-CTX-zein/gliadin). The gluten group was switched to a gluten-containing diet (GCD) for 14 days (d), while the zein-control group remained on GFD. In vivo analysis of blood pressure (BP) and metabolic cage (MC) assessments were performed on selected mice at both the beginning and end of the treatment. Transthoracic echocardiography (TTE) was conducted solely at the conclusion of the treatment, followed by ex vivo analysis (for detailed information, please refer to the methods section). (B) Villous height to crypt depth ratios (VH:CrD) in distal duodenum (for villous length and crypt depth s. Suppl. Fig. S3). (C) Systolic and (D) diastolic blood pressure (BP) measured by tail-cuff at the end of the treatment (day 14), (E) body weight gain during the treatment (day 14 – day 0), (F) serum total cholesterol levels and (G) non-fasting blood glucose levels were evaluated in whole blood ex vivo (day 15).
(B) Unpaired t-test; n(Ctr) = 7, n(G) = 7, (C)–(D) Unpaired t-test; n(Ctr) = 16, n(G) = 15, (E) Unpaired t-test; n(Ctr) = 17, n(G) = 19, (F) Unpaired t-test; n(Ctr) = 10, n(G) = 8, (G) Unpaired t-test; n(Ctr) = 16, n(G) = 20. Data are means ± SEM. **P < 0.01; ***P < 0.001.
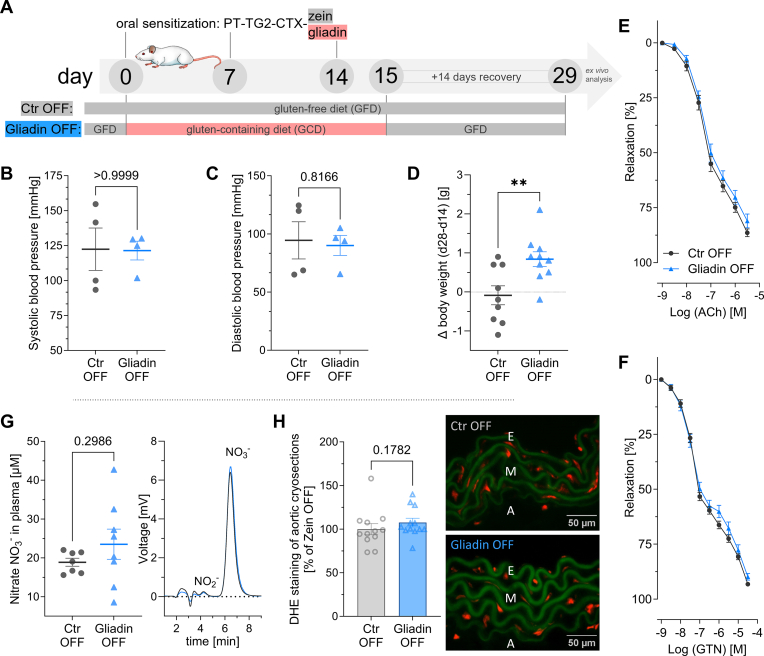
Fig. 6.
Remission of celiac disease after 14 days on a gluten-free diet restores the normal cardiovascular phenotype.
(A) OFF-study treatment scheme; on top of the already described treatments of C
tr and gluten group (compare Fig. 1A), additional 14 days of recovery were given. The now 10–14-week-old males switched or remained on a gluten-free diet (GFD) without any further treatments, completely off any gliadin or gluten exposure. (B) Systolic and (C) diastolic blood pressure were measured by tail-cuff on day 28 (compare Fig. 1C–D). (D) Body weight gain during 14 days of recovery (day 28 – day 14; (compare Fig. 1E). (E) Endothelium-dependent (acetylcholine, ACh) relaxation and (F) endothelium-independent (nitroglycerin, GTN) relaxation (compare Fig. 4A-B). (G) High-performance liquid chromatography (HPLC) was used to detect nitrate (NO3−) and nitrite (NO2−) in plasma. Representative chromatograms are shown beside the quantification graph (compare Fig. 4E). (H) Aortic cryosections stained with dihydroethidium (DHE) were used to detect reactive oxygen species in the vascular wall (compare Fig. 4G).
(B) Mann-Whitney test; n(Ctr) = 4, n(G) = 4, (C) Unpaired t-test; n(Ctr) = 4, n(G) = 4, (D) Unpaired t-test; n(Ctr) = 9, n(G) = 10, (E)–(F) two-way ANOVA and Bonferroni multiple comparison test; n(Ctr) = 12, n(G) = 12, (G) unpaired t-test; n(Ctr) = 7, n(G) = 8, (H) Mann-Whitney test; n(Ctr) = 12, n(G) = 12. Data are means ± SEM. **P < 0.01.
2.4. Tissue sampling/sacrifice
Animals were sacrificed a day after the third sensitization under deep anesthesia (i.p injection with 120 mg/kg BW Ketamine and 16 mg/kg BW Xylazine). Blood, heart, aorta, small intestine, brain, and adipose tissue were collected. Tissue aliquots were snap-frozen, fixed, or processed. Protein extraction involved homogenization and centrifugation. Protein content was quantified, and blood glucose and cholesterol were measured. Heart weight and body weight changes were monitored. For a detailed description, see Online Supplement.
2.5. Non-invasive blood pressure recordings (NIBP)
Blood pressure was measured non-invasively using tail-cuff occlusion and volume pressure recording sensor technology. Systolic and diastolic blood pressures were recorded using the CODA High Throughput System. Mice were acclimated with training runs before measurements, and baseline readings were taken before the study. Mean values from multiple measurements were used for data analysis. For a detailed description, see Online Supplement.
2.6. High-frequency ultrasound (HFUS) transthoracic echocardiography (TTE)
High-resolution echocardiography was performed on anesthetized mice using the Vevo 3100 imaging system. B-mode and M-mode images of the heart were acquired to assess cardiac function, including ejection fraction and cardiac output. Strain analysis was conducted using Vevo Strain measurement software to evaluate myocardial contractility. For a detailed description, see Online Supplement.
2.7. Metabolic cages
Mice were individually housed in metabolic cages to assess food and water intake. Urine and feces were collected, and food and water consumption were monitored for 24-h intervals. The setup accounted for circadian rhythms, and the mice had ad libitum access to food and water during this period. For a detailed description, see Online Supplement.
2.8. Detection of oxidative stress
Oxidative stress was assessed through blood oxidative burst using zymosan A stimulation. ROS formation was measured by L-012-enhanced chemiluminescence. Left ventricles and aortic ring segments were assessed for oxidative stress using dihydroethidium (DHE) staining and subsequent fluorescence quantification. For a detailed description, see Online Supplement.
2.9. Dot blot analysis
Cardiac protein homogenates were analyzed for oxidative stress markers using dot blot analysis. 3-Nitrotyrosine (3-NT) and 4-hydroxy-2-nonenal (4-HNE) specific antibodies were used, and densitometric quantification of dots was performed. For a detailed description, see Online Supplement.
2.10. Immunohistochemical staining and densitometric analysis
Aortic ring segments (3-NT, 06–284, Merck, Germany) and distal duodenal samples (CD3, ab5690; CD4, ab183685; CD68, 97778S; Ki67, ab16667, from abcam or Cell Signaling) were stained with specific antibodies. Densitometric analysis of stained proteins and cells was either performed by counting or using Image J software. For a detailed description, see Online Supplement.
2.11. Reverse transcription polymerase chain reaction (qRT-PCR)
RNA isolation was performed using the acid guanidinium thiocyanate–phenol–chloroform extraction method. Real-time RT-PCR was conducted to analyze gene expression levels using specific TaqMan Gene Expression assays. For a detailed description, see Online Supplement.
2.12. Determination of nitrate and nitrite
Nitrate and nitrite levels were measured using an ENO-20 NOx Analyzer based on the liquid chromatography method with post-column derivatization with Griess reagent. For a detailed description, see Online Supplement.
2.13. Enzyme-linked immunosorbent assay
Interleukin-17A in plasma was quantified using a commercial ELISA kit (Mouse IL-17A Flex Set, 560283). For a detailed description, see Online Supplement.
2.14. Isometric tension recordings
Vascular reactivity studies were performed on thoracic aortic rings using isometric tension recordings. Concentration-relaxation and concentration-contraction curves were recorded in response to acetylcholine (ACh), nitroglycerin (GTN) and potassium chloride (KCl). For a detailed description, see Online Supplement.
2.15. Flow cytometry
Aortic vessels were prepared for single-cell suspensions, surface-stained with CD45-APC-eFluor 780, NK1.1-PE-Cy7 (eBiosciences, CA, USA), TCR-β V450, CD11b-PE, (BD Biosciences, NJ, USA), and analyzed using flow cytometry to study cellular markers. For a detailed description, see Online Supplement.
2.16. Plasma proteomics
Plasma proteomics analysis was conducted using the Target 96 Mouse Exploratory Panel (95380). The normalized protein expression units were quantified using Olink technology and analyzed for protein concentrations. For a detailed description, see Online Supplement.
2.17. Statistics
Data are expressed as means ± standard error of mean (SEM). Statistical calculations were performed with GraphPad Prism version 10.0.0 for Windows (GraphPad Software Inc., MA, USA). Outliers were identified and subsequently removed based on the ROUT Test with a significance level of Q = 1 %. Continuous data was analyzed for normal distribution using the Shapiro-Wilk test. In the case of normal distribution, the unpaired student's t-test was used to compare two groups, otherwise Mann-Whitney test was used. In the case of three groups ordinary one-way ANOVA (with Dunnett correction for comparison of multiple means) was used. Two-way ANOVA (with Bonferroni's correction for comparison of multiple means) was used for comparisons of the whole blood oxidative burst and the dose-response relations of the isometric tension recordings. All data was assumed to be two-tailed. P values < 0.05 were considered statistically significant and marked by asterisks (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
3. Results
3.1. Gluten feeding of gliadin-sensitized NOD.DQ8 mice induces celiac disease and leads to hypertension and hypercholesterolemia
Humanized NOD.DQ8 mice were sensitized with pepsin-trypsin (PT)-digested and tissue transglutaminase (TG2)-treated gliadin (PT/TG2-gliadin) or with PT/TG2-treated zein and set on a gluten-containing diet (GCD) or gluten-free diet (GFD), respectively (Fig. 1A). CeD was induced in the gluten group but not in the control group, as assessed by measurement of villous height to crypt depth ratios (VH:CrD) in the duodenum, the histological hallmark of CeD (Fig. 1B, Suppl. Fig. S1) [18]. This was associated with increased systolic and diastolic blood pressure, which was not associated with the development of cardiac hypertrophy (Fig. 1C-D, Suppl. Fig. S4A). Gluten mice showed a trend of reduced weight gain compared to the control mice, whereas serum total cholesterol levels were statistically significantly increased (Fig. 1E and F). Glucose homeostasis, as assessed by non-fasting blood glucose levels, was not affected in the mice with CeD (Fig. 1G).
Additionally, mice were kept in metabolic cages for 24 h before the treatment initiation (day 0) and at the end of the treatment period (day 14) to closely monitor food and water intake, which revealed no differences between the groups (Suppl. Figs. S2B–D).
3.2. Immunological effects of gluten challenge on the small intestine of NOD.DQ8
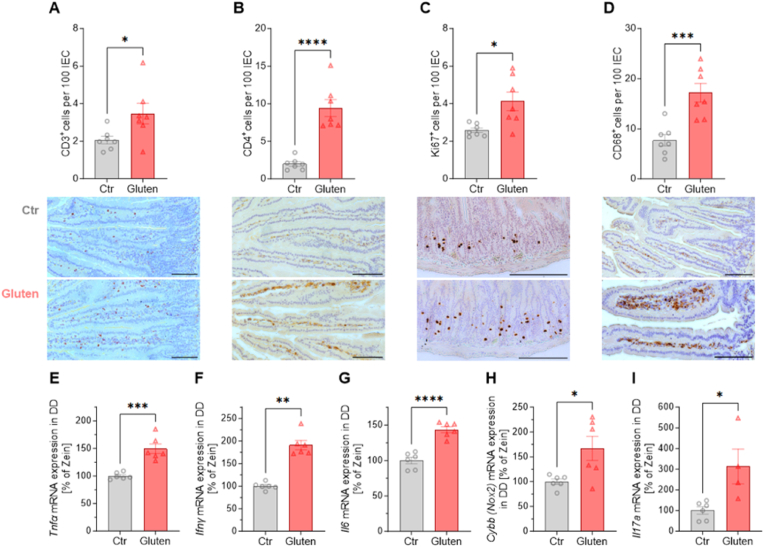
Quantitative immunohistochemistry was performed on duodenal sections of gluten-challenged NOD.DQ8 mice (gluten group) compared to the zein-sensitized NOD.DQ8 controls (ctr group). As in human CeD, CD3+ intraepithelial lymphocytes, lamina propria CD4+ T cells and CD68+ macrophages were significantly increased compared to the controls, as was crypt cell hyperplasia by prominent Ki67 staining, an indicator of enhanced proliferation (Fig. 2A–D). Notably, these gluten-induced changes are hallmarks of human CeD, confirming that our model is valid mimicking the human disease [19,20]. Expression of inflammatory cytokines, tumor necrosis factor (Tnfα), interferon gamma (Ifng), interleukin-6 (Il6), and interleukin-17A (Il17a) mRNA in the duodenum were significantly elevated in gluten mice compared to the controls. Furthermore, transcript levels of cytochrome b-245 (Cybb), encoding for the heavy chain of the superoxide-generating enzyme NADPH oxidase 2 (Nox2) were upregulated indicating enhanced small intestinal oxidative stress (Fig. 2E–I).
Fig. 2.
Immunological effects of gluten challenge on the small intestine of NOD.DQ8.
Distal duodenal (DD) immunohistochemical analysis (IHC) of (A) CD3+ positive intraepithelial lymphocytes (IELs) (B) CD4+ T cells (C) Ki67+ proliferating cells (D) CD68+ cells per 100 intestinal epithelial cells (IEC) together with representative images (scale bars 100 μm). (E) Tumor necrosis factor α (Tnfα), (F) Interferon-gamma (Ifng), (G) Interleukin-6 (Il6), (H)Cybb (encoding for Nox2), (I) Interleukin-17A (Il17a) mRNA expression was measured by qRT-PCR in distal duodenum.
(A)–(D) Unpaired t-test; n(Ctr) = 7, n(G) = 7, (E) Unpaired t-test; n(Ctr) = 6, n(G) = 6, (F) Mann-Whitney test; n(Ctr) = 6, n(G) = 6, (G)–(H) Unpaired t-test; n(Ctr) = 6, n(G) = 6, (I) Unpaired t-test; n(Ctr) = 6, n(G) = 4. Data are means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Taken together, in addition to histological changes typical of CeD, two weeks of gluten challenge in sensitized genetically predisposed NOD.DQ8 mice led to CeD specific immune cell infiltration in the duodenal epithelium and the underlying lamina propria which was accompanied by enhanced expression of pro-inflammatory cytokines.
3.3. Effects of active murine celiac disease on cardiac function, mRNA expression and oxidative and nitro-oxidative stress
Overall, after 2 weeks of gluten challenge cardiac function was not significantly affected, since neither the longitudinal strain of epicardium and endocardium, nor the cardiac ejection fraction and stroke volume were significantly impaired (Fig. 3A–E). However, transthoracic echocardiography (TTE) indicated a trend for an impaired cardiac diastolic volume and cardiac output (Suppl. Fig. S4B).
Fig. 3.
Effects of active murine celiac disease on cardiac function, gene expression and oxidative and nitro-oxidative stress.
Analysis of transthoracic echocardiography (TTE) at day 14 of the treatment (A) longitudinal strain epicardium and (B) longitudinal strain endocardium, (C) ejection fraction, (D) stroke volume, (E) representative M-mode parasternal long axis (PLAX)-images with their left ventricular end-systolic (LVESD) and left ventricular end-diastolic diameter (LVEDD), as well as their anterior (AW) and posterior wall (PW) displayed (for additional parameters, see suppl. Fig. S4B. (F) Vascular cell adhesion molecule-1 (Vcam1) and (G)Cybb (encoding for Nox2) mRNA expression was measured by qRT-PCR with cardiac left ventricular tissue. (H) Cardiac formation of ROS was visualized by dihydroethidium (DHE) staining in cryosections of the left ventricle. Exemplary photomicrographs (red fluorescence indicating superoxide formation) are shown beside the densitometrical analysis. (I) 4-Hydroxynonenal (4-HNE) and (J) 3-nitrotyrosine (3-NT) positive/modified proteins in heart tissue. Representative dot blot images are shown below the densitometrical analysis.
(A)–(D) Unpaired t-test; n(Ctr) = 5, n(G) = 6, (F) Unpaired t-test; n(Ctr) = 12, n(G) = 12, (G) Mann-Whitney test; n(Ctr) = 12, n(G) = 12, (H) Mann-Whitney test; n(Ctr) = 19, n(G) = 20, (I) Unpaired t-test; n(Ctr) = 12, n(G) = 12, (J) Mann-Whitney test; n(Ctr) = 16, n(G) = 16. Data are means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Transcript levels of vascular cell adhesion molecule 1 (Vcam1) and Cybb (encoding for Nox2) in the left ventricle were increased in mice with CeD (Fig. 3F and G). This was accompanied by elevated levels of ROS as detected by superoxide-derived fluorescence of dihydroethidium (DHE)-stained cardiac cryosections, and the abundance of 4-hydroxy-2-nonenal (4-HNE) and 3-nitrotyrosine (3-NT) modified proteins in left ventricular cardiac tissue (Fig. 3H–J). Thus, acute CeD in mice increased cardiac inflammation and nitro-oxidative stress burden, while cardiac function was not significantly impaired.
3.4. Effect of active celiac disease on endothelial-dependent and -independent vascular function, and on vascular and whole blood nitro-oxidative stress
Gluten challenge of gliadin-sensitized NOD.DQ8 mice compared to zein challenged control mice led to impaired vascular function as determined by isometric tension studies. Endothelial-dependent relaxation after acetylcholine (ACh) dosing, but also endothelial-independent relaxation after nitroglycerin (GTN) dosing were reduced in gluten mice. While the contraction curves indicate a statistically significant difference at 25 mM potassium chloride (KCl), the overall curve progression of both groups is comparable (Fig. 4A–C).
Fig. 4.
Effect of active celiac disease on endothelial-dependent and -independent vascular function, and on vascular and whole blood nitro-oxidative stress.
Isometric tension studies of (A) Endothelium-dependent (acetylcholine, ACh) and (B) Endothelium-independent (nitroglycerine, GTN) relaxation and (C) Contraction (potassium chloride, KCl) of thoracic aorta rings. (D) Time course of whole blood oxidative burst determined by enhanced chemiluminescence (L-012 ECL) after zymosan A stimulation. (E) High-performance liquid chromatography (HPLC) was used to detect nitrate (NO3−) and nitrite (NO2−) in plasma. Representative chromatograms are shown beside the quantification graph of NO3−. (F) Densitometrical quantification of immunohistochemistry (IHC) of thoracic aortic rings for 3-NT positive cells/protein together with a representative image. (G) Aortic cryosections stained with dihydroethidium (DHE) were used to detect ROS in the vascular wall. Exemplary photomicrographs are shown below the densitometrical analysis. Laminae (green autofluorescence); superoxide formation (red fluorescence; E-endothelium; M-tunica media; A-tunica adventitia).
(A)–(C) two-way ANOVA and Bonferroni multiple comparison test; n(Ctr) = 30, n(G) = 36, (D) two-way ANOVA and Bonferroni multiple comparison test; n(Ctr) = 2, n(G) = 2, (E) Unpaired t-test; n(Ctr) = 7, n(G) = 7, (F) Mann-Whitney test; n(Ctr) = 11, n(G) = 10, (G) Mann-Whitney test; n(Ctr) = 19, n(G) = 20. Data are means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Whole blood oxidative burst time course after zymosan A stimulation (L-012) was increased in the gluten mice indicating enhanced Nox2 activation in leukocytes (Fig. 4D), accompanied by elevation of plasma levels of nitrite (NO2−, data not shown) and especially nitrate (NO3−, Fig. 4E) compatible with higher levels of aortic iNOS (Fig. 5B). Semi-quantitative immunohistochemistry of the aortic wall revealed increased protein-tyrosine nitration (3-NT) as a consequence of excessive superoxide and nitric oxide formation, and excess overall ROS formation (DHE) (Fig. 4F and G). This demonstrates that CeD in mice has adverse effects on endothelial-dependent and -independent vascular function, and on vascular and whole blood nitro-oxidative stress.
Fig. 5.
CeD drives aortic immune cell infiltration and inflammatory transcript expression.
(A) Vascular cell adhesion molecule-1 (Vcam1), (B)Nos2 (encoding for iNOS), (C) Tumor necrosis factor α (Tnfα), and (D)Cybb (encoding for Nox2) mRNA was measured by qRT-PCR in aortic tissue. (E) Flow cytometry of aortic single-cell suspensions showed CD11b+ myeloid cells (per cm) within the aortic wall (CD11b marker against side scatter area (SSC-A)). Exemplary original plots are shown beside the quantification bar graph.
(A) Unpaired t-test; n(Ctr) = 10, n(G) = 12, (B) Unpaired t-test; n(Ctr) = 13, n(G) = 15, (C) Unpaired t-test; n(Ctr) = 14, n(G) = 16, (D) Mann-Whitney test; n(Ctr) = 14, n(G) = 16, (E) unpaired t-test; n(Ctr) = 6, n(G) = 5. Data are means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
3.5. CeD drives aortic immune cell infiltration and inflammatory transcript expression
Transcripts of vascular cell adhesion molecule 1 (Vcam1), Nos2 (encoding for iNOS), Tnfα and Cybb (Nox2) were increased in the gluten group (Fig. 5A–D), as was aortic wall infiltration by CD11b+ myeloid cells by using flow cytometry (Fig. 5E; for gating strategy, see Suppl. Fig. S5). Increased aortic inflammation was also reflected by elevated transcript levels of Cd11b and Lep (Leptin) in perivascular adipose tissue (PVAT), while no changes were observed in epididymal white adipose tissue (EWAT) (Suppl. Figs. S6A–D). Pro-inflammatory and oxidative stress markers like Tnfα, Cybb (Nox2) and Nos1 (neuronal NOS) and Nos2 (iNOS) were also elevated in the brain tissue of gluten mice (Suppl. Figs. S6E–H).
Thus, active CeD in gluten mice increased not only CD11b+ myeloid cell infiltration and pro-inflammatory markers in the aorta and its surrounding adipose tissue, but also caused inflammatory changes in the brain.
3.6. Remission of celiac disease after 14 days on a gluten-free diet restores a normal cardiovascular phenotype
In addition to the 2-weeks treatment on the gluten-containing (GCD) or gluten-free control diet (GFD) after respective sensitization, mice were allowed an additional 14 days of GFD, which should allow some recovery of the gluten-fed group. (OFF-Study, Fig. 6A; compare Fig. 1A, for diet compositions see Suppl. Fig. S1).
At sacrifice, systolic and diastolic blood pressure were not different between the original control and gluten group (Fig. 6B-C, compare Fig. 1C and D). The reduced weight gain, which resulted from the initial gluten challenge was regained after the diet switch (Fig. 6D, compare Fig. 1E).
Additionally, endothelial-independent (nitroglycerin, GTN) and -dependent (acetylcholine, ACh) relaxation, plasma levels of nitrite (NO2−) and nitrate (NO3−), as well as oxidative stress (DHE staining in aortic cryosections) were restored to normal after the CeD mice were off gluten for 2 weeks (Fig. 6E-H, compare Fig. 4A and B and Fig. 4E + G).
Thus, already within 14 days after diet switch to the GFD, body weight, endothelial dysfunction and markers of nitro-oxidative stress in vessels and blood were restored in the gluten mice.
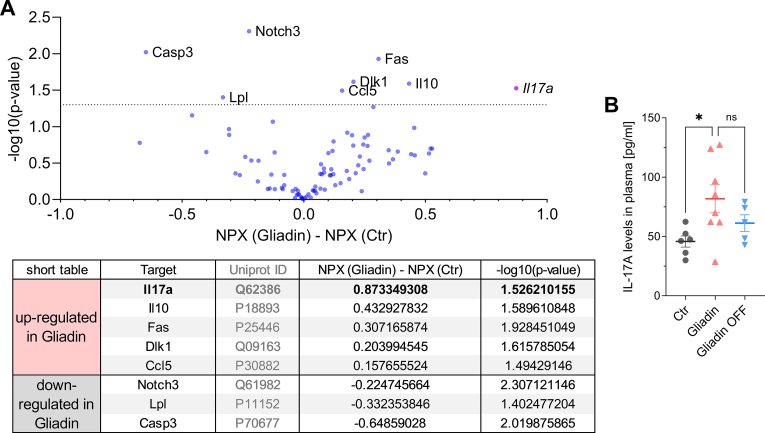
3.7. Plasma proteomics indicate a role of interleukin-17A as a link between intestinal and vascular inflammation
We performed Olink plasma proteomics, to show an upregulation of interleukin-17A (IL-17A) in the gluten mice vs the control mice. The overall proteomics results are shown as a volcano plot, with the most statistically significant proteins listed in the table below (Fig. 7A). Other plasma proteins that were upregulated in the gluten mice vs the controls include IL10, Fas, Dlk1, and CCL5, although the degree of their upregulation was not as prominent as IL-17A. Other plasma proteins were decreased in the gluten mice, including Notch3, Lpl, and Casp3 (Fig. 7A; for complete proteomics results see Suppl. Table S1). The pronounced upregulation of IL-17A in the gluten-fed NOD.DQ8 mice, as well as a subsequent decline by trend of IL-17A plasma levels after the remission phase was confirmed by a mouse IL-17A ELISA (Fig. 7B). Both solidifies the findings from the proteomics analysis and the role of IL-17A as potential link between intestinal and vascular inflammation.
Fig. 7.
Plasma proteomics indicate a role of interleukin-17A as a link between intestinal and vascular inflammation.
(A) Volcano plot of olink plasma proteomics with its most statistically significant (-log10(p-value)>1.3) up- and down-regulated proteins shown in the table below (normalized protein expression, NPX). (B) Plasma levels of IL-17A after 14 days of control and gluten treatment and after 14 days of gluten-free remission (ns, P = 0.2717).
(A) unpaired t-test; n(Ctr) = 11, n(G) = 13, (B)one-way ANOVA and Dunnetts comparison test; n(Ctr) = 6, n(G) = 8; n(G,OFF) = 5. Data are means ± SEM. *P < 0.05.
4. Discussion
With the present study, we demonstrate negative effects of active CeD on the cardiovascular system in a humanized murine model. CeD has been extensively characterized and studied in relation to the intestinal system [[21], [22], [23]]. CeD is induced by certain dietary gluten peptides that escape digestion, to be antigenically presented via HLA-DQ2 or -DQ8 in the intestinal lamina propria where they elicit activation and expansion of gluten-specific aggressive Th1 CD4+ T cells. Moreover, the CeD autoantigen, TG2, deamidates and crosslinks these peptides which increases their immunogenic potential. There is a great interest in therapeutic approaches beyond a GFD, such as the inhibition of TG2 that prevented gluten-induced intestinal damage in the CEC-3 trial [24]. Notably, despite evidence of increased cardiovascular mortality in patients with CeD [11,12], there is no patho-mechanistic link that would explain such effect of (active) CeD on the cardiovascular system, and these epidemiological data have been ignored by current guidelines.
We used a novel human transgenic mouse model of CeD that replicates the central mechanistic and immunological features of the disease [25,26]. Feeding these mice for 2 weeks did not only cause CeD, but also unfavorable cardiovascular effects, including arterial hypertension, impaired endothelium-dependent and -independent vasodilatation, and nitro-oxidative stress in vascular and cardiac tissue, as well as in whole blood. This phenotype evolved without the development of cardiac hypertrophy or detectable loss of cardiac function, nor impacted glucose homeostasis. Instead, it led to reduced weight gain which is associated with the clinical picture of CeD and malabsorption. In contrast, the same mice when exposed to the non-gluten protein zein from maize did not show intestinal damage or any adverse cardiovascular effects. In line with the increased risk of cardiovascular events demonstrated for CeD patients [11,12], the present study supports an interorgan interaction between the intestinal- and cardiovascular system in CeD.
Arterial hypertension has been recognized as an auto-inflammatory disease and a major cardiovascular risk factor [27]. Here, cells of the innate and the adaptive immune system infiltrate the vascular wall and promote vascular inflammation and dysfunction. Myeloid cells [28], but also CD8+ T cells [29] and γδ T cells [30] have been demonstrated to migrate into the vascular wall. CD11b+ myeloid cells are capable of producing high amounts of ROS by the Nox2-type NADPH oxidase. ROS are detrimental for vascular function [31] and are not only generated by myeloid cells but also endothelial, smooth muscle and T cells. Vascular inflammation and oxidative stress subsequently lead to uncoupling of the endothelial nitric oxide synthase (eNOS) and reduction in nitric oxide (NO) bioavailability [32]. In our murine model of active CeD, in which we observe elevated blood pressure associated with inflammation of the cardiovascular system, endothelium-dependent relaxation in the animals’ aortas is significantly impaired after 14 days of the gluten-containing diet in the gluten-sensitized NOD.DQ8 mice. Simultaneously, we find markedly increased oxidative stress in vascular tissues (3-NT and DHE staining in aortic tissue), as well as elevated nitrate levels in plasma. This suggests an excessive nitric oxide formation by upregulation of Nos2 (iNOS) in plasma and an enhanced oxidative break-down of vascular nitric oxide by superoxide [33]. The present data suggests that CD11b+ cells and concomitant Cybb (Nox2) upregulation in the vessel wall are predominantly responsible for the increased cardiovascular oxidative stress in active CeD. The present evidence of altered endothelial function is in line with clinical studies that showed impaired flow-mediated dilation in CeD patients compared to healthy controls [34]. In this vein, other studies showed elevated levels of soluble vascular adhesion molecule-1, soluble intercellular adhesion molecule-1 and soluble endothelial selectin [35] and plasmatic oxidative stress [36] in CeD subjects.
We indicate in our CeD model that a diet induced inflammation within the duodenum and involving CD3+, and CD4+ T cells, and CD68+ monocytes-macrophages, promotes inflammation in another organ system, i.e., the cardiovascular system. Other inflammatory organ-to-organ communications have been demonstrated previously for other tissues and (autoimmune) diseases. Severe psoriasis is meanwhile considered an independent risk factor for cardiovascular mortality [37]. As another example, rheumatoid arthritis is a chronic inflammatory disorder that increases the risk of developing hypertension and cardiovascular disease [5]. In line with our present data for CeD, we have shown that psoriasis and rheumatoid arthritis are also associated with endothelial dysfunction, vascular inflammation and oxidative stress [5,38]. Psoriasis itself and its cardiovascular consequences such as atherosclerosis are mediated by increased IL-17A levels [[39], [40], [41]]. We therefore suggest an inflammatory gut-to-vascular axis based on our data on the observed link between active CeD and vascular inflammation and dysfunction. Notably, the small intestinal inflammation leads to an enhanced production and secretion of key pro-inflammatory cytokines, including TNF-α, IL-1β and IL-17A, which enter the bloodstream and stimulate, recruit, and proliferate immune cells also in extra-intestinal tissues, including the vasculature. Alternatively, activated immune cells, especially monocytes and monocytes-macrophages, can leave the intestinal lamina propria and migrate to extraintestinal sites to fuel inflammation, as previously demonstrated for the myeloid TLR4 which is stimulated by another class of wheat proteins, amylase trypsin inhibitors (ATIs) [[42], [43], [44]]. The thus stimulated vascular immune cell activation, here in CeD then result in the upregulation of adhesion molecules and chemokines, promoting the recruitment of leukocytes and their migration into the vessel wall and other tissues, reflected by higher transcript levels of pro-inflammatory markers in the heart, aorta, surrounding perivascular adipose tissue (PVAT) and the brain. Once there, leukocytes can release further pro-inflammatory mediators and reactive oxygen species, contributing to the propagation of inflammation, tissue destruction and remodeling, and the development of endothelial dysfunction [45,46]. Notably, while all these features already appear after 14 days of gluten feeding, they have disappeared after another 14 days of switching back to the GFD. It can be assumed that longer duration of a gluten containing diet might worsen or prolong the cardiovascular impact. This study underlines not only the importance of the GFD in the treatment of the intestinal inflammation in CeD [47], but also for the suppression of remote inflammation, prominently of the cardiovascular system. The demonstrated proteomics data indicate that CeD-mediated inflammation in the intestine activates pro-inflammatory and counteracting anti-inflammatory pathways like CCL5 or IL-10. But also, pro-apoptotic and pathways of cell differentiation seem to be involved (Fas, Dlk1). The impact on systemic inflammatory pathways indicates a link to inflammasomes.
The NLRP3 is the most studied inflammasome in humans and involved in atherosclerosis as well as hypertension. Its activation leads to immune cell activation and migration. This pro-inflammatory cascade induces IL-17 release by CD4+ T helper 17 cells and γδ T cells which has been reported to be involved in the pathogenesis of arterial hypertension [48]. Vice versa, and more important in celiac disease, high-levels of activated γδ T cells release IL-17 activate NLRP3 with concomitant systemic inflammation in remote organs [49]. Therefore, celiac disease might induce a viscous or amplifying circle of cytokine release (IL-17) from the intestine with subsequent activation of NLRP3, which itself promotes inflammation and immune cell recruitment in remote organs. Blockade of the NLRP3 inflammasome might be a potential therapeutic target to interrupt this viscous circle and prevent vascular inflammation in celiac disease as it has been reported in basic and clinical studies [48].
Our findings of elevated proinflammatory markers (vascular cell adhesion molecule 1 (Vcam1) and Cybb (encoding for Nox2)) and oxidative stress (4-HNE, 3-NT and DHE) are relevant, since it was recently shown that young human subjects with newly diagnosed CeD show left ventricular concentric remodeling, diastolic dysfunction and altered circumferential strain [50]. We assume that a longer gluten-containing diet would also lead to a significantly compromised global longitudinal strain and/or diastolic dysfunction in our mouse model.
The NOD.DQ8 mice used in this study have an increased risk of developing type 1 diabetes [51], which, however, occurs only at advanced age. Our mice were between the ages of 10 and 15 weeks at the time of sacrifice, during which no abnormal blood glucose levels were observed. Furthermore, the reversibility of the cardiovascular phenotype under the GFD supports the gluten and small intestinal inflammation dependent mechanism. A limitation of the study is that we did not specifically examine a potential impact of malnutrition, including micronutrient deficiencies (such as vitamins A, B, D, and E) or nutritional antioxidants, that might have led to increased cardiovascular risk [52]. However, our diets were highly supplemented with such micronutrients and the duration of gluten exposure was very short (2 weeks), too short to cause significant micronutrient deficiencies.
Besides intestinal inflammation by autoimmunity or deficiency of micronutrients, metabolites from a changed microbiome by gluten-containing/free diet should also be considered to be involved in the proinflammatory response of the cardiovascular system as demonstrated in this study. Generally, gut microbiota can facilitate angiotensin–II–induced vascular dysfunction and hypertension by an MCP-1/IL-17-driven immune cell infiltration [53]. Furthermore, the metabolites of microbiota trimethylamine N-oxide (TMAO) and short-chain fatty acid (SCFA) have immune-modulatory and pro-inflammatory effects that might also directly impact vascular function and inflammation in celiac disease. The gut microbiota is implicated in the initiation and perpetuation of intestinal inflammation across various chronic diseases. Instances of intestinal dysbiosis have been documented in patients with CeD, whether they are untreated or undergoing a GFD. Numerous studies have identified distinct bacterial populations associated with CeD patients and healthy individuals. Nevertheless, the causative or consequential relationship between intestinal dysbiosis and CeD remains unclear [54].
Until now, and despite some epidemiological studies to the contrary, CeD has not been considered a cardiovascular risk factor. However, our data indicates that this inflammatory disease with autoimmune characteristics shares immunological features with other autoinflammatory non-cardiovascular diseases such as rheumatoid arthritis and psoriasis. Now, we would suggest including active CeD among those autoinflammatory diseases that seems to adversely affect various organ systems, most notably the cardiovascular system. In addition, may suggest that also other intestinal inflammatory diseases and conditions may affect cardiovascular health.
Based on our data, in-depth mechanistic and functional clinical studies are justified to further investigate the immunological relationship between CeD and cardiovascular risk. Such studies should also extend to other inflammatory diseases of the intestinal tract.
Sources of funding
This study was supported by a grant of the German Research Foundation (DFG STE2528/21, STE2528/41 and DFG INST 371/47-1 FUGG) and by a grant of the Else-Kröner-Fresenius Foundation (Excellence Stipend, 2021_EKES.04) to SS. KK, LS, HU, IK, DM and KVM received PhD fellowships by the TransMed PhD Program at the University Medical Center Mainz. DS received project related support by the German Research Foundation (DFG Schu 646/201/2, Schu 646/24 project B08). SK and JW are supported by the Boehringer Ingelheim Foundation “Novel and neglected cardiovascular risk factors: molecular mechanisms and therapeutic implications”. SK is supported by TRR156/2–246807620 (“The Skin as Sensor and Effector Organ Orchestrating Local and Systemic Immune Responses”, DFG). SK is funded by the Deutsche Herzstiftung and by the German Centre for Cardiovascular Research (DZHK) Excellence Programme (“Interleukin-17A mediated inflammation in myocardial infarction in a mouse model of psoriasis-like skin disease”).
CRediT authorship contribution statement
Karin Keppeler: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Aline Pesi: Writing – original draft, Visualization, Methodology. Simon Lange: Methodology. Johanna Helmstädter: Writing – review & editing, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Lea Strohm: Methodology, Investigation. Henning Ubbens: Methodology, Investigation. Marin Kuntić: Methodology, Investigation. Ivana Kuntić: Methodology, Investigation. Dominika Mihaliková: Methodology, Investigation. Ksenija Vujačić-Mirski: Methodology, Investigation. Alexandra Rosenberger: Methodology, Investigation, Data curation. Leonie Küster: Methodology, Investigation. Charlotte Frank: Methodology. Matthias Oelze: Writing – review & editing, Validation, Supervision, Software, Resources, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation. Stefanie Finger: Writing – review & editing, Resources, Methodology. Agnieszka Zakrzewska: Methodology. Elena Verdu: Resources. Johannes Wild: Resources. Susanne Karbach: Writing – review & editing, Resources, Methodology, Formal analysis. Philip Wenzel: Supervision, Software, Resources, Methodology, Data curation. Philipp Wild: Supervision, Resources, Methodology. David Leistner: Writing – review & editing. Thomas Münzel: Writing – review & editing, Supervision, Resources, Project administration, Funding acquisition, Conceptualization. Andreas Daiber: Writing – review & editing, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Detlef Schuppan: Writing – review & editing, Writing – original draft, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization. Sebastian Steven: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
All authors declared no conflicts of interest.
Acknowledgments
We are indebted to Jörg Schreiner, Nicole Glas and Angelica Karpi for their expert technical assistance and to Swenja Kröller-Schön, Mirka Kvandová, Sanela Rajlic, Maria Teresa Bayo Jimenez, Paul Stamm, Alexander Czarnowski, Tristan Junglas, Agnieszka Zakrzewska, Stefan Chlopicki, Julia Ringen, Tanja Knopp, Katharina Perius, Susann Kahl, Manjusha Neerukonda, Manuel Encalada, Michelle Wiegel, Paul Frankenbach and Julian Ruhnau for their valuable collaboration and support. This publication contains results that are part of the doctoral thesis of Karin Keppeler in affiliation with the Faculty of Biology at Johannes Gutenberg-University Mainz.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2024.103071.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.Murray C.J., Atkinson C., Bhalla K., Birbeck G., Burstein R., Chou D., Dellavalle R., Danaei G., Ezzati M., Fahimi A., Flaxman D., Foreman, Gabriel S., Gakidou E., Kassebaum N., Khatibzadeh S., Lim S., Lipshultz S.E., London S., Lopez, MacIntyre M.F., Mokdad A.H., Moran A., Moran A.E., Mozaffarian D., Murphy T., Naghavi M., Pope C., Roberts T., Salomon J., Schwebel D.C., Shahraz S., Sleet D.A., Murray, Abraham J., Ali M.K., Atkinson C., Bartels D.H., Bhalla K., Birbeck G., Burstein R., Chen H., Criqui M.H., Dahodwala Jarlais, Ding E.L., Dorsey E.R., Ebel B.E., Ezzati M., Fahami, Flaxman S., Flaxman A.D., Gonzalez-Medina D., Grant B., Hagan H., Hoffman H., Kassebaum N., Khatibzadeh S., Leasher J.L., Lin J., Lipshultz S.E., Lozano R., Lu Y., Mallinger L., McDermott M.M., Micha R., Miller T.R., Mokdad A.A., Mokdad A.H., Mozaffarian D., Naghavi M., Narayan K.M., Omer S.B., Pelizzari P.M., Phillips D., Ranganathan D., Rivara F.P., Roberts T., Sampson U., Sanman E., Sapkota A., Schwebel D.C., Sharaz S., Shivakoti R., Singh G.M., Singh D., Tavakkoli M., Towbin J.A., Wilkinson J.D., Zabetian A., Murray, Abraham J., Ali M.K., Alvardo M., Atkinson C., Baddour L.M., Benjamin E.J., Bhalla K., Birbeck G., Bolliger I., Burstein R., Carnahan E., Chou D., Chugh S.S., Cohen A., Colson K.E., Cooper L.T., Couser W., Criqui M.H., Dabhadkar K.C., Dellavalle R.P., Jarlais, Dicker D., Dorsey E.R., Duber H., Ebel B.E., Engell R.E., Ezzati M., Felson D.T., Finucane M.M., Flaxman S., Flaxman A.D., Fleming T., Foreman, Forouzanfar M.H., Freedman G., Freeman M.K., Gakidou E., Gillum R.F., Gonzalez-Medina D., Gosselin R., Gutierrez H.R., Hagan H., Havmoeller R., Hoffman H., Jacobsen K.H., James S.L., Jasrasaria R., Jayarman S., Johns N., Kassebaum N., Khatibzadeh S., Lan Q., Leasher J.L., Lim S., Lipshultz S.E., London S., Lopez, Lozano R., Lu Y., Mallinger L., Meltzer M., Mensah G.A., Michaud C., Miller T.R., Mock C., Moffitt T.E., Mokdad A.A., Mokdad A.H., Moran A., Naghavi M., Narayan K.M., Nelson R.G., Olives C., Omer S.B., Ortblad K., Ostro B., Pelizzari P.M., Phillips D., Raju M., Razavi H., Ritz B., Roberts T., Sacco R.L., Salomon J., Sampson U., Schwebel D.C., Shahraz S., Shibuya K., Silberberg D., Singh J.A., Steenland K., Taylor J.A., Thurston G.D., Vavilala M.S., Vos T., Wagner G.R., Weinstock M.A., Weisskopf M.G., Wulf S., Murray & Collaborators U.S. B.o.D. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collaborators G.B.D.R.F. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vavlukis M., Pop-Gjorcevab D., Poposka L., Sandevska E., Kedev S. Myocardial infarction in systemic lupus erythematosus - the Sex-specific risk profile. Curr. Pharmaceut. Des. 2021;27:3221–3228. doi: 10.2174/1381612826666201210110809. [DOI] [PubMed] [Google Scholar]
- 4.Zwain A., Aldiwani M., Taqi H. The association between psoriasis and cardiovascular diseases. Eur. Cardiol. 2021;16:e19. doi: 10.15420/ecr.2020.15.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skeoch S., Bruce I.N. Atherosclerosis in rheumatoid arthritis: is it all about inflammation? Nat. Rev. Rheumatol. 2015;11:390–400. doi: 10.1038/nrrheum.2015.40. [DOI] [PubMed] [Google Scholar]
- 6.Lundin K.E., Sollid L.M. Advances in coeliac disease. Curr. Opin. Gastroenterol. 2014;30:154–162. doi: 10.1097/MOG.0000000000000041. [DOI] [PubMed] [Google Scholar]
- 7.Lebwohl B., Sanders D.S., Green P.H.R. Coeliac disease. Lancet. 2018;391:70–81. doi: 10.1016/S0140-6736(17)31796-8. [DOI] [PubMed] [Google Scholar]
- 8.Green P.H., Lebwohl B., Greywoode R. Celiac disease. J. Allergy Clin. Immunol. 2015;135:1099–1106. doi: 10.1016/j.jaci.2015.01.044. quiz 1107. [DOI] [PubMed] [Google Scholar]
- 9.van Gils T., Nijeboer P., van Wanrooij R.L., Bouma G., Mulder C.J. Mechanisms and management of refractory coeliac disease. Nat. Rev. Gastroenterol. Hepatol. 2015;12:572–579. doi: 10.1038/nrgastro.2015.155. [DOI] [PubMed] [Google Scholar]
- 10.Ciaccio E.J., Lewis S.K., Biviano A.B., Iyer V., Garan H., Green P.H. Cardiovascular involvement in celiac disease. World J. Cardiol. 2017;9:652–666. doi: 10.4330/wjc.v9.i8.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ludvigsson J.F., James S., Askling J., Stenestrand U., Ingelsson E. Nationwide cohort study of risk of ischemic heart disease in patients with celiac disease. Circulation. 2011;123:483–490. doi: 10.1161/CIRCULATIONAHA.110.965624. [DOI] [PubMed] [Google Scholar]
- 12.Lebwohl B., Green P.H.R., Soderling J., Roelstraete B., Ludvigsson J.F. Association between celiac disease and mortality risk in a Swedish population. JAMA. 2020;323:1277–1285. doi: 10.1001/jama.2020.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolia R., Srivastava A., Kapoor A., Yachha S.K., Poddar U. Children with untreated coeliac disease have sub-clinical cardiac dysfunction: a longitudinal observational analysis. Scand. J. Gastroenterol. 2018;53:803–808. doi: 10.1080/00365521.2018.1473483. [DOI] [PubMed] [Google Scholar]
- 14.Ventura A., Magazzu G., Greco L. Duration of exposure to gluten and risk for autoimmune disorders in patients with celiac disease. SIGEP Study Group for Autoimmune Disorders in Celiac Disease. Gastroenterology. 1999;117:297–303. doi: 10.1053/gast.1999.0029900297. [DOI] [PubMed] [Google Scholar]
- 15.Cosnes J., Cellier C., Viola S., Colombel J.F., Michaud L., Sarles J., Hugot J.P., Ginies J.L., Dabadie A., Mouterde O., Allez M., Nion-Larmurier I., Groupe D'Etude et de Recherche Sur la Maladie, C Incidence of autoimmune diseases in celiac disease: protective effect of the gluten-free diet. Clin. Gastroenterol. Hepatol. 2008;6:753–758. doi: 10.1016/j.cgh.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 16.Molberg O., McAdam S.N., Korner R., Quarsten H., Kristiansen C., Madsen L., Fugger L., Scott H., Noren O., Roepstorff P., Lundin K.E., Sjostrom H., Sollid L.M. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat Med. 1998;4:713–717. doi: 10.1038/nm0698-713. [DOI] [PubMed] [Google Scholar]
- 17.Galipeau H.J., McCarville J.L., Huebener S., Litwin O., Meisel M., Jabri B., Sanz Y., Murray J.A., Jordana M., Alaedini A., Chirdo F.G., Verdu E.F. Intestinal microbiota modulates gluten-induced immunopathology in humanized mice. Am. J. Pathol. 2015;185:2969–2982. doi: 10.1016/j.ajpath.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marsh M.N. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity ('celiac sprue') Gastroenterology. 1992;102:330–354. [PubMed] [Google Scholar]
- 19.ter Steege J., Buurman W., Arends J.W., Forget P. Presence of inducible nitric oxide synthase, nitrotyrosine, CD68, and CD14 in the small intestine in celiac disease. Lab. Invest. 1997;77:29–36. [PubMed] [Google Scholar]
- 20.Moss S.F., Attia L., Scholes J.V., Walters J.R., Holt P.R. Increased small intestinal apoptosis in coeliac disease. Gut. 1996;39:811–817. doi: 10.1136/gut.39.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green P.H., Cellier C. Celiac disease. N. Engl. J. Med. 2007;357:1731–1743. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 22.Schuppan D., Junker Y., Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology. 2009;137:1912–1933. doi: 10.1053/j.gastro.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Verdu E.F., Schuppan D. Co-Factors, microbes, and immunogenetics in celiac disease to Guide novel approaches for diagnosis and treatment. Gastroenterology. 2021;161:1395–1411. doi: 10.1053/j.gastro.2021.08.016. e1394. [DOI] [PubMed] [Google Scholar]
- 24.Schuppan D., Maki M., Lundin K.E.A., Isola J., Friesing-Sosnik T., Taavela J., Popp A., Koskenpato J., Langhorst J., Hovde O., Lahdeaho M.L., Fusco S., Schumann M., Torok H.P., Kupcinskas J., Zopf Y., Lohse A.W., Scheinin M., Kull K., Biedermann L., Byrnes V., Stallmach A., Jahnsen J., Zeitz J., Mohrbacher R., Greinwald R., Group C.E.C.T. A randomized trial of a transglutaminase 2 inhibitor for celiac disease. N. Engl. J. Med. 2021;385:35–45. doi: 10.1056/NEJMoa2032441. [DOI] [PubMed] [Google Scholar]
- 25.Galipeau H.J., Rulli N.E., Jury J., Huang X., Araya R., Murray J.A., David C.S., Chirdo F.G., McCoy K.D., Verdu E.F. Sensitization to gliadin induces moderate enteropathy and insulitis in nonobese diabetic-DQ8 mice. J. Immunol. 2011;187:4338–4346. doi: 10.4049/jimmunol.1100854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamas B., Hernandez-Galan L., Galipeau H.J., Constante M., Clarizio A., Jury J., Breyner N.M., Caminero A., Rueda G., Hayes C.L., McCarville J.L., Bermudez Brito M., Planchais J., Rolhion N., Murray J.A., Langella P., Loonen L.M.P., Wells J.M., Bercik P., Sokol H., Verdu E.F. Aryl hydrocarbon receptor ligand production by the gut microbiota is decreased in celiac disease leading to intestinal inflammation. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.aba0624. [DOI] [PubMed] [Google Scholar]
- 27.Wenzel U.O., Bode M., Kurts C., Ehmke H. Salt, inflammation, IL-17 and hypertension. Br. J. Pharmacol. 2019;176:1853–1863. doi: 10.1111/bph.14359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wenzel P., Knorr M., Kossmann S., Stratmann J., Hausding M., Schuhmacher S., Karbach S.H., Schwenk M., Yogev N., Schulz E., Oelze M., Grabbe S., Jonuleit H., Becker C., Daiber A., Waisman A., Munzel T. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation. 2011;124:1370–1381. doi: 10.1161/CIRCULATIONAHA.111.034470. [DOI] [PubMed] [Google Scholar]
- 29.Youn J.C., Yu H.T., Lim B.J., Koh M.J., Lee J., Chang D.Y., Choi Y.S., Lee S.H., Kang S.M., Jang Y., Yoo O.J., Shin E.C., Park S. Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension. 2013;62:126–133. doi: 10.1161/HYPERTENSIONAHA.113.00689. [DOI] [PubMed] [Google Scholar]
- 30.Caillon A., Mian M.O.R., Fraulob-Aquino J.C., Huo K.G., Barhoumi T., Ouerd S., Sinnaeve P.R., Paradis P., Schiffrin E.L. Gammadelta T cells mediate angiotensin II-induced hypertension and vascular injury. Circulation. 2017;135:2155–2162. doi: 10.1161/CIRCULATIONAHA.116.027058. [DOI] [PubMed] [Google Scholar]
- 31.Cave A.C., Brewer A.C., Narayanapanicker A., Ray R., Grieve D.J., Walker S., Shah A.M. NADPH oxidases in cardiovascular health and disease. Antioxidants Redox Signal. 2006;8:691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- 32.Forstermann U., Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 33.Kossmann S., Hu H., Steven S., Schonfelder T., Fraccarollo D., Mikhed Y., Brahler M., Knorr M., Brandt M., Karbach S.H., Becker C., Oelze M., Bauersachs J., Widder J., Munzel T., Daiber A., Wenzel P. Inflammatory monocytes determine endothelial nitric-oxide synthase uncoupling and nitro-oxidative stress induced by angiotensin II. J. Biol. Chem. 2014;289:27540–27550. doi: 10.1074/jbc.M114.604231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sari C., Bayram N.A., Dogan F.E., Bastug S., Bolat A.D., Sari S.O., Ersoy O., Bozkurt E. The evaluation of endothelial functions in patients with celiac disease. Echocardiography. 2012;29:471–477. doi: 10.1111/j.1540-8175.2011.01598.x. [DOI] [PubMed] [Google Scholar]
- 35.Comba A., Caltepe G., Yank K., Gor U., Yuce O., Kalayc A.G. Assessment of endothelial dysfunction with adhesion molecules in patients with celiac disease. J. Pediatr. Gastroenterol. Nutr. 2016;63:247–252. doi: 10.1097/MPG.0000000000001138. [DOI] [PubMed] [Google Scholar]
- 36.Moretti S., Mrakic-Sposta S., Roncoroni L., Vezzoli A., Dellanoce C., Monguzzi E., Branchi F., Ferretti F., Lombardo V., Doneda L., Scricciolo A., Elli L. Oxidative stress as a biomarker for monitoring treated celiac disease. Clin. Transl. Gastroenterol. 2018;9:157. doi: 10.1038/s41424-018-0031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehta N.N., Azfar R.S., Shin D.B., Neimann A.L., Troxel A.B., Gelfand J.M. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur. Heart J. 2010;31:1000–1006. doi: 10.1093/eurheartj/ehp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karbach S., Croxford A.L., Oelze M., Schuler R., Minwegen D., Wegner J., Koukes L., Yogev N., Nikolaev A., Reissig S., Ullmann A., Knorr M., Waldner M., Neurath M.F., Li H., Wu Z., Brochhausen C., Scheller J., Rose-John S., Piotrowski C., Bechmann I., Radsak M., Wild P., Daiber A., von Stebut E., Wenzel P., Waisman A., Munzel T. Interleukin 17 drives vascular inflammation, endothelial dysfunction, and arterial hypertension in psoriasis-like skin disease. Arterioscler. Thromb. Vasc. Biol. 2014;34:2658–2668. doi: 10.1161/ATVBAHA.114.304108. [DOI] [PubMed] [Google Scholar]
- 39.Schuler R., Brand A., Klebow S., Wild J., Veras F.P., Ullmann E., Roohani S., Kolbinger F., Kossmann S., Wohn C., Daiber A., Munzel T., Wenzel P., Waisman A., Clausen B.E., Karbach S. Antagonization of IL-17a attenuates skin inflammation and vascular dysfunction in mouse models of psoriasis. J. Invest. Dermatol. 2019;139:638–647. doi: 10.1016/j.jid.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 40.Czubkowski P., Osiecki M., Szymanska E., Kierkus J. The risk of cardiovascular complications in inflammatory bowel disease. Clin. Exp. Med. 2020;20:481–491. doi: 10.1007/s10238-020-00639-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Stebut E., Boehncke W.H., Ghoreschi K., Gori T., Kaya Z., Thaci D., Schaffler A. IL-17A in psoriasis and beyond: cardiovascular and metabolic implications. Front. Immunol. 2019;10:3096. doi: 10.3389/fimmu.2019.03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zevallos V.F., Raker V., Tenzer S., Jimenez-Calvente C., Ashfaq-Khan M., Russel N., Pickert G., Schild H., Steinbrink K., Schuppan D. Nutritional wheat amylase-trypsin inhibitors promote intestinal inflammation via activation of myeloid cells. Gastroenterology. 2017;152:1100–1113. doi: 10.1053/j.gastro.2016.12.006. e1112. [DOI] [PubMed] [Google Scholar]
- 43.Zevallos V.F., Raker V.K., Maxeiner J., Scholtes P., Steinbrink K., Schuppan D. Dietary wheat amylase trypsin inhibitors exacerbate murine allergic airway inflammation. Eur. J. Nutr. 2019;58:1507–1514. doi: 10.1007/s00394-018-1681-6. [DOI] [PubMed] [Google Scholar]
- 44.Dos Santos Guilherme M., Zevallos V.F., Pesi A., Stoye N.M., Nguyen V.T.T., Radyushkin K., Schwiertz A., Schmitt U., Schuppan D., Endres K. Dietary wheat amylase trypsin inhibitors impact alzheimer's disease pathology in 5xFAD model mice. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21176288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turner M.D., Nedjai B., Hurst T., Pennington D.J. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta. 2014;1843:2563–2582. doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 46.Aboukhater D., Morad B., Nasrallah N., Nasser S.A., Sahebkar A., Kobeissy F., Boudaka A., Eid A.H. Inflammation and hypertension: underlying mechanisms and emerging understandings. J. Cell. Physiol. 2023 doi: 10.1002/jcp.31019. [DOI] [PubMed] [Google Scholar]
- 47.Ghazanfar H., Javed N., Lee S., Shaban M., Cordero D., Acherjee T., Hasan K.Z., Jyala A., Kandhi S., Hussain A.N., Patel H. Novel therapies for celiac disease: a clinical review article. Cureus. 2023;15 doi: 10.7759/cureus.39004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olsen M.B., Gregersen I., Sandanger O., Yang K., Sokolova M., Halvorsen B.E., Gullestad L., Broch K., Aukrust P., Louwe M.C. Targeting the inflammasome in cardiovascular disease. JACC Basic Transl Sci. 2022;7:84–98. doi: 10.1016/j.jacbts.2021.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang L., Liu P., Wen W., Bai X., Zhang Y., Liu M., Wang L., Wu Y., Yuan Z., Zhou J. IL-17A contributes to myocardial ischemic injury by activating NLRP3 inflammasome in macrophages through AMPKalpha/p38MAPK/ERK1/2 signal pathway in mice. Mol. Immunol. 2019;105:240–250. doi: 10.1016/j.molimm.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 50.Sciatti E., Bernardi N., Dallapellegrina L., Valentini F., Fabbricatore D., Scodro M., Cotugno A., Alonge M., Munari F., Zanini B., Ricci C., Vizzardi E. Evaluation of systo-diastolic cardiac function and arterial stiffness in subjects with new diagnosis of coeliac disease without cardiovascular risk factors. Intern Emerg Med. 2020;15:981–988. doi: 10.1007/s11739-019-02261-7. [DOI] [PubMed] [Google Scholar]
- 51.Luce S., Guinoiseau S., Gadault A., Letourneur F., Blondeau B., Nitschke P., Pasmant E., Vidaud M., Lemonnier F., Boitard C. Humanized mouse model to study type 1 diabetes. Diabetes. 2018;67:1816–1829. doi: 10.2337/db18-0202. [DOI] [PubMed] [Google Scholar]
- 52.Conroy M., Allen N., Lacey B., Soilleux E., Littlejohns T. Association between coeliac disease and cardiovascular disease: prospective analysis of UK Biobank data. BMJ Med. 2023;2 doi: 10.1136/bmjmed-2022-000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karbach S.H., Schonfelder T., Brandao I., Wilms E., Hormann N., Jackel S., Schuler R., Finger S., Knorr M., Lagrange J., Brandt M., Waisman A., Kossmann S., Schafer K., Munzel T., Reinhardt C., Wenzel P. Gut microbiota promote angiotensin II-induced arterial hypertension and vascular dysfunction. J. Am. Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chibbar R., Dieleman L.A. The gut microbiota in celiac disease and probiotics. Nutrients. 2019;11 doi: 10.3390/nu11102375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.