Abstract
Somatostatin analogues have recently been used as therapeutic targets for metastatic or surgically unresectable gastroenteropancreatic (GEP) neuroendocrine tumors (NETs), and associated somatostatin receptor (SSTR) expression has been well demonstrated in most GEP NETs, with the exception of rectal NETs. SSTR2 immunohistochemical expressions were evaluated in 350 surgically or endoscopically resected rectal NETs and compared to clinicopathologic factors. SSTR2 expression was observed in 234 (66.9%) rectal NET cases and associated tumors with smaller size (p = 0.001), low pT classification (p = 0.030), low AJCC tumor stage (p = 0.012), and absence of chromogranin expression (p = 0.009). Patients with rectal NET and SSTR2 expression had significantly better overall survival than those without SSTR2 expression both by univariable (p = 0.006) and multivariable (p = 0.014) analyses. In summary, approximately two-thirds of rectal NETs expressed SSTR2. SSTR2 expression was significantly associated with favorable behavior and good overall survival in patients with rectal NETs. Furthermore, SSTR2 expression can be used as prognostic factors. When metastatic disease occurs, SSTR2 expression can be used a possible target for somatostatin analogues.
Keywords: Somatostatin, Receptor 2, Rectum, Neuroendocrine tumor, Prognosis
Subject terms: Biotechnology, Cancer, Gastroenterology, Medical research
Introduction
Rectal neuroendocrine tumors (NETs) are becoming more common among gastroenteropancreatic (GEP) NETs, owing to improved screening quality with colonoscopy1–3. They account for the majority of GEP-NETs in the Korea, Japan, United States, and Taiwan1–3. Despite the fact that L-cell type rectal NETs were considered to have uncertain malignant potential in the 2010 World Health Organization (WHO) grading system, all rectal NETs are now classified as malignant according to the 2019 WHO grading scheme4,5. This change may indicate that a subset of L-cell type rectal NETs exhibit malignant characteristics, such as lymph node or distant metastasis6–8. As a result, novel prognostic factors and therapeutic targets for metastatic or surgically unresectable rectal NETs are required.
Somatostatin (SST) is a peptide hormone that binds to somatostatin receptors (SSTRs) and which can be found in many organs, including the pancreas, central nervous system, and gastrointestinal tract9–12. It inhibits endocrine and exocrine secretion, as well as angiogenesis and cellular proliferation13. The physiologic functions of SST are mediated by interactions with SSTRs. SSTRs have five subtypes of G-protein-coupled transmembrane receptors (SSTR1–5) that mediate different biologic function of SST14–16. SSTR2 is the most frequently expressed subtypes in both GEP-NETs and also in normal tissue17. However, the frequency and expression pattern are different according to tumor types, location and patient characteristics18.
Somatostatin analogues (SSAs), such as octreotide and lanreotide, have been used to treat GEP NETs19,20. By binding to SSTR, SSAs provide symptomatic relief by inhibiting hormone hypersecretion and cellular proliferation of NETs21. In addition, radiolabeled SSAs are used to find the location and staging of NETs by SSTR-targeting scintigraphy or positron emission tomography (PET)/computed tomography (CT)21,22. SSTR expression, particularly SSTR2 and SSTR5 expression, has been used as a surrogate marker of peptide receptor radionuclide therapy (PRRT) for NENs23–25. SSTR2 expression, in particular, has been shown to be useful in several GEPNENs, with their clinicopathologic correlation, prognostic significance and utility of SSAs as therapeutic tools26,27. In the previous studies, most rectal NETs, particularly those of small size (≤ 1 cm), showed a favorable prognosis6,7,28. Complete endoscopic or surgical removal of these lesions is thus curative in the absence of additional PRRT or radiologic diagnostic tools. However, a small proportion of rectal NETs have showed aggressive behavior, such as advanced pT classification, presence of lymphovascular and perineural invasion, lymph node metastasis and even distant metastasis6. Thus, there is need for evaluating SSTR expression status for additional treatment or diagnostic modality in a subset of advanced or aggressive rectal NETs. SSAs currently available have a high affinity for SSTR2A, and immunohistochemical expression of SSTR2 in pancreatic NETs was linked to therapeutic effect of SSAs29,30. However, the association of SSTR2 expression status with clinicopathologic significances in rectal NETs with a large cohort has not been evaluated.
In this study, we evaluated SSTR2 immunohistochemical expression in rectal NETs and correlated with clinicopathologic factors, including patients’ survival.
Materials and methods
Case collection
After approval from the Institutional Review Board (approval number: 2014-0580) with a waiver of patients’ consent, a total of 350 surgically or endoscopically resected rectal NETs) between 2000 and 2014 were selected from Asan Medical Center, Seoul, Republic of Korea. Clinical data, such as patients’ age, sex, clinical procedure and survival outcome, were reviewed from electronic medical records. The institutional review board of Asan Medical Center, Seoul, Republic of Korea approved this study with waiver of informed consent (approval number: 2014-0580). All procedures were performed in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Pathologic study
Pathologic data, including tumor size, location, depth of invasion, mitotic count, Ki-67 labeling index, lymphovascular and perineural invasion, lymph node and distant metastasis, resection marginal status and immunohistochemical results of synaptophysin and chromogranin expression, was reviewed. Cases were classified as NET grade 1 and grade 2 according to 2019 WHO classification on the basis of mitotic count and Ki-67 labeling index5. The TNM stage was classified based on the 8th American Joint Committee on Cancer (AJCC) cancer staging manual31.
Tissue microarray and immunohistochemistry
All hematoxylin and eosin slides of each case were reviewed and representative slides and paraffin blocks were retrieved. Tissue microarrays (TMAs) were constructed form representative paraffin blocks with tissue microarrayer (Uni TMA Co Ltd, Seoul, Republic of Korea). One 2.0 mm core was punched from donor tumor blocks and replaced into recipient blocks. Immunohistochemical staining was performed as previously described6. Briefly, from each TMA block, 4 μm thick sections were cut and deparaffinized and hydrated in xylene and ethanol. Endogenous peroxidase was blocked and heat-induced antigen retrieval was done. Sections were incubated with primary rabbit monoclonal antibody for SSTR2 (1:6400, ab134152, Abcam, Cambridge, UK).An OptiView DAB Detection Kit (Ventana Medical Systems) was used for the brown chromogen of SSTR2. Slides were counterstained with hematoxylin and dehydrated with ethanol. The result of SSTR2 immunohistochemical staining was graded into 4 groups on the basis of the extent of membranous staining (0, ≤ 5%; 1+, 6–25%; 2+, 26–50%; 3+, 51–75%; 4+, ≥ 76%). The cases were reclassified to 0 as negative group and 1+, 2+, and 3+ as positive groups as previously described6,29.
Statistical analyses
Chi-squared test and Fisher’s exact test were performed to analysis the association between SSTR2 expression and clinicopathologic factors. The overall and recurrence free survival was evaluated with the Kaplan–Meier analysis with the log-rank test. The prognostic significance of SSTR2 was evaluated using the Cox proportional hazards regression model. P value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
Results
Clinicopathologic characteristics of rectal NETs
The characteristics of rectal NETs are summarized in Table 1. The mean age was 48.5 ± 11.4 years (range 22–77 years) with a male to female ratio of 1.1:1. The median follow up period was 66.5 months (range 1–213 months). There were 324 (92.6%) NET grade 1 and 26 (7.4%) NET grade 2 cases. No grade 3 NET was included. The mean tumor size was 0.6 ± 0.4 cm (range 0.1–3.5 cm). When the NET size was dichotomized, sizes 1 cm or smaller were observed in 316 cases (90.3%) and sizes larger than 1 cm were noted in 34 cases (9.7%). The number of mitosis in 2 mm2 was < 2 in 335 (95.7%) and ≥ 2 in 15 (4.3%) cases. Seven cases received surgical resection and 343 cases were endoscopically resected. Proper muscle invasion was identified in 3 (42.9%) cases. Endoscopically resected rectal NETs (343 cases) were not applicable for proper muscle invasion and lymph node metastasis, as endoscopically resected specimens contained mucosa and submucosa only. Lymphovascular and perineural invasion was identified in 8 (2.3%) and 4 (1.1%) cases, respectively. Lymph node and metachronous distant metastasis was observed in 3 (42.9%), and 1 (0.3%) cases, respectively. According to the AJCC staging, there were 341 (97.4%) pT1, 7 pT2 (2.0%), 1 pT3 (0.3%), and 1 pT4 (0.3%) tumors, respectively and 338 (96.6%) stage I, 5 (1.4%) stage II, 5 (1.4%) stage III, and 2 (0.6%) stage IV cases, respectively. The median follow-up period was 67 months (range 1–214 months).
Table 1.
SSTR2a expression and correlation with clinicopathologic factors of rectal neuroendocrine tumors.
| Clinicopathologic factors | N (%) | SSTR2 expression | ||
|---|---|---|---|---|
| Absent (%) | Present (%) | p value | ||
| Age (years) | 0.325 | |||
| ≤ 50 | 200 (57.1) | 62 (31.0) | 138 (69.0) | |
| > 50 | 150 (42.8) | 54 (36.0) | 96 (64.0) | |
| Sex | 0.645 | |||
| Male | 187 (53.4) | 64 (34.2) | 123 (65.8) | |
| Female | 163 (46.6) | 52 (31.9) | 111 (68.1) | |
| Grade | 0.868 | |||
| G1 | 324 (92.6) | 107 (33.0) | 217 (67.0) | |
| G2 | 26 (7.4) | 9 (34.6) | 17 (65.4) | |
| Size | 0.001* | |||
| ≤ 1 cm | 316 (90.3) | 96 (30.4) | 220 (69.6) | |
| > 1 cm | 34 (9.7) | 20 (58.8) | 14 (41.2) | |
| Proper muscle invasion (n = 7)a | 0.809 | |||
| Absent | 4 (57.1) | 3 (75.0) | 1 (25.0) | |
| Present | 3 (42.9) | 2 (66.7) | 1 (33.3) | |
| Lymphovascular invasion | 0.306 | |||
| Absent | 342 (97.7) | 112 (32.7) | 230 (67.3) | |
| Present | 8 (2.3) | 4 (50.0) | 4 (50.0) | |
| Perineural invasion | 0.074 | |||
| Absent | 346 (98.9) | 113 (32.7) | 233 (67.3) | |
| Present | 4 (1.1) | 3 (75.0) | 1 (25.0) | |
| Lymph node metastasis (n = 7)a | 0.147 | |||
| Absent | 4 (57.1) | 2 (50.0) | 2 (50.0) | |
| Present | 3 (42.9) | 3 (100.0) | 0 (0.0) | |
| Distant metastasis | 0.155 | |||
| Absent | 349 (99.7) | 115 (33.0) | 234 (67.0) | |
| Present | 1 (0.3) | 1 (100.0) | 0 (0.0) | |
| pT classification | 0.030* | |||
| pT1 | 341 (97.4) | 110 (32.3) | 231 (67.7) | |
| pT2-4 | 9 (2.6) | 6 (66.7) | 3 (33.3) | |
| AJCC stage group | 0.012* | |||
| I | 338 (96.6) | 108 (32.0) | 230 (68.0) | |
| II–IV | 12 (3.4) | 8 (66.7) | 4 (33.3) | |
| Chromogranin expression | 0.009* | |||
| Absent | 289 (82.6) | 87 (30.1) | 202 (69.9) | |
| Present | 61 (17.4) | 29 (47.5) | 32 (52.5) | |
| Somatostatin expression | 0.070 | |||
| Absent | 324 (92.6) | 105 (32.4) | 219 (67.6) | |
| Present | 19 (5.4) | 10 (52.6) | 9 (47.4) | |
SSTR somatostatin receptor, AJCC American Joint Committee on Cancer.
*Statistically significant at p < 0.05.
aCases of endodscopically resected neuroendocrine tumors were not applicable for proper muscle invasion and lymph node metastasis and were excluded from this analysis.
SSTR2 expression in rectal NETs
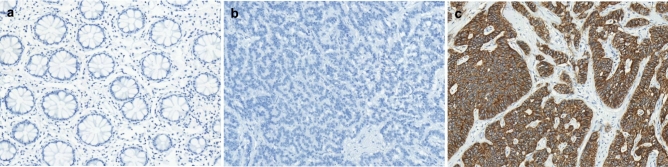
Representative images of SSTR2 expression in normal rectal mucosa and rectal NETs are illustrated in Figs. 1 and 2. Peritumoral non-neoplastic colonic mucosa was evaluated in 83 cases and none of them showed SSTR2 expression. In contrast, SSTR2 expression was observed in 234 (66.9%) rectal NETs. SSTR2 expression was significantly associated with small tumor size (p = 0.001), low pT classification (p = 0.030), low AJCC stage (p = 0.012), and absence of chromogranin expression (p = 0.009; Table 1).
Figure 1.
Representative images of SSTR2 immunohistochemical staining. (a) Normal rectal mucosa does not show SSTR2 expression. (b) Negative and (c) positive SSTR2 expression in rectal neuroendocrine tumors.
Figure 2.
Representative images of rectal neuroendocrine tumors based on the SSTR2 immunohistochemical grading. SSTR2 immunohistochemical (a) grade 1, (b) grade 2, (c) grade 3, and (d) grade 4.
Survival analyses according to SSTR2 expression in rectal NETs
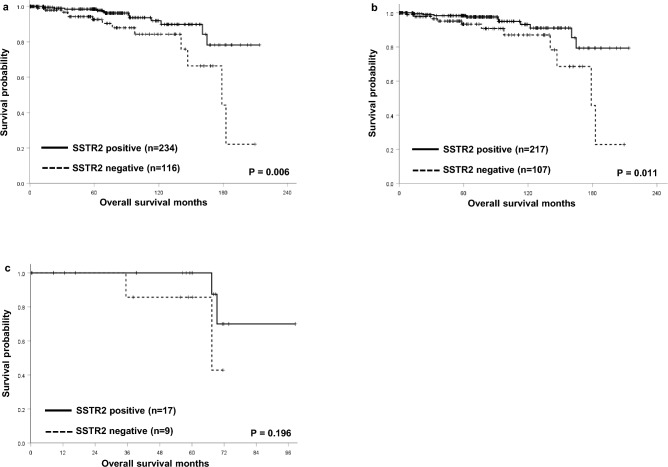
The overall survival of rectal NET patients with SSTR2 expression was significantly better than those without SSTR2 expression [hazard ration (HR) 0.346; 95% confidential interval (CI) 0.157–0.759; p = 0.006]. The 5-year survival rate of rectal NET patients with SSTR2 expression was significantly better than those without SSTR2 expression (98.5% vs. 92.6%, p = 0.006; Fig. 3a). Subgroup analysis based on tumor grade showed that grade 1 rectal NET patients with SSTR2 expression had significant better overall survival than those without SSTR2 expression (5-year survival rate, 98.4% vs. 93.4%, p = 0.011; Fig. 3b). In contrast, the overall 5-year survival rate of grade 2 rectal NET patients with SSTR2 expression was better than those without SSTR2 expression though statistically not significant (100.0% vs. 85.7%, p = 0.196; Fig. 3c).
Figure 3.
Kaplan–Meier survival analyses of rectal neuroendocrine tumors. (a) The overall 5-year survival rate of patients with SSTR2 expression was significantly better than those without expression (98.5% vs. 92.6%, p = 0.006). (b) Patients with grade 1 rectal NET and SSTR2 expression had significant better overall 5-year survival rate than those without SSTR2 expression (98.4% vs. 93.4%, p = 0.011). (c) The overall 5-year survival rate of grade 2 rectal NET patients with SSTR2 expression was better than those without SSTR2 expression though statistically not significant (100.0% vs. 85.7%, p = 0.196).
Univariable and multivariable survival analysis
Clinicopathologic factors including low grade (HR 5.719, CI 1.793–18.243, p = 0.001), absence of lymphovascular invasion (HR 4.455, CI 1.027–19.317, p = 0.029), absence of lymph node metastasis (HR 6.371, CI 0.826–49.136, p = 0.041), and absence of distant metastasis (HR 72.812, CI 8.132–651.909, p < 0.001) were significantly correlated with better overall survival in rectal NET patients by univariable analyses (Table 2).
Table 2.
Univariate and multivariate analyses.
| Clinicopathologic factors | 5YSR (%) | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | ||
| Age (years) | 0.054 | ||||||
| ≤ 50 | 96.6 | 1.00 | |||||
| > 50 | 96.9 | 2.167 | 0.967–4.856 | ||||
| Sex | 0.784 | ||||||
| Male | 98.0 | 1.00 | |||||
| Female | 95.4 | 1.116 | 0.507–2.456 | ||||
| Grade | 0.001* | 0.002* | |||||
| NET G1 | 96.9 | 1.00 | 1.00 | ||||
| NET G2 | 95.2 | 5.719 | 1.793–18.243 | 6.633 | 2.023–21.746 | ||
| Size | 0.685 | ||||||
| ≤ 1 cm | 96.8 | 1.00 | |||||
| > 1 cm | 96.2 | 1.354 | 0.311–5.889 | ||||
| Proper muscle invasion | 0.232 | ||||||
| Absent or NAa | 96.7 | 1.00 | |||||
| Present | 75.0 | 3.210 | 0.424–24.296 | ||||
| Lymphovascular invasion | 0.029* | 0.492 | |||||
| Absent | 97.0 | 1.00 | 1.00 | ||||
| Present | 87.5 | 4.455 | 1.027–19.317 | 1.789 | 0.341–9.390 | ||
| Perineural invasion | 0.668 | ||||||
| Absent | 96.7 | 1.00 | |||||
| Present | # | ||||||
| Lymph node metastasis | 0.041* | 0.633 | |||||
| Absent or NAa | 96.7 | 1.00 | 1.00 | ||||
| Present | 50.0 | 6.371 | 0.826–49.136 | 0.542 | 0.044–6.702 | ||
| Distant metastasis | < 0.001* | < 0.001* | |||||
| Absent | 97.1 | 1.00 | 1.00 | ||||
| Present | 0 | 72.812 | 8.132–651.909 | 58.519 | 6.116–559.950 | ||
| pT classification | 0.388 | ||||||
| pT1 | 96.6 | 1.00 | |||||
| pT2–4 | 80.0 | 2.374 | 0.314–17.942 | ||||
| AJCC stage group | 0.058 | ||||||
| I | 97.0 | 1.00 | |||||
| II–IV | 90.9 | 3.775 | 0.862–16.537 | ||||
| Chromogranin expression | 0.900 | ||||||
| Absent | 96.0 | 1.00 | |||||
| Present | 96.3 | 0.934 | 0.320–2.726 | ||||
| SSTR2 expression | 0.006* | 0.014* | |||||
| Absent | 92.6 | 1.00 | 1.00 | ||||
| Present | 98.5 | 0.346 | 0.157–0.759 | 0.365 | 0.164–0.815 | ||
*Statistically significant at p < 0.05.
aEndoscopically resected neuroendocrine tumors were regarded as not applicable cases.
#All cases are censored so statistics are not calculated.
SSTR somatostatin receptor, NA not applicable, AJCC American Joint Committee on Cancer, HR hazard ratio, CI confidence interval.
Multivariable analyses were conducted with factors that were shown by the univariable analyses to be significant (Table 3). SSTR2 expression (p = 0.014), low tumor grade (p = 0.002), and absence of distant metastasis (p < 0.001) were an independent prognostic factors in rectal NET patients (Table 2).
Discussion
SSAs have been used to treat NETs, and radiolabeled SSAs have been increasingly used for imaging and therapy31–34. The expression of SSTR in NETs is the rationale for these clinical applications35. Among five subtypes of the SSTRs, SSTR2 is the most commonly expressed subtype in the NETs36–38. SSTR2 expression has previously been reported in GEP NETs, but it has not been specifically reported in rectal NETs. To the best of our knowledge, this is the first study on SSTR2 expression and its clinicopathologic correlation in large cohort of rectal NETs, including patients’ survival.
SSTR2 expression was identified in approximately 70% of rectal NETs in the present study. Previous studies demonstrated that SSTR2 expression in a small cohort of rectal NETs (range 3–13 patients) and the majority of them were included as a component of left colon or colorectal NET cohorts18,38–41. The proportion of SSTR2 expression in rectal NETs ranged from 14 to 100% in the previous studies18,38,39,41. The prevalence (70%) of SSTR2 expression in rectal NETs in the present study is similar with that of Oana et al., and they reported 53.8% (7 of 13 cases) of rectal NETs with SSTR2 expression39. Previously reported prevalence of SSTR2 expression in rectal NETs is variable18,38,39,41. Surprisingly, one study reported 3 rectal NETs with 100% SSTR positivity41. Hirofumi et al. reported SSTR2 expression in 10 out of 71 (14.1%) rectal NETs40. The low prevalence of SSTR2 expression in previous study may be due to different proportion of high grade (grade 2 and 3) rectal NETs. The present study include 324 (92.6%) cases of grade 1 and 26 (7.4%) cases of grade 2 rectal NETs, while previous study include 51 (71.8%) cases of grade 1 and 20 (28.2%) cases of grade 2 and 3 rectal NETs40.
SSTR2 expression in rectal NETs was significantly correlated with favorable clinicopathologic factors, such as small size, absence of lymph node metastasis, low pT classification, low AJCC stage group, and negative chromogranin immunohistochemical expression. In addition, SSTR2 expression was significantly associated with favorable survival and an independent good prognostic factor in rectal NET patients. There have been a few studies of SSTR2 expression and compared their clinicopathologic correlation, including patients’ survival in the pancreatic NETs, but no previous large cohort studies in rectal NETs. SSTR2 expression was significantly correlated with improved survival rate and an independent good prognostic factor in previous studies with pancreatic NETs26,27,42, which was consistent with the present study.
To the best of our knowledge, this is the first large-scale study to evaluate the significance of SSTR2 immunohistochemistry in rectal NETs. However, we did not evaluate the value of SSTR2 expressions for SSTR-targeting PET/CT and PRRT in terms of clinical outcomes. Further studies with clinical value of PRRT or imaging modalities using SSTR expression is recommended to assess the overall effects of SSTR expressions in treatment of rectal NET patients.
In conclusion, approximately two-thirds of rectal NETs expressed SSTR2. SSTR2 expression were significantly associated with favorable behavior and good overall survival in patients with rectal NETs. Furthermore, SSTR2 expression can be used as prognostic factors. When metastatic disease occurs, SSTR2 expression can be used a possible target for somatostatin analogues.
Author contributions
Conceptualization: J.Y.K., S.-M.H. Data curation: J.K., Y.-i.K., D.-H.Y., C.Y., I.J.P., B.-Y.R., J.-S.R. Formal analysis: J.Y.K., J.K., S.-M.H. Methodology: Y.-i.K., D.-H.Y., C.Y., I.J.P., B.-Y.R., J.-S.R. Writing—review and editing: J.Y.K., S.-M.H., Y.-i.K.
Data availability
All relevant data are within the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cho MY, Kim JM, Sohn JH, Kim MJ, Kim KM, et al. Current trends of the incidence and pathological diagnosis of gastroenteropancreatic neuroendocrine tumors (GEP-NETs) in Korea 2000–2009: Multicenter study. Cancer Res Treat. 2012;44(3):157–165. doi: 10.4143/crt.2012.44.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsai HJ, Wu CC, Tsai CR, Lin SF, Chen LT, Chang JS. The epidemiology of neuroendocrine tumors in Taiwan: A nation-wide cancer registry-based study. PLoS One. 2013;8(4):e62487. doi: 10.1371/journal.pone.0062487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fraenkel M, Kim M, Faggiano A, de Herder WW, Valk GD, Knowledge N. Incidence of gastroenteropancreatic neuroendocrine tumours: A systematic review of the literature. Endocr. Relat. Cancer. 2014;21(3):R153–R163. doi: 10.1530/ERC-13-0125. [DOI] [PubMed] [Google Scholar]
- 4.Bosman FT, Carneiro F, Hruban RH, Theise ND. In: WHO Classification of Tumours of the Digestive System. 4. Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. International Agency for Research on Cancer; 2010. [Google Scholar]
- 5.Arends MJ, Fukayama M, Klimstra DS, et al., editors. WHO Classification of Tumours of the Digestive System. 5. International Agency for Research on Cancer; 2019. [Google Scholar]
- 6.Kim JY, Kim KS, Kim KJ, Park IJ, Lee JL, Myung SJ, et al. Non-L-cell immunophenotype and large tumor size in rectal neuroendocrine tumors are associated with aggressive clinical behavior and worse prognosis. Am. J. Surg. Pathol. 2015;39(5):632–643. doi: 10.1097/PAS.0000000000000400. [DOI] [PubMed] [Google Scholar]
- 7.Lee SH, Kim BC, Chang HJ, Sohn DK, Han KS, Hong CW, et al. Rectal neuroendocrine and L-cell tumors: Diagnostic dilemma and therapeutic strategy. Am. J. Surg. Pathol. 2013;37(7):1044–1052. doi: 10.1097/PAS.0b013e3182819f0f. [DOI] [PubMed] [Google Scholar]
- 8.Gastrointestinal Pathology Study Group of Korean Society of P. Sohn JH, Cho MY, Park Y, Kim H, Kim WH, et al. Prognostic significance of defining L-cell type on the biologic behavior of rectal neuroendocrine tumors in relation with pathological parameters. Cancer Res. Treat. 2015;47(4):813–822. doi: 10.4143/crt.2014.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filopanti M, Ronchi C, Ballare E, Bondioni S, Lania AG, Losa M, et al. Analysis of somatostatin receptors 2 and 5 polymorphisms in patients with acromegaly. J. Clin. Endocrinol. Metab. 2005;90(8):4824–4828. doi: 10.1210/jc.2005-0132. [DOI] [PubMed] [Google Scholar]
- 10.Guillermet-Guibert J, Lahlou H, Cordelier P, Bousquet C, Pyronnet S, Susini C. Physiology of somatostatin receptors. J. Endocrinol. Invest. 2005;28(11):5–9. [PubMed] [Google Scholar]
- 11.Dalm VA, van Hagen PM, van Koetsveld PM, Achilefu S, Houtsmuller AB, Pols DH, et al. Expression of somatostatin, cortistatin, and somatostatin receptors in human monocytes, macrophages, and dendritic cells. Am. J. Physiol. Endocrinol. Metab. 2003;285(2):E344–E353. doi: 10.1152/ajpendo.00048.2003. [DOI] [PubMed] [Google Scholar]
- 12.Ampofo E, Nalbach L, Menger MD, Laschke MW. Regulatory mechanisms of somatostatin expression. Int J Mol Sci. 2020;21:11. doi: 10.3390/ijms21114170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel YC. Somatostatin and its receptor family. Front. Neuroendocrinol. 1999;20(3):157–198. doi: 10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- 14.Yamada Y, Reisine T, Law SF, Ihara Y, Kubota A, Kagimoto S, et al. Somatostatin receptors, an expanding gene family: Cloning and functional characterization of human, a protein coupled to adenylyl cyclase. Mol. Endocrinol. 1992;6(12):2136–2142. doi: 10.1210/mend.6.12.1337145. [DOI] [PubMed] [Google Scholar]
- 15.Yamada Y, Post SR, Wang K, Tager HS, Bell GI, Seino S. Cloning and functional characterization of a family of human and mouse somatostatin receptors expressed in brain, gastrointestinal tract, and kidney. Proc. Natl. Acad. Sci. USA. 1992;89(1):251–255. doi: 10.1073/pnas.89.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corleto VD, Nasoni S, Panzuto F, Cassetta S, Delle FG. Somatostatin receptor subtypes: Basic pharmacology and tissue distribution. Dig. Liver Dis. 2004;36(Suppl 1):S8–16. doi: 10.1016/j.dld.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Cakir M, Dworakowska D, Grossman A. Somatostatin receptor biology in neuroendocrine and pituitary tumours: Part 1—molecular pathways. J. Cell Mol. Med. 2010;14(11):2570–2584. doi: 10.1111/j.1582-4934.2010.01125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zamora V, Cabanne A, Salanova R, Bestani C, Domenichini E, Marmissolle F, et al. Immunohistochemical expression of somatostatin receptors in digestive endocrine tumours. Dig. Liver Dis. 2010;42(3):220–225. doi: 10.1016/j.dld.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 19.Oberg KE. Gastrointestinal neuroendocrine tumors. Ann. Oncol. 2010;21(Suppl 7):72–80. doi: 10.1093/annonc/mdq290. [DOI] [PubMed] [Google Scholar]
- 20.Martin-Gago P, Rol A, Todorovski T, Aragon E, Martin-Malpartida P, Verdaguer X, et al. Peptide aromatic interactions modulated by fluorinated residues: Synthesis, structure and biological activity of Somatostatin analogs containing 3-(3',5'difluorophenyl)-alanine. Sci. Rep. 2016;6:27285. doi: 10.1038/srep27285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalkner KM, Janson ET, Nilsson S, Carlsson S, Oberg K, Westlin JE. Somatostatin receptor scintigraphy in patients with carcinoid tumors: Comparison between radioligand uptake and tumor markers. Cancer Res. 1995;55(23 Suppl):5801s–s5804. [PubMed] [Google Scholar]
- 22.Johnbeck CB, Knigge U, Kjaer A. PET tracers for somatostatin receptor imaging of neuroendocrine tumors: Current status and review of the literature. Future Oncol. 2014;10(14):2259–2277. doi: 10.2217/fon.14.139. [DOI] [PubMed] [Google Scholar]
- 23.Waldherr C, Pless M, Maecke HR, Schumacher T, Crazzolara A, Nitzsche EU, et al. Tumor response and clinical benefit in neuroendocrine tumors after 7.4 GBq (90)Y-DOTATOC. J. Nucl. Med. 2002;43(5):610–6. [PubMed] [Google Scholar]
- 24.Bartsch DK, Scherubl H. Neuroendocrine tumors of the gastrointestinal tract. Visc. Med. 2017;33(5):321–322. doi: 10.1159/000481766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaemmerer D, Peter L, Lupp A, Schulz S, Sanger J, Prasad V, et al. Molecular imaging with (6)(8)Ga-SSTR PET/CT and correlation to immunohistochemistry of somatostatin receptors in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging. 2011;38(9):1659–1668. doi: 10.1007/s00259-011-1846-5. [DOI] [PubMed] [Google Scholar]
- 26.Okuwaki K, Kida M, Mikami T, Yamauchi H, Imaizumi H, Miyazawa S, et al. Clinicopathologic characteristics of pancreatic neuroendocrine tumors and relation of somatostatin receptor type 2A to outcomes. Cancer. 2013;119(23):4094–4102. doi: 10.1002/cncr.28341. [DOI] [PubMed] [Google Scholar]
- 27.Mehta S, de Reuver PR, Gill P, Andrici J, D'Urso L, Mittal A, et al. Somatostatin receptor SSTR-2a expression is a stronger predictor for survival than Ki-67 in pancreatic neuroendocrine tumors. Medicine (Baltimore) 2015;94(40):e1281. doi: 10.1097/MD.0000000000001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim GU, Kim KJ, Hong SM, Yu ES, Yang DH, Jung KW, et al. Clinical outcomes of rectal neuroendocrine tumors </= 10 mm following endoscopic resection. Endoscopy. 2013;45(12):1018–1023. doi: 10.1055/s-0033-1344860. [DOI] [PubMed] [Google Scholar]
- 29.Volante M, Brizzi MP, Faggiano A, La Rosa S, Rapa I, Ferrero A, et al. Somatostatin receptor type 2A immunohistochemistry in neuroendocrine tumors: A proposal of scoring system correlated with somatostatin receptor scintigraphy. Mod. Pathol. 2007;20(11):1172–1182. doi: 10.1038/modpathol.3800954. [DOI] [PubMed] [Google Scholar]
- 30.Kasajima A, Papotti M, Ito W, Brizzi MP, La Salvia A, Rapa I, et al. High interlaboratory and interobserver agreement of somatostatin receptor immunohistochemical determination and correlation with response to somatostatin analogs. Hum. Pathol. 2018;72:144–152. doi: 10.1016/j.humpath.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Amin MB, Edge SB, Greene FL, Byrd DR, Compton CC, Fritz AG, et al. AJCC Cancer Staging Manual. 8. Springer; 2017. [Google Scholar]
- 32.Hofland LJ, Breeman WA, Krenning EP, de Jong M, Waaijers M, van Koetsveld PM, et al. Internalization of [DOTA degrees,125I-Tyr3]Octreotide by somatostatin receptor-positive cells in vitro and in vivo: Implications for somatostatin receptor-targeted radio-guided surgery. Proc. Assoc. Am. Physicians. 1999;111(1):63–69. doi: 10.1046/j.1525-1381.1999.09110.x. [DOI] [PubMed] [Google Scholar]
- 33.Slooter GD, Mearadji A, Breeman WA, Marquet RL, de Jong M, Krenning EP, et al. Somatostatin receptor imaging, therapy and new strategies in patients with neuroendocrine tumours. Br. J. Surg. 2001;88(1):31–40. doi: 10.1046/j.1365-2168.2001.01644.x. [DOI] [PubMed] [Google Scholar]
- 34.Park S, Parihar AS, Bodei L, Hope TA, Mallak N, Millo C, et al. Somatostatin receptor imaging and theranostics: Current practice and future prospects. J. Nucl. Med. 2021;62(10):1323–1329. doi: 10.2967/jnumed.120.251512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schonbrunn A. Somatostatin receptors present knowledge and future directions. Ann. Oncol. 1999;10(Suppl 2):S17–21. doi: 10.1093/annonc/10.suppl_2.s17. [DOI] [PubMed] [Google Scholar]
- 36.Reubi JC, Waser B, Schaer JC, Laissue JA. Somatostatin receptor sst1-sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur. J. Nucl. Med. 2001;28(7):836–846. doi: 10.1007/s002590100541. [DOI] [PubMed] [Google Scholar]
- 37.Reubi JC, Laissue J, Waser B, Horisberger U, Schaer JC. Expression of somatostatin receptors in normal, inflamed, and neoplastic human gastrointestinal tissues. Ann. N Y Acad. Sci. 1994;733:122–137. doi: 10.1111/j.1749-6632.1994.tb17262.x. [DOI] [PubMed] [Google Scholar]
- 38.Papotti M, Bongiovanni M, Volante M, Allia E, Landolfi S, Helboe L, et al. Expression of somatostatin receptor types 1–5 in 81 cases of gastrointestinal and pancreatic endocrine tumors. A correlative immunohistochemical and reverse-transcriptase polymerase chain reaction analysis. Virch. Arch. 2002;440(5):461–75. doi: 10.1007/s00428-002-0609-x. [DOI] [PubMed] [Google Scholar]
- 39.Popa O, Taban SM, Pantea S, Plopeanu AD, Barna RA, Cornianu M, et al. The new WHO classification of gastrointestinal neuroendocrine tumors and immunohistochemical expression of somatostatin receptor 2 and 5. Exp. Ther. Med. 2021;22(4):1179. doi: 10.3892/etm.2021.10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watanabe H, Fujishima F, Komoto I, Imamura M, Hijioka S, Hara K, et al. Somatostatin receptor 2 expression profiles and their correlation with the efficacy of somatostatin analogues in gastrointestinal neuroendocrine tumors. Cancers (Basel) 2022;14:3. doi: 10.3390/cancers14030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yerci O, Sehitoglu I, Ugras N, Cubukcu E, Yuce S, Bedir R, et al. Somatostatin receptor 2 and 5 expressions in gastroenteropancreatic neuroendocrine tumors in Turkey. Asian Pac. J. Cancer Prev. 2015;16(10):4377–4381. doi: 10.7314/apjcp.2015.16.10.4377. [DOI] [PubMed] [Google Scholar]
- 42.Song KB, Kim SC, Kim JH, Seo DW, Hong SM, Park KM, et al. Prognostic value of somatostatin receptor subtypes in pancreatic neuroendocrine tumors. Pancreas. 2016;45(2):187–192. doi: 10.1097/MPA.0000000000000493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.