Abstract
Background
Neoadjuvant chemo-immunotherapy combination has shown remarkable advances in the management of esophageal squamous cell carcinoma (ESCC). However, the identification of a reliable biomarker for predicting the response to this chemo-immunotherapy regimen remains elusive. While computed tomography (CT) is widely utilized for response evaluation, its inherent limitations in terms of accuracy are well recognized. Therefore, in this study, we present a novel technique to predict the response of ESCC patients before receiving chemo-immunotherapy by testing volatile organic compounds (VOCs) in exhaled breath.
Methods
This study employed a prospective-specimen-collection, retrospective-blinded-evaluation design. Patients’ baseline breath samples were collected and analyzed using high-pressure photon ionization time-of-flight mass spectrometry (HPPI-TOFMS). Subsequently, patients were categorized as responders or non-responders based on the evaluation of therapeutic response using pathology (for patients who underwent surgery) or CT images (for patients who did not receive surgery).
Results
A total of 133 patients were included in this study, with 91 responders who achieved either a complete response (CR) or a partial response (PR), and 42 non-responders who had stable disease (SD) or progressive disease (PD). Among 83 participants who underwent both evaluations with CT and pathology, the paired t-test revealed significant differences between the two methods (p < 0.05). For the breath test prediction model using breath test data from all participants, the validation set demonstrated mean area under the curve (AUC) of 0.86 ± 0.06. For 83 patients with pathological reports, the breath test achieved mean AUC of 0.845 ± 0.123.
Conclusions
Since CT has inherent weakness in hollow organ assessment and no other ideal biomarker has been found, our study provided a noninvasive, feasible, and inexpensive tool that could precisely predict ESCC patients’ response to neoadjuvant chemo-immunotherapy combination using breath test based on HPPI-TOFMS.
Subject terms: Predictive markers, Oesophageal cancer
Introduction
Esophageal squamous cell carcinoma (ESCC) is the most common pathological type of esophageal cancer (EC) in China, with the majority of patients being diagnosed at a late stage [1]. Although neoadjuvant immunotherapy combined with chemotherapy has shown significant progress in treating ESCC [2], there is a subset of patients who do not benefit from this combination, which poses risks of adverse events and economic losses [3]. Therefore, there is a pressing need for biomarkers that can predict the response to chemo-immunotherapy combination before initiating the treatment [4]. Some potential biomarkers considered for immunotherapy response in ESCC include tumor mutation burden (TMB), programmed death receptor-1 (PD-1) or programmed death ligand-1 (PD-L1) expression levels, circulating tumor DNA, microsatellite instability, and gut microbiome composition. However, these biomarkers have yet to provide satisfactory results in immunotherapy, let alone reliable biomarkers for chemo-immunotherapy in ESCC [5].
For locally advanced ESCC, chemo-immunotherapy followed by surgical resection is considered the optimal treatment [6]. The commonly used tool for evaluating treatment response before surgery is CT-based modified Response Evaluation Criteria in Solid Tumors (iRECIST) [7]. However, CT has limitations when assessing hollow organs without lumen distension, such as gastrointestinal tumors, potentially leading to imprecise results based on subjective clinician judgment [8]. Consequently, endoscopic ultrasonography (EUS) and positron emission tomography (PET) are recommended for staging and therapeutic evaluation, but their invasiveness and cost restrict their widespread use [8, 9].
Human exhaled breath contains thousands of volatile organic compounds (VOCs), which are generated during various biological or pathological processes, including lipid peroxidation, cytochrome P450 family, dysregulated cellular metabolism, or microbiome interactions [10]. VOC profiles have been closely associated with individuals’ biological or pathological status [11]. In this study, our hypothesis is that ESCC patients who will respond or not respond to the chemo-immunotherapy may exhibit distinct baseline VOC profiles that can be detected using high-pressure proximal ionization time-of-flight mass spectrometry (HPPI-TOFMS). HPPI-TOFMS is a direct and online mass spectrometry technique that ionizes air samples directly, eliminating the need for pre-treatment. Its efficacy in EC detection has been confirmed in our previous study [12].
The objective of this study is to investigate whether a breath test using HPPI-TOFMS can accurately predict therapeutic response among ESCC patients before receiving the combination therapy.
Methods
Patients and sample collection
This study was conducted using a prospective-specimen-collection, retrospective-blinded-evaluation design [13]. The data analysis team were blind to the clinical evaluations and clinicians who performed surgery and endoscopy were also blind to results of breath testing.
Patients at The First Affiliated Hospital of Zhengzhou University were recruited according to the following inclusion criteria: 1) age >18, 2) diagnosed with ESCC and scheduled to receive chemo-immunotherapy combination, 3) no history of other cancer within 5 years, and no prior anti-cancer treatment. Patients meeting any of the following criteria were excluded: 1) active infections, 2) liver or kidney dysfunction.
Baseline exhaled breath samples were collected prior to the initiation of any treatments. Trained researchers followed a standardized protocol, ensuring all participants fasted for a minimum of 6 h and abstained from consuming spicy foods, alcohol, or coffee the previous night. To maintain consistency, participants first rinsed their mouths with pure water, followed by a deep nasal inhalation and a complete exhalation into a Tedlar air bag. To eliminate background interference from sampling components (breathing bags) and room air, breath samples were obtained within a controlled environment, and samples of the surrounding air were also collected before and after the participants exhaled. All air bags were promptly delivered to the laboratory and analyzed using HPPI-TOFMS within a maximum timeframe of 4 h. Moreover, to ensure accurate analysis and quality control, we performed calibration tests using standardized samples to verify the instrument’s operational status and validate the accuracy of the exhaled breath samples.
The study conformed to the principles of the Reporting recommendations for tumor MARKer prognostic studies (REMARK) [11] and fulfil the requirements for study design, conduct, analysis and evaluation in predictive biomarkers [14]. This study has been approved by the Ethic Committee of the First Affiliated Hospital of Zhengzhou University and has been registered in the Chinese Clinical Trial Registry (www.chictr.org.cn, registry ID: hiCTR2000040966). A written signed informed consent was obtained from all patients before entering any study procedure.
Mass spectrometry detection
Analysis process of exhaled breath is through HPPI-TOFMS as previously described [12]. Briefly, exhaled breath air was directly introduced into the ionization region through a 250 μm i.d. 0.60 m long stainless-steel capillary from Tedlar bags. The HPPI-TOFMS consisted of a VUV lamp (Heraeus, Germany)-based HPPI ion source and an orthogonal acceleration TOF (oa-TOF) mass analyzer, and the TOF mass analyzer had a mass resolution of 4000 (FWHM) at m/z = 92, which was achieved with a 0.4 m field-free drift tube. The TOF signals were recorded by a 400 ps time-to-digital converter rate at 25 kHz, and all the mass spectra were accumulated for 60 seconds. The pressure in the HPPI ion source was set at 500 Pa, and two capillaries were arranged in the ion source. In order to eliminate condensation of exhaled VOCs and minimize possible surface adsorption, the stainless-steel capillary was heated to 100°Cand the HPPI ion source was heated to 60°C. Mass spectrum peaks with a m/z less than 350 were recorded.
Data collection
Exhaled breath data obtained from various machines and time points were subjected to a batch-effect-removal algorithm to eliminate any system-specific biases. During data preprocessing, we delineated five steps for spectral: smoothing, baseline correction, peak detection, peak alignment, and area-under-peak estimation. Initially, the Savitzky-Golay filter [15] is judiciously employed for noise abatement on individual spectra. Subsequently, given the observed incongruities in baseline values across different measurements, the Top-Hat algorithm [16] is invoked to synchronize the baseline of all spectra to a unified standard. During the phase of peak identification, by comparing the intensity of each spectral point with its neighbors, significant peaks are identified, concurrently eliminating those whose prominence falls short of ten units. After that, leveraging identified peaks as benchmarks, spectra are linearly scaled and coalesced with a canonical template. The culmination of this procedure entails the computation of the area beneath each peak, designated as its characteristic value. Upon this rigorous refinement, each spectrum emerges endowed with a repertoire of 252 distinctive features.
For clinical data analysis, we utilized the Becker criteria for tumor regression grading (TRG) to assess therapeutic response using resected tissues [17]. Patients who were unable to undergo surgery due to poor efficacy or other complications were evaluated based on CT reports using the iRECIST criteria [7]. The participants were categorized as Responders if they achieved complete or partial response (CR or PR), while non-Responders were defined as those showing stable disease or progressive disease (SD or PD).
Statistical analysis
Python (version 3.10.4) were used for data analysis. Descriptive statistics were expressed as mean + SD if data were normally distributed and as median (interquartile range) if data were non-normally distributed.
We evaluated CT-evaluation and pathology-evaluation by paired t-tests and linearly weighted Kappa was used to measure the agreement between the two evaluations.
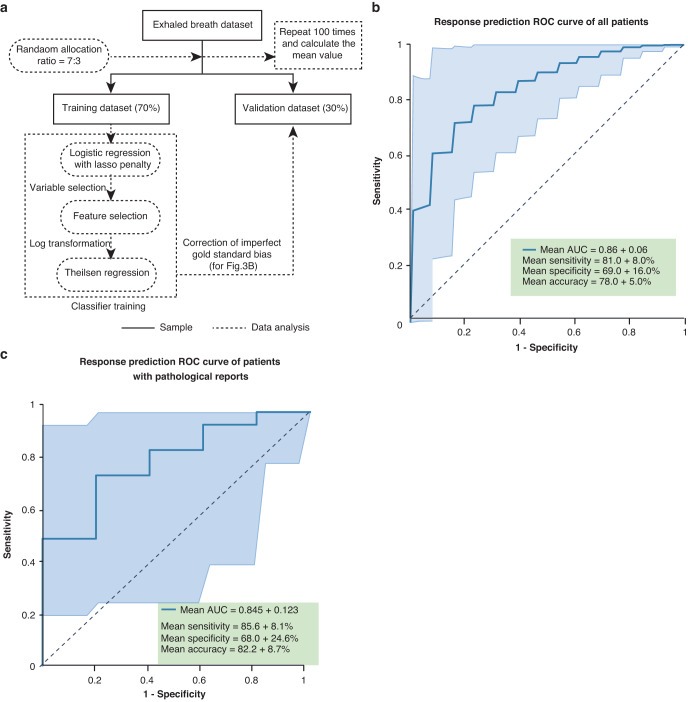
The analysis process involved two steps. In the first step, we extracted the exhaled breath variables of all participants. The response of patients to chemo-immunotherapy was taken into account as a label for the treatment. The data were partitioned into training and validation sets in a 7:3 ratio [18]. Due to observed data imbalance, the Synthetic Minority Over-sampling Technique (SMOTE) was applied to the training set. Subsequent variable selection employed logistic regression with Lasso penalty [19]. Data were log-transformed to minimize outlier effects, and Theilsen Regression was used in model construction for its robustness in handling outliers [20], modeling 19 selected variables. Once the model was established, the test set was engaged. We added an imperfect gold standard bias to the modeling in order to strengthen the robustness of the results [21]. After 100 repetitions, average values and standard deviations were computed, and the receiver operator characteristic (ROC) curve was illustrated, anchored by the mean and its 95% confidence interval (CI).
The two sub-groups of participants with pathological reports and evaluable tumor lesions in CT were extracted separately, and the above process was repeated in order to validate the prediction model and determine whether the different classification had an impact on the prediction.
Results
Patient characteristics
A total of 133 eligible patients were enrolled in this study. The median age was 66 years, with the majority being male (64.5%). All patients received a combination treatment of anti-PD-1 immune checkpoint inhibitors and platinum-based doublet chemotherapies. Among them, 92 patients achieved CR or PR and were categorized as Responders, while 41 patients demonstrated either SD or PD were categorized as non-Responders. The recruitment process and clinical characteristics of the subjects have been presented in Fig. 1 and summarized in Table 1, respectively.
Fig. 1. The flow chart shows recruitment and allocation process.
*iRECIST modified Response Evaluation Criteria in Solid Tumours 1.1 for immune based therapeutics; TRG tumor regression grading.
Table 1.
Patient Demographics and Characteristics (n = 133).
| Response | non-Responsea | P | |
|---|---|---|---|
| n | 91 | 42 | |
| Mean age(y), mean ± SDa | 65.0 ± 8.2 | 63.7 ± 7.2 | 0.39 |
| Sex (male), % | 65.9 | 76.1 | 0.23 |
| BMIa (kg/m2), mean ± SD | 23.4 ± 3.1 | 22.7 ± 2.4 | 0.65 |
| Smoking | 0.30 | ||
| Smoking history, n (%) | 39 (42.8%) | 22 (52.3%) | |
| Never, n (%) | 52 (57.1%) | 20 (47.6%) | |
| Alcohol use | 0.92 | ||
| Drinking history n (%) | 36 (39.5%) | 17 (40.4%) | |
| Never, n (%) | 55 (60.4%) | 25 (59.5%) | |
| Histological Subtypes | 0.79 | ||
| ESCC, n (%) | 88 (96.7%) | 41 (97.6%) | |
| EAC, n (%) | 2 (2.1%) | 1 (2.3%) | |
| Other, n (%) | 1 (1.0%) | 0 | |
| Anti-PD-1a agent, n (%) | 0.29 | ||
| Camrelizumab, n (%) | 74 (81.3%) | 38 (90.4%) | |
| Pembrolizumab, n (%) | 6 (6.5%) | 1 (2.3%) | |
| Sintilimab, n (%) | 6 (6.5%) | 0 | |
| Tislelizumab, n (%) | 4(4.3%) | 3(7.1%) | |
| Toripalimab, n (%) | 1(1.0%) | 0 |
aSD standard deviation, BMI body mass index, PD-1 programmed death receptor 1.
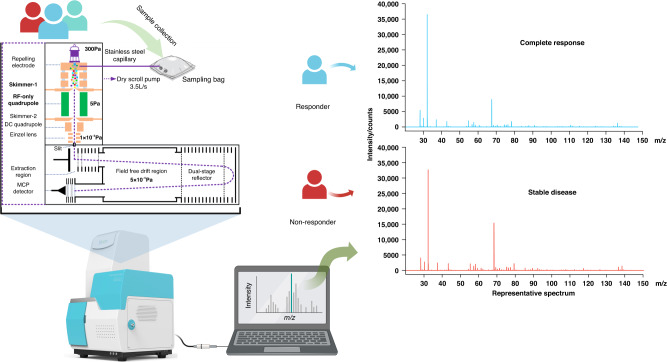
Additionally, we have provided a graphical representation of the sample collection and detection process, along with two representative spectra from a responder and a non-responder, in Fig. 2.
Fig. 2. Sample collection and VOCs detection.
We collected and detected baseline exhaled breath from patients by HPPI-TOFMS. We illustrate two representative spectrum from a responder and a non-responder. The upper panel is the spectrum of a patient achieved complete response. The lower panel is the spectrum of a patient achieved stable disease. VOCs, volatile organic compounds.
CT is not accurate for therapeutic response evaluation
We evaluated the correlation between CT and pathology evaluations in the context of therapeutic response evaluation. Among the 83 participants who underwent both evaluations (as shown in Table 2), the paired t-test revealed a significant difference between the two methods, with a p value of less than 0.05 (Table 3). Furthermore, the result of the linearly weighted Kappa coefficient (k = 0.507) indicated incomplete agreement between CT-evaluation and pathology-evaluation (Table 3). These findings strongly suggest that the therapeutic response evaluation conducted by CT-based iRECIST may not be considered a precise and perfect gold standard.
Table 2.
Clinical Characteristics of Patients with both CT-evaluation and Pathology-evaluation (n = 83).
| Response | Non-responsea | P | |
|---|---|---|---|
| n | 66 | 17 | |
| Mean age(y), mean ± SDa | 64.3 ± 7.9 | 64.2 ± 6.0 | 0.96 |
| Sex (male), % | 72.7 | 88.2 | 0.18 |
| BMIa (kg/m2), mean ± SD | 23.4 ± 3.1 | 24.6 ± 3.0 | 0.14 |
| Smoking | 0.45 | ||
| Smoking history, n (%) | 33 (50.0%) | 10 (58.8%) | |
| Never, n (%) | 33 (50.0%) | 7 (41.1%) | |
| Alcohol use | 0.52 | ||
| Drinking history n (%) | 30 (45.4%) | 6 (35.2%) | |
| Never, n (%) | 36 (54.5%) | 11 (64.7%) | |
| Histological Subtypes | |||
| ESCC, n (%) | 66 (100%) | 17 (100%) | |
| EAC, n (%) | 0 | 0 | |
| Other, n (%) | 0 | 0 | |
| Anti-PD-1a agent, n (%) | 0.30 | ||
| Camrelizumab, n (%) | 54 (81.8%) | 16 (94.1%) | |
| Pembrolizumab, n (%) | 5 (7.5%) | 0 | |
| Sintilimab, n (%) | 3 (4.5%) | 0 | |
| Tislelizumab, n (%) | 3 (4.5%) | 1 (5.8%) | |
| Toripalimab, n (%) | 1 (1.5%) | 0 |
aSD standard deviation, BMI body mass index, PD-1 programmed death receptor 1.
Table 3.
CT-evaluation and pathology-evaluation results of therapeutic response.
| CT evaluation | Pathology evaluation | |
|---|---|---|
| Response | Non-response | |
| Response | 57 | 3 |
| Non-response | 9 | 14 |
Prediction model construction based on breath test
We initially adjusted the imperfect response evaluated by CT. Subsequently, we employed the pathological and adjusted CT evaluations as labels to test the predictive performance of the breath test. All 133 participants were included in the analysis, with 93 patients randomly assigned to the training set and 40 patients to the validation set (Fig. 3a). As described in Methods section, after 100 repetitions, the mean value was calculated and the ROC curve was plotted (Fig. 3b). The breath test prediction model reached a mean AUC of 0.86 ± 0.06, sensitivity of 81.0% ± 8.0%, specificity of 69.0% ± 16.0%, accuracy of 78.0% ± 5.0%, and precision of 87.0% ± 6.0% in the validation set.
Fig. 3. Prediction model construction based on breath test.
a The flow chart shows the data analysis process. All 133 patients’ data set recorded by HPPI-TOFMS were randomly allocated into training set and test set in a ratio of 7:3. b Receiver operator characteristic curve (ROC) curve (with 95%CI) for the selection of responders in the validation set of all patients, c patients with pathological reports. CI confidence interval.
We further selected 83 patients (Table 2) who had undergone surgery and evaluated with pathological reports, then analyzed breath data of the 83 patients with the same procedure shown in Fig. 3a. As a result, the breath test achieved a mean AUC of 0.845 ± 0.123, sensitivity of 85.6% ± 8.1%, specificity of 68.0% ± 24.6%, accuracy of 82.2% ± 8.7%, and precision of 91.7% ± 6.2% in the validation set (Fig. 3c).
Discussion
PD-L1 expression and TMB are widely recognized as prominent biomarkers for PD-1/PD-L1 treatment. However, these biomarkers are imperfect in identifying ESCC patients who will benefit from such treatment. Previous studies have reported that EC patients with high T-cell receptor clonality and low molecular tumor burden index have better survival outcomes [22]. patients who are PD-L1 positive and have tumor infiltrating lymphocytes are more likely to benefit from immunotherapy in various tumors [23]. Additionally, tertiary lymphoid structures have been found to predict immunotherapy response independent of PD-L1 status [24]. In a study by Cristescu et al. [25], it was observed that the group with high T-cell inflammatory gene expression profile and high TMB showed the strongest therapeutic response. Other factors such as serum RNA (including miRNA [26], LncRNA [27] and circRNA [28]), CpGs methylation level [29], host microbiota and its derivative metabolites [30] have also been associated with immunotherapy response.
However, while these biomarkers have been extensively studied in the context of immunotherapy, none of them have achieved accurate response prediction specifically in ESCC. With the growing evidence supporting chemo-immunotherapy as a first-line treatment for ESCC [31, 32], there is a need for an ideal biomarker that is minimally invasive, easy to collect, reliable, inexpensive, and capable of accurately identifying treatment response. To the best of our knowledge, this is the first study to test exhaled breath by HPPI-TOFMS to assess chemo-immunotherapy response of ESCC patients.
An ideal biomarker should minimally invasive, easy to collect, reliable, inexpensive and can be used to accurately identify a treatment response. Breath test process is relatively simple, requiring only the collection of exhaled breath into a gas bag without the need for other testing reagents and equipment. And the data analysis software was developed in collaboration with our teams, so there is no additional cost required. Therefore, the cost of our testing mainly comes from the sampling gas bag. Each Tedlar bag costs $15 but can be reused for about 30 times. As a result, the average cost per patient is only $0.5.
However, there are limitations to our study. Due to various factors such as death, changes in treatment modality or medical center, a significant number of advanced ESCC patients with poor response to chemo-immunotherapy were lost to follow-up. As a result, the proportion of participants rated as PD was very low, while a high proportion of participants were rated as CR or PR among all enrolled patients. Therefore, the interpretation of our results should be cautious since this is a single-center study, and further studies with multiple centers and independent validation are needed to test the predictive performance of the breath test.
In our study, we confirmed that using HPPI-TOFMS to test exhaled breath could accurately predict the response of ESCC patients to chemo-immunotherapy before it is performed, suggesting the potential clinical application of the breath test. In several studies, breath tests were shown to effectively predict responses to immunotherapy in non-small-cell carcinoma (NSCLC) [33] and malignant pleural mesothelioma [34], providing a quicker and noninvasive method compared to RECIST [33, 35]. Nevertheless, neither our study nor those mentioned above have identified specific chemical compounds that are linked to the prediction model. To further investigate this, we will continue our research by expanding our sample size and enhancing the HPPI-TOFMS technique for the identification of characteristic compounds.
Considering the advantages of noninvasiveness, low cost, and high accuracy, the breath test based on HPPI-TOFMS shows promise as a triage test for ESCC patients before chemo-immunotherapy is applied, thus facilitating clinical decision making. It has been demonstrated CT has poor accuracy for assessment of response to neoadjuvant therapy in patients with EC, compared with EUS and PET [8, 9].
In our study, we only analyzed baseline breath data collected before the application of chemo-immunotherapy. The composition of VOCs in breath may vary according to the tumor metabolic status and microenvironment [36]. These dynamic changes may also occur during the development of ESCC and the course of chemo-immunotherapy. The inherent advantages of breath testing and the data from our study underscore the clinical value of breath testing for dynamic therapeutic monitoring during chemo-immunotherapy, especially for ESCC patients with unevaluable primary lesions.
Hence, sample collection at different time points may be more helpful in elucidating the role of exhaled breath testing and exploring the possibility of replacing CT testing in predicting treatment efficacy. We have designed and implemented a new study in which patients’ VOCs were collected and analyzed at the same time points as each CT scan. We expect to identify changes in VOCs at different time points during treatment and demonstrate the advantages of breath testing over CT.
According to current evidence, several factors have been proved to be associated with response of neoadjuvant chemo-immunotherapy, such as L-arginine [37] and microbiota [38]. It has been reported that circulating L-arginine is associated response to immunotherapy [37]. And breath includes thousands small VOCs and breath metabolomics also show metabolic pattern of an individual patients [38]. The composition of intratumoral microbiota in ESCC is a potential predictive biomarker for regulating the response to chemo-immunotherapy [39]. The study conducted by Bhandari et al. reveals that testing exhaled breath VOCs can help identify volatile metabolites and evaluate the functional influence of the microbiome on gastric cancer [40]. Thus, it is plausible to predict response to neoadjuvant chemo-immunotherapy by breath testing.
In conclusion, ESCC patients who responded or did not respond to chemo-immunotherapy exhibited distinct VOC mass spectrum profiles. HPPI-TOFMS-based breath testing could accurately distinguish between these two groups by detecting these differences and could potentially monitor treatment response dynamically. Therefore, exhaled breath testing may be a superior method to CT and other biomarkers for distinguishing responders from non-responders, and it may serve as a valuable tool for clinical decision making.
Acknowledgements
This work is partially supported by the National Natural Science Foundation of China (82303567, 82173386), Peking University People’s Hospital Research and Development Founds (RDH2021-07 and RZ2022-04), Beijing Nova Program (20230484314) and Research Project of Shenzhen Second People’s Hospital (20213357024).
Author contributions
MQ: Conceptualization, Methodology, Supervision, Writing- Reviewing and Editing, Funding acquisition. QH: Resources, Data curation, Writing- Original draft preparation. ZL: Writing – Original draft preparation, Investigation, Visualization. YY: Formal analysis, Software, Validation. ZR: Data curation, Software. PW: Data curation. SW: Resources. HW: Clinical interpretation, Editing. XY: Writing- Reviewing and Editing, Methodology. WCC: Writing – Reviewing and Editing. TM: Data curation. JL: Data curation. JZ: Data curation. XL: Conceptualization, Methodology, Supervision. YH: Conceptualization, Methodology, Supervision.
Competing interests
The authors declare no conflict of interest.
Ethics approval
This study has been approved by the Ethic Committee of the First Affiliated Hospital of Zhengzhou University and has been registered in the Chinese Clinical Trial Registry (www.chictr.org.cn, registry ID: hiCTR2000040966). A written signed informed consent was obtained from all patients before entering any study procedure. The study was performed in accordance with the Declaration of Helsinki.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Qi Huang, Zheng Liu, Yipei Yu.
Contributor Information
Mantang Qiu, Email: qiumantang@163.com.
Yan Hou, Email: houyan@bjmu.edu.cn.
Xiangnan Li, Email: lxn-2000@163.com.
References
- 1.Abnet CC, Arnold M, Wei WQ. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology. 2018;154:360–73. doi: 10.1053/j.gastro.2017.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398:759–71. doi: 10.1016/S0140-6736(21)01234-4. [DOI] [PubMed] [Google Scholar]
- 3.Topalian SL, Taube JM, Pardoll DM. Science. 2020;367:eaax0182. doi: 10.1126/science.aax0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He S, Xu J, Liu X, Zhen Y. Advances and challenges in the treatment of esophageal cancer. Front Oncol. 2020;10:763. doi: 10.1016/j.apsb.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sundar R, Smyth EC, Peng S, Yeong JPS, Tan P. Predictive biomarkers of immune checkpoint inhibition in gastroesophageal cancers. Front Oncol. 2020;10:763. doi: 10.3389/fonc.2020.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leng XF, Daiko H, Han YT, Mao YS. Optimal preoperative neoadjuvant therapy for resectable locally advanced esophageal squamous cell carcinoma. Ann N. Y Acad Sci. 2020;1482:213–24. doi: 10.1111/nyas.14508. [DOI] [PubMed] [Google Scholar]
- 7.Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–e152. doi: 10.1016/S1470-2045(17)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halvorsen RA, Thompson WA. Computed tomography of the gastroesophageal junction. Crit Rev Diagn Imaging. 1984;21:183–228. [PubMed] [Google Scholar]
- 9.Ulla M, Gentile EM, Cavadas D, Yeyati EL, Frank L, Argerich JI, et al. Esophageal cancer characterization with pneumo-64-MDCT. Abdom Imaging. 2012;37:501–11. doi: 10.1007/s00261-011-9784-z. [DOI] [PubMed] [Google Scholar]
- 10.Horváth I, Lázár Z, Gyulai N, Kollai M, Losonczy G. Exhaled biomarkers in lung cancer. Eur Respir J. 2009;34:261–75. doi: 10.1183/09031936.00142508. [DOI] [PubMed] [Google Scholar]
- 11.Gaspar EM, Lucena AF, Duro da Costa J, Chaves das Neves H. Organic metabolites in exhaled human breath-a multivariate approach for identification of biomarkers in lung disorders. J Chromatogr A. 2009;1216:2749–56. doi: 10.1016/j.chroma.2008.10.125. [DOI] [PubMed] [Google Scholar]
- 12.Huang Q, Wang S, Li Q, Wang P, Li J, Meng S, et al. Assessment of breathomics testing using high-pressure photon ionization time-of-flight mass spectrometry to detect esophageal cancer. JAMA Netw Open. 2021;4:e2127042. doi: 10.1001/jamanetworkopen.2021.27042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pepe MS, Feng Z, Janes H, Bossuyt PM, Potter JD. Pivotal evaluation of the accuracy of a biomarker used for classification or prediction: standards for study design. J Natl Cancer Inst. 2008;100:1432–8. doi: 10.1093/jnci/djn326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon RM, Paik S, Hayes DF. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst. 2009;101:1446–52. doi: 10.1093/jnci/djp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savitzky A, Golay MJE. Smoothing and differentiation of data by simplified least squares procedures. Anal Chem. 1964;36:1627–39. doi: 10.1021/ac60214a047. [DOI] [Google Scholar]
- 16.Zeng M, Jianxun L, Zhang P. The design of Top-Hat morphological filter and application to infrared target detection. Infrared Phys Technol. 2006;48:67–76. doi: 10.1016/j.infrared.2005.04.006. [DOI] [Google Scholar]
- 17.Jiang H, Yu X, Li N, Kong M, Ma Z, Zhou D, et al. Efficacy and safety of neoadjuvant sintilimab, oxaliplatin and capecitabine in patients with locally advanced, resectable gastric or gastroesophageal junction adenocarcinoma: early results of a phase 2 study. J Immunother Cancer. 2022;10:e003635. doi: 10.1136/jitc-2021-003635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang S, Cai N, Pacheco PP, Narrandes S, Wang Y, Xu W. Applications of Support Vector Machine (SVM) Learning in Cancer Genomics. Cancer Genomics Proteom. 2018;15:41–51. doi: 10.21873/cgp.20063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simon N, Friedman J, Hastie T, Tibshirani R. Regularization Paths for Cox’s Proportional Hazards Model via Coordinate Descent. J Stat Softw. 2011;39:1–13. doi: 10.18637/jss.v039.i05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lavagnini I, Badocco D, Pastore P, Magno F. Theil-Sen nonparametric regression technique on univariate calibration, inverse regression and detection limits. Talanta. 2011;87:180–8. doi: 10.1016/j.talanta.2011.09.059. [DOI] [PubMed] [Google Scholar]
- 21.Limmathurotsakul D, Turner EL, Wuthiekanun V, Thaipadungpanit J, Suputtamongkol Y, Chierakul W, et al. Fool’s gold: Why imperfect reference tests are undermining the evaluation of novel diagnostics: a reevaluation of 5 diagnostic tests for leptospirosis. Clin Infect Dis. 2012;55:322–31. doi: 10.1093/cid/cis403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu J, Li Y, Fan Q, Shu Y, Yang L, Cui T, et al. Clinical and biomarker analyses of sintilimab versus chemotherapy as second-line therapy for advanced or metastatic esophageal squamous cell carcinoma: a randomized, open-label phase 2 study (ORIENT-2) Nat Commun. 2022;13:857. doi: 10.1038/s41467-022-28408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell Infiltration and PD-L1. Cancer Res. 2015;75:2139–45. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577:549–55. doi: 10.1038/s41586-019-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. 2018;362:eaar3593. doi: 10.1126/science.aar3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, et al. Identification of serum microRNAs predicting the response of esophageal squamous-cell carcinoma to nivolumab. Jpn J Clin Oncol. 2020;50:114–21. doi: 10.1093/jjco/hyz146. [DOI] [PubMed] [Google Scholar]
- 27.Huang S, Zhang J, Lai X, Zhuang L, Wu J. Identification of Novel Tumor Microenvironment-Related Long Noncoding RNAs to Determine the Prognosis and Response to Immunotherapy of Hepatocellular Carcinoma Patients. Front Mol Biosci. 2021;8:781307. doi: 10.3389/fmolb.2021.781307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo YH, Yang YP, Chien CS, Yarmishyn AA, Ishola AA, Chien Y, et al. Plasma Level of Circular RNA hsa_circ_0000190 Correlates with Tumor Progression and Poor Treatment Response in Advanced Lung Cancers. Cancers (Basel) 2020;12:1740. doi: 10.3390/cancers12071740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JY, Choi JK, Jung H. Genome-wide methylation patterns predict clinical benefit of immunotherapy in lung cancer. Clin Epigenetics. 2020;12:119. doi: 10.1186/s13148-020-00907-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malczewski AB, Navarro S, Coward JI, Ketheesan N. Microbiome-derived metabolome as a potential predictor of response to cancer immunotherapy. J Immunother Cancer. 2020;8:e001383. doi: 10.1136/jitc-2020-001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang W, Xing X, Yeung SJ, Wang S, Chen W, Bao Y, et al. Neoadjuvant programmed cell death 1 blockade combined with chemotherapy for resectable esophageal squamous cell carcinoma. J Immunother Cancer. 2022;10:e003497. doi: 10.1136/jitc-2021-003497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Z, Sun N, He J. Adjuvant immunotherapy in resected esophageal squamous cell carcinoma: a gospel to the non-pCRs. Signal Transduct Target Ther. 2021;6:314. doi: 10.1038/s41392-021-00722-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Vries R, Muller M, van der Noort V, Theelen WSME, Schouten RD, Hummelink K, et al. Prediction of response to anti-PD-1 therapy in patients with non-small-cell lung cancer by electronic nose analysis of exhaled breath. Ann Oncol. 2019;30:1660–6. doi: 10.1093/annonc/mdz279. [DOI] [PubMed] [Google Scholar]
- 34.Disselhorst MJ, de Vries R, Quispel-Janssen J, Wolf-Lansdorf M, Sterk PJ, Baas P. Nose in malignant mesothelioma-Prediction of response to immune checkpoint inhibitor treatment. Eur J Cancer. 2021;152:60–67. doi: 10.1016/j.ejca.2021.04.024. [DOI] [PubMed] [Google Scholar]
- 35.Nardi-Agmon I, Abud-Hawa M, Liran O, Gai-Mor N, Ilouze M, Onn A, et al. Exhaled breath analysis for monitoring response to treatment in advanced lung cancer. J Thorac Oncol. 2016;11:827–37. doi: 10.1016/j.jtho.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 36.Lubes G, Goodarzi M. GC-MS based metabolomics used for the identification of cancer volatile organic compounds as biomarkers. J Pharm Biomed Anal. 2018;147:313–22. doi: 10.1016/j.jpba.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Peyraud F, Guégan JP, Bodet D, Nafia I, Fontan L, Auzanneau C, et al. Circulating L-arginine predicts the survival of cancer patients treated with immune checkpoint inhibitors. Ann Oncol. 2022;33:1041–51. doi: 10.1016/j.annonc.2022.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Boots AW, van Berkel JJ, Dallinga JW, Smolinska A, Wouters EF, van Schooten FJ. The versatile use of exhaled volatile organic compounds in human health and disease. J Breath Res. 2012;6:027108. doi: 10.1088/1752-7155/6/2/027108. [DOI] [PubMed] [Google Scholar]
- 39.Wu H, Leng X, Liu Q, Mao T, Jiang T, Liu Y, et al. Intratumoral Microbiota Composition Regulates Chemoimmunotherapy Response in Esophageal Squamous Cell Carcinoma. Cancer Res. 2023;83:3131–44. doi: 10.1158/0008-5472.CAN-22-2593. [DOI] [PubMed] [Google Scholar]
- 40.Bhandari MP, Polaka I, Vangravs R, Mezmale L, Veliks V, Kirshners A, et al. Volatile Markers for Cancer in Exhaled Breath-Could They Be the Signature of the Gut Microbiota? Molecules. 2023;28:3488. doi: 10.3390/molecules28083488. [DOI] [PMC free article] [PubMed] [Google Scholar]