Abstract
Flow cytometry for the intracellular detection of T-cell cytokines was performed for 15 Gabonese patients during acute uncomplicated Plasmodium falciparum malaria. A striking expansion of CD4+ and CD8+ T cells producing gamma interferon (IFN-γ) was found during drug-induced clearance of parasitemia, paralleled by a decrease of interleukin-2 (IL-2) production. The frequency of IL-4- and IL-13-producing CD4+ cells gradually decreased, whereas the frequency of T cells producing IL-2+–IFN-γ+, IL-4−–IL-5+, and IL-4+–IL-5+ cytokines as well as IL-4+–IFN-γ+ and IL-13+–IFN-γ+ cytokines was not significantly altered. The capacity for IL-10 production within the CD4+ subset increased due to an expansion of both IL-10+–IFN-γ− and IL-10+–IFN-γ+ cytokine-expressing cells. Thus, a more pronounced Th2-driven immune response during acute untreated P. falciparum infection with a shift towards Th1 responsiveness induced by parasite clearance is suggested.
The course of Plasmodium falciparum malaria is characterized by a complex interaction of host immune responses and parasite survival strategies. T lymphocytes and their products are essential both in regulating specific antibody formation and in inducing antibody-independent immunity to Plasmodium species (reviewed in reference 29). The CD4 T-cell subset is of major importance for the induction of blood-stage immunity, while the CD8 subset has been shown to be cytolytic against liver stages of the parasite. It has also been demonstrated, however, that the number of T cells as well as their response to antigenic stimulation is lower in the peripheral circulation during acute malaria (7, 11, 12). This cellular hyporesponsiveness has been associated with the sequestration of activated T cells expressing the adhesion molecule leukocyte function-associated molecule-1 (LFA-1) on their surfaces (11, 12). After the clearance of parasitemia, previously sequestered cells reemerge into the periphery, with subsequent restoration of immune responsiveness (7, 11). If this reallocation hypothesis is true, it would be of particular interest to identify the cytokine pattern displayed by these T cells during the course of disease, as Th1 cytokines, such as gamma interferon (IFN-γ) and interleukin-2 (IL-2), as well as Th2 cytokines, such as IL-4, appear to have substantial impact on disease outcome and the development of protection in infection due to Plasmodium spp. (10, 26).
By using flow cytometric analysis for the intracellular detection of cytokines, we sought to characterize the phenotypes and the frequency of cytokine-producing T cells during the course of drug-treated P. falciparum malaria from a region in Central Africa where the disease is hyperendemic.
Study population.
The study was conducted in the Albert Schweitzer Hospital in Lambaréné, Gabon, where P. falciparum malaria is predominantly hyperendemic (32). Patients attending the outpatient clinic during November 1997 were enrolled if they met the following study criteria: infection with P. falciparum, no recent antimalarial treatment, and no other systemic infection. Fifteen patients (nine female) were included. The median age was 12 years (range, 4 to 35 years). The mean parasitemia was 32,100/μl (range, 1,500 to 158,500/μl) before treatment, decreased to 85/μl (range, 0 to 500/μl) on day 3, and was below the limit of detection in all patients on day 10. All patients were treated as outpatients with the combination of sulfadoxine and pyrimethamine, which has been shown to be curative in this area (22). The patients or their parents provided informed consent, and the study was approved by the Ethics Committee of the International Foundation of the Albert Schweitzer Hospital in Lambaréné.
Peripheral blood mononuclear cell cultures and intracellular cytokine detection by flow cytometry.
Flow cytometric assessment of T-cell cytokine production was performed essentially according to the technique described by Jung et al. and modified by Willheim et al. (15, 33). Peripheral blood mononuclear cells were isolated from heparinized blood by Ficoll-diatrizoate centrifugation. The cells were then cultured in Ultra Culture medium (BioWhittaker, Walkersville, Md.) supplemented with l-glutamine (2 mM/liter; Sigma, St. Louis, Mo.), gentamicin (170 mg/liter; Sigma), and 2-mercaptoethanol (3.5 μl/liter; Merck, Darmstadt, Germany) and were stimulated with phorbol 12-myristate 13-acetate (10 ng/ml; Sigma) and ionomycin (1.25 μM; Sigma) in the presence of monensin (1 μM; Sigma) for 4 h at 37°C in 5% CO2. The cells were then harvested on ice, washed twice in phosphate-buffered saline (PBS), and fixed with 2% formaldehyde (1 ml per 2 × 106 cells; Merck) for 20 min. After two additional washes in PBS, the cells were resuspended in Hank’s balanced salt solution (supplemented with 0.3% bovine serum albumin and 0.1% sodium-azide) and stored at 4°C in the dark until they were stained. The fixed cells were washed twice with PBS and made permeable with saponin (0.1%; Sigma), resuspended with 50 μl of saponin buffer-diluted antibodies, and incubated for 25 min at room temperature in the dark. The following monoclonal antibodies (MAbs) were used: cytokine-specific mouse anti-human MAb (fluorescein-isothiocyanate [FITC]-labeled IFN-γ [clone B27]) and rat anti-human MAb (phycoerythrin [PE]-conjugated IL-2 [MQ1-17H12], PE-labeled IL-4 [MP4-25D2], FITC-labeled IL-4 [MP4-25D2], PE-labeled IL-5 [TRFK5], PE-labeled IL-10 [JES3-9D7], and PE-labeled IL-13 [JES10-5A2]). All MAbs were purchased from Pharmingen (San Diego, Calif.). The anti-CD4 MAb and the anti-CD8 MAb were allophycocyanin and peridinin chlorophyll labeled, respectively (Becton Dickinson, Mountain View, Calif.). Four-color staining was performed, and at least 104 cells were analyzed on a FACSCalibur (Becton Dickinson) equipped with a two-laser system (488- and 630-nm wavelength, respectively). All cytokine combinations (IL-2–IFN-γ, IL-4–IFN-γ, IL-10–IFN-γ, IL-13–IFN-γ, and IL-4–IL-5) were stained in conjunction with CD4 and CD8. The data were analyzed with CELLQuest software (Becton Dickinson), and the results were expressed as the percentage of cytokine-producing cells in each CD4+- or CD8+-cell population (Fig. 1 and 2).
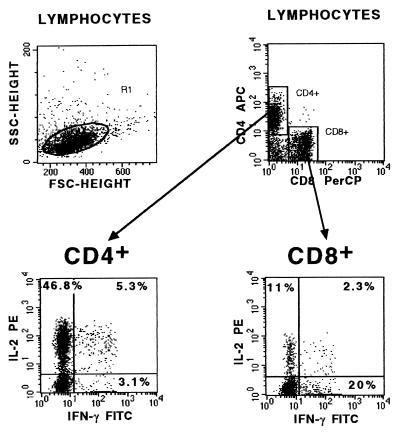
FIG. 1.
Cytokine-specific anti-human MAbs (either FITC labeled or PE conjugated) were used in combination with allophycocyanin (APC)-labeled anti-CD4 MAb and peridinin chlorophyll (PerCP)-labeled anti-CD8 MAb for intracellular staining of cells within the lymphocyte scatter gate. The numbers in each quadrant represent the percentage of gated cytokine-producing cells within the CD4+- or CD8+-cell population. The dot plots from a representative child donor show that 46.8% of gated CD4+ cells were IL-2+–IFN-γ−, 5.3% were IL-2+–IFN-γ+, and 3.1% were IL-2−–IFN-γ+. Eleven percent of CD8+ cells produced IL-2+–IFN-γ− cytokines, 2.3% produced IL-2+–IFN-γ+ cytokines, and 20% produced IL-2−–IFN-γ+ cytokines.
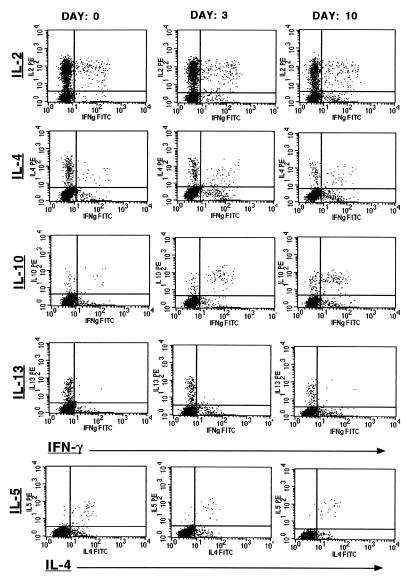
FIG. 2.
Representative two-parameter dot plots displaying the kinetics of the frequency of cytokine-producing CD4+ cells during the course of antimalarial treatment. Each vertical row depicts the results of the cytometric analyses performed for one representative patient before treatment (day 0), during clearance of parasites (day 3), and after resolution of parasitemia (day 10). IFN-γ (IFNg; x axis) was stained in combination with IL-2, IL-4, IL-10, and IL-13 (all y axis). IL-4 (x axis) was also combined with IL-5 (y axis). The quadrant statistics were set on the basis of the corresponding negative controls. Corresponding data and statistics are displayed in Fig. 3 and its legend.
Statistical methods.
Statistical analysis was performed with a standard statistical package (SPSS 7.5 for Windows; SPSS Inc., Chicago, Ill.). The general linear model repeated measures was applied for the analysis of dependent variables within the observation period. Bivariate correlations were done by computing Pearson’s correlation coefficient. A P value of 0.05 was considered significant.
Patients.
Fifteen patients with P. falciparum infection were studied. Sulfadoxine-pyrimethamine-induced clearance of parasitemia was rapid in eight patients, having eliminated their parasites on day 3. In all patients parasitemia was below the level of detection on day 10. Further clinical follow-up disclosed recrudescent P. falciparum malaria in the study participants; no side effects clearly attributable to the antimalarial treatment were observed.
Frequency of CD4+ and CD8+ T cells during parasite clearance.
The percentage of cells within the lymphocyte scatter gate positively stained for CD4+ (mean ± standard error of the mean [SEM]: day 0, 50% ± 2%; day 3, 52% ± 1%; day 10, 49% ± 2%) or CD8+ (mean ± SEM: day 0, 19% ± 1%; day 3, 18% ± 1%; day 10, 19% ± 1%) did not change significantly during the observation period.
Frequency of Th1-cytokine-producing CD4+ and CD8+ T cells.
The frequency of T cells producing IFN-γ or IL-2 or coproducing both cytokines was investigated (Fig. 1 to 3). With respect to the CD4+ subset, a gradually increased capacity for IL-2−–IFN-γ+ cytokine production was found until day 10 (Fig. 2 and 3), and the frequency of IL-2+–IFN-γ− cytokine-expressing cells within the same subset was significantly lower after parasite clearance. The frequency of T cells coproducing both cytokines (IL-2+–IFN-γ+) was not significantly different at any time (Fig. 2 and 3). Within the CD8+ subset a significantly higher frequency of IL-2−–IFN-γ+ cytokine-producing cells and a significantly decreased frequency of IL-2+–IFN-γ− cytokine-producing cells was found after parasite clearance. The percentages of IL-2−–IFN-γ+ cells (mean ± SEM) were as follows: day 0, 29% ± 3%; day 3, 28% ± 2%; and day 10, 36% ± 3%; P < 0.05. The percentages of IL-2+–IFN-γ− cells (mean ± SEM) were as follows: day 0, 7% ± 1%; day 3, 6% ± 0.5%; and day 10, 4% ± 0.3%; P < 0.01.
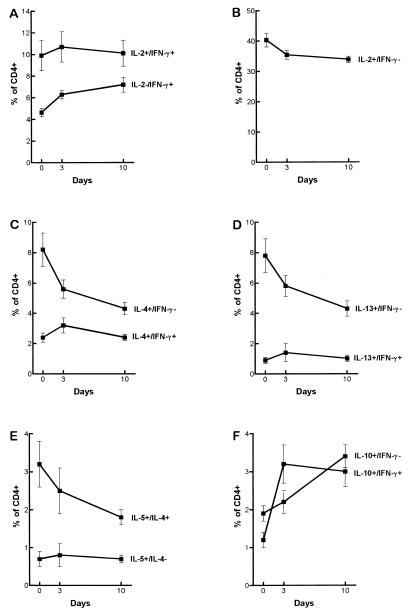
FIG. 3.
Frequency of cytokine-producing CD4+ cells following admission (day 0), during clearance of parasitemia (day 3), and after resolution of parasitemia (day 10) in 15 patients with uncomplicated P. falciparum malaria. The data points indicate the mean percentage of CD4+ cells ± SEM. The differences of mean frequencies throughout the observation period within the CD4+ subset were as follows: (A) IL-2−–IFN-γ+, P < 0.001 and IL-2+–IFN+, not significant (n.s.); (B) IL-2+–IFN−, P < 0.01; (C) IL-4+–IFN−, P < 0.01 and IL-4+–IFN+, n.s.; (D) IL-13+–IFN−, P < 0.01 and IL-13+–IFN+, n.s.; (E) IL-5+–IL-4−, n.s. and IL-5+–IL-4+, n.s.; (F) IL-10+–IFN−, P < 0.001 and IL-10+–IFN+, P < 0.001.
Frequency of Th2-cytokine-producing CD4+ and CD8+ T cells.
The frequencies of IL-4-, IL-5-, and IL-13-producing CD4+ and CD8+ T cells were assessed (Fig. 2 and 3). In addition, the capacities of CD4+ and CD8+ T cells to coproduce IL-4 and IL-5 were studied (Fig. 2 and 3). While IL-4+–IFN-γ− and IL-13+–IFN-γ− cytokine-producing CD4+ cells decreased significantly during antimalarial treatment, the frequencies of IL-4−–IL-5+ and IL-4+–IL-5+ cytokine-expressing T cells were not significantly different at any time (Fig. 2 and 3). No differences were seen in the IL-4+–IFN-γ−, IL-13+–IFN-γ−, IL-4−–IL-5+, and IL-4+–IL-5+ CD8+ subpopulations, with each of them representing less than 1% of the CD8+ subset (data not shown).
Frequency of IL-10+–IFN-γ− and of IL-10+–IFN-γ+ cytokine-producing CD4+ and CD8+ T cells.
A marked increase was found in the IL-10+–IFN-γ− and the IL-10+–IFN-γ+ cytokine-producing CD4+ subpopulations until day 10 (Fig. 2 and 3). IL-10+–IFN-γ− and IL-10+–IFN-γ+ cytokine expression was found in less than 1% of CD8+ cells, and the frequency was not significantly different at any time.
Frequency of CD4+ and CD8+ T cells producing a Th0-type pattern of cytokines.
The capacity of CD4+ cells to coproduce Th1 and Th2 cytokines as well as that of CD8+ cells to coproduce Tc1 and Tc2 cytokines (IL-4+–IFN-γ+ and IL-13+–IFN-γ+) was not significantly altered during the clearance of parasitemia (Fig. 2 and 3).
Decreased relative and absolute numbers of T cells in the peripheral circulation due to enhanced sequestration as well as defective production (e.g., of IL-2) have been considered responsible for the marked hyporesponsiveness to specific and nonspecific antigenic stimulation in acute P. falciparum malaria (7, 9, 11, 12, 17, 25). In addition, it has been shown that the clearance of parasitemia is paralleled by a restoration of immune responsiveness and the rapid reemergence of the previously sequestered T cells into the periphery (11). The relative frequency of CD4+ and CD8+ cells was not altered during the course of disease in our study patients; the cytokine production capacities of these subsets, however, identified at the single-cell level, showed striking differences. A consistent pattern, with increased expression of IFN-γ in both CD4+ and CD8+ T cells, was found during resolution of P. falciparum parasitemia, paralleled by a decreased IL-2 production capacity of both subsets. This discordant expression of IL-2 and IFN-γ in T cells during the course of malaria suggests different regulatory mechanisms for each Th1 cytokine. It is tempting to speculate that the T cells producing only IL-2 are committed to a more Th1-biased phenotype during drug-induced clearance of parasitemia, and some indirect support for the possibility that IL-12 promotes this Th1 development comes from the finding of a marked increase in CD4+ cells coproducing IFN-γ and IL-10 in our patients during parasite clearance. The coproduction of these cytokines has been found to be particularly induced by IL-12 (34). However, the relative lack of IFN-γ-producing T cells in the acutely ill patients may also represent their enhanced disease-induced sequestration, with subsequent redistribution into the periphery after drug cure.
Before the initiation of antimalarial treatment, a considerable frequency of IL-4- and IL-13-producing CD4+ cells was found, which markedly decreased with the resolution of parasitemia. This downregulation of the Th2 response obviously discriminates successfully treated uncomplicated P. falciparum malaria from the murine model of infection with Plasmodium chabaudi chabaudi AS, where a Th2-dominated immune response has been shown to be essential in preventing recrudescent malaria during the course of disease (24, 28). Taking both Th1 and Th2 data together, a more pronounced Th2-driven response (a lower ratio of IFN-γ expression to IL-4 expression) during acute untreated P. falciparum malaria was replaced by a shift towards a Th1-biased response (a higher ratio of IFN-γ expression to IL-4 expression) paralleling the clearance of parasitemia in peripheral-blood T cells. High IFN-γ production as part of a Th1-driven immune response has been associated with a more favorable outcome in most animal models of malaria (13, 14, 20, 21, 23, 25), and treatment of the otherwise-lethal murine Plasmodium vinckei infection with IFN-γ greatly enhanced the effect of antimalarial chemotherapy (8, 16, 21). This effect has been attributed to the monocyte/macrophage-activating capacities of IFN-γ, with rapid killing of the malarial blood-stage parasites by reactive oxygen and nitrogen intermediates (1, 27). Our findings emphasize the role of IFN-γ as a key molecule in human antimalarial host defense and do not support a direct involvement of IL-4 or IL-13 in the clearance of P. falciparum parasites, especially since IL-4 has been shown to suppress macrophage anti-P. falciparum activity in vitro (18). Some differences between the activities of IL-4 and IL-13 within the Th2 response against gastrointestinal nematodes have recently been reported (30); our results, however, suggest an apparent conformity in the regulation of both IL-13 and IL-4 in response to P. falciparum infection.
The role of IL-10 in malaria is still not well understood, yet a striking increase in its expression within the CD4+ subset was found during the course of disease in our study patients with uncomplicated P. falciparum infection. This might be an indirect-feedback inhibition of IFN-γ expression, since IL-10 suppresses antigen-presenting-cell function by downregulating class II major histocompatibility complex antigens, costimulatory signaling through CD80, and by blocking IL-12 production (2, 4, 6). IL-10, however, is also a potent inhibitor of Th2-cell functions, and its expansion within the CD4 subset during parasite clearance clearly discriminates its regulation from that of the Th2 cytokines, IL-4 and IL-13 (5). Its expression in IL-4-deficient mice challenged with P. chabaudi chabaudi was not affected (31), and in models of experimental cerebral malaria IL-10 was protective, with inhibition of tumor necrosis factor production (3). In addition, its absence in mutant mice with targeted disruption of the IL-10 gene was accompanied by an enhanced IFN-γ response and increased mortality, suggesting that it plays a prominent role in limiting potentially harmful inflammatory effects on the host (19).
In summary, the data presented indicate a shift from a Th2-biased response to a more pronounced Th1-regulated immune responsiveness during acute, uncomplicated, successfully managed P. falciparum malaria, when the immune response is assessed at the single-cell level in peripheral-blood T cells. While IFN-γ appears essential for the resolution of parasitemia, IL-10 appears to be a key molecule in the control of inflammatory responses that otherwise may lead to tissue damage rather than to clearance of parasitemia.
Acknowledgments
This work was supported by a grant from the Austrian Society of Chemotherapy, from the Medizinisch-Wissenschaftlicher Fonds des Bürgermeisters der Bundeshauptstadt Wien (grant 1516), and from the Fortüne Programme, Medical Faculty, University of Tübingen.
REFERENCES
- 1.Clark I A, Hunt N H. Evidence for reactive oxygen intermediates causing hemolysis and parasite death in malaria. Infect Immun. 1983;39:1–6. doi: 10.1128/iai.39.1.1-6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Andrea A, Aste-Amezaga M, Valianta N M, Ma X, Kubin M, Trinchieri G. IL-10 inhibits human lymphocyte IFN-γ-production by suppressing IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Kossodo S, Monso C, Juillard P, Velu T, Goldman M, Grau G E. Interleukin-10 modulates susceptibility in experimental cerebral malaria. Immunology. 1997;91:536–540. doi: 10.1046/j.1365-2567.1997.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Waal Malefijt R, Haanen R J, Spits H, Roncarolo M G, te Velde A, Figdor C, Johnson K, Kastelein R, Yssel H, de Vries J E. IL-10 and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via down-regulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Waal Malefijt R, Yssel H, de Vries J E. Direct effects of IL-10 on subsets of human CD4 T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J Immunol. 1993;150:4754–4765. [PubMed] [Google Scholar]
- 6.Ding L, Linsley P S, Huang L Y, Germain R N, Shevach E M. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–1234. [PubMed] [Google Scholar]
- 7.Elhassan I M, Hviid L, Satti G, Akerstrom B, Jacobsen P H, Jensen J B, Theander T G. Evidence of endothelial inflammation, T cell activation, and T cell reallocation in uncomplicated Plasmodium falciparum malaria. Am J Trop Med Hyg. 1994;51:372–379. doi: 10.4269/ajtmh.1994.51.372. [DOI] [PubMed] [Google Scholar]
- 8.Finnemann S, Kremsner P G, Chaves M F, Schumacher C, Neifer S, Bienzle U. Antibody response in Plasmodium vinckei malaria after treatment with chloroquine and adjuvant interferon-gamma. Parasitol Res. 1992;78:629–634. doi: 10.1007/BF00931511. [DOI] [PubMed] [Google Scholar]
- 9.Ho M, Webster H K, Green B, Looareesuwan S, Kongchareon S, White N J. Defective production of and response to IL-2 in acute human falciparum malaria. J Immunol. 1988;141:2755–2759. [PubMed] [Google Scholar]
- 10.Hommel M. Modulation of host cell receptors: a mechanism for the survival of malaria parasites. Parasitology. 1997;115:45–54. doi: 10.1017/s0031182097002345. [DOI] [PubMed] [Google Scholar]
- 11.Hviid L, Kurtzhals J A L, Goka B Q, Oliver-Commey J O, Nkrumah F K, Theander T G. Rapid reemergence of T cells into peripheral circulation following treatment of severe and uncomplicated Plasmodium falciparum malaria. Infect Immun. 1997;65:4090–4093. doi: 10.1128/iai.65.10.4090-4093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hviid L, Theander T G, Abdulhadi N H, Abu-Zeid A, Bayoumi R A, Jensen J B. Transient depletion of T cells with high LFA-1 expression from peripheral circulation during acute Plasmodium falciparum malaria. Eur J Immunol. 1991;21:1249–1253. doi: 10.1002/eji.1830210523. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs P, Radzioch D, Stevenson M M. A Th1-associated increase in tumor necrosis factor alpha expression in the spleen correlates with resistance to blood-stage malaria in mice. Infect Immun. 1996;64:535–541. doi: 10.1128/iai.64.2.535-541.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobs P, Radzioch D, Stevenson M M. In vivo regulation of nitric oxide production by tumor necrosis factor alpha and gamma interferon, but not by interleukin-4, during blood stage malaria in mice. Infect Immun. 1996;64:44–49. doi: 10.1128/iai.64.1.44-49.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung T, Schauer U, Heusser C, Neumann C, Rieger C. Detection of intracellular cytokines by flow cytometry. J Immunol Methods. 1993;159:197–207. doi: 10.1016/0022-1759(93)90158-4. [DOI] [PubMed] [Google Scholar]
- 16.Kremsner P G, Neifer S, Schermuck S, Ferreira Chaves M, Sliwa K, Bienzle U. Interferon-γ enhances the effect of antimalarial chemotherapy in murine Plasmodium vinckei malaria. J Infect Dis. 1991;163:1161–1163. doi: 10.1093/infdis/163.5.1161. [DOI] [PubMed] [Google Scholar]
- 17.Kremsner P G, Zotter G M, Feldmeier H, Graninger W, Rocha R M, Jansen-Rosseck R, Bienzle U. Immune response during and after Plasmodium falciparum infection. J Infect Dis. 1990;161:1025–1028. doi: 10.1093/infdis/161.5.1025. [DOI] [PubMed] [Google Scholar]
- 18.Kumaratilake L M, Ferrante A. IL-4 inhibits macrophage-mediated killing of Plasmodium falciparum in vitro. A possible parasite-immune evasion mechanism. J Immunol. 1992;149:194–199. [PubMed] [Google Scholar]
- 19.Linke A, Kühn R, Müller W, Honarvar N, Li C, Langhorne J. Plasmodium chabaudi chabaudi: differential susceptibility of gene-targeted mice deficient in IL-10 to an erythrocytic-stage infection. Exp Parasitol. 1996;84:253–263. doi: 10.1006/expr.1996.0111. [DOI] [PubMed] [Google Scholar]
- 20.Mohan K, Moulin P, Stevenson M M. Natural killer cell cytokine production, not cytotoxicity, contributes to resistance against blood-stage Plasmodium chabaudi AS infection. J Immunol. 1997;159:4990–4998. [PubMed] [Google Scholar]
- 21.Perlmann H, Kumar S, Vinetz J M, Kullberg M, Miller L H, Perlmann P. Cellular mechanisms in the immune response to malaria in Plasmodium vinckei-infected mice. Infect Immun. 1995;63:3987–3993. doi: 10.1128/iai.63.10.3987-3993.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt-Ott R, Luckner D, Lehman L G, Lell B, Matousek P, Greve B, Kremsner P G. Sulfadoxine/pyrimethamine for treating uncomplicated Plasmodium falciparum malaria in young children in Gabon. Trans R Soc Trop Med Hyg. 1997;91:578–579. doi: 10.1016/s0035-9203(97)90033-x. [DOI] [PubMed] [Google Scholar]
- 23.Sedegah M, Finkelman F, Hoffman S L. Interleukin 12 induction of interferon γ-dependent protection against malaria. Proc Natl Acad Sci USA. 1994;91:10700–10702. doi: 10.1073/pnas.91.22.10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevenson M M, Tam M F. Differential induction of helper T cell subsets during blood-stage Plasmodium chabaudi AS infection in resistant and susceptible mice. Clin Exp Immunol. 1993;92:77–83. doi: 10.1111/j.1365-2249.1993.tb05951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevenson M M, Tam M F, Wolf S F, Sher A. IL-12-induced protection against blood-stage Plasmodium chabaudi AS requires IFN-γ and TNF-α and occurs via a nitric oxide-dependent mechanism. J Immunol. 1995;155:2545–2556. [PubMed] [Google Scholar]
- 26.Taylor-Robinson A W. Immunoregulation of malarial infection: balancing the vices and virtues. Int J Parasitol. 1998;28:135–148. doi: 10.1016/s0020-7519(97)00173-2. [DOI] [PubMed] [Google Scholar]
- 27.Taylor-Robinson A W, Looker M. Sensitivity of malaria parasites to nitric oxide at low oxygen tensions. Lancet. 1998;351:1630. doi: 10.1016/s0140-6736(05)77685-6. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 28.Taylor-Robinson A W, Phillips R S, Severn A, Moncada S, Liew F Y. The role of Th1 and Th2 cells in a rodent malaria infection. Science. 1993;260:1931–1934. doi: 10.1126/science.8100366. [DOI] [PubMed] [Google Scholar]
- 29.Troye-Blomberg M, Berzins K, Perlmann P. T-cell control of immunity to the asexual blood stages of the malaria parasite. Crit Rev Immunol. 1994;14:131–155. doi: 10.1615/critrevimmunol.v14.i2.20. [DOI] [PubMed] [Google Scholar]
- 30.Urban J F, Noben-Trauth N, Donaldson D D, Madden K B, Morris S C, Collins M, Finkelman F D. IL-13, IL-4Rα, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity. 1998;8:255–264. doi: 10.1016/s1074-7613(00)80477-x. [DOI] [PubMed] [Google Scholar]
- 31.Von der Weid T, Kopf M, Köhler G, Langhorne J. The immune response to Plasmodium chabaudi malaria in interleukin-4-deficient mice. Eur J Immunol. 1994;24:2285–2293. doi: 10.1002/eji.1830241004. [DOI] [PubMed] [Google Scholar]
- 32.Wildling E, Winkler S, Kremsner P G, Brandts C, Jenne L, Wernsdorfer W H. Malaria epidemiology in the province of Moyen Ogooué, Gabon. Trop Med Parasitol. 1995;46:77–82. [PubMed] [Google Scholar]
- 33.Willheim M, Ebner C, Baier K, Kern W, Schrattbauer K, Thien R, Kraft D, Breiteneder H, Reinisch W, Scheiner O. Cell surface characterization of T lymphocytes and allergen-specific T cell clones: correlation of CD26 expression with Th1 subsets. J Allergy Clin Immunol. 1997;100:348–355. doi: 10.1016/s0091-6749(97)70248-3. [DOI] [PubMed] [Google Scholar]
- 34.Windhagen A, Anderson D E, Carrizosa A, Williams R E, Hafler D A. IL-12 induces human T cells secreting IL-10 with IFN-γ. J Immunol. 1996;157:1127–1131. [PubMed] [Google Scholar]