Abstract
Background
Pancreatic ductal adenocarcinoma (PDAC) is frequently accompanied by perineural invasion (PNI), which is associated with excruciating neuropathic pain and malignant progression. However, the relationship between PNI and tumour stromal cells has not been clarified.
Methods
The dorsal root ganglia or sciatic nerves nerve model was used to observe the paracrine interaction and the activation effect among Schwann cells, tumour-associated macrophages (TAMs), and pancreatic cancer cells in vitro. Next generation sequencing, enzyme-linked immunosorbent assay and chromatin immunoprecipitation were used to explore the specific paracrine signalling between TAMs and Schwann cells.
Results
We demonstrated that more macrophages were expressed around nerves that have been infiltrated by pancreatic cancer cells compared with normal nerves in murine and human PNI specimens. In addition, high expression of CD68 or GFAP is associated with an increased incidence of PNI and indicates a poor 5-year survival rate in patients with PDAC. Mechanistically, tumour-associated macrophages (TAMs) activate Schwann cells via the bFGF/PI3K/Akt/c-myc/GFAP pathway. Schwann cells secrete IL-33 to recruit macrophages into the perineural milieu and facilitate the M2 pro-tumourigenic polarisation of macrophages.
Conclusions
Our study demonstrates that the bFGF/IL-33 positive feedback loop between Schwann cells and TAMs is essential in the process of PNI of PDAC. The bFGF/PI3K/Akt/c-myc/GFAP pathway would open potential avenues for targeted therapy of PDAC.
Subject terms: Pancreatic cancer, Cancer microenvironment
Background
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive disease with a relatively poor prognosis and a 5-year survival rate of less than 10% [1]. The clinical outcomes of patients with PDAC have improved little with modern therapeutics, mainly due to late diagnosis in the nonsurgical period with distant metastasis, local infiltration, or distant metastasis [2]. Distinct invasion and migration of cancer cells along nerves is a pathological process known as perineural invasion [3]. perineural invasion (PNI) is detected in 70–100% of PDAC cases and contributes to dismal prognosis and neuropathic pain [4]. Although PNI is an important metastatic route in neurotropic pancreatic cancer, its exact mechanisms are poorly understood.
A characteristic feature of PDAC is the exceedingly dense desmoplastic inflammatory microenvironment, which consists predominantly of immune cells, activated fibroblasts, glial cells, adipocytes, lymphatic endothelial cells, epithelial cells, pericytes, vascular cells, and the extracellular matrix [5]. As main glial cells of the peripheral nervous system, Schwann cells are involved in multiple biological processes of tumour progression, including cancer dissemination, PNI, immunomodulation, and PDAC-associated neural remodelling, rather than just being bystanders [6, 7].
Accumulating evidence has confirmed that tumour cells and tumour-associated macrophages (TAMs) or Schwann cells cooperate to facilitate malignant tumour progression [8–11]. Several preliminary studies have explored the relationship between macrophages, Schwann cells, and PNI. Schwann cells recruit inflammatory macrophage precursors and promote tumour invasion by disrupting the perineurium [12]. Perineural macrophages express GDNF in the perineural microenvironment to mediate nerve invasion in PDAC [13]. This indicates that Schwann cells can recruit macrophages into the perineural microenvironment and promote the PNI of PDAC. However, heterotypic interactions between Schwann cells and TAMs via reciprocal signalling and their precise roles in perineural invasion are poorly understood.
In the current study, we demonstrated that the bFGF/IL-33 positive feedback loop between Schwann cells and TAMs is essential for PNI in PDAC.
Materials and methods
Cell culture
PANC-1, Mia PaCa-2, and BxPC-3 pancreatic cancer cell lines, hTERT-HPNE pancreatic epithelial cells, THP-1, RSC96, sNF96.2, RAW264.7 were purchased from ATCC. All cell lines were identified. Cells were cultured in culture chamber with 5% CO2 at 37 °C. The medium is Dulbecco’s modified Eagle’s medium (DMEM, Biological Industries, Beit Haemek, Israel) containing 10% fetal bovine serum (Biological Industries, Beit Haemek, Israel) and 50 U/ml penicillin/streptomycin (Biological Industries, Beit Haemek, Israel).
Isolation of peripheral blood monocytes (PBMs) from pancreatic cancer patients or healthy donors by density-gradient centrifugation using Ficoll-Hypaque (Pharmacia, Peapack, NJ) and the acquired cells were CD14+ monocytes were determined by FACS analysis. For in vitro activation, PBMs at 1 × 106 cells/mL were treated for 7 days with 45 ng/ml recombinant human interleukin-4 (IL-4, Peprotech, New Jersey, USA) or 25 μg/ml lipopolysaccharides (LPS, Peprotech, New Jersey, USA). All the cell lines were monthly tested for STR profiling and mycoplasma, and cells are authenticated and mycoplasma-free.
Prepare and coculture DRGs
A DRG/sciatic nerve model was used to observe the paracrine interaction and the effect among neurons, macrophages, and cancer cells in vitro. Dorsal root ganglia (DRGs) or sciatic nerves were isolated from newborn male BALB/c mice and placed twice in phosphate-buffered saline. DRGs or sciatic nerves were implanted in 12-well culture plates with 20 μl Matrigel matrix and grown in DMEM or other condition culture media containing 10% FBS at 37 °C and 5%CO2. We detected morphological changes and calculated the area when the DRGs or sciatic nerves were incubated from 0 to 5 days.
Murine sciatic nerve invasion model
This mouse model of sciatic nerve invasion has been widely used to observe the effects of perineural invasion [14]. Six-week-old female SCID mice were randomly divided into four groups (n = 5): (i) MiaPaCa-2 + PBS, (ii) MiaPaCa-2+TAMs, (iii) MiaPaCa-2+bFGF, and (iv) MiaPaCa-2+TAMs+anti-bFGF. The mice were anaesthetised with 4% chloral hydrate (85 μl/10 g, intraperitoneal injection) and diethyl ether inhalation. The left sciatic nerve was surgically exposed. The cells were mixed with 3 μl PBS and injected into the sciatic nerve. The wounds of the mice were closed using sutures. The recovery of the mice was recorded daily for 72 h. Signs of nerve invasion appeared approximately two weeks after the injection.
Patients and clinical samples
Pancreatic tissue samples were obtained from patients diagnosed with PDAC who underwent surgery at the Sun Yat-sen Memorial Hospital of Sun Yat-sen University between February 2008 and February 2016. The surgical specimens and diagnosis of PDAC were confirmed by two independent professional pathologists. For RNA and protein extraction, tissue specimens were immediately frozen in liquid nitrogen and stored at −80 °C until required. For immunohistochemistry (IHC), tissue specimens were fixed in 10% (v/v) neutral-buffered formalin, dehydrated in 70% ethanol, and embedded in paraffin. The detailed clinicopathological characteristics of the patients are summarised in Supplementary Table 1. Biological samples, antibodies, primer sequence and siRNA sequences are summarised in Supplementary Tables 3–5.
Statistical analysis
All statistical analyses were performed using SPSS software (version 13.0; IBM Corp., Armonk, NY, USA). Two-tailed Student’s t tests were used for comparisons between two groups. One-way ANOVA with Bonferroni’s test was used for multiple comparisons. Survival curves were plotted using the Kaplan–Meier method, and the data were compared using a log-rank test. Survival data were evaluated using a multivariate Cox regression analysis. Student’s t tests were used to calculate the P values for most of the in vitro and animal experiments, Student’s t tests were used to calculate the p-value. All experiments were repeated three times, and the final data are presented as the mean ± S.D. of at least three independent experiments. Results were considered statistically significant if the P value was <0.05.
Results
Macrophage expression in human and murine perineural invasion specimens
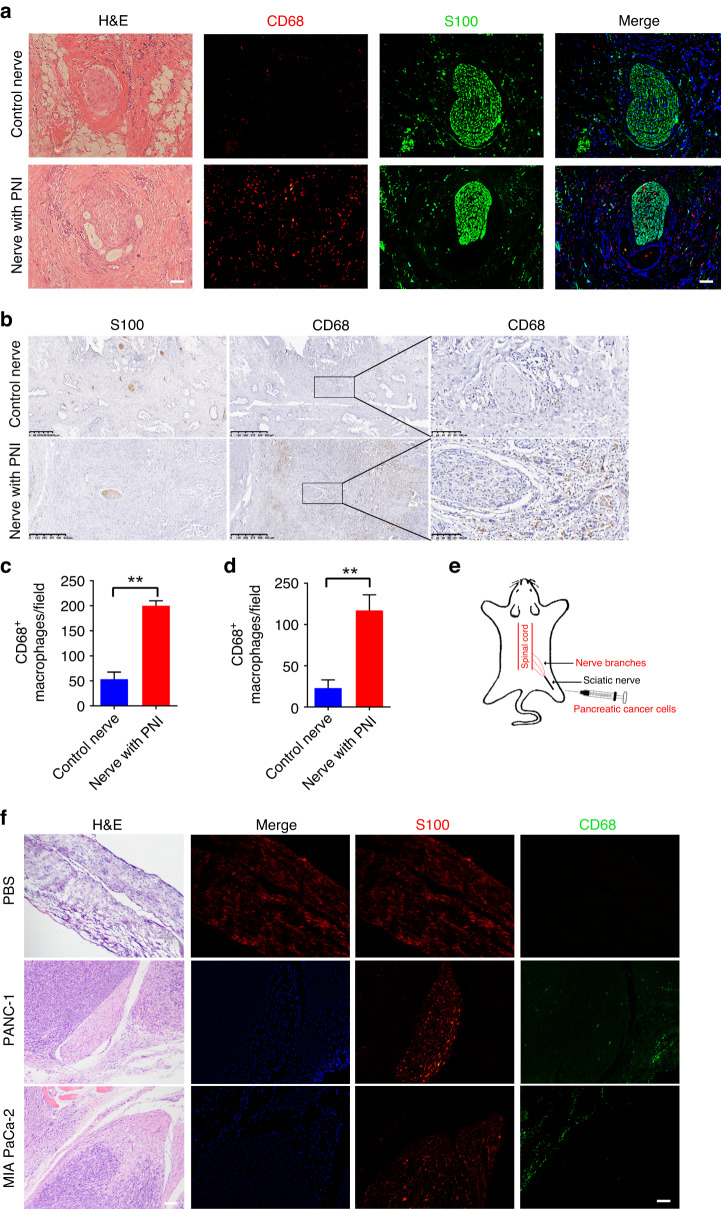
We first verified macrophage expression in the neural microenvironment of PDAC patients with PNI and healthy controls. Immunofluorescence analysis of these slides revealed that CD68+ macrophages were concentrated in the perineural niche of the invaded nerves (Fig. 1a, c and Supplementary Fig. 1). Immunohistochemistry (IHC) of these samples showed the same results as immunofluorescence (Fig. 1b, d). After injecting cancer cells into the sciatic nerve of mice (Fig. 1e), we observed that more CD68+ macrophages were recruited to the perineural niche (Fig. 1e). Collectively, these experiments indicated an increase in macrophages in perineural invasion samples.
Fig. 1. Macrophage expression in murine and human perineural invasion samples.
a Representative images of nerve sections with CD68 (red, bio-marker of macrophages) and S100 (green, bio-marker of nerves) staining and corresponding H&E sections in PDAC patients. Control nerve section from the same patient in the field without PNI.These results showed that CD68+ macrophages were concentrated in the perineural niche of the invaded nerves. Scale bar, 200 μm. b Representative IHC images of nerve sections with CD68 staining in PDAC patients showed that CD68+ macrophages concentrated in PDAC with PNI. c Quantification of CD68-positive macrophages expressed by percentage per slide tumour sections in immunofluorescence images (**P < 0.01). d Quantification of CD68-positive macrophages expressed by percentage per slide tumour sections in IHC images (**P < 0.01). e Schematic diagram of a model of a murine sciatic nerve invasion. Pancreatic cancer cells (PANC-1 and MIA PaCa-2) were injected into the distal sciatic nerve. f Representative images of nerve sections with S100(red) and CD68(green) staining and corresponding H&E sections in normal nerves and murine sciatic nerve model. This indicated CD68+ macrophages were concentrated in the perineural niche of the invaded nerves in model of a murine sciatic nerve invasion. Scale bar, 200 μm.
TAMs promote the proliferation and migration of Schwann cells
Macrophages promote perineural invasion by disrupting the perineurium in a mouse model [14]. Moreover, Schwann cells promote cancer cell invasion and dispersion to promote perineural invasion [6]. However, the relationship between Schwann cells and macrophages is still unknown in the perineural milieu remains unclear. We treated human monocyte-derived macrophages (MDMs) with the conditioned medium (CM) of PDAC to obtain polarised tumour-associated macrophages (pol-TAMs). Schwann cells were co-cultured with different TAMs in a Transwell system [15]. We evaluated the effect of TAMs on nerve outgrowth using in vitro nerve invasion coculture models and found that treating pol-TAMs increased the DRG area compared with MDMs or DRG alone (Fig. 2a). We then assessed the migration and proliferation of Schwann cells in explanted sciatic nerves of mice. We found that treatment with pol-TAMs increased the Schwann cell number compared with MDMs or DRG alone (Fig. 2b). Our results showed that treatment with pol-TAMs significantly promoted Schwann cell migration (Fig. 2c, d). Taken together, our data showed that TAMs facilitate the migration and proliferation of Schwann cells.
Fig. 2. TAMs promote the migration and proliferation of Schwann cells.
a Representative images of DRGs alone or cocultured with different macrophages supernatant (SN). Relative DRG area were calculated at 4 days of cocultivation (*P < 0.05, **P < 0.01, ***P < 0.001, ns, no significant difference). Scale bar, 200 μm. b Representative images of sciatic nerves alone or cocultured with different macrophages supernatant. The number of Schwann cells derived from sciatic nerves at three days of cocultivation (*P < 0.05, ***P < 0.001, ns, no significant difference). Scale bar, 200 μm. c Representative Cell trajectories images of Schwann cell line sNF96.2 treated with control medium or other macrophages supernatant. Schwann cell movement distance and speed Analysis was assessed (*P < 0.05, ***P < 0.001, ns, no significant difference). d Representative images of the migration assay on Schwann cell line sNF96.2 treated with control medium or other macrophages supernatant. Quantitative summary of the number of Schwann cells counted individually per visual field (*P < 0.05, **P < 0.01, ns, no significant difference). Scale bar, 200 μm.
TAMs promote the activation of Schwann cells by upregulating the expression of GFAP
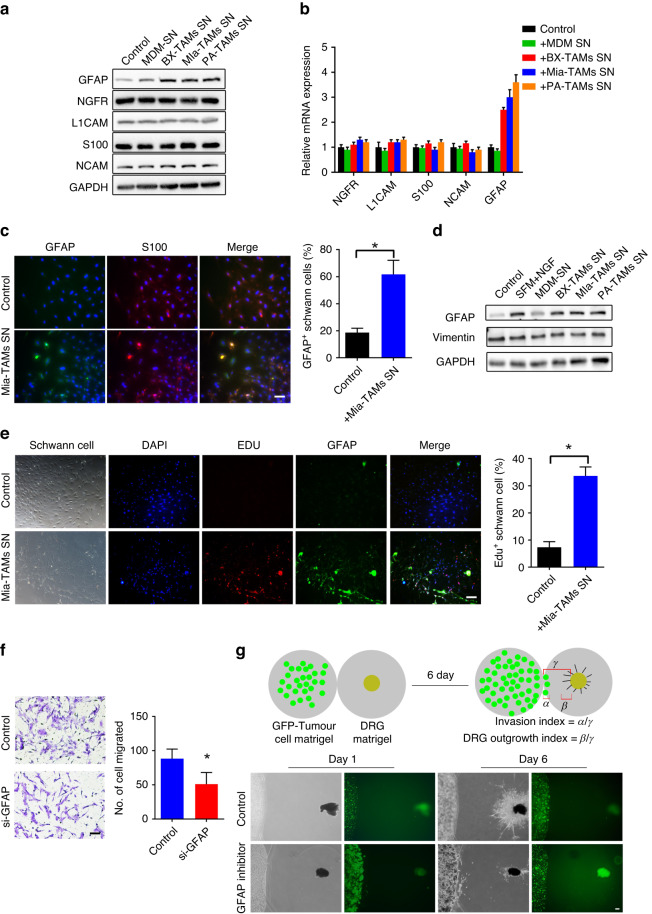
To further identify the features of these Schwann cells in the neural microenvironment of PDAC patients, we detected the biomarkers (GFAP, NCAM, S100, L1CAM, and NGFR) that are involved in the interactions between cancer and Schwann cells. Glial fibrillary acidic protein (GFAP) is a marker of activated Schwann cells [6, 9, 16]. Dedifferentiated GFAP+ Schwann cells display vital migration and proliferation [6, 17]. We found that GFAP expression was upregulated in Schwann cells treated with pol-TAMs versus those treated with MDMs or ordinary medium (Fig. 3a, b). Next, we verified the overexpression of GFAP in Schwann cells treated with pol-TAMs by immunofluorescent staining (Fig. 3c) and immunoblotting (Fig. 3d). Furthermore, we isolated primary Schwann cells from mouse sciatic nerves and confirmed their purity of primary Schwann cells (Supplementary Fig. 2). The results showed that GFAP proteins and EDU staining were overexpressed in primary Schwann cells treated with pol-TAMs (Fig. 3e).
Fig. 3. TAMs promote the activation of Schwann cells by upregulating the expression of GFAP.
a Western blot analysis of expression of biomarkers (GFAP, NCAM, S100, L1CAM, NGFR) in Schwann cell line (sNF96.2) treated with control medium or other macrophages supernatant. b Quantitative polymerase chain reaction of Schwann cell line (sNF96.2) treated with control medium or other macrophages supernatant. c Representative immunofluorescence images of Schwann cell line SNF96.2 with S100(red) and GFAP (green) staining. Quantitative summary of the ratio of GFAP+ Schwann cells per visual field. Scale bar, 100 μm. d Western blot analysis of expression of GFAP and vimentin in Schwann cell treated with control medium or other macrophages supernatant. Vimentin expression serving as control intermediate filament protein (*P < 0.05). e Representative immunofluorescence images of primary Schwann cell with DAPI (blue), Edu(red), and GFAP (green) staining. Quantitative summary of the ratio of Edu+ Schwann cells per visual field (*P < 0.05). Scale bar, 200 μm. f Representative images of the migration assay on Schwann cell line SNF96.2 treated with siRNA-induced knockdown of GFAP, normalised to values obtained with nontargeting control siRNA (*P < 0.05). Scale bar, 100 μm. g Schematic diagram of the 3D coculture model of DRGs and pancreatic cancer cells. Pancreatic cancer cells and DRG attract each other. The GFP+ pancreatic cancer cell shows the initial location of the tumour cells at day 1. the black DRG shows the initial location of DRG at day1. Nerve invasion index (α/γ) and DRG outgrowth index (β/γ) were calculated at day 6 in different coculture groups. The DRG outgrowth index decreased in DRG treated with the GFAP inhibitor Zilganersen. Scale bar, 200 μm.
To investigate whether TAMs promote Schwann cell activation through GFAP expression, we silenced GFAP expression in Schwann cells using short interfering RNA (siRNA). GFAP silencing significantly suppressed the migration of Schwann cells (Fig. 3f). Furthermore, the effects of TAMs on Schwann cell migration and nerve outgrowth were evaluated using in vitro nerve invasion co-culture models (Supplementary Fig. 3A). We assessed the capacity of the DRG axons to approach cancer cells and the invasion capacity of cancer cells to DRG through DRG outgrowth index and nerve invasion index, respectively (Fig. 3g and Supplementary Fig. 3B, C). The DRG outgrowth index decreased in DRG treated with the GFAP inhibitor. Collectively, these results suggest that TAMs promote Schwann cell activation through a GFAP-dependent mechanism.
TAMs promote the activation of Schwann cells in vivo
To further confirm the relationship between TAMs and Schwann cells, immunofluorescence staining for GFAP (activated Schwann cell marker), S100 (total Schwann cell marker), CD68 (TAM marker), and CK19 (tumour cell marker) was performed, revealing enrichment of GFAP and CD68 in the PNI sections of PDAC patients (Fig. 4a, b and Supplementary Fig. 4). Correlation analysis revealed that CD68 and GFAP were co-expressed in PDAC tissues (Fig. 4c). Next, we detected GFAP and CD68 expression in the sciatic nerve model with tumour injection, and we observed more GFAP+ Schwann cells and CD68+ TAMs in the sciatic nerve than the control nerve in the sciatic nerve model (Fig. 4d). In patients with pancreatic intraepithelial neoplasia (precancerous lesion of pancreatic cancer), we found that the nerve with TAMs possessed more GFAP+ Schwann cells, while the nerve without TAMs but surrounded by cancer cells showed fewer GFAP+ Schwann cells (Supplementary Fig. 5A). Similar results were observed in patients with pancreatic cancer (Supplementary Fig. 5B). This indicates that during tumourigenesis, TAMs activate Schwann cells in human PDAC patients.
Fig. 4. TAMs promote the activation of Schwann cells in vivo.
a Representative images of serial nerve sections with CD68, CK19(red), and GFAP, S100 (green) staining in PDAC patients. These revealed enrichment of GFAP and CD68 in the PNI sections of PDAC patients. Scale bar, 200 μm. b Quantification of GFAP-positive nerve by percentage per slide tumour sections in immunofluorescence images, These revealed enrichment of GFAP+ nerves in the PNI sections of PDAC patients (**P < 0.01). c The percentage of CD68-high or CD68-low pancreatic cancer cells among GFAP-high or GFAP-low pancreatic cancer cell populations, respectively, were shown. These indicated that CD68 and GFAP were co-expressed in PDAC tissues. (Spearman’s correlation coefficient for ranked data, R = 0.44, P < 0.001). d Representative images of serial nerve sections with GFAP (red) and CD68 (green) staining in murine sciatic nerve invasion model. These indicated that CD68 and GFAP were co-expressed in murine sciatic nerve invasion model. Scale bar, 200 μm.
TAMs activate Schwann cells by secreting bFGF
To understand the mechanism by which TAMs activate Schwann cells, we performed next-generation sequencing (NGS) to compare the mRNA expression profiles of pol-TAMs and MDMs (Fig. 5a left panel and Supplementary Fig. 6). KEGG and G.O. enrichment analysis suggested that the differentially expressed genes were mainly enriched in chemokine receptor binding and cytokine–cytokine receptor interaction (Supplementary Fig. 7). We selected the top 18 cytokines upregulated in pol-TAMs for further confirmation (Fig. 5a, right panel). Among the top 18 cytokines, we found that 17 cytokines were upregulated in both PA-TAMs and Mia-TAMs (TAM co-cultured with PANC-1 or MIA PaCa-2). Among these, bFGF was the most upregulated (5.6 and 3.5 times, respectively). We then confirmed the upregulation of bFGF in pol-TAMs using qRT-PCR and ELISA (Fig. 5c, d). Treatment of Schwann cells with bFGF enhanced the ratio of GFAP+ Schwann cells (Fig. 5e and Supplementary Fig. 8A) and promoted the migration of Schwann cells (Fig. 5f and Supplementary Fig. 8B). In addition, treating the cocultures of pol-TAMs and Schwann cells with neutralising antibodies against bFGF potently abrogated the effects of TAMs on Schwann cells (Supplementary Fig. 8C, D). To confirm the relationship between bFGF and GFAP, we found that bFGF facilitated GFAP expression at both the mRNA and protein levels (Fig. 5g). Exogenous bFGF accelerated the migration of Schwann cells (Fig. 5h and Supplementary Fig. 8E). Together, our data showed that TAMs secrete bFGF to activate Schwann cells.
Fig. 5. TAMs activate Schwann cells by secreting factor bFGF.
a The heatmap showed the differential gene expression between MDMs and pol-TAMs (Pa-TAMs and Mia-TAMs; left panel). The heatmap represented Top 18 cytokines upregulated in pol-TAMs (right panel). b Venn diagram showed the overlap upregulated cytokines related gene between Pa-TAMs and Mia-TAMs. The quantitative polymerase chain reaction of the upregulated cytokines related gene in pol-TAMs. c Quantitative polymerase chain reaction detected the mRNA level of bFGF in pol-TAMs, which indicated the upregulation of bFGF in pol-TAMs using qRT-PCR (*P < 0.05, **P < 0.01). d ELISA detected the protein level of bFGF in pol-TAMs, which indicated the upregulation of bFGF in pol-TAMs using ELISA (*P < 0.05, **P < 0.01, ***P < 0.001). e Immunofluorescence showed that the ratio of GFAP+ Schwann cells increased with bFGF stimulation. f 3D coculture model of DRGs showed that DRG area increased at 4 days with bFGF stimulation. g Western blot analysis and quantitative polymerase chain reaction detected the expression of GFAP treated with bFGF or anti-bFGF. These showed that bFGF promoted the expression of GFAP in vitro. h Migration assay showed exogenous bFGF accelerated the migration of Schwann cells.
TAMs activate Schwann cells through bFGF/PI3K/Akt/c-myc/GFAP pathway
To unravel the signalling pathways activated in Schwann cells, we performed next-generation sequencing (NGS) of Schwann cells treated with TAMs and Schwann cells alone. The results showed that the PI3K-Akt pathway was enriched in Schwann cells treated with pol-TAM (Fig. 6a). Gene set enrichment analysis (GSEA) revealed enrichment of the PI3K-Akt pathway in Schwann cells treated with pol-TAMs (Fig. 6b). Moreover, we verified activation of the PI3K/Akt/c-myc pathway at the protein level. Neutralising antibodies for bFGF efficiently abrogated the activation of the PI3K/Akt/c-myc pathway, while exogenous bFGF promoted this pathway in Schwann cells (Fig. 6c, d). These data suggest that TAMs activate Schwann cells through the bFGF/PI3K/Akt/c-myc pathway.
Fig. 6. TAMs activate Schwann cells through bFGF/PI3K/Akt/c-myc/GFAP pathway.
a KEGG top10 of pathway enrichment differentially expressed genes between Schwann cells treated with MDMs and pol-TAMs (left panel: Bx-TAMs; right panel: Mia-TAMs). b GSEA analysis revealed an enrichment of PI3K-Akt target genes in the Schwann cells treated with pol-TAMs. c Western blot of Schwann cells treated with bFGF or anti-bFGF blotted for key molecules in common signalling pathways (PI3K, AKT, P38, ERK). d Western blot of Schwann cells treated with different pol-TAMs and anti-bFGF. e MYC-binding elements on the promoters of GFAP genes were predicted by JASPAR. f Localisations of myc to the promoters of GFAP genes in indicated Schwann cells were analysed by ChIP assay using anti-myc Ab. g Luciferase reporter assays in Schwann cell treated with different pol-TAMs and AKT inhibitor MK-2206, neutralising antibody for bFGF and c-myc silence (*P < 0.05, **P < 0.01, ***P < 0.001). h Western blot, qPCR and migration assay of Schwann cells treated with AKT inhibitor MK-2206, neutralising antibody for bFGF and c-myc silence.
To further understand the mechanisms of high PI3K/Akt activity in activated Schwann cells, we found that the promoters of GFAP genes contained MYC-binding elements using JASPAR (Fig. 6e). Additionally, chromatin immunoprecipitation (ChIP) analyses were performed to confirm binding between c-Myc and GFAP promoters. We found that pol-TAM enhanced c-Myc occupancy at the GFAP promoter (Fig. 6f). In agreement with this, enhanced c-myc transcription activities were observed in Schwann cells treated with pol-TAMs, which can be abrogated by the AKT inhibitor MK-2206, a neutralising antibody for bFGF and c-myc silencing (Fig. 6g). To further confirm the relationship between c-Myc and GFAP, we found that the AKT inhibitor MK-2206, a neutralising antibody for bFGF and c-Myc silencing, abrogated the overexpression of GFAP in Schwann cells treated with TAMs at the protein and mRNA levels (Fig. 6h). In agreement with this, the AKT inhibitor MK-2206, a neutralising antibody for bFGF and c-myc silencing, inhibited GFAP expression and migration of Schwann cells. These data suggest that TAMs activate Schwann cells through bFGF the PI3K/Akt/c-myc/GFAP pathway.
Loss of bFGF decreases PNI in vivo
To assess the role of bFGF in perineural invasion, we injected MIA PaCa-2 cells with PBS or TAMs into sciatic nerves and detected nerve invasion by tumour size and histological analysis (Fig. 7a). To evaluate the effect of nerve invasion on limb function in mice, sciatic nerve function index and sciatic nerve function score were evaluated as previously described [18]. Mice were divided into four groups (MIA PaCa-2 + PBS, MIA PaCa-2 + TAMs, MIA PaCa-2 + bFGF, or MIA PaCa-2 + TAMs + anti-bFGF). Mice treated with TAMs or bFGF developed paralysis, whereas mice injected with PBS retained better function. In contrast, mice treated with anti-bFGF were only partially paralysed after cancer cell injection (Fig. 7b). The spread length between the first and fifth toes of the hind limbs of the mice was calculated as the sciatic nerve function index. The sciatic nerve function index showed results similar to those of the sciatic nerve function score (Fig. 7c). The length of cancer cell invasion measured by H&E staining suggested decreased invasion in mice treated with neutralising antibodies against bFGF (Fig. 7d, e). In orthotopic pancreatic tumours, we found that p-Akt was overexpressed in cells treated with TAMs, and that the overexpression of p-Akt could be abrogated by the neutralising antibody of bFGF (Fig. 7f). We concluded that TAMs activate Schwann cells through bFGF the PI3K/Akt pathway in vivo.
Fig. 7. Loss of bFGF decreases perineural invasion in vivo.
a Surgical images of sciatic nerve PNI. Pancreatic cancer cells were injected into the perineurium of sciatic nerves of SCID mice. Mice treated with MIA PaCa-2 + PBS, MIA PaCa-2 + TAMs, MIA PaCa-2 + bFGF or MIA PaCa-2 + TAMs + anti-bFGF (5 mice per group). Tumours exhibited obvious PNI. Scale bar, 100 μm. b Sciatic nerve function score of mice treated with MIA PaCa-2 + PBS, MIA PaCa-2 + TAMs, MIA PaCa-2 + bFGF or MIA PaCa-2 + TAMs + anti-bFGF. (*P < 0.05, **P < 0.01). c Sciatic nerve function index of mice treated with MIA PaCa-2 + PBS, MIA PaCa-2 + TAMs, MIA PaCa-2 + bFGF or MIA PaCa-2 + TAMs + anti-bFGF(*P < 0.05, **P < 0.01). d The length of cancer cell invasion of mice treated with MIA PaCa-2 + PBS, MIA PaCa-2 + TAMs, MIA PaCa-2 + bFGF or MIA PaCa-2 + TAMs + anti-bFGF. e Representative H&E images of the sciatic nerve to evaluate the length of invasion. Cancer cell invasion detected by analysis of H&E staining in mice treated with MIA PaCa-2 + PBS, MIA PaCa-2 + TAMs, MIA PaCa-2 + bFGF or MIA PaCa-2 + TAMs + anti-bFGF. The black arrows indicate the site of injection and the distal end of tumour invasion. The number indicates the length of invasion in different group (mm). Scale bar: 50um (*P < 0.05, **P < 0.01). Scale bar, 50 μm. f Representative images in orthotopic pancreatic tumours with p-AKT and p-ERK staining showed that p-AKT and p-ERK were over-expressed in pancreatic cancer with TAM stimulation.
PDAC-TAMs-stimulated Schwann cells (PTs-SCs) recruit and polarise M2 pro-tumourigenic macrophages through IL-33 paracrine signalling
To evaluate the effect of Schwann cells on macrophages, we performed NGS to evaluate the differential mRNA expression profiles of the PTs-SCs. The top 10 upregulated and downregulated mRNA target genes are listed by p-value, among which IL-33 was the most upregulated gene (Supplementary Fig. 9A–D). Although previous studies have confirmed that IL-33 plays a pivotal role in immune regulation, the exact role of IL-33 in the phenotypic and functional regulation of monocytes/macrophages in PNI remains relatively unexplored [19]. Immunofluorescent cell-binding assays indicated that IL-33 blocking antibody markedly restrained the binding of monocytes to PTs-SCs compared to the control or IgG group. Correspondingly, stimulation with recombinant human IL-33 facilitated the binding of monocytes to Schwann cells in vitro (Supplementary Fig. 9E, F). Our transwell assay illustrated that Schwann cell co-culture or Schwann cell-CM treatment markedly promoted RAW264.7 macrophage migration compared with the control group. Conversely, blocking antibodies against IL-33 dramatically attenuated RAW264.7 cell migration in comparison with the control or IgG group, whereas rh-IL-33 stimulation had the opposite effect (Supplementary Fig. 9G, H).
To further explore the biological effect of IL-33 derived from PTs-SCs on macrophage polarisation, undifferentiated macrophages incubated with PTs-SCs, CM derived from PTs-SCs, or rh-IL-33 were more spindle-shaped in appearance and morphologically distinct from the control or anti-IL-33 group (Supplementary Fig. 9I). Accordingly, compared with the control or anti-IL-33 group, undifferentiated macrophages incubated with PTs-SCs, CM derived from PTs-SCs, or rh-IL-33 showed elevated mRNA expression of the M2 macrophage markers CCL18, CCL22, IL10, CD206, and CD163 (Supplementary Fig. 9J), indicating a significantly higher level of CCL18, IL10, and CCL22 (Supplementary Fig. 9K). Collectively, we conclude that IL-33 derived from PTs-SCs might recruit and polarise M2 pro-tumourigenic macrophages, orchestrating an inflammatory microenvironmental milieu that promotes the PNI of PDACs.
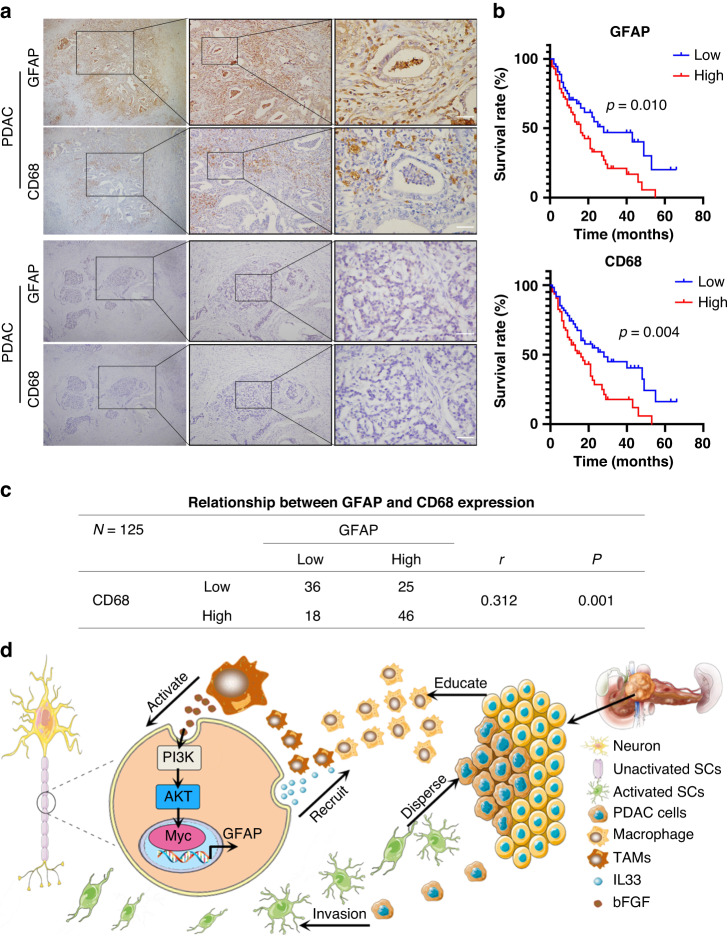
Clinicopathological correlation of GFAP and CD68 expression in human samples
To further confirm the relationship between TAMs and Schwann cells, IHC was used to assess the correlation between GFAP and CD68 expression in PDAC tissues. Among 125 PDAC patients, 71 (56.8%) were GFAP-positive, while 64 cases were CD68 positive (Fig. 8a, c). A positive correlation between GFAP and CD68 was observed in PDAC patients (r = 0.312, P = 0.001; Fig. 8c). Moreover, clinicopathological analysis indicated that GFAP expression was related to tumour size and nerve invasion, while CD68 expression was related to nerve invasion and TNM stage (Supplementary Table 1). Univariate and multivariate analyses indicated that GFAP expression and TNM stage were independent prognostic factors in PDAC patients (Supplementary Table 2). Kaplan–Meier analysis showed that the prognosis of patients with high expression of GFAP and CD68 was worse (P = 0.01 and P = 0.004, respectively; Fig. 8b). These data revealed that GFAP expression was positively correlated with CD68 levels and was an independent prognostic factor in patients with PDAC.
Fig. 8. Clinicopathological correlation of GFAP and CD68 expression in human samples.
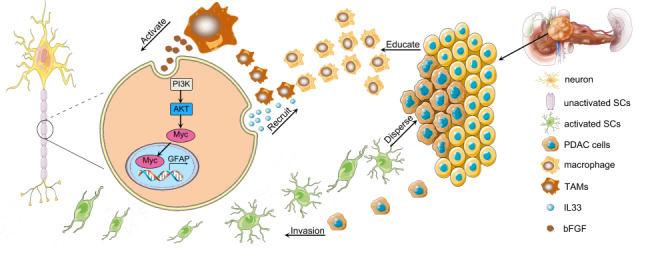
a Representative IHC images of serial sections with CD68 and GFAP staining in PDAC patients. CD68 and GFAP were co expressed in PDAC patients. Scale bar, 100 μm. b Kaplan–Meier survival analysis of PDAC patients according to the protein level of GFAP and CD68 (log-rank test). c The relevance between GFAP and CD68 expression in PDAC (Spearman rank correlation analysis, r = 0.312, P = 0.001). d A schematic summary of the molecular mechanism of TAM and Schwann cell on promoting PNI in PDAC.We have improved the specific process of PNI: Schwann cells recruit and polarise M2 protumourigenic macrophages. Subsequently, M2 pro-tumourigenic macrophages or TAMs activate Schwann cells through the bFGF/PI3K/Akt/c-myc/GFAP pathway. Finally, activated Schwann cells and educated macrophages orchestrate the invasion of cancer cells into nerves. Collectively, the process of PNI starts with Schwann cells and then recruits TAMs to form a positive feedback loop to promote the PNI process.
Discussion
Perineural invasion consists of cancer cells in nerves or surrounding or pass-through nerves, tumour cells close to the nerve and surrounding at least 33% of the nerve periphery, or tumour cells invading any of the three layers of the neurolemma structure [20]. PNI is a significant determinant of the recurrence and prognosis of pancreatic cancer [21]. Current studies suggest that Schwann cells play a pivotal role in the development of PNI [22–29]. However, interactions between TAMs and Schwann cells through reciprocal signalling and their precise roles in perineural invasion are poorly understood. This study shows that macrophages and Schwann cells promote PNI through bFGF a IL-33 positive feedback loop. We confirmed that the process of PNI requires an interaction between TAMs and Schwann cells (Fig. 8d). This means that intervention of the interaction between TAMs and Schwann cells may be a potential way to prevent PNI in PDAC patients.
PNI refers to the invasion of cancer cells into nerves, which influences the pathological characteristics of malignant tumours [12]. During the PNI process, Schwann cells remain in an active, undifferentiated state in the tumour-infiltrated nerves. Activated Schwann cells accelerate cancer cell protrusion and increase the isolation of tumour cells [6]. Schwann cells at PNI release CCL2 to recruit inflammatory monocytes to nerves [14]. This raises two questions: (1) What activates and recruits Schwann cells in the PNI? 2, What is the role of macrophages in the perineural microenvironment, in addition to promoting tumour PNI directly? This study showed that macrophages activate Schwann cells through the bFGF/PI3K/Akt/c-myc/GFAP pathway. Combined with previous studies, we have improved the specific process of PNI: Schwann cells recruit and polarise M2 protumourigenic macrophages. Subsequently, M2 pro-tumourigenic macrophages or TAMs activate Schwann cells through the bFGF/PI3K/Akt/c-myc/GFAP pathway. Finally, activated Schwann cells and educated macrophages orchestrate the invasion of cancer cells into nerves. Collectively, the process of PNI starts with Schwann cells and then recruits TAMs to form a positive feedback loop to promote the PNI process.
To further explore the time sequence and critical cells of Schwann cell activation, IHC was performed in pancreatic intraepithelial neoplasia (precancerous lesion of pancreatic cancer) patients and it was found that the nerve with macrophages possessed more GFAP+ Schwann cells, while the nerve without macrophages but surrounded with intraepithelial neoplasia cells showed less GFAP+ Schwann cells (Supplementary Fig. 5A). A similar result was observed in patients with PDAC (Supplementary Fig. 5B). These data indicate that macrophages, but not tumour cells, activate Schwann cells. Schwann cells recruit macrophages, which activate Schwann cells in precancerous lesions of pancreatic cancer. This suggests that early interference with macrophage recruitment and Schwann cell activation may inhibit the PNI process and promote patient prognosis.
Our study had some limitations. First, we used the precancerous lesion of pancreatic cancer to explore the time sequence and critical cells involved in Schwann cell activation. Further studies are required to verify the occurrence of PNI in the KPC mouse model. Second, the dominance of the autocrine or paracrine effects of bFGF and IL-33 remains to be determined. Furthermore, the specific mechanism by which c-Myc signalling upregulates GFAP expression in Schwann cells remains to be explored. Finally, our research focused on TAMs and Schwann cells expressed in the perineural niche, and other cells may be involved in cancer–nerve interactions, including tumour-associated fibroblasts, neurons, endothelial cells, and neutrophils.
Supplementary information
Acknowledgements
We thank all study participants and their families for supporting our research.
Author contributions
QZ and YL conceived of the study. BZ, XG, and HY experimented on the function and mechanisms and analysed the data. LH conducted the experiment, and analysed the data. BZ and LH revised the manuscript, and YZ, ZL, DS, LL, and PZ conducted the IHC assay. HY conceived of the study and guided the revision of manuscripts. The manuscript was written by QZ, BZ, XG, HY, LH, and YL. All authors made comments on the results and manuscript.
Funding
This research was supported by grants from the Natural Science Foundation of China (82073149, 81672807, 82003073, 82203526), the Natural Science Foundation of Guangdong Province, China (No.2020A1515011296, 2020A1515111135, 2021A1515012357, 2022A1515220219), Guangdong Science and Technology Department (No.2020B1212060018), and Guangdong Provincial Clinical Research Center for Digestive Diseases (2020B1111170004).
Data availability
The data generated in this study are publicly available in Gene Expression Omnibus (GEO) at GSE194191 and GSE194205. Other supporting data are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted in accordance with the principles of the Declaration of Helsinki principles. This study was approved by the Institutional Animal Care and Use Committees of Sun Yat-sen University(SYSU-IACUC-2020-B0229) and Ethics Committee of Sun Yat-sen Memorial Hospital (SYSEC-KY-KS-2021-172).
Consent for publication
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Bin Zhang, Xiaofeng Guo, Leyi Huang.
Contributor Information
Huilin Ye, Email: yehlin3@mail.sysu.edu.cn.
Yanan Lu, Email: luynan@mail.sysu.edu.cn.
Quanbo Zhou, Email: zhouqbo@mail.sysu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-023-02539-w.
References
- 1.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA-Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008–20. doi: 10.1016/S0140-6736(20)30974-0. [DOI] [PubMed] [Google Scholar]
- 3.Azam SH, Pecot CV. Cancer’s got nerve: Schwann cells drive perineural invasion. J Clin Invest. 2016;126:1242–4. doi: 10.1172/JCI86801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amit M, Na’Ara S, Gil Z. Mechanisms of cancer dissemination along nerves. Nat Rev Cancer. 2016;16:399–408. doi: 10.1038/nrc.2016.38. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Kim J, Yang S, Wang H, Wu CJ, Sugimoto H, et al. Type I collagen deletion in alphaSMA(+) myofibroblasts augments immune suppression and accelerates progression of pancreatic cancer. Cancer Cell. 2021;39:548–65. doi: 10.1016/j.ccell.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deborde S, Omelchenko T, Lyubchik A, Zhou Y, He S, McNamara WF, et al. Schwann cells induce cancer cell dispersion and invasion. J Clin Invest. 2016;126:1538–54. doi: 10.1172/JCI82658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monk KR, Feltri ML, Taveggia C. New insights on Schwann cell development. Glia. 2015;63:1376–93. doi: 10.1002/glia.22852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavel O, Shomron O, Shabtay A, Vital J, Trejo-Leider L, Weizman N, et al. Endoneurial macrophages induce perineural invasion of pancreatic cancer cells by secretion of GDNF and activation of RET tyrosine kinase receptor. Cancer Res. 2012;72:5733–43. doi: 10.1158/0008-5472.CAN-12-0764. [DOI] [PubMed] [Google Scholar]
- 9.Deborde S, Wong RJ. How Schwann cells facilitate cancer progression in nerves. Cell Mol Life Sci. 2017;74:4405–20. doi: 10.1007/s00018-017-2578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su S, Liu Q, Chen J, Chen J, Chen F, He C, et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell. 2014;25:605–20. doi: 10.1016/j.ccr.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 11.Zhang B, Ye H, Ren X, Zheng S, Zhou Q, Chen C, et al. Macrophage-expressed CD51 promotes cancer stem cell properties via the TGF-beta1/smad2/3 axis in pancreatic cancer. Cancer Lett. 2019;459:204–15. doi: 10.1016/j.canlet.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Dwivedi S, Krishnan A. Neural invasion: a scenic trail for the nervous tumor and hidden therapeutic opportunity. Am J Cancer Res. 2020;10:2258–70. [PMC free article] [PubMed] [Google Scholar]
- 13.Fang M, Li Y, Huang K, Qi S, Zhang J, Zgodzinski W, et al. IL33 promotes colon cancer cell stemness via JNK activation and macrophage recruitment. Cancer Res. 2017;77:2735–45. doi: 10.1158/0008-5472.CAN-16-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakst RL, Xiong H, Chen CH, Deborde S, Lyubchik A, Zhou Y, et al. Inflammatory monocytes promote perineural invasion via CCL2-mediated recruitment and cathepsin B expression. Cancer Res. 2017;77:6400–14. doi: 10.1158/0008-5472.CAN-17-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen F, Chen J, Yang L, Liu J, Zhang X, Zhang Y, et al. Extracellular vesicle-packaged HIF-1alpha-stabilizing lncRNA from tumour-associated macrophages regulates aerobic glycolysis of breast cancer cells. Nat Cell Biol. 2019;21:498–510. doi: 10.1038/s41556-019-0299-0. [DOI] [PubMed] [Google Scholar]
- 16.Na’Ara S, Amit M, Gil Z. L1CAM induces perineural invasion of pancreas cancer cells by upregulation of metalloproteinase expression. Oncogene. 2019;38:596–608. doi: 10.1038/s41388-018-0458-y. [DOI] [PubMed] [Google Scholar]
- 17.Demir IE, Tieftrunk E, Schorn S, Saricaoglu OC, Pfitzinger PL, Teller S, et al. Activated Schwann cells in pancreatic cancer are linked to analgesia via suppression of spinal astroglia and microglia. Gut. 2016;65:1001–14. doi: 10.1136/gutjnl-2015-309784. [DOI] [PubMed] [Google Scholar]
- 18.Gil Z, Cavel O, Kelly K, Brader P, Rein A, Gao SP, et al. Paracrine regulation of pancreatic cancer cell invasion by peripheral nerves. J Natl Cancer Inst. 2010;102:107–18. doi: 10.1093/jnci/djp456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y, Andersson P, Hosaka K, Zhang Y, Cao R, Iwamoto H, et al. The PDGF-BB-SOX7 axis-modulated IL-33 in pericytes and stromal cells promotes metastasis through tumour-associated macrophages. Nat Commun. 2016;7:11385. doi: 10.1038/ncomms11385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boilly B, Faulkner S, Jobling P, Hondermarck H. Nerve dependence: from regeneration to cancer. Cancer Cell. 2017;31:342–54. doi: 10.1016/j.ccell.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Crippa S, Pergolini I, Javed AA, Honselmann KC, Weiss MJ, Di Salvo F, et al. Implications of perineural invasion on disease recurrence and survival after pancreatectomy for pancreatic head ductal adenocarcinoma. Ann Surg. 2020;276:378–85. [DOI] [PubMed]
- 22.Qin T, Xiao Y, Qian W, Wang X, Gong M, Wang Q, et al. HGF/c-Met pathway facilitates the perineural invasion of pancreatic cancer by activating the mTOR/NGF axis. Cell Death Dis. 2022;13:387. doi: 10.1038/s41419-022-04799-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roger E, Martel S, Bertrand-Chapel A, Depollier A, Chuvin N, Pommier RM, et al. Schwann cells support oncogenic potential of pancreatic cancer cells through TGFβ signaling. Cell Death Dis. 2019;10:886. doi: 10.1038/s41419-019-2116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shurin GV, Kruglov O, Ding F, Lin Y, Hao X, Keskinov AA, et al. Melanoma-induced reprogramming of schwann cell signaling aids tumor growth. Cancer Res. 2019;79:2736–47. doi: 10.1158/0008-5472.CAN-18-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian Z, Ou G, Su M, Li R, Pan L, Lin X, et al. TIMP1 derived from pancreatic cancer cells stimulates Schwann cells and promotes the occurrence of perineural invasion. Cancer Lett. 2022;546:215863. doi: 10.1016/j.canlet.2022.215863. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Jia R, Zhao T, Li X, Lang M, Lan C, et al. HIF-1α mediates tumor-nerve interactions through the up-regulation of GM-CSF in pancreatic ductal adenocarcinoma. Cancer Lett. 2019;453:10–20. doi: 10.1016/j.canlet.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 27.Yin L, Li J, Wang J, Pu T, Wei J, Li Q, et al. MAOA promotes prostate cancer cell perineural invasion through SEMA3C/PlexinA2/NRP1–cMET signaling. Oncogene. 2021;40:1362–74. doi: 10.1038/s41388-020-01615-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Tao L, Yang M, Xu D, Jiang S, Fu X, et al. CD74 promotes perineural invasion of cancer cells and mediates neuroplasticity via the AKT/EGR-1/GDNF axis in pancreatic ductal adenocarcinoma. Cancer Lett. 2021;508:47–58. doi: 10.1016/j.canlet.2021.03.016. [DOI] [PubMed] [Google Scholar]
- 29.Zhang W, He R, Yang W, Zhang Y, Yuan Q, Wang J, et al. Autophagic Schwann cells promote perineural invasion mediated by the NGF/ATG7 paracrine pathway in pancreatic cancer. J Exp Clin Cancer Res. 2022;41:48. doi: 10.1186/s13046-021-02198-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are publicly available in Gene Expression Omnibus (GEO) at GSE194191 and GSE194205. Other supporting data are available from the corresponding author upon reasonable request.