Abstract
Background
Imatinib has become an exceptionally effective targeted drug for treating gastrointestinal stromal tumors (GISTs). Despite its efficacy, the resistance to imatinib is common in GIST patients, posing a significant challenge to the effective treatment.
Methods
The expression profiling of TRIM21, USP15, and ACSL4 in GIST patients was evaluated using Western blot and immunohistochemistry. To silence gene expression, shRNA was utilized. Biological function of TRIM21, USP15, and ACSL4 was examined through various methods, including resistance index calculation, colony formation, shRNA interference, and xenograft mouse model. The molecular mechanism of TRIM21 and USP15 in GIST was determined by conducting Western blot, co-immunoprecipitation, and quantitative real-time PCR (qPCR) analyses.
Results
Here we demonstrated that downregulation of ACSL4 is associated with imatinib (IM) resistance in GIST. Moreover, clinical data showed that higher levels of ACSL4 expression are positively correlated with favorable clinical outcomes. Mechanistic investigations further indicated that the reduced expression of ACSL4 in GIST is attributed to excessive protein degradation mediated by the E3 ligase TRIM21 and the deubiquitinase USP15.
Conclusion
These findings demonstrate that the TRIM21 and USP15 control ACSL4 stability to maintain the IM sensitive/resistant status of GIST.
Subject terms: Gastrointestinal cancer, Cancer therapeutic resistance
Background
Gastrointestinal stromal tumors (GISTs) are a type of mesenchymal tumor found in the gastrointestinal tract and arise from interstitial cells of Cajal (ICCs) or other precursor cells. These tumors are primarily caused by mutations in either the KIT or PDGFRA [1, 2]. Imatinib is considered as a first-generation tyrosine kinase inhibitor (TKI) that exhibits outstanding efficacy as a primary targeted agent in the treatment of GIST. It serves as the primary therapy for high-risk and completely resected GISTs, as well as advanced, relapsed, unresectable, and metastatic GISTs [3, 4]. Although the significant advances made in GIST treatment through imatinib, approximately half of patients with GIST still suffer from secondary drug resistance within two years [5]. Secondary mutations partially explain imatinib resistance in GISTs with secondary imatinib resistance. However, approximately 40% of patients with no secondary mutations experience limited benefit from second- and third-line drugs such as sunitinib and regorafenib [6–8]. Currently, there are limited treatment options available for imatinib-resistant tumors without secondary mutations. The overall efficacy of these therapies is poor. Therefore, analyzing the mechanisms responsible for IM resistance is crucial, which must be characterized to address this failure to provide potential significant pathways for better treatment.
Ferroptosis is a non-apoptotic form of cell death that depends on iron and occurs when cells lose their ability to repair lipid peroxides through the glutathione peroxidase 4 (GPX4). It is characterized by the oxidation of polyunsaturated fatty acids and the presence of redox-active iron [9–11]. Utilizing ferroptosis as a strategy to sensitize cancer cells toward their first-line treatment (targeted therapy and chemotherapy) has been widely applied [12–15]. Primary or secondary resistance to imatinib continues to be a significant issue in managing GISTs. Recently, the pivotal role played by ferroptosis in the progression of GISTs has been discovered [16]. ACSL4 is a crucial enzyme that facilitates the conversion of fatty acids to fatty acyl-CoA esters, thereby regulating lipid biosynthesis. It has recently been uncovered as an indispensable component in the execution of ferroptosis [17]. However, there is no available research to clarify the effects of ACSL4 induced ferroptoisis on secondary resistance to imatinib in GISTs.
Ubiquitination is a fundamental process in post-translational protein modification that significantly regulates nearly all cellular activities, including signal transduction pathways and protein-protein interactions [18–20], and dysregulation of the ubiquitination process is critical in the development of various diseases [21]. Deubiquitinases (DUBs) can reverse the ubiquitin-mediated control over these processes by removing ubiquitin from substrates modified by E3 ubiquitin ligases and by depolymerizing polyubiquitin chains [22]. Although existing clues suggest that UPS is involved in mediating ACSL4 stability [23–25], studies on ACSL4 ubiquitination are in a preliminary stage. Moreover, there is a dearth of studies available regarding the deubiquitination process of ACSL4.
The present study elucidates the intricate process of ubiquitination and deubiquitination modifications of ACSL4 in GISTs. Our findings indicate that the E3 ligase TRIM21 and deubiquitinase USP15 regulate ACSL4 protein stability and play a role in regulating drug resistance in GIST cells. In conclusion, the findings suggest that the TRIM21/USP15-ACSL4 axis plays a crucial role in the progression of IM resistance, and this finding offers a potential approach to treat GIST.
Materials and methods
Cell culture
In this study, the human GIST cell lines, GIST-T1 with 57-nucleotide inframe deletion in KIT exon 11, and GIST-882 cells with K642E mutation in exon 13 of KIT were purchased from Biowit Technologies (Shenzhen, China). The GIST cells were cultivated in DMEM medium fortified with 10% fetal bovine serum and 1% penicillin-streptomycin at 37 °C in a humid atmosphere containing 5% CO2.
Establishment of resistant cell lines
To establish IM resistant cell lines, drug-containing medium with intermittently increasing IM (Glivec, Novartis AG, Switzerland) concentrations (from 5 μM and gradually increasing to 50 μM) was added to culture medium when GIST cells in the exponential phase. After culturing for 48 h, the IM-containing medium was replaced. The cells were then passaged when cell fusion. After two years of screening, the stable resistant GIST cell lines (GIST-T1R and GIST-882R) were successfully established. Through next-generation sequencing technology, it has been established that there were no secondary mutations identified in these cell lines. The sequencing results of GIST cells are shown in Supplementary Tables 4 and 5.
Antibodies and reagents
The following antibodies were used: rabbit monoclonal anti-ACSL4 (ab155282; Abcam, Cambridge, UK); rabbit polyclonal anti-USP15 (ab71713; Abcam); rabbit monoclonal anti-SLC7A11 (ab175186; Abcam); rabbit monoclonal anti-GPx4 (ab125066; Abcam); rabbit polyclonal anti-GAPDH (ab9485; Abcam); rabbit monoclonal anti-TRIM21 (ab207728; Abcam); rabbit monoclonal anti-Myc (ab32072; Abcam); rabbit monoclonal anti-GST (ab138491; Abcam); rabbit monoclonal anti-Flag (14793; Cell Signaling Technology); rabbit monoclonal anti-HA (ab236632; Abcam); and, mouse monoclonal anti-Ubiquitin (646302; Biolegend, San Diego, USA). Ferrostatin-1(Fer-1), MG132 proteasome inhibitor and cycloheximide (CHX) protein synthesis inhibitor were purchased from Selleck Chemicals (Houston, TX, USA) and Cell Signaling Technology, respectively.
Plasmids, lentiviral vectors, shRNA, and transfection
Lentiviral expression vectors with Flag-labeled USP15, Flag-labeled USP15 C269A, Flag-labeled TRIM21, Flag-labeled TRIM21CA and His-labeled ACSL4 were generated by inserting the relevant genes with the Flag or His tag into the pCDH-CMV-MCS-EF1α-Puro vector. HA-labeled ubiquitin-Lys48(K48) and HA-labeled ubiquitin-Lys63(K63) were obtained from Addgene (Watertown, USA), in which one Lys (K48 or K63) was retained and the rest were replaced with Arg residues. All constructs were verified by sequencing. USP15, TRIM21, ACSL4 and control shRNA were acquired from Dharmacon (Shanghai, China). The sequences of shRNAs are shown in Supplementary Table 2. Transfection of plasmid and shRNA was performed with Lipo3000 (Invitrogen, Carlsbad, USA) following the manufacturer’s instructions. LV-Flag-labeled USP15, LV-Flag-labeled USP15 C269A, LV-Flag-labeled TRIM21, LV-Flag-labeled TRIM21CA and LV-His-labeled ACSL4 were seeded in cells with a multiplicity of infection. Transfection with an empty vector was used as a control. After puromycin screening for 5–7 days until a final concentration of 2 μg/mL was reached, the cells that overexpressed Flag-labeled USP15, Flag-labeled USP15 C269A, Flag-labeled TRIM21, Flag-labeled TRIM21CA, His-labeled ACSL4, and control cells were harvested. ACSL4 knockout GIST-882 and GIST-T1 cell lines were screened with puromycin two days after lentivirus infection.
RNA isolation and real-time RT-PCR
The Total RNA Purification Kit (Norgen, Thorold, Canada) was used to isolate total RNA from both cell lines and tissues. To determine targeted mRNA levels, quantitative real-time RT-PCR (qRT-PCR) was performed on a StepOnePlus™ Real-Time PCR System (ThermoFisher Scientific, Waltham, USA). In order to standardize the results, the levels of GAPDH were used as a reference. Supplementary Table 3 provides a list of primers used for qPCR.
IC50 and resistance index calculation
To determine the IC50 and resistance index of GIST cell lines after exposure to Imatinib, the CCK-8 assay (CK04, Dojindo, Tokyo, Japan) was utilized. Briefly, the GIST cells were seeded into 96-well plates and incubated with 100 μL of DMEM medium containing 10 μL CCK-8 reagent for an additional 2 h. The absorbance was measured at a wavelength of 450 nm using a microplate reader (ThermoFisher Scientific, USA) spectrophotometer. The assays were performed in duplicate at least three times.
Patients and tissues
Samples of imatinib-sensitive GIST (n = 15) were obtained from patients who underwent curative surgery at the First Affiliated Hospital of Nanjing Medical University, China, between February 2017 and February 2022. After the patient is diagnosed with gastrointestinal stromal tumor, imaging evaluation is conducted every 3 months during the oral IM process to understand the mass situation, and PR is determined according to the Choi standard. Conduct multidisciplinary discussions before proceeding with surgery. Surgery was performed on patients to obtain GIST tissue, and genetic testing showed mutations in exon 11 without secondary mutations. And all patients received imatinib treatment after surgery, and no tumor recurrence was observed on CT scans at least one year after surgery. Samples of imatinib-resistant GIST (n = 15) were obtained either through surgery or CT-guided needle biopsy from patients with disease progression while receiving imatinib therapy. Immediately after resection, all tissues were stored in liquid nitrogen. The collection of human samples adhered to the guidelines of the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University. The clinical information of GIST patients is shown in Supplementary Table 1.
5‐HETE assay
This study evaluated 5-hydroxyeicosatetraenoic acid (5-HETE), a product of arachidonic acid oxidation mediated by ACSL4, in lysates of cells [26], using an enzyme-linked immunosorbent assay (ELISA) kit (catalog number CED739Ge, Uscnlife, Hubei, China). The manufacturer’s recommended protocol was followed to measure 5-HETE levels.
12/15-HETE assay
12/15-HETE levels were assessed using 12/15-HETE ELISA kits (catalog numbers ab133034/ab133035, Abcam) according to the manufacturer’s instructions.
Iron assay
Intracellular ferrous iron (Fe2+) level was determined using the iron assay kit (ab83366, Abcam) according to the manufacturer’s instructions.
GSH assay
Reduced glutathione (GSH) was detected by GSH and GSSG Assay Kits (Beyotime, S0053, Shanghai, China).
Lipid ROS assay
Lipid ROS was determined using an ROS Assay Kit (Beyotime, S0033S, Shanghai, China).
Colony formation assay
Cells were cultured at a density of 1000 cells per well in 6-well plates. Crystal violet (Kaigen, China) was used to stain the fixed cells, which were then washed with PBS. Subsequently, the number of colonies was counted.
Western blot
Total protein was extracted from GIST cells and tissues. The extracted proteins were subjected to SDS-PAGE and transferred to PVDF membranes (Millipore, Massachusetts, USA). The membranes were blocked in TBST buffer with 5% skimmed milk and incubated overnight at 4 °C with primary antibodies. After washing, the membranes were incubated with secondary antibodies for 2 h at room temperature. Thereafter, the membranes were processed with the Super ECL Plus Kit (US EVERBRIGHT INC, Suzhou, China). Cutoff values to define the high and low expression of targets were chosen on the basis of a measurement of heterogeneity with the log-rank test statistic with respect to progression-free survival.
Co-immunoprecipitation
Cells (1 × 107) were washed with PBS twice and then lysed on ice for 30 min with 500 μL of NP40 lysis buffer (Beyotime, Haimen, China). The lysates were centrifuged at 12,000 × g for 15 min at 4 °C, and the supernatant was incubated with the targeted antibody. The samples were then incubated at 4 °C overnight. Protein A/G Plus-Agarose (Santa Cruz Biotechnology) was added to the samples, which were rotated at 4 °C overnight. The Protein A/G Plus-Agarose was washed three times with washing buffer. After removing the washing buffer, it was eluted by adding 2X SDS-PAGE Sample Buffer (Beyotime) and boiled. All ubiquitin assays in our study were performed under denaturing conditions. Ubiquitination was detected using denature-ip.
In vitro ubiquitylation of ACSL4
For preparation of ubiquitinated ACSL4 as the substrate for the in vitro ubiquitination assay, HEK293T cells were transfected with both His-ACSL4 and HA-ubiquitin and were treated with 20 μM MG132 for 8 h. Ubiquitylated ACSL4 was purified from the cell extracts with anti-His affinity column (Thermo Fisher Scientific) and then incubated with the recombinant protein in a buffer for 2 h at 37 °C. Reactions were subjected to IB analysis.
Cycloheximide chase assay and protein half-life analysis
For the ACSL4 half-life assay, cyclohexamide (CHX; MedChemExpress; HY-12320, Monmouth Junction, NJ, USA) was added to cells at a final concentration of 40 μg/mL to block new protein synthesis. Cells were collected at the indicated time points for western blot analysis.
Transmission electron microscopy
GIST cells cultured in a 6-well plate were digested with trypsin, centrifuged at 300 g for 5 min, and resuspended with serum in a 1.5 mL microcentrifuge tube. The cells were fixed in EM fixation buffer overnight at 4 °C. After staining with OsO4 (1%), the samples were sliced into ultrafine sections and subjected to electron microscopy (JEM-F200, Japan).
Immunohistochemistry assay
Fresh-frozen human GIST tissue samples and GIST xenografts, which had been stored at −80 °C, were fixed in 4% formalin and embedded in paraffin for immunohistochemistry (IHC). The 4 μm-thick sections derived from paraffin-embedded tissues were stained with the indicated primary antibody at 4 °C overnight, followed by incubation with secondary HRP-conjugated antibody at 37 °C for 1 h in the dark. The tissues were counterstained with Hoechst nuclear dye (Thermo Fisher Scientific, MA, USA) at room temperature for 1 min, and the sections were imaged with a fluorescence microscope at 40x magnification. Immunofluorescence analysis was performed using ImageJ software (Version 1.46; National Institutes of Health, MD, USA).
Animal studies
All animal procedures were approved by the Nanjing Medical University Institutional Animal Care and Use Committee. For subcutaneous tumour models, logarithmically growing GIST cells (6 × 106 cells per mouse) that were resuspended in 100 μL phosphate-buffered saline (PBS) were injected subcutaneously into the flanks of 6-week-old female nude mice for tumor growth assays. Tumors were measured every 4 days. When the tumor volume reached 300 mm3, treatment was initiated. All mice were monitored daily for the development of subcutaneous tumors. when the tumors grew to the required size, mice were euthanized.
Statistical analysis
All results are presented as the mean ± standard deviation (SD) or as the mean ± standard error of the mean (SEM). The statistical analyses were performed using GraphPad Prism 9.0 and SPSS 20.0 software. All statistical tests were either a 2-tailed Student’s t-test or a one-way ANOVA, with a P value of less than 0.05 indicating a statistically significant difference.
Results
ACSL4 is aberrantly downregulated in IM resistance GIST
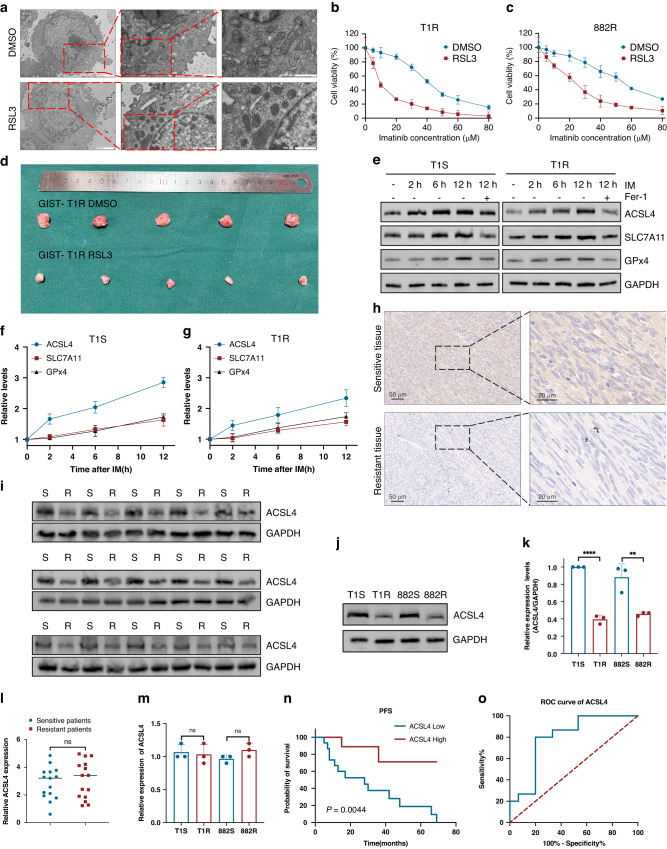
Previous research has highlighted the critical role of ferroptosis in the development of resistance to IM in GIST [16, 27]. The small molecule RSL3 functions as a ferroptosis inducer and has been shown to promote cell death through lipid peroxidation [28]. To further investigate the correlation between ferroptosis and IM resistance in Gastrointestinal stromal tumors (GISTs), transmission electron microscopy (TEM) showed that GIST cells, after treatment with RSL3, exhibited shrunken mitochondria accompanied by an increase in membrane density (Fig. 1A). The morphological changes are typical of ferroptosis. Additionally, RSL3 was administered to GIST-T1R and GIST-882R cells which led to a considerable reduction in IC50 values for IM (Fig. 1B, C). To evaluate the therapeutic effects of ferroptosis activation in GIST, xenograft models were employed. We administered a single dose of RSL3(50 mg/kg/day), which considerably reduced the growth of xenograft tumors in nude mice (Figs. 1D and S1E). Our analysis revealed that the single dose treatment of RSL3 remarkably suppressed tumor regrowth. Subsequently, we attempted to investigate the impact of ferroptosis on IM resistance. We initially investigated the expression levels of a number of vital constituents that participate in ferroptosis pathways upon stimulation with IM. The expression of both SLC7A11 and GPX4 was induced by IM (Fig. 1E). Considering the inhibitory functions of both SLC7A11 and GPX4 on ferroptosis, it is improbable that induction of SLC7A11 or GPX4 expression by IM would contribute to the occurrence of ferroptosis. Intriguingly, we detected that IM also markedly increased the expression of ACSL4 (Fig. 1E), which facilitates ferroptosis by promoting the conversion of fatty acids to fatty acyl-CoA esters. The expression of ACSL4 induced by IM was observed prior to the induction of GPX4 and SLC7A11 (Fig. 1F, G), indicating the probable role of SLC7A11 and GPX4 in restoring cell survival through a negative feedback mechanism after IM treatment. The expression of the positive regulator, ACSL4, was found decreased in IM resistance cells as compared to that in sensitive cells (Fig. S1A). However, the expression of two negative regulators, namely GPx4 and SLC7A11, have no difference (Fig. S1B, C).
Fig. 1. ACSL4 is aberrantly downregulated in GIST.
A Ferroptosis were observed by transmission electron microscopy in T1R cells cultured with or without RSL3. B, C CCK8 assays of IM-resistant cells cultured in IM treated with or without RSL3 (150 nM for 3 h). D Representative images of tumors in nude mice formed by the GIST-T1R cells in the different subgroups (n = 5 mice/group). E Western blot analysis of ACSL4, SLC7A11, and GPX4 expression in GIST cells at 2 h, 6 h, and 12 h after exposure to IM with or without ferrostatin-1 (Fer-1) (20 μM 12 h). F, G Quantification of ACSL4, SLC7A11, and GPX4 protein level in GIST cells. H IHC for ACSL4. I Protein level of ACSL4 in human GIST tissue from IM-resistant and IM-sensitive patients. J, K Western blot analysis and quantification of ACSL4 protein level in GIST cells. L RT-qPCR analyses of ACSL4 mRNA levels in sensitive and resistant GIST tissues. M Quantitative RT-PCR analysis of ACSL4 target genes from IM-sensitive and IM-resistant cells. N Kaplan-Meier plot of progression-free survival by ACSL4 expression. O Receiver operating characteristic analysis of the risk of patients with IM-sensitive and IM-resistant GIST. Error bars represent the mean (n = 3) ± S.D. *P < 0.05, **P < 0.01, ***P < 0.001.
In the present study, we investigated the possible involvement of ACSL4 in IM resistance. Immunohistochemistry (IHC) was performed to examine the expression of ACSL4, which indicated a negative association between ACSL4 expression and IM resistance in GIST (Fig. 1H). Next, analyses of protein and mRNA levels demonstrated a significant decrease in ACSL4 protein in IM-resistant GIST cells and tissues, without a corresponding decrease in mRNA levels (Fig. 1I–M). This suggests that the decrease in ACSL4 protein is not due to a transcriptional down-regulation of ACSL4 mRNA. Then, we employed receiver operating characteristic curve analysis as a means of evaluating the significance of ACSL4 in predicting patient prognosis. According to our findings, ACSL4 has the potential to be a sensitive biomarker for diagnosing IM resistance in GIST as evidenced by the 0.7956 area under the curve (95% CI, 0.6274–0.9637) (Fig. 1N, O).
ACSL4 mitigates imatinib resistance of GIST by promoting ferroptosis in vivo and in vitro
To investigate the potential correlation between the expression of ACSL4 and IM resistance in GIST, we introduced short hairpin RNA (shRNA) for ACSL4 or ACSL4 overexpression plasmid into GIST-T1 and GIST-882 cells (Fig. 2A–D). The overexpression of ACSL4 resulted in a significant decrease in the IC50 values for IM. Conversely, ACSL4 knockdown resulted in an elevation in the IC50 values of GIST-T1 and GIST-882 cells (Fig. 2E, F). In addition, the outcomes of colony formation support the previous findings, which underscore the critical function of ACSL4 in suppressing proliferation in GIST (Fig. 2G). To investigate the tumor-suppressive impact of ACSL4 in GIST, xenograft models were utilized. The ACSL4 overexpression resulted in a noteworthy reduction in the growth of xenograft tumors in nude mice (Figs. 2H and S1F). In conclusion, the preceding data highlights that ACSL4 mitigates IM resistance in GIST cells.
Fig. 2. ACSL4 mitigates imatinib resistance of GIST by promoting ferroptosis in vivo and in vitro.
A–D Western blot analysis of ACSL4 overexpressing and knockdown efficiency in GIST cells. E, F CCK8 proliferation assay verified the effect of ACSL4 expression level on the sensitivity of GIST cells to IM. G The effect of ACSL4 expression levels on the proliferation of GIST cells was verified by clone formation assay. H Representative images of tumors in nude mice bearing T1R cells in different groups (n = 5 mice/group). Scale bars: 1 cm. I The ferroptosis in GIST cells were detected by transmission electron microscopy. J 5-HETE levels were detected using a 5-HETE kit in GIST cells. K 12-HETE levels were detected using a 12-HETE kit in GIST cells. L 15-HETE levels were detected using a 15-HETE kit in GIST cells. Error bars represent the mean (n = 3) ± S.D. *P < 0.05, **P < 0.01, ***P < 0.001.
ACSL4 has been suggested to play a key role in both the process of ferroptosis and proliferation in several other animal models [29]. However, the question of whether ACSL4 modulates ferroptosis in GIST cells remains unclear. The administration of Fer-1 and IM in combination was observed to result in higher cell viability when contrasted with IM administered alone (Fig. S1D). In addition, transmission electron microscopy (TEM) analysis was employed to examine ferroptosis and revealed that overexpression of ACSL4 resulted in increased levels of ferroptosis (Fig. 2I). The overexpression of ACSL4 markedly altered additional indicators of ferroptosis (Figs. 2J–L and S1G–I). The increased concentrations of hydroxyeicosatetraenoic acids (HETEs) are positively correlated with the progression of ferroptosis [11]. The findings indicate that the overexpression of ACSL4 is associated with an upregulation of ferroptosis. Knockdown of ACSL4 resulted in a significant decrease in the expression levels of 5-HETE, 12-HETE, and 15-HETE (Fig. 2J–L). These findings indicate that knockdown of ACSL4 leads to a diminished production of ferroptosis markers. Consequently, the downregulation of ACSL4 was observed to significantly decrease the level of ferroptosis. As such, it was concluded that ACSL4 plays a critical role in the regulation of ferroptosis in GIST cells.
The E3 Ligase TRIM21 interacts with ACSL4 and targets it for ubiquitination, leading to its degradation
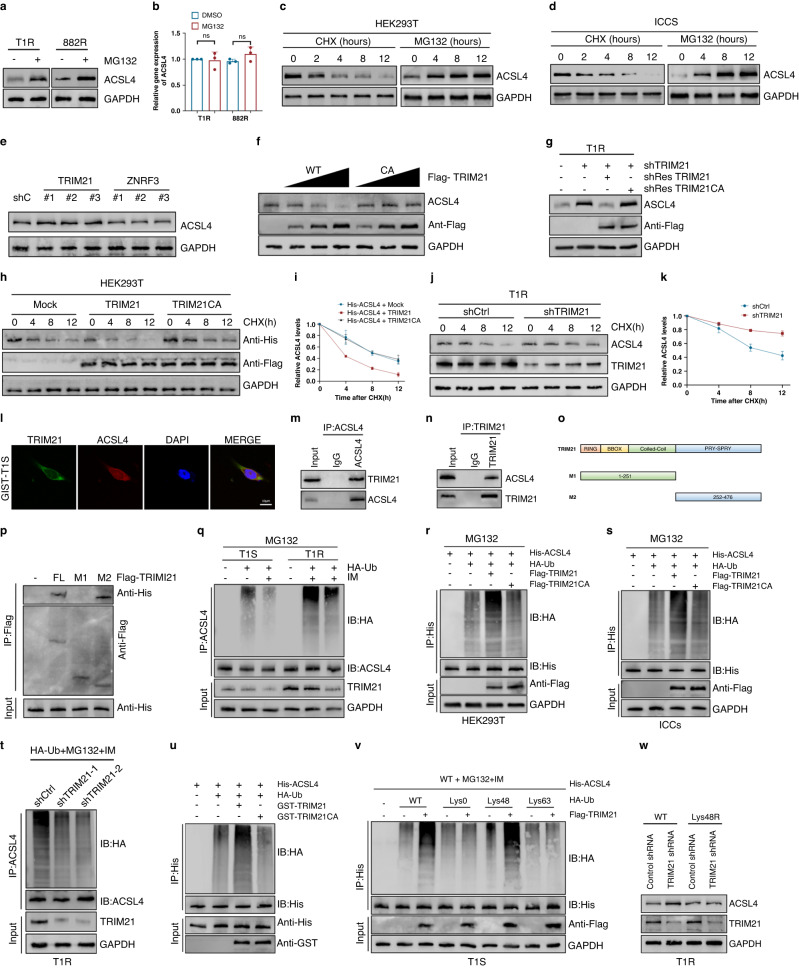
Considering the significant influence of post-translational modifications on protein stability and drug resistance [30–32], it is necessary to conduct further research on the ubiquitination regulation of ACSL4 in GIST. To achieve this objective, we subjected T1R and 882 R cells to treatment with the proteasome inhibitor MG132. Our findings demonstrated a significant increase in the protein expression levels of ACSL4 in response to MG132 treatment (Fig. 3A). The qRT-PCR assay established that there was no significant change in the mRNA expression levels of ACSL4 after MG132 treatment (Fig. 3B). In order to inhibit protein synthesis by de novo means, we utilized CHX, resulting in a gradual degradation of ACSL4 protein, ultimately reaching undetectable levels within 12 h. Additionally, treatment of cells with the proteasome inhibitor MG132 produced a notable elevation in levels of ACSL4 protein (Fig. 3C, D). Based on these observations, we postulated that the regulation of ACSL4 occurs at the post-translational level via the ubiquitin-proteasome system. To identify the specific E3 ligase and DUB responsible for regulating ACSL4 stability, an assessment was conducted on ACSL4-associated proteins using MS analysis, leading to the identification of two E3 ligases and three DUBs that interacted with ACSL4 (Fig. S2A).
Fig. 3. The E3 ligase TRIM21 interacts with and ubiquitinates ACSL4 to promote its degradation.
A Western blot analysis of ACSL4 expression in T1R and 882 R cells treated with DMSO or MG132 for 8 h. B qRT-PCR analysis of ACSL4 expression in the indicated cells treated with DMSO or MG132. C, D Western blot analysis of ACSL4 expression in HEK293T and ICCs cells after treatment with CHX (40 µg/ml) or MG-132 (20 µM). E Western blot analysis of ACSL4 expression in T1R cells expressing the indicated shRNAs. F Increasing amounts of Flag-labeled wild-type TRIM21 or TRIM21CA were transfected into HEK293T cells, and cell lysates were analyzed by western blotting with antibody against ACSL4. G Western blot analysis of ACSL4 levels in GIST-T1R cells transfected with TRIM21 shRNA together with either shRNA-resistant (sh-res) Flag-labeled wild-type TRIM21 or TRIM21CA. H, I HEK293T cells were co-transfected with His-labeled ACSL4 and Flag-labeled wild-type TRIM21 or TRIM21CA, treated with CHX (100 μg/ml), collected at the indicated times, and then subjected to western blotting with antibodies against His and Flag. Quantification of ACSL4 levels relative to GAPDH are shown. J, K GIST-T1R cells stably expressing control shRNA or shRNA-TRIM21 were treated with CHX (100 μg/ml), harvested at the indicated times, and then subjected to western blotting with antibodies against ACSL4 and TRIM21. Quantification of ACSL4 levels relative to GAPDH are shown. L Confocal images showing colocalization of TRIM21 (green) and ACSL4 (red) in GIST cells. Nuclei were counterstained with DAPI (blue). Scale bar: 20 μm. M, N Cell lysates from GIST-T1R cellls were analyzed by IP using antibodies against TRIM21 and ACSL4, then subjected to western blotting analysis. IgG was used as the isotype control. O Schematic representation of full-length (FL) Flag-labeled TRIM21 and various deletion mutants. P HEK293T cells were co-transfected with His-ACSL4 and FL Flag-labeled TRIM21 or deletion mutants. Cell lysates were analyzed by IP with Flag beads followed by western blotting with antibodies against His and Flag. Q GIST-T1S and GIST-T1R were co-transfected with HA-ubiquitin (HA-Ub), and cell lysates were subjected to denature-IP with ACSL4 antibody, followed by western blotting with antibodies against HA and ACSL4. Cells were treated with 20 μM MG-132 and with or without low-dose IM for eight hours before harvesting. R, S HEK293T cells or ICCs were cotransfected with His-ACSL4, HA-ubiquitin (HA-Ub), and Flag-labeled wild-type TRIM21 or TRIM21CA, and cell lysates were subjected to denature-IP with His beads followed by western blotting with antibodies against HA and His. Cells were treated with 20 μM MG-132 for eight hours before harvesting. T GIST-T1R was cotransfected with the indicated shRNA and HA-Ub, and cell lysates were subjected to denature-IP with ACSL4 antibody, followed by western blotting with antibodies against HA and ACSL4. Cells were treated with 20 μM MG-132 for eight hours before harvesting. U Unubiquitylated or ubiquitylated His-ACSL4 was incubated with wild-type GST-TRIM21 or GST-TRIM21CA coupled to glutathione-sepharose beads. His-ACSL4 was subjected to denature-IP with His beads followed by western blotting with antibodies against HA and His. Recombinant GST-TRIM21 or GST-TRIM21CA were analyzed by SDS-PAGE. V GIST-T1S cells were co-transfected with His-ACSL4, Flag-TRIM21, and HA-Ub Lys0, Lys48-only, or Lys63-only plasmids, and then the ACSL4 ubiquitylation linkage was analyzed. W GIST-T1R cells transfected with wild-type Ub or Ub-Lys48R were cultured for 72 h in the presence of control shRNA or TRIM21 shRNA. Cell lysates were analyzed by western blotting using antibodies against ACSL4 and TRIM21.Error bars represent the mean (n = 3) ± S.D. *P < 0.05, **P < 0.01, ***P < 0.001.
Subsequently, we selected two E3 ligases, which exhibited a significant association with ACSL4, for further investigation. Three distinct shRNA were developed to reduce TRIM21 or ZNRF3 expression in T1R cells. Our findings demonstrated that only the three shRNA sequences targeting TRIM21 significantly increased protein expression levels of ACSL4, while leaving its mRNA expression levels unaffected (Figs. 3E and S2B, C). The enzymatic activity of E3 ligases depends crucially on the RING finger domain [32]. We demonstrated that the overexpression of the wild-type TRIM21, but not the catalytically inactive mutant TRIM21CA (C16A/C31A/H33W), led to a dose-dependent reduction in the endogenous ACSL4 protein level, without any noticeable effect on its mRNA expression levels (Figs. 3F and S2D), indicating that TRIM21 modulates ACSL4 via its ubiquitinating activity. The depletion of TRIM21 resulted in a concurrent increase in the expression of ACSL4. This effect was mitigated by the overexpression of wild-type TRIM21, but not by the TRIM21CA mutant (Figs. 3G and S2E). Moreover, CHX pulse-chase experiments showed that in HEK293T cells, overexpression of TRIM21 markedly reduced the stability of ACSL4, while the TRIM21CA mutant failed to yield any such impact (Fig. 3H, I). Conversely, knockdown of TRIM21 in resistant cell lines led to an increased stability of the ACSL4 protein (Figs. 3J, K and S2F, G). Collectively, these results provide evidence that TRIM21 plays a crucial role in the modulation of ACSL4 stability.
Next, we analyzed the subcellular colocalization of the two elements. Our findings, as observed through confocal images, revealed the green (TRIM21) and red (ACSL4) coexistence within the cytoplasm of GIST cells (Figs. 3L and S2H). Similarly, we confirmed the physical association between endogenous TRIM21 and ACSL4 protein in GIST cells (Figs. 3M, N and S2I, J). To identify the precise ACSL4-binding region on TRIM21, several Flag-labelled TRIM21 were evaluated (Fig. 3O). IP assays indicated that the primary interaction-mediating zone of TRIM21 is the M2 region, comprising amino acids 252–476 (Fig. 3P). Whether TRIM21 acts as a bona fide E3 ligase for ubiquitinating ACSL4, was investigated further. Results indicated that ACSL4 ubiquitination levels were higher in resistant cells, compared to sensitive ones. Moreover, the addition of IM alone decreased the ubiquitination of ACSL4. Correspondingly, the concentration of TRIM21 was also elevated in drug-resistant cells, and the addition of the drug alone led to a decrease in TRIM21 levels (Figs. 3Q and S2K). To examine if TRIM21 ubiquitinates ACSL4, wild-type or the TRIM21CA were co-transfected into HEK293T cells. Results from immunoprecipitation analysis demonstrated that ACSL4 was heavily ubiquitinated in cells treated with MG132. Strikingly, co-transfection with wild-type TRIM21 significantly increased ACSL4 ubiquitination, whereas TRIM21CA did not have the same effect (Figs. 3R and 3S). In line with these findings, ACSL4 ubiquitination was found to be eliminated by shTRIM21 in T1R and 882 R cells (Figs. 3T and S2L). To examine the direct relationship between ACSL4 and TRIM21, a cell-free system was utilized to incubate purified TRIM21 and ubiquitinated ACSL4. Results showed that the in vitro increase of ACSL4 polyubiquitination was only observed when using the purified wild-type TRIM21, but not the catalytically inactive mutant TRIM21CA (Fig. 3U). Consequently, TRIM21 directly ubiquitinates ACSL4. Two main forms of polyubiquitin chains, which involve Lys48(K48) or Lys63(K63) linkages have been categorized [33]. To determine which type of polyubiquitin modifications on ACSL4 are affected by TRIM21, a series of ubiquitin mutants were used. These mutations retained one Lys residue while replacing the remainder with Arg residues. The results indicated that when TRIM21 was expressed, Lys48(K48)-linked ubiquitination of ACSL4 was promoted, but not Lys63(K63)-linked ubiquitination (Figs. 3V and S2M). Furthermore, we observed that the expression of ACSL4 was elevated through the use of wild type (WT) ubiquitin rather than the enforced expression of a Lys48-resistant (K48R) version of ubiquitin (Figs. 3W and S2N). Collectively, this study unveils that TRIM21 facilitates the degradation of ACSL4 by utilizing K48-linked polyubiquitin chains.
TRIM21 promotes IM-resistance by impeding the onset of ferroptosis in vitro and in vivo
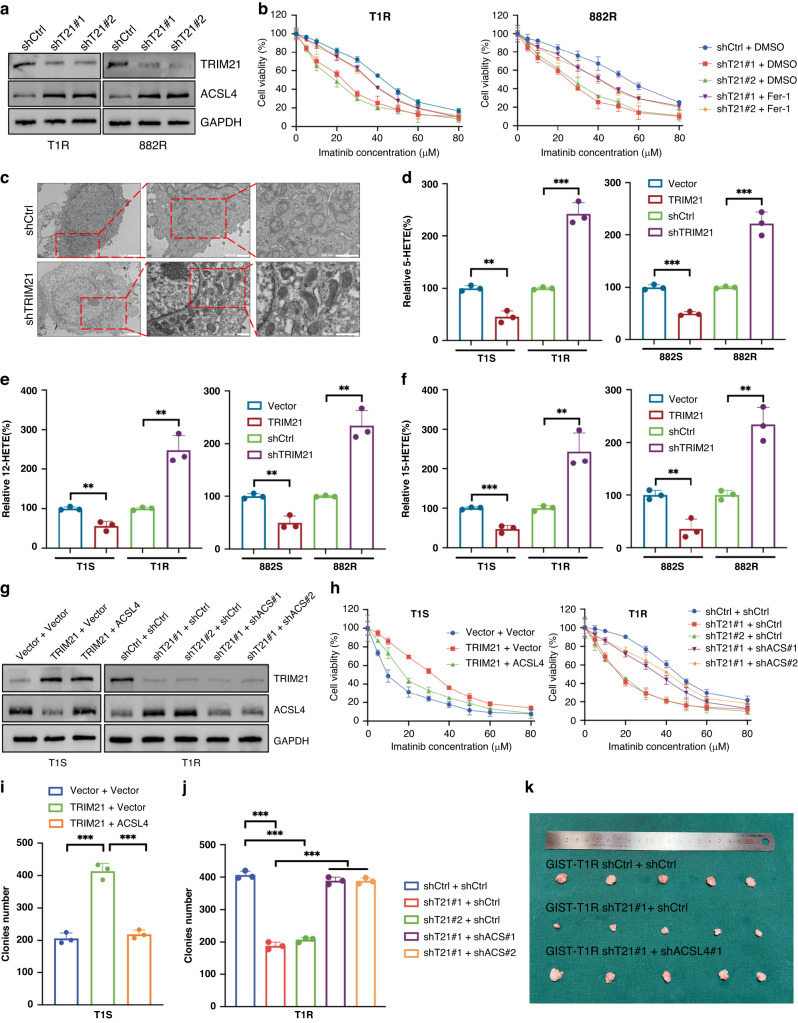
In order to explore the role of TRIM21 in GIST, we employed a knockdown approach to suppress TRIM21 expression specifically in T1R and 882 R cells (Fig. 4A). The concurrent application of Fer-1 and IM resulted in improved cell viability compared to IM monotherapy (Fig. 4B) which supports the proposition that TRIM21 impedes ferroptosis and thereby enhances IM resistance. Furthermore, the utilization of transmission electron microscopy (TEM) was employed to investigate ferroptosis, revealing that the decrease of TRIM21 activity led to an increase in the level of ferroptosis (Fig. 4C). In addition, various markers that are indicative of ferroptosis were analyzed. The results showed that elevating the expression of TRIM21 in IM-sensitive cells inhibited ferroptosis, whereas reducing TRIM21 expression in IM-resistant cells had the opposite effect (Figs. 4D–F and S2O-Q).
Fig. 4. TRIM21 promotes IM-resistance by inhibiting ferroptosis in vitro and in vivo.
A Western blot analysis of TRIM21 and ACSL4 expression in GIST cells expressing the indicated shRNAs. B Cell viability of GIST cells expressing the indicated shRNAs after treatment are indicated. C The ferroptosis in GIST cells were detected by transmission electron microscopy. D 5-HETE levels were detected using a 5-HETE kit in GIST cells. E 12-HETE levels were detected using a 12-HETE kit in GIST cells. F 15-HETE levels were detected using a 15-HETE kit in GIST cells. G Western blot analysis of TRIM21 and ACSL4 in GIST cells expressing Vector, TRIM21, ACSL4 or the indicated shRNAs. H Cell viability of GIST cells after treatment as indicated. I, J The effect of TRIM21 expression level on the proliferation of GIST cells was examined by clone formation assay. K Representative images of tumors in nude mice formed by the GIST-T1R cells in the different subgroups. Error bars represent the mean (n = 3) ± S.D. *P < 0.05, **P < 0.01, ***P < 0.001, respectively.
To confirm that the activities of TRIM21 are implemented through ACSL4, we overexpressed or knocked down TRIM21 in T1S or T1R cells (Fig. 4G). The results of both CCK-8 and colony formation assays demonstrated that TRIM21 depletion suppressed IM resistance. However, such inhibition could be ameliorated through further depletion of ACSL4 (Fig. 4H, J). Conversly, Overexpressed TRIM21 promoted IM resistance, which could be effectively counteracted by upregulation of ACSL4 (Fig. 4H, I). In summary, these findings provide evidence that TRIM21 exerts an inhibitory effect on ferroptosis-mediated IM sensitivity in GIST cells.
Based on the preceding in vitro findings, we conducted murine GIST xenograft experiments utilizing T1R cells expressing shRNAs that target TRIM21 and administering IM to the mice. The depletion of TRIM21 resulted in a significant decrease in T1R cell growth and an increase in the sensitivity of tumors to IM. Moreover, further depletion of ACSL4 restored the effect engendered by TRIM21 loss (Figs. 4K and S2R). Taken as a whole, our results demonstrate that knockdown of TRIM21 leads to an attenuated IM resistance of GIST by upregulating ACSL4 protein expression.
USP15 interacts with and deubiquitinates ACSL4
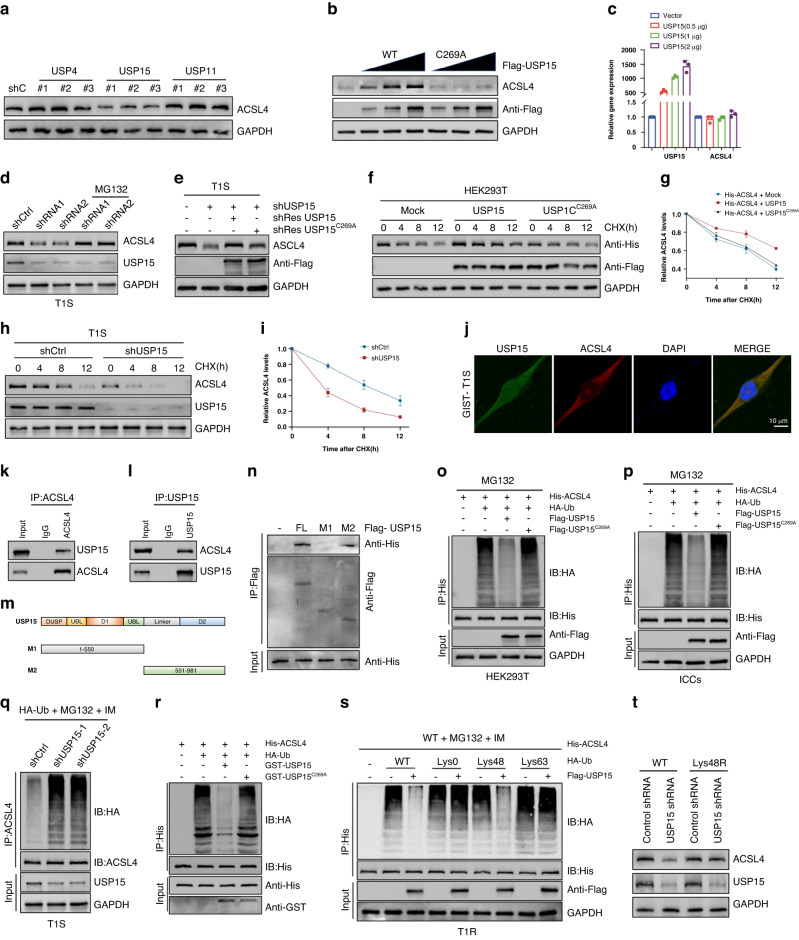
Deubiquitinating enzymes have been identified as promising therapeutic targets for the treatment of drug-resistant cancers [34, 35]. Therefore, continued investigation into these enzymes, and their involvement in the regulation of ACSL4 may lead to the development of novel treatments to overcome drug resistance. The MS analysis also demonstrated the association of three deubiquitinases with ACSL4 (Fig. S2A). In order to confirm the modulation of USP4, USP15 and USP11 on ACSL4, we employed three distinct shRNA sequences to knock down the expression of USP4, USP15, or USP11 in T1S cells and examined the endogenous ACSL4 protein level. ACSL4 expression was found to be significantly decreased upon USP15 depletion. however, USP4 and USP11 depletion did not result in any noticeable downregulation of ACSL4 (Fig. 5A). It is worth noting that the mRNA expression level of ACSL4 did not change following knockdown of USP4, USP15, or USP11 (Fig. S3A–C). Furthermore, it was observed that increasing amounts of wild-type Flag-USP15 transfection gradually increased the endogenous ACSL4 protein level, while having no effect on its mRNA level. In contrast, the transfection of mutant-type USP15C269A did not lead to any increase in the ACSL4 protein level despite the increasing amount of transfection (Fig. 5B, C), indicating that USP15 modulates ACSL4 via its deubiquitinating activity. The loss of USP15 resulted in a reduction of ACSL4 expression, which was further mitigated by treatment with MG132 or through the overexpression of wild-type USP15. However, the USP15-C269A mutant did not provide any attenuation of ACSL4 expression (Figs. 5D, E and S3D, E). Additionally, CHX pulse-chase experiments demonstrated that the stability of ACSL4 was considerably enhanced in HEK293T cells with the overexpression of USP15, but not the USP15-C269A mutant (Fig. 5F, G). In contrast, USP15 knockdown in sensitive cell lines led to destabilization of the ACSL4 protein (Figs. 5H, I and S3F, G). In summary, the aforementioned findings provide evidence that the stability of ACSL4 is modulated by USP15.
Fig. 5. USP15 interacts with and deubiquitinates ACSL4.
A Western blot analysis of ACSL4 expression in T1S cells expressing the indicated shRNAs. B Increasing amounts of Flag-labeled wild-type USP15 or USP15-C269A were transfected into HEK293T cells, and cell lysates were analyzed by western blotting with antibody against ACSL4. C qRT-PCR analysis of ACSL4 mRNA expression in 293T cells transfected with increasing amounts of USP15. D GIST-T1S cells transfected with two independent USP15 shRNA were treated with or without the proteasome inhibitor MG-132 (20 μM for 8 h) and then USP15 and ACSL4 were analyzed. E Western blot analysis of ACSL4 levels in GIST-T1S cells transfected with USP15 shRNA together with either shRNA-resistant (sh-res) Flag-labeled wild-type USP15 or USP15-C269A. F, G HEK293T cells were co-transfected with His-labeled ACSL4 and Flag-labeled wild-type USP15 or USP15-C269A, treated with CHX (100 μg/ml), collected at the indicated times, and then subjected to western blotting with antibodies against His and Flag. Quantification of ACSL4 levels relative to GAPDH are shown. H, I GIST-T1S cells stably expressing control shRNA or shRNA-USP15 were treated with CHX (100 μg/ml), harvested at the indicated times, and then subjected to western blotting with antibodies against ACSL4 and USP15. Quantification of ACSL4 levels relative to GAPDH are shown. J Confocal images showing colocalization of USP15 (green) and ACSL4 (red) in GIST cells. Nuclei were counterstained with DAPI (blue). Scale bar: 20 μm. K, L Cell lysates from GIST-T1S cellls were analyzed by IP using antibodies against USP15 and ACSL4, then subjected to western blotting analysis. IgG was used as the isotype control. M Schematic representation of full-length (FL) Flag-labeled USP15 and various deletion mutants. N HEK293T cells were co-transfected with His-ACSL4 and FL Flag-labeled USP15 or deletion mutants. Cell lysates were analyzed by IP with Flag beads followed by western blotting with antibodies against His and Flag. O, P HEK293T cells or ICCs were cotransfected with His-ACSL4, HA-ubiquitin (HA-Ub), and Flag-labeled wild-type USP15 or USP15-C269A, and cell lysates were subjected to denature-IP with His beads followed by western blotting with antibodies against HA and His. Cells were treated with 20 μM MG-132 for eight hours before harvesting. Q GIST-T1S was cotransfected with the indicated shRNA and HA-Ub, and cell lysates were subjected to denature-IP with ACSL4 antibody, followed by western blotting with antibodies against HA and ACSL4. Cells were treated with 20 μM MG-132 for eight hours before harvesting. R Unubiquitylated or ubiquitylated His-ACSL4 was incubated with wild-type GST-USP15 or GST-USP15-C269A coupled to glutathione-sepharose beads. His-ACSL4 was subjected to denature-IP with His beads followed by western blotting with antibodies against HA and His. Recombinant GST-USP15 or GST-USP15-C269A were analyzed by SDS-PAGE. S GIST-T1S cells were co-transfected with His-ACSL4, Flag-USP15, and HA-Ub Lys0, Lys48-only, or Lys63-only plasmids, and then the ACSL4 ubiquitylation linkage was analyzed. T GIST-T1S cells transfected with wild-type Ub or Ub-Lys48R were cultured for 72 h in the presence of control shRNA or USP15 shRNA. Cell lysates were analyzed by western blotting using antibodies against ACSL4 and USP15.Error bars represent the mean (n = 3) ± S.D. *P < 0.05, **P < 0.01, ***P < 0.001.
Next, we analyzed the subcellular localization of USP15 and ACSL4. Through the use of confocal imaging, we observed colocalization of green (USP15) and red (ACSL4) within the cytoplasm of GIST cells (Figs. 5J and S3H). Furthermore, our analysis revealed that endogenous USP15 physically interacted with ACSL4 protein within T1R cells (Figs. 5K, L and S3I, J). To determine the specific ACSL4-binding region on USP15, various Flag-labelled USP15 were conducted (Fig. 5M). IP assays provided evidence that the M2 region of USP15, specifically amino acids 551–981, is primarily responsible for mediating the interaction (Fig. 5N). In order to determine whether USP15 is responsible for deubiquitinating ACSL4, we co-transfected HEK293T cells with either wild-type or C269A mutant forms of USP15. IP analysis of MG132-treated cells showed that ACSL4 was heavily ubiquitinated. However, co-transfection with wild-type USP15 resulted in a significant reduction in ACSL4 ubiquitination, while co-transfection with USP15-C269A had no effect (Fig. 5O, P). The findings support the notion that shUSP15 enhances ACSL4 ubiquitination in sensitive cells (Figs. 5Q and S3K). In order to establish if ACSL4 serves as a direct substrate of USP15, a cell-free system was utilized in which purified USP15 and ubiquitinated ACSL4 were incubated. The results showed that the wild-type USP15, but not the catalytically inactive C269A mutant, led to a reduction in ACSL4 polyubiquitination in vitro (Fig. 5R). Consequently, the findings indicate that USP15 directly deubiquitinates ACSL4. we observed that the expression of USP15 inhibited the Lys48(K48)-linked ubiquitination of ACSL4, while leaving the Lys63(K63)-linked ubiquitination unaffected (Figs. 5S and S3L). Additionally, it was demonstrated that the enforced expression of a Lys48-resistant (K48R) version of ubiquitin led to the attenuation of ACSL4 expression (Figs. 5T and S3M). In summary, our findings demonstrate that USP15 contributes to the stabilization of ACSL4 via K48-linked polyubiquitin chains.
USP15 prevents IM-resistance via ferroptosis in vitro and in vivo
To explore the role of USP15 in GIST, we conducted USP15 overexpression experiments in T1R and 882 R cell lines (Fig. 6A). The co-administration of Fer-1 and IM resulted in a significant increase in cell viability compared to IM alone (Fig. 6B), indicating that USP15 may play a crucial role in eliminating IM resistance by facilitating ferroptosis. In addition, TEM analysis was employed to investigate the role of USP15 in ferroptosis, and the results indicated that overexpression of USP15 promoted ferroptosis (Fig. 6C). Furthermore, several other indicators of ferroptosis were evaluated. The findings revealed that knockdown of USP15 in IM-sensitive cells suppressed ferroptosis, while overexpression of USP15 in IM-resistant cells had an opposing impact (Figs. 6D–F and S3N-P).
Fig. 6. USP15 prevents IM-resistance via ferroptosis in vitro and in vivo.
A Western blot analysis of USP15 and ACSL4 expression in GIST cells expressing Vector or USP15. B Cell viability of GIST cells expressing Vector or USP15 after treatment are indicated. C The ferroptosis in GIST cells were detected by transmission electron microscopy. D 5-HETE levels were detected using a 5-HETE kit in GIST cells. E 12-HETE levels were detected using a 12-HETE kit in GIST cells. F 15-HETE levels were detected using a 15-HETE kit in GIST cells. G Western blot analysis of USP15 and ACSL4 in GIST cells expressing Vector, USP15, ACSL4 or the indicated shRNAs. H, I Cell viability of GIST cells after treatment as indicated. J, K The effect of USP15 expression level on the proliferation of GIST cells was examined by clone formation assay. L Representative images of tumors in nude mice formed by the GIST-T1R cells in the different subgroups. Error bars represent the mean (n = 3) ± S.D. *P < 0.05, **P < 0.01, ***P < 0.001, respectively.
To corroborate that the actions of USP15 are conveyed through ACSL4, we depleted ACSL4 in T1R cells that were overexpressing USP15 (Fig. 6G). Both CCK-8 and colony formation showed that the overexpression of USP15 inhibits IM resistance, which could be rescued by further ACSL4 depletion (Fig. 6I, K). conversely, USP15 knockdown promoted IM resistance, which were largely reversed by overexpression of ACSL4 in T1S cells (Fig. 6H, J). Taken together, these findings indicate that USP15 induces ferroptosis in GIST cells, resulting in decreased resistance to IM.
Based on the preceding in vitro findings, murine GIST xenograft experiments were carried out utilizing T1R cells that expressed ectopic USP15. Concurrently, the mice were administered with IM. The augmented USP15 level conspicuously impeded T1R cell growth and amplified the sensitivity of the tumors to IM. The reversibility of this effect was noted upon knockdown of ACSL4 (Figs. 6L and S3Q). Collectively, these data indicate that the amplification of USP15 decreases IM resistance of GIST by upregulating the protein expression of ACSL4.
We then proceeded to explore whether the c-KIT signaling pathway impacts the expression levels of TRIM21 and USP15. Hence, treating GIST cells with imatinib caused a rapid decrease in the protein levels of pKIT in imatinib-sensitive GIST cells, while the levels remained almost unchanged in imatinib-resistant GIST cells. To investigate whether activation of c-KIT signaling is associated with TRIM21/USP15-ACSL4 axis expression, we analyzed the expression levels of TRIM21, USP15, and ACSL4 proteins in GIST-T1S and GIST-T1R cells treated with imatinib at the indicated time points. As indicated below, the addition of IM alone augmented the concentration of USP15 and ACSL4. Correspondingly, the concentration of TRIM21 was reduced in drug-sensitive cells. At the same time, it showed almost no changes in imatinib resistant GIST cells (Fig. S4A). In summary, these results suggest that activation of the c-KIT signaling pathway triggers an upregulation in TRIM21 expression and a decline in USP15 expression, ultimately resulting in the degradation of ACSL4.
Correlation between TRIM21, USP15 and ACSL4 expression and association with GIST patient progression-free survival
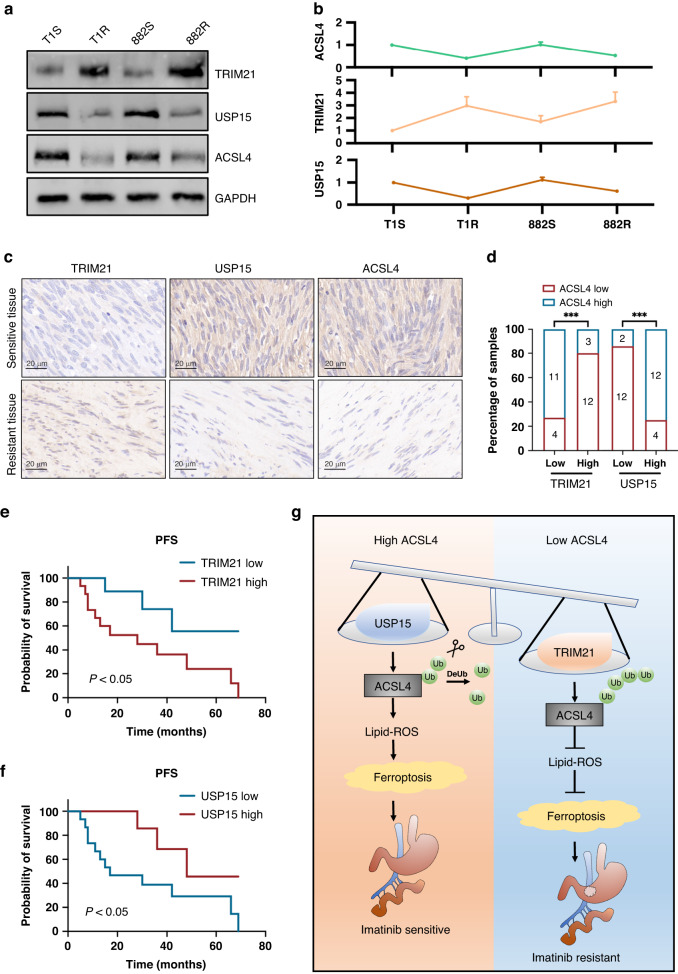
In light of the prior findings that indicate significant regulation of TRIM21 and USP15 on the stability of ACSL4 protein, we endeavored to verify the expression correlations of these three proteins in both GIST cells and samples. To accomplish this, we investigated the expression levels of TRIM21, USP15, and ACSL4. The Western blot analysis uncovered a negative correlation between the expression of TRIM21 and ACSL4 protein level. Contrarily, the expression level of USP15 exhibited a positive correlation with the expression level of ACSL4 (Fig. 7A, B). In this study, we evaluated the expression of TRIM21, USP15, and ACSL4 by immunohistochemistry in a sample of 30 gastrointestinal stromal tumor specimens. The objective was to investigate the possible clinical significance of our results. Our findings revealed a substantial upregulation of USP15 and ACSL4 in sensitive tumors when compared to resistant tumors. In contrast, TRIM21 exhibited a significant downregulation (Fig. 7C). Besides, the outcomes of this study unveiled that there exists a correlation among the expression levels of each of the proteins under scrutiny (Fig. 7D). The presence of elevated TRIM21 expression levels or decreased USP15 expression levels relates significantly to inferior prognoses and lower progression-free survival rates in GIST patients (Fig. 7E, F). Additionally, the development of secondary resistance to imatinib is correlated with these high/low expression levels. These findings strongly suggest the potential of these proteins as a therapeutic target.
Fig. 7. Correlation between TRIM21, USP15 and ACSL4 expression and association with GIST patient progression-free survival.
A, B Western blot analysis and correlation analysis of TRIM21, USP15, and ACSL4 expression in the indicated cell lines. C Representative images of IHC staining of TRIM21, USP15, and ACSL4 in clinical GIST samples. Scale bars, 20 µm. D Correlation analyses of IHC data of GIST in C. Statistical significance was determined by the chisquare test. A total of 30 GIST specimens were analyzed. E, F Kaplan-Meier analyses for GIST in C. A total of 30 GIST specimens were analyzed. G The mechanistic scheme of this study.
Discussion
Imatinib has emerged as the go-to treatment option for GIST patients [4, 36, 37]. Despite the promising response rates among patients receiving imatinib, the emergence of secondary resistance to this therapy remains a significant challenge in the clinical setting [38, 39]. Hence, elucidation of the molecular mechanisms of IM resistance is pivotal for targeted GIST therapy. The induction of ferroptosis is a commonly employed approach to overcome drug resistance in tumor cells subjected to targeted therapies [15, 40, 41]. Nevertheless, the precise molecular mechanisms that govern the development of imatinib resistance via ferroptosis in Gastrointestinal Stromal Tumors (GISTs) are not yet adequately characterized. Currently, three primary molecules, namely ACSL4, SLC7A11, and GPx4, have been shown to hold significant regulatory sway over ferroptosis [42–44]. Of these, ACSL4 has garnered significant attention as a crucial regulatory molecule in ferroptosis. A member of the acyl-CoA synthetase (ACS) family, which converts fatty acids into fatty acyl-CoA esters, ACSL4 exhibits substrate selectivity for arachidonic acid (AA). Due to its role in regulating lipid metabolism, ACSL4 has been identified as a regulator of ferroptosis. Recent findings have demonstrated that genetic deletion of ACSL4 protects cells from iron-mediated death by eliminating ferroptosis. Additionally, miR-20a-5p has been identified as a negative regulator of acyl-CoA synthetase long-chain family member 4 (ACSL4) by targeting the 3’ untranslated region of ACSL4 mRNA, thereby inhibiting ACSL4-dependent ferroptosis [45]. Furthermore, ACSL4 was found to be a direct E2F target gene and is crucial to RB1 loss-induced ferroptosis sensitization [46]. However, it remains unknown whether ACSL4 influences ferroptosis in GIST. Therefore, the current study was conducted to determine whether the induction of ferroptosis and targeting of ACSL4 would serve as promising strategies to overcome resistance to IM in GIST.
The ubiquitin-proteasome system (UPS) plays a vital role in protein degradation and is responsible for approximately 80–90% of cellular proteolysis. This pathway comprises E3 ligases and DUBs that target oncogenes or tumor suppressors, proteins that affect oncogenesis in various ways. Identifying these regulatory proteins holds great promise for the diagnosis and treatment of malignancies. In ferroptosis regulation, the UPS system controls the stability of several key proteins involved in ferroptosis. A previous study indicated that TRIM59 could promote ferroptosis in NAFLD via enhancing GPX4 ubiquitination [47]. In addition, high expression of USP8 promoted the progression and inhibited ferroptosis though its deubiquitylation activity [48]. However, the specific mechanism through which the UPS regulates ACSL4 stability remains largely unknown. Thus, this study aimed to investigate the effect of UPS on ACSL4 in Gastrointestinal Stromal Tumors (GIST). Our findings revealed that UPS controls ACSL4 protein levels, leading to aberrant activation of ferroptosis, enhanced GIST cell survival, and resistance to IM.
Aberrant UPS activity is a common occurrence in human cancers, and as a result, numerous molecular targeted drugs have been developed to combat cancer. These drugs are designed to target potential therapeutic targets identified based on such UPS activity [49–51]. Despite the sequential involvement of E3 ligases, E1, and E2 in ubiquitination, the specificity of this modification is primarily determined by E3 ligases. To identify the key E3 ligase responsible for ACSL4 ubiquitination, we conducted MS analysis to evaluate the association between ACSL4 and E3 ligases in GIST cells. Resultantly, we identified two E3 ligases that specifically interact with ACSL4. However, our subsequent studies demonstrated that only TRIM21 substantially affects the degradation of ACSL4. Furthermore, TRIM21’s control of ACSL4 depends on its enzymatic activity. Out of the USP family members, only USP15 displayed a noticeable impact on the stabilization of ACSL4. These findings suggest that the expression levels of TRIM21 and USP15 may be decisive factors in the stability of ACSL4. While TRIM21 has been shown to play both tumor-suppressive and oncogenic roles in prior studies, the mechanisms underlying these seemingly contradictory roles remain unclear in the context of cancer [52–55]. The results of our study indicate that TRIM21 can promote IM resistance by degrading ACSL4, thus highlighting the crucial role of TRIM21 in GIST. In contrast, USP15 is a key DUB that performs diverse functions depending on the context, including but not limited to disease-associated inflammation and tumorigenesis in various cancers [56–58]. Based on the crucial function of USP15 in stabilizing ACSL4, it was hypothesized that USP15 might function as a significant tumor suppressor in GIST IM resistance. Our in vitro and in vivo experiments corroborated this hypothesis, demonstrating that USP15 inhibits GIST IM resistance, and this effect is mediated by ACSL4.
Conclusion
The current study underscores the importance of regulatory mechanisms that balance ACSL4 activity in GIST cells. The ubiquitination-dependent regulation of ACSL4 by TRIM21 and USP15 demonstrated in our study highlights the complex interplay between the ubiquitin-proteasome system and tumor development, which could have implications for the development of novel anti-cancer therapies. Our study provides important insights into the role of TRIM21 and USP15 in regulating ACSL4 activity and promoting imatinib resistance in GISTs, and suggests that targeting ACSL4 stabilization could be a promising therapeutic approach in the treatment of drug-resistant GISTs.
Supplementary information
Author contributions
Conception and design: ZWC; data acquisition, analysis, and interpretation: ZWC, HYS, TTX, HX, BWL, FYL; investigation: ZWC, ZSG, CL, TTX, YBB, JNZ, TYL, QZZ, ZYH; acquisition of patient specimens: ZSG, CL, ZHL, THG, ZKX and HX; article drafting and revising: ZWC, HYS, ZSG, CL and TTX; and article writing: ZWC. All authors approved the final version of the manuscript.
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The collection of specimens and animal handling for the study have been reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Zhiwei Cui, Haoyu Sun, Zhishuang Gao, Chao Li, Tingting Xiao.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-023-02562-x.
References
- 1.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–80. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 2.Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen C-J, Joseph N, et al. PDGFRA Activating Mutations in Gastrointestinal Stromal Tumors. Science. 2003;299:708–10. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 3.Chen H, Isozaki K, Kinoshita K, Ohashi A, Shinomura Y, Matsuzawa Y, et al. Imatinib inhibits various types of activating mutant kit found in gastrointestinal stromal tumors. Int J Cancer. 2003;105:130–5. doi: 10.1002/ijc.11025. [DOI] [PubMed] [Google Scholar]
- 4.Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl J Med. 2002;347:472–80. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 5.Soreide K, Sandvik OM, Soreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 2016;40:39–46. doi: 10.1016/j.canep.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 6.Klug LR, Khosroyani HM, Kent JD, Heinrich MC. New treatment strategies for advanced-stage gastrointestinal stromal tumours. Nat Rev Clin Oncol. 2022;19:328–41. doi: 10.1038/s41571-022-00606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahadevan D, Cooke L, Riley C, Swart R, Simons B, Della Croce K, et al. A novel tyrosine kinase switch is a mechanism of imatinib resistance in gastrointestinal stromal tumors. Oncogene. 2007;26:3909–19. doi: 10.1038/sj.onc.1210173. [DOI] [PubMed] [Google Scholar]
- 8.Debiec-Rychter M, Cools J, Dumez H, Sciot R, Stul M, Mentens N, et al. Mechanisms of resistance to imatinib mesylate in gastrointestinal stromal tumors and activity of the PKC412 inhibitor against imatinib-resistant mutants. Gastroenterology. 2005;128:270–9. doi: 10.1053/j.gastro.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 9.Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017;171:273–85. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, et al. Ferroptosis: process and function. Cell Death Differ. 2016;23:369–79. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassannia B, Vandenabeele P, Vanden Berghe T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell. 2019;35:830–49. doi: 10.1016/j.ccell.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Shen Z, Song J, Yung BC, Zhou Z, Wu A, Chen X. Emerging Strategies of Cancer Therapy Based on Ferroptosis. Adv Mater. 2018;30:e1704007. doi: 10.1002/adma.201704007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu B, Chen XB, Ying MD, He QJ, Cao J, Yang B. The Role of Ferroptosis in Cancer Development and Treatment Response. Front Pharm. 2017;8:992. doi: 10.3389/fphar.2017.00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang T, Sun B, Zhong C, Xu K, Wang Z, Hofman P, et al. Targeting histone deacetylase enhances the therapeutic effect of Erastin-induced ferroptosis in EGFR-activating mutant lung adenocarcinoma. Transl Lung Cancer Res. 2021;10:1857–72. doi: 10.21037/tlcr-21-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delvaux M, Hague P, Craciun L, Wozniak A, Demetter P, Schoffski P, et al. Ferroptosis Induction and YAP Inhibition as New Therapeutic Targets in Gastrointestinal Stromal Tumors (GISTs) Cancers. 2022;14:5050. doi: 10.3390/cancers14205050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mann M, Jensen ON. Proteomic analysis of post-translational modifications. Nat Biotechnol. 2003;21:255–61. doi: 10.1038/nbt0303-255. [DOI] [PubMed] [Google Scholar]
- 19.Popovic D, Vucic D, Dikic I. Ubiquitination in disease pathogenesis and treatment. Nat Med. 2014;20:1242–53. doi: 10.1038/nm.3739. [DOI] [PubMed] [Google Scholar]
- 20.Harhaj EW, Dixit VM. Deubiquitinases in the regulation of NF-κB signaling. Cell Res. 2010;21:22–39. doi: 10.1038/cr.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du J, Fu L, Sui Y, Zhang L. The function and regulation of OTU deubiquitinases. Front Med. 2020;14:542–63. doi: 10.1007/s11684-019-0734-4. [DOI] [PubMed] [Google Scholar]
- 22.Komander D, Clague MJ, Urbe S. Breaking the chains: structure and function of the deubiquitinases. Nat Rev Mol Cell Biol. 2009;10:550–63. doi: 10.1038/nrm2731. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen KT, Mun SH, Yang J, Lee J, Seok OH, Kim E, et al. The MARCHF6 E3 ubiquitin ligase acts as an NADPH sensor for the regulation of ferroptosis. Nat Cell Biol. 2022;24:1239–51. doi: 10.1038/s41556-022-00973-1. [DOI] [PubMed] [Google Scholar]
- 24.Bao Z, Liu Y, Chen B, Miao Z, Tu Y, Li C, et al. Prokineticin-2 prevents neuronal cell deaths in a model of traumatic brain injury. Nat Commun. 2021;12:4220. doi: 10.1038/s41467-021-24469-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen C, Yang Y, Guo Y, He J, Chen Z, Qiu S, et al. CYP1B1 inhibits ferroptosis and induces anti-PD-1 resistance by degrading ACSL4 in colorectal cancer. Cell Death Dis. 2023;14:271. doi: 10.1038/s41419-023-05803-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan H, Li X, Zhang X, Kang R, Tang D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun. 2016;478:1338–43. doi: 10.1016/j.bbrc.2016.08.124. [DOI] [PubMed] [Google Scholar]
- 27.Ishida T, Takahashi T, Kurokawa Y, Nishida T, Hirota S, Serada S, et al. Targeted therapy for drug-tolerant persister cells after imatinib treatment for gastrointestinal stromal tumours. Br J Cancer. 2021;125:1511–22. doi: 10.1038/s41416-021-01566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neefjes J, Jongsma MML, Berlin I. Stop or Go? Endosome Positioning in the Establishment of Compartment Architecture, Dynamics, and Function. Trends Cell Biol. 2017;27:580–94. doi: 10.1016/j.tcb.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Feng D, Wang Z, Zhao Y, Sun R, Tian D, et al. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ. 2019;26:2284–99. doi: 10.1038/s41418-019-0299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narayanan S, Cai C-Y, Assaraf YG, Guo H-Q, Cui Q, Wei L, et al. Targeting the ubiquitin-proteasome pathway to overcome anti-cancer drug resistance. Drug Resistance Updates. 2020;48:100663. doi: 10.1016/j.drup.2019.100663. [DOI] [PubMed] [Google Scholar]
- 31.Chen S, Xu K, Kong D, Wu L, Chen Q, Ma X, et al. Ubiquitin ligase OsRINGzf1 regulates drought resistance by controlling the turnover of OsPIP2;1. Plant Biotechnol J. 2022;20:1743–55. doi: 10.1111/pbi.13857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Itahana K, Mao H, Jin A, Itahana Y, Clegg HV, Lindstrom MS, et al. Targeted inactivation of Mdm2 RING finger E3 ubiquitin ligase activity in the mouse reveals mechanistic insights into p53 regulation. Cancer Cell. 2007;12:355–66. doi: 10.1016/j.ccr.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Dikic I, Schulman BA. An expanded lexicon for the ubiquitin code. Nat Rev Mol Cell Biol. 2023;24:273–87. doi: 10.1038/s41580-022-00543-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu X, Zhang Y, Luo Q, Wu X, Huang F, Shu T, et al. The deubiquitinase USP11 promotes ovarian cancer chemoresistance by stabilizing BIP. Signal Transduct Target Ther. 2021;6:264. doi: 10.1038/s41392-021-00580-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nie ZY, Yao M, Yang Z, Yang L, Liu XJ, Yu J, et al. De-regulated STAT5A/miR-202-5p/USP15/Caspase-6 regulatory axis suppresses CML cell apoptosis and contributes to Imatinib resistance. J Exp Clin Cancer Res. 2020;39:17. doi: 10.1186/s13046-019-1502-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Oosterom AT, Judson I, Verweij J, Stroobants S, Donato di Paola E, Dimitrijevic S, et al. Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet. 2001;358:1421–3. doi: 10.1016/S0140-6736(01)06535-7. [DOI] [PubMed] [Google Scholar]
- 37.Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364:1127–34. doi: 10.1016/S0140-6736(04)17098-0. [DOI] [PubMed] [Google Scholar]
- 38.Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626–32. doi: 10.1200/JCO.2007.13.4452. [DOI] [PubMed] [Google Scholar]
- 39.Poveda A, Garcia Del Muro X, Lopez-Guerrero JA, Cubedo R, Martinez V, Romero I, et al. GEIS guidelines for gastrointestinal sarcomas (GIST) Cancer Treat Rev. 2017;55:107–19. doi: 10.1016/j.ctrv.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 40.Sun X, Niu X, Chen R, He W, Chen D, Kang R, et al. Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis. Hepatology. 2016;64:488–500. doi: 10.1002/hep.28574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Markowitsch SD, Schupp P, Lauckner J, Vakhrusheva O, Slade KS, Mager R, et al. Artesunate Inhibits Growth of Sunitinib-Resistant Renal Cell Carcinoma Cells through Cell Cycle Arrest and Induction of Ferroptosis. Cancers. 2020;12:3150. doi: 10.3390/cancers12113150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife. 2014;3:e02523. doi: 10.7554/eLife.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L, Liu Y, Du T, Yang H, Lei L, Guo M, et al. ATF3 promotes erastin-induced ferroptosis by suppressing system Xc() Cell Death Differ. 2020;27:662–75. doi: 10.1038/s41418-019-0380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao FJ, Zhang D, Wu Y, Jia QH, Zhang L, Li YX, et al. miRNA-17-92 protects endothelial cells from erastin-induced ferroptosis through targeting the A20-ACSL4 axis. Biochem Biophys Res Commun. 2019;515:448–54. doi: 10.1016/j.bbrc.2019.05.147. [DOI] [PubMed] [Google Scholar]
- 45.Shi L, Song Z, Li Y, Huang J, Zhao F, Luo Y, et al. MiR-20a-5p alleviates kidney ischemia/reperfusion injury by targeting ACSL4-dependent ferroptosis. Am J Transpl. 2023;23:11–25. doi: 10.1016/j.ajt.2022.09.003. [DOI] [PubMed] [Google Scholar]
- 46.Wang ME, Chen J, Lu Y, Bawcom AR, Wu J, Ou J, et al. RB1-deficient prostate tumor growth and metastasis are vulnerable to ferroptosis induction via the E2F/ACSL4 axis. J Clin Investig. 2023;133:e166647. doi: 10.1172/JCI166647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J, Xie H, Yao J, Jin W, Pan H, Pan Z, et al. TRIM59 promotes steatosis and ferroptosis in non-alcoholic fatty liver disease via enhancing GPX4 ubiquitination. Hum Cell. 2023;36:209–22. doi: 10.1007/s13577-022-00820-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang J, Long G, Xiao L, Zhou L. USP8 positively regulates hepatocellular carcinoma tumorigenesis and confers ferroptosis resistance through beta-catenin stabilization. Cell Death Dis. 2023;14:360. doi: 10.1038/s41419-023-05747-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoeller D, Dikic I. Targeting the ubiquitin system in cancer therapy. Nature. 2009;458:438–44. doi: 10.1038/nature07960. [DOI] [PubMed] [Google Scholar]
- 50.Veggiani G, Gerpe MCR, Sidhu SS, Zhang W. Emerging drug development technologies targeting ubiquitination for cancer therapeutics. Pharm Ther. 2019;199:139–54. doi: 10.1016/j.pharmthera.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang D, Ma L, Wang B, Liu J, Wei W. E3 ubiquitin ligases in cancer and implications for therapies. Cancer Metastasis Rev. 2017;36:683–702. doi: 10.1007/s10555-017-9703-z. [DOI] [PubMed] [Google Scholar]
- 52.Zhou W, Zhang Y, Zhong C, Hu J, Hu H, Zhou D, et al. Decreased expression of TRIM21 indicates unfavorable outcome and promotes cell growth in breast cancer. Cancer Manag Res. 2018;10:3687–96. doi: 10.2147/CMAR.S175470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang F, Zhang Y, Shen J, Yang B, Dai W, Yan J, et al. The Ubiquitin E3 Ligase TRIM21 Promotes Hepatocarcinogenesis by Suppressing the p62-Keap1-Nrf2 Antioxidant Pathway. Cell Mol Gastroenterol Hepatol. 2021;11:1369–85. doi: 10.1016/j.jcmgh.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Si W, Zhou J, Zhao Y, Zheng J, Cui L. SET7/9 promotes multiple malignant processes in breast cancer development via RUNX2 activation and is negatively regulated by TRIM21. Cell Death Dis. 2020;11:151. doi: 10.1038/s41419-020-2350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou G, Wu H, Lin J, Lin R, Feng B, Liu Z. TRIM21 Is Decreased in Colitis-associated Cancer and Negatively Regulates Epithelial Carcinogenesis. Inflamm Bowel Dis. 2021;27:458–68. doi: 10.1093/ibd/izaa229. [DOI] [PubMed] [Google Scholar]
- 56.Lu Y, Qiu Y, Chen P, Chang H, Guo L, Zhang F, et al. ER-localized Hrd1 ubiquitinates and inactivates Usp15 to promote TLR4-induced inflammation during bacterial infection. Nat Microbiol. 2019;4:2331–46. doi: 10.1038/s41564-019-0542-2. [DOI] [PubMed] [Google Scholar]
- 57.Torre S, Polyak MJ, Langlais D, Fodil N, Kennedy JM, Radovanovic I, et al. USP15 regulates type I interferon response and is required for pathogenesis of neuroinflammation. Nat Immunol. 2017;18:54–63. doi: 10.1038/ni.3581. [DOI] [PubMed] [Google Scholar]
- 58.Zou Q, Jin J, Hu H, Li HS, Romano S, Xiao Y, et al. USP15 stabilizes MDM2 to mediate cancer-cell survival and inhibit antitumor T cell responses. Nat Immunol. 2014;15:562–70. doi: 10.1038/ni.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.