Abstract
The Saccharomyces cerevisiae ubiquitin ligase SCFMet30 is essential for cell cycle progression. To identify and characterize SCFMet30-dependent cell cycle steps, we used temperature-sensitive met30 mutants in cell cycle synchrony experiments. These experiments revealed a requirement for Met30 during both G1/S transition and M phase, while progression through S phase was unaffected by loss of Met30 function. Expression of the G1-specific transcripts CLN1, CLN2, and CLB5 was very low in met30 mutants, whereas expression of CLN3 was unaffected. However, overexpression of Cln2 could not overcome the G1 arrest. Interestingly, overexpression of Clb5 could induce DNA replication in met30 mutants, albeit very inefficiently. Increased levels of Clb5 could not, however, suppress the cell proliferation defect of met30 mutants. Consistent with the DNA replication defects, chromatin immunoprecipitation experiments revealed significantly lower levels of the replication factors Mcm4, Mcm7, and Cdc45 at replication origins in met30 mutants than in wild-type cells. These data suggest that Met30 regulates several aspects of the cell cycle, including G1-specific transcription, initiation of DNA replication, and progression through M phase.
In all organisms the cell division cycle is tightly regulated in response to internal and external signals. In the budding yeast Saccharomyces cerevisiae, as in most metazoans, integration of these signals occurs during the G1 phase of the cell cycle (26, 47). Cells can either choose to commit to a new round of mitotic division or to enter an alternative developmental pathway. The event in late G1 phase at which cells irreversibly commit to a new round of cell division is called Start in yeast and restriction point in mammalian cells. As in other eukaryotes, the yeast cyclin-dependent kinase Cdc28 associates with several G1 cyclins to control major events in G1. Start depends on the activation of a G1-specific transcription program during which more than 200 G1 transcripts are expressed, among them the G1 cyclins CLN1/2 as well as the B-type cyclins CLB5/6 (4, 26). Activation of this transcription program is dependent on the G1 cyclin Cln3. Two recent studies demonstrated Cln3/Cdc28-dependent phosphorylation of Whi5, an inhibitor of the G1-specific transcription program analogous to the mammalian retinoblastoma protein RB (5, 6, 34).
Activation of Cln1/Cdc28 and Cln2/Cdc28 induces several G1-specific events, such as polarization of the actin cytoskeleton to the future bud site, budding, spindle pole body duplication, and DNA replication (47). Initiation of DNA replication is triggered by phosphorylation of the Cdk inhibitor Sic1 by Cln1-2/Cdc28 (36, 46). Sic1 specifically inhibits activation of Cdc28 by B-type cyclins, and its presence during G1 prevents premature S-phase initiation. Once phosphorylated, Sic1 is rapidly ubiquitylated and degraded, which in turn leads to activation of the S-phase-specific Cdk complex Clb5/Cdc28 (45). Clb5/Cdc28 together with another cyclin-dependent kinase, Dbf4/Cdc7, start DNA replication (8).
The importance of Sic1 proteolysis illustrates the significance of the ubiquitin proteasome pathway in the regulation of cell cycle progression (32). Ubiquitin is a small protein that is covalently attached to lysine residues of substrate proteins. Additional ubiquitin units can be linked to substrate-bound ubiquitin to form a growing polyubiquitin chain. Generally these ubiquitin chains are recognized by the 26S proteasome and thereby induce substrate proteolysis. Substrate ubiquitination is a highly regulated process and involves a series of enzymes for ubiquitin activation (E1 enzyme) and conjugation (E2 and E3 enzymes). E3 enzymes, also called ubiquitin ligases, are responsible for recognition of substrates (18, 19). Several groups of ubiquitin ligases have been identified. One large group is formed by the SCF ubiquitin ligases, which regulate various biological processes and seem to be particularly important for regulation of the cell cycle. SCF ubiquitin ligases are multisubunit complexes with similar architectures in yeast and higher eukaryotes (7). The budding yeast SCF consists of Skp1, the scaffold protein Cdc53, the RING-finger protein Rbx1/Roc1/Hrt1, and one member of the family of F-box proteins. F-box proteins directly recruit substrates and are therefore the determinants of SCF substrate specificity (43). Most yeast SCF ubiquitin ligases analyzed so far appear to depend on the E2 enzyme Cdc34, which is essential for cell proliferation.
Among the more than 15 F-box proteins in budding yeast, two are essential for cell cycle progression, namely Cdc4 and Met30. SCFCdc4 ubiquitinates Sic1 and regulates its degradation (11, 38). The essential function of SCFMet30 is inactivation of the transcription factor Met4, since deletion of MET4 suppresses the cell cycle defect of met30 mutants (20, 30). Interestingly, Met4 ubiquitylation by Cdc34/SCFMet30 is sufficient for Met4 repression, and proteolysis does not appear to play an important role in Met4 inactivation (12, 20). Genetic experiments indicate that Met4 regulation primarily responds to intracellular levels of the principal methyl-group donor S-adenosylmethionine (SAM) (41). During normal growth conditions, Cdc34/SCFMet30 maintains Met4 in its inactive, ubiquitylated form. However, when the intracellular SAM level is low, Met4 is no longer ubiquitylated and thus activated. Deubiquitylated Met4 together with several other transcription factors (Cbf1, Met28, Met31, and Met32) induce expression of a large number of genes, including genes required for synthesis of sulfur amino acids and SAM (41). Met4 activation therefore restores adequate intracellular SAM levels. Fully activated, deubiquitylated Met4 also induces a cell cycle arrest that might be important to maintain cellular integrity under conditions of SAM limitation.
The Met4-induced cell cycle arrest underlies the lethality of met30 mutants, since they are unable to keep Met4 in its ubiquitylated, inactive form (12, 20, 23, 30). Deletion of MET4 as well as deletion of MET32 suppress the lethality of met30Δ mutants (20, 30). Both Met4 and Met32 function as transcription factors (41). In addition, another transcription factor, Cbf1, can partially suppress the proliferation defect of temperature-sensitive met30 mutants (20). Therefore, it is likely that inappropriate induction or repression of Met4-regulated genes underlies the cell cycle defect of met30 mutants.
Conditional overexpression of Met4 from the inducible GAL1 promoter in met30Δ mutants was used previously to characterize this cell cycle arrest (30). That study demonstrated that met4::GAL1-MET4 met30Δ cells when shifted to galactose medium arrest as large unbudded cells with 1C DNA content (30). The arrest point was mapped at or after the pheromone arrest point but prior to budding and DNA replication (30). Growth rate and protein synthesis were normal for at least 100 min after induction of GAL1-MET4 in met30Δ mutants but slowed down somewhat after prolonged cell cycle arrest. This delay in growth rate reduction after induction of the cell cycle arrest indicates that the protein synthesis defects of met30 mutants are secondary consequences of the arrest but do not cause the cell cycle block (30). Interestingly, CLN1, CLN2, PCL1, and PCL2 but not CLN3 transcripts were undetectable in met4::GAL1-MET4 met30Δ mutants when shifted to galactose medium, possibly explaining the G1 arrest of met30 mutants (30). Remarkably, CLN2 transcripts were undetectable even when expressed under control of the constitutive ADH1 promoter, which prompted the authors to suggest that hyperactive Met4 in met30Δ mutants induces a potent G1 cyclin mRNA degradation mechanism, which might be responsible for the G1 arrest observed with met30 mutants (30).
In this study we characterize the cell cycle arrest of temperature-sensitive met30 mutants. We demonstrate that lack of G1 cyclin expression does not cause the cell cycle arrest of met30 mutants. We further show that overexpression of the S-phase cyclin Clb5 can initiate DNA replication in met30 mutants, suggesting that Met30 is important for activation of prereplication complexes. In addition, we identified a Met30-dependent cell cycle step outside of G1. These data indicate that Met30 is involved in multiple steps of cell cycle regulation.
MATERIALS AND METHODS
Yeast strains and growth conditions.
Yeast strains used in this study are listed in Table 1. All strains used in this study are isogenic to 15DaubΔ, a derivative of BF264-15D (33). Yeast strains were grown in standard culture media, and standard yeast genetic methods were used (15). For galactose induction, cells were grown in medium containing 2% raffinose, and galactose was added to a final concentration of 2%. Growth of mutants was assayed at different temperatures after spotting of serial dilutions on agar plates using a pin tool (V&P Scientific, San Diego, CA).
TABLE 1.
Yeast strains used in this study
| Strain | Relevant genotype | Source or reference |
|---|---|---|
| 15Daub | abar1Δ ura3Δns ade1 his2 leu2-3,112 trp1-1 | 33 |
| PY283 | abar1 met30-6::KAN | 20 |
| PY340 | abar1::LEU2 met30-6::KAN sic1::URA3 | This study |
| PY344 | abar1::LEU2 sic1::URA3 | This study |
| PY560 | abar1 GAL1-CLN2-HA3::LEU2 | This study |
| PY561 | abar1 met30-6::KAN GAL1-CLN2-HA3::LEU2 | This study |
| PY607 | abar1 SWI4-6myc::LEU2 SWI6-HA3::LEU2 pep4::URA3 | This study |
| PY822 | abar1 met30-6::KAN SWI4-6myc::LEU2 SWI6-HA3::LEU2 pep4::URA3 | This study |
| PY833 | α met30-6::KAN swi6::TRP1 <SWI6::URA3>CEN | This study |
| PY834 | α swi6::TRP1 <SWI6::URA3>CEN | This study |
| PY835 | α met30-6::KAN <SWI6::URA3>CEN | This study |
| PY836 | α met30::KAN met4::KAN swi6::TRP1 <SWI6::URA3>CEN | This study |
| PY837 | α met4::KAN swi6::TRP1 <SWI6::URA3>CEN | This study |
| PY838 | abar1 CLN2-HA3::URA3 CLN3-18myc::TRP1 | This study |
| PY839 | abar1 met30-6::KAN CLN2-HA3::URA3 CLN3-18myc::TRP1 | This study |
| PY1058 | abar1 MCM4-HA3::TRP1 | This study |
| PY1059 | abar1 met30-6::KAN MCM4-HA3::TRP1 | This study |
| PY1090 | abar1 GAL1-CLB5-9myc::URA3 | This study |
| PY1091 | abar1 met30-6::KAN GAL1-CLB5-9myc::URA3 | This study |
| PY1108 | abar1 sic1::HYG GAL1-CLB5-9myc::URA3 | This study |
| PY1109 | abar1 met30-6::KAN sic1::HYG GAL1-CLB5-9myc::URA3 | This study |
| PY1202 | abar1 CDC45-HA3::TRP1 | This study |
| PY1203 | abar1 met30-6::KAN CDC45-HA3::TRP1 | This study |
| PY1204 | abar1 MCM7-HA3::TRP1 | This study |
| PY1207 | abar1 met30-6::KAN MCM7-HA3::TRP1 | This study |
Cell cycle synchronization and flow cytometry.
Cells were synchronized in G1 phase with the mating pheromone α-factor (20-ng/ml final concentration), in early S-phase with hydroxyurea (0.4 M final concentration), and in metaphase with nocodazole (15-μg/ml final concentration). Cells were arrested at 25°C until ≥90% of cells were unbudded (for α-factor) or budded (for hydroxyurea and nocodazole arrest). Cells were then shifted to 37°C for 90 min to inactivate the temperature-sensitive met30 alleles. Cells were washed in prewarmed medium (37°C) and incubated at 37°C in fresh medium to release cells from the cell cycle block. Cells were prepared for flow cytometry and stained with SYTOX green (Molecular Probes, Inc., Eugene, OR) as described previously (16).
Protein analyses and kinase assays.
For immunoblot analysis, protein extracts were prepared in urea buffer (8M urea, 200 mM NaCl, 100 mM Tris [pH 7.5], 0.2% sodium dodecyl sulfate [SDS], 10 mM Na pyrophosphate, 5 mM EDTA, 5 mM EGTA, 50 mM NaF, 0.1 mM orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 1 μg/ml [each] aprotenin, leupeptin, and pepstatin). Cells were broken with glass beads for 80 s at setting 4.5 in a FastPrep FP120 (Qbiogene, Carlsbad, CA), and cell debris was removed by centrifugation for 10 min at 13,000 rpm. Protein lysates were diluted to a final concentration of 4 M urea before separation by SDS-polyacrylamide gel electrophoresis.
Separated proteins were transferred to a polyvinylidene difluoride membrane, and the membrane was probed with the antibodies indicated. Primary antibodies were used at the following dilutions: anti-hemagglutinin (HA) and anti-Myc, 1:2,000 (Covance, Princeton, NJ); anti-Rad53, 1:1,000 (SC-6749; Santa Cruz Biotechnology, Santa Cruz, CA).
Cln2-associated kinase was analyzed in strains expressing Cln2-HA3 under control of the GAL1 promoter. Cell lysates were prepared in a buffer containing 50 mM Tris-HCl (pH 7.5), 250 mM NaCl, 0.1% Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride, 1 μg/ml (each) aprotenin, leupeptin, and pepstatin. Cln2-HA3 immunocomplexes were purified from 200 μg protein lysates using anti-HA (12CA5) mouse ascites fluid and protein A beads. Kinase assays were performed as described previously (2).
RNA analyses and PCR.
RNA was isolated with the RNeasy kit (QIAGEN, Germantown, MD) following the manufacturer's protocol with the following modification. Cell pellets (1 × 107 to 2 × 107 cells) were broken with glass beads in buffer RLT for four times 40 s at setting 4.5 in a FastPrep FP120 (Qbiogene, Carlsbad, CA) with 1-min breaks between the runs. Cell debris was removed by centrifugation for 2 min at 13,000 rpm.
Northern blot analysis was performed as described previously (39). The membrane was hybridized with radiolabeled probes (HiPrime; Roche) in hybridization buffer (100 mM sodium phosphate buffer [pH 7.0], 400 mM NaCl, 5 mM EDTA, 1% SDS, 10% dextran sulfate, and 0.1 mg/ml denatured salmon sperm DNA). Northern blots were analyzed by phosphorimaging.
For RNA analysis by real-time reverse transcription (RT)-PCR, first-strand cDNA synthesis was performed with SuperScriptII following the manufacturer's recommendations, with the exception that 1.5 μg of RNA was used in a 10-μl reaction with 0.3 μl SuperScriptII. One-hundredth of the cDNA preparation was used in real-time PCRs on an iCycler iQ (Bio-Rad, Hercules, CA), using iQ SybrGreen Supermix (Bio-Rad, Hercules, CA). Primers for real-time PCR were designed to amplify 100- to 150-bp fragments using Beacon Designer 2.1 software (Biosoft International, Palo Alto, Calif.). For each experiment a standard curve was generated using fivefold dilutions of cDNA. The first dilution in the series was set arbitrarily to a copy number of 3,000. Only when PCR products were falling within the range of the standard curve, the amount of cDNAs was calculated relative to the standard curve and normalized to the control (ACT1) samples. Samples were run in duplicates in a PCR program with an initial 3-min 95°C step, followed by 40 cycles of 10 s at 95°C and 45 s at 55°C. After each run a melting curve was run to ensure that no primer dimers or secondary products were formed.
The ratio of nuclear to mitochondrial DNA content was analyzed by amplification of ACT1, [COX1], and [COX3]. Total DNA was amplified with iQ SybrGreen Supermix with an iCycler iQ real-time PCR detection system.
Primer sequences are available upon request from N.Y.S. (ningyuas@uci.edu).
ChIPs.
Chromatin immunoprecipitation (ChIP) assays were performed as described previously (20) with the following modifications. After breaking cells, the lysates were separated from cell debris and subjected to sonication. DNA was fragmented to approximately 200 to 800 bp using a Misonix 3000 sonicator with the cup horn device (5.5 in.) (Misonix, Farmingdale, NY). The initial output level was set at 10, with a total process time of 6 min. The pulse time was 30 s with a 2-min pause between pulses. After clarification, the HA-tagged proteins were immunoprecipitated with 12CA5 ascites fluid (a generous gift from Ian Wilson) and protein A beads for 4 h at 4°C. Protein-DNA complexes were eluted with 100 μl elution buffer (50 mM Tris [pH 8.0], 10 mM EDTA, 1% SDS), and DNA-protein cross-linking was reversed in 1% SDS-Tris-EDTA at 65°C overnight. DNA was purified on QIAQuick PCR columns (QIAGEN, Germantown, MD) according to the manufacturer's instructions. Real-time PCR was performed as described above for RT-PCR. One microliter of the eluted DNA (IP and input DNA) was used in a 20 μl PCR. All reactions were run in triplicates. For each experiment a standard curve was generated using fivefold dilutions of input DNA. ChIPs were normalized to the input DNA, and background obtained from samples expressing untagged proteins (normalized to input DNA) was subtracted from the ChIPs. Primer sequences used for detection of ARS305 and ARS501 are available upon request from N.Y.S. (ningyuas@uci.edu).
Cell biology.
For visualization of F-actin, cells were fixed by addition of formaldehyde (3.7% final concentration) directly to the culture. Fixed cells were incubated overnight at 4°C and washed with phosphate-buffered saline (PBS), and approximately 107 cells were resuspended in 100 μl PBS. Cells were stained with rhodamine-phalloidin (Molecular Probes, Inc., Eugene, OR) as described previously (15). To stain cells with 4′,6′diamidino-2-phenylindole, nocodazole-arrested cells were fixed in ethanol, washed in PBS, and stained with 4′,6′diamidino-2-phenylindole, and the number of nuclei/cell was determined microscopically.
RESULTS
Met30 is required at several positions in the cell cycle.
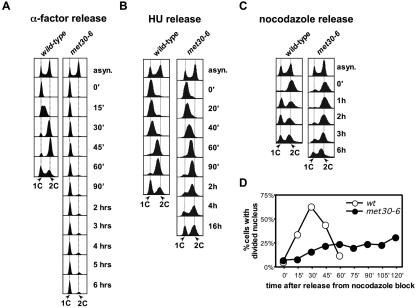
When temperature-sensitive met30-6 mutants were grown to early log phase and shifted to the restrictive temperature, the cell number remained constant after the temperature shift, indicating an immediate cell cycle arrest upon inactivation of Met30 (Fig. 1A). To determine the position in the cell cycle at which met30-6 mutants arrest, we analyzed the same samples by flow cytometry. The S-phase population of met30-6 mutants rapidly disappeared at restrictive temperature, and cells with 1C and 2C DNA content accumulated (Fig. 1B). At later time points the 2C peak decreased, since some of the cells leaked through this arrest point to end up in G1 (Fig. 1B). Similar results were obtained with a different temperature-sensitive allele (met30-9) (data not shown). Previous experiments indicated that the Cdk-inhibitory kinase Swe1 is stabilized in met30-6 mutants, possibly by an indirect mechanism (21, 25). Hyperactivation of Swe1 cannot explain the cell cycle defect of met30 mutants, since deletion of SWE1 does not overcome the arrest phenotype of met30 mutants (21). However, since hyperactivation of Swe1 specifically inhibits Cdc28 complexed with mitotic cyclins, it was possible that hyperactive Swe1 caused the postreplication arrest of met30-6 mutants. Therefore, we compared the budding indices of met30-6 mutants and met30-6 swe1Δ double mutants. After 6 h at the restrictive temperature, the fraction of budded cells was similar in met30-6 and met30-6 swe1Δ mutants (32% and 28%, respectively), indicating that Swe1 stabilization is not responsible for the arrest of met30-6 mutants with 2C DNA content.
FIG. 1.
Cell cycle arrest of temperature sensitive met30 mutants: (A) Wild-type cells and temperature sensitive met30-6 mutants were grown in yeast extract-peptone-dextrose at 25°C to an optical density at 600 nm of 0.2, the temperature was shifted to 37°C and samples were taken at the time intervals indicated. For each sample cell number was counted. (B) DNA content of the samples as in panel A was analyzed by flow cytometry. (C) Wild-type cells and met30-6 mutants were grown in yeast extract-peptone-dextrose at 25°C to an optical density at 600 nm of 0.2 and shifted to a semipermissive temperature (30°C) for 2 h, and filamentous actin stained with rhodamine-phalloidin was visualized by fluorescence microscopy.
Microscopic observation showed that met30-6 mutants were larger and the cell shape was rounder than that of wild-type cells, indicating a possible problem in polarized growth. We therefore grew cells at the semipermissive temperature for met30-6 mutants (30°C) and stained filamentous actin with rhodamine-phalloidin (Fig. 1C). Consistent with the round cell shape, most of the actin filaments were depolarized in met30-6 cells (Fig. 1C). Similar defects in actin polarization were observed in met30-9 mutants (data not shown).
These experiments suggested that Met30 function is required for the transition from the G1 phase of the cell cycle to S phase but not for progression through S phase. In addition, the accumulation of cells with 2C DNA content indicated that Met30 is important for some aspects of M phase. The essential function of Met30 for the G1/S transition is consistent with results obtained upon overexpression of Met4 from the GAL1 promoter in met30Δ met4Δ double mutants. However, induction of GAL1-MET4 did not affect M phase in these mutants (30).
To further characterize the cell cycle arrest of met30-6 mutants, we followed cell cycle progression of cells presynchronized at different cell cycle stages. First we analyzed cells that were released from a G1 block. After the cells were arrested with mating pheromone, the temperature was shifted to 37°C for 90 min to inactivate met30-6. Cells were then released from the pheromone block at 37°C, and cell cycle progression was monitored by flow cytometry (Fig. 2A). met30-6 mutants remained in G1, confirming that Met30 is required to enter S phase (Fig. 2A). Microscopic analysis of the cells revealed that met30-6 mutants failed to initiate budding. However, consistent with previous results (30), cell mass increased even though cell cycle progression was blocked, which excludes a major metabolic defect as cause for the cell cycle arrest of met30-6 mutants (data not shown). We next synchronized cells at early S phase with hydroxyurea, shifted cells to the restrictive temperature, and then released cells from the S-phase block at the restrictive temperature (Fig. 2B). met30-6 mutants progressed through S phase with kinetics similar to that of wild-type cells, confirming that Met30 function is not required for DNA replication. However, met30-6 mutants showed a significant delay of several hours in mitosis (Fig. 2B). Even after 16 h, more than 50% of the cells had not finished the cell cycle, which was evident from constant cell numbers throughout the time course and a significant number of cells with 2C DNA content (Fig. 2B; also data not shown). We also applied the same protocol to cells synchronized in metaphase with the spindle toxin nocodazole. met30-6 mutants showed a severe delay in progression through M phase (Fig. 2C). Although we cannot exclude that this delay is caused by a defect in recovery from the spindle checkpoint rather than by problems during M phase, both the hydroxyurea release experiment (Fig. 2B) and the temperature shift experiment shown in Fig. 1 are consistent with a requirement for Met30 in M phase. To determine what step in mitoses is dependent on Met30, we analyzed kinetics of nuclear division of cells released from a nocodazole block (Fig. 2D). Cells were synchronized in metaphase with nocodazole and shifted to 37°C for 90 min to inactivate met30-6. Cells were then released from the nocodazole block in fresh medium at 37°C, and nuclear division was monitored (Fig. 2D). Nuclear division in wild-type cells started shortly after the cells were released, whereas the majority of met30 mutants failed to progress into anaphase as indicated by undivided nuclei.
FIG. 2.
Met30 is required for G1/S transition and M-phase, but not for S-phase: (A) Wild-type cells and met30-6 mutants were grown in yeast extract-peptone-dextrose at 25°C to an optical density at 600 nm of 0.2, arrested with α-factor (20 ng/ml final concentration) until the fraction of budded cells was less than 10%. The culture was shifted to 37°C and incubation in the presence of α-factor was continued for 90 min. For the release, the cells were washed in prewarmed (37°C) yeast extract-peptone-dextrose and incubated in fresh prewarmed yeast extract-peptone-dextrose medium at 37°C. Samples were taken at the time intervals indicated and analyzed by flow cytometry. (B) Wild-type cells and met30-6 mutants were grown as for panel A, but instead of α-factor, hydroxyurea (400 mM final concentration) was added. When >90% of cells showed a “large-budded” morphology, cells were shifted to 37°C and incubation in the presence of hydroxyurea was continued for 90 min. Cells were released and analyzed from the hydroxyurea block as described for panel A. (C and D) Same as for panel B, but nocodazole (15-μg/ml final concentration) was used to arrest cells instead of hydroxyurea. Nuclear division was scored by counting the number of nuclei/cell.
Taken together, these results indicate that the F-box protein Met30 is required for entry into S phase and for the transition from metaphase to anaphase but not for progression through S phase.
Met30 is required for efficient expression of G1 cyclins.
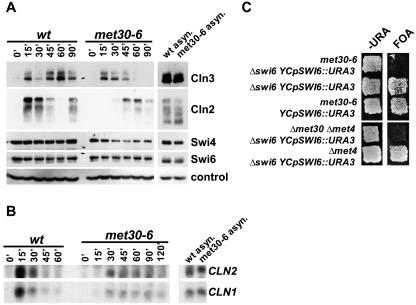
To characterize the G1/S transition defect of met30-6 mutants, we analyzed several key regulators of Start. To this end we synchronized cells in G1 with mating pheromone, shifted the cells to the restrictive temperature, and released them from the pheromone block at the restrictive temperature. Samples were taken at various time intervals after the release and analyzed by immunoblotting (Fig. 3A). The levels of the G1 cyclin Cln2 were significantly lower and induced later in met30-6 mutants than in wild-type cells (Fig. 3A). The lower Cln2 protein levels were a reflection of decreased CLN2 mRNA (Fig. 3B). Expression of another G1 cyclin, CLN1, was also severely affected in met30-6 mutants (Fig. 3B). CLN1 and CLN2 expression depend on a third G1 cyclin, Cln3 (9, 40, 44). Interestingly, Cln3 levels were induced with kinetics similar to that of wild-type cells after release from the pheromone block (Fig. 3A), indicating that the CLN1/CLN2 expression defect of met30-6 mutants is downstream of Cln3. We therefore analyzed Swi4 and Swi6, the two main transcription factors that regulate CLN1/CLN2 expression (4). Swi6 levels were unaffected by the met30-6 mutation (Fig. 3A). Swi4 levels were somewhat lower than wild-type levels at the later time points. However, initial levels of Swi4 after release from the pheromone block were comparable to that in wild-type cells, yet CLN1/CLN2 expression was not induced (Fig. 3B). Genetic experiments revealed that deletion of SWI6 is synthetic lethal with met30-6 mutants (Fig. 3C). No synthetic interaction between met30-6 and deletion of SWI4 was observed (data not shown). Surprisingly, swi6 met30 met4 triple mutants were inviable (Fig. 3C). This was unexpected, because deletion of MET4 rescues the cell cycle defect of met30 mutants (20, 30). The synthetic-lethal phenotype of swi6 met30 met4 therefore suggests a Met4-independent requirement for Met30.
FIG. 3.
Efficient expression of G1 cyclins requires Met30. (A) α-Factor arrest and release were as described in the legend to Fig. 2A. Samples were analyzed by immunoblotting: wild-type cells and met30-6 mutants expressing both Cln2-HA3 and Cln3-18myc were used to detect Cln2 and Cln3; strains expressing both Swi4-6myc and Swi6-HA3 for detection of Swi4 and Swi6. Cdc28 was detected with anti-PSTAIRE antibodies as a loading control. (B) Total RNA was analyzed by Northern blotting with radiolabeled probes to detect CLN1 and CLN2, respectively. (C) Strains as indicated were replica plated to plates lacking uracil and plates containing 5-fluoro-orotic acid and incubated at 25°C.
Genetic experiments have suggested that in addition to G1 cyclins, the protein kinase Gin4 is an essential transcription target for Swi6. Indeed, Gin4 overexpression, like Cln2 overexpression, rescues a swi6Δ bck2Δ mutant (13). Overexpression of Gin4 alone or in combination with Cln2 could not overcome the cell cycle arrest of met30-6 mutants even when analyzed just above the permissive temperature for met30-6 (data not shown).
Recent results demonstrated the importance of Whi5 in regulation of G1-specific transcription (5, 6). Because Whi5 acts as an inhibitor of the G1-specific transcription program, we tested whether deletion of WHI5 suppresses the CLN2 expression defect of met30-6 mutants. To this end, we compared CLN2 RNA levels of met30-6 and met30-6 whi5Δ mutants in a pheromone block/release experiment similar to that shown in Fig. 3A. Real-time RT-PCR analysis showed no significant change in absolute CLN2 levels upon deletion of WHI5; however, the low level of CLN2 expression observed in met30-6 mutants was induced about 15 min earlier in met30-6 whi5Δ double mutants (data not shown). Analysis of cell cycle progression of pheromone-synchronized met30-6 whi5Δ double mutants by flow cytometry revealed that deletion of WHI5 did not suppress the G1/S transition defect of met30 mutants (data not shown).
Defects in expression of G1 cyclins are not causing the cell cycle arrest of met30 mutants.
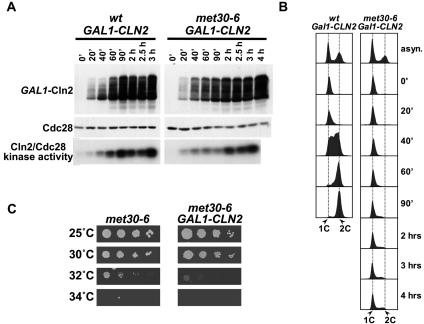
The defect in G1 cyclin expression of met30-6 mutants is consistent with results obtained by overexpression of Met4 from the GAL1 promoter in met30Δ met4Δ double mutants (30). These results prompted the authors to suggest that met30 mutants show a cell cycle arrest because of a lack of G1 cyclins. We tested this idea by overexpression of the G1 cyclin Cln2 from the strong GAL1 promoter. met30-6 mutants were arrested in G1 with mating pheromone, shifted to 37°C to inactivate met30-6, and then released from the pheromone block under conditions that allowed overexpression of Cln2. Immunoblot analysis demonstrated that Cln2 accumulated to high levels in both wild-type cells and met30-6 mutants (Fig. 4A). Cln2/Cdc28 kinase activity towards histone H1 correlated well with Cln2 levels (Fig. 4A). Although Cln2 levels and Cln2/Cdc28 kinase activity were somewhat delayed in met30-6 mutants, they eventually reached levels that were much higher than that observed at the time point when wild-type cells entered S phase (Fig. 4A, wt, 40 min). Surprisingly, despite Cln2/Cdc28 kinase activity, met30-6 mutants failed to enter S phase (Fig. 4B) or initiate budding (data not shown). Accordingly, overexpression of Cln2 from the GAL1 promoter did not suppress the cell cycle arrest of met30-6 mutants even when analyzed slightly above the permissive temperature (Fig. 4C).
FIG. 4.
Cln2 overexpression does not overcome the G1 arrest of met30 mutants. (A) Wild-type cells and met30-6 mutants harboring a GAL1-CLN2-HA3 allele integrated at the LEU2 locus were grown in yeast extract-peptose-raffinose at 25°C to an optical density at 600 nm of 0.2, arrested with α-factor until the fraction of budded cells was below 10%. The culture was shifted to 37°C, and incubation in the presence of α-factor was continued for 90 min. For the release, the cells were washed in prewarmed (37°C) yeast extract-peptose-raffinose and incubated in fresh prewarmed yeast extract-peptone-galactose (2% galactose, final concentration) at 37°C. Samples were taken at the time intervals indicated and analyzed by immunoblotting with anti-HA (12CA5) antibodies to detect Cln2-HA3 and anti-PSTAIRE antibodies for detection of Cdc28. Cln2-associated kinase activity was assayed using anti-HA-associated immunocomplexes to phosphorylate histone H1. (B) Samples from the experiments described for panel A were analyzed by flow cytometry. (C) Serial dilutions of the strains used panel A were spotted on yeast extract-peptone-galactose plates and incubated at the temperatures indicated.
Expression of Cln2 in cells lacking all other G1 cyclins is sufficient for passage through Start (1). Therefore, these results indicate that although Met30 is required for efficient expression of G1 cyclins, the reduced expression of G1 cyclins in met30 mutants cannot explain their defect in G1/S transition.
Deletion of Sic1 cannot suppress the cell cycle arrest of met30 mutants.
The Cdk inhibitor Sic1 controls G1/S transition (26). Sic1 inhibits B-type cyclin-associated kinase activity and prevents accumulation of the S-phase-promoting Clb5/Cdc28 activity (28, 36). Sic1 degradation by the ubiquitin proteasome system is triggered by its phosphorylation by G1 cyclin/Cdc28 kinase (45). This phosphorylation step is the only nonredundant essential function for G1 cyclins, which is demonstrated by suppression of the lethality of cln1,2,3Δ triple mutants by deletion of SIC1 (10, 35, 42). We asked whether deletion of SIC1 could suppress the cell cycle defect of met30 mutants. To this end we compared colony formation of serial dilutions of met30-6 and sic1Δ met30-6 mutants at different temperatures (Fig. 5A). Deletion of SIC1 did not suppress the proliferation defect of met30-6 mutants (Fig. 5A). It was possible that deletion of SIC1 suppressed only some aspects of the cell cycle defects of met30 mutants but not others. Since Sic1 is particularly important for regulation of S-phase initiation, we compared cell cycle progression of sic1Δ mutants and sic1Δ met30-6 double mutants (Fig. 5B). Cells were arrested with pheromone in G1, shifted to the restrictive temperature for met30-6 mutants, and released from the pheromone block at restrictive temperature. sic1Δ mutants progressed through the cell cycle, whereas the majority of sic1Δ met30-6 mutants failed to enter S phase (Fig. 5B). However, at later time points (3 h and 4 h [Fig. 5B]) a small but significant number of sic1Δ met30-6 double mutants seemed to have entered S phase. An increase in the number of cells with 2C DNA content was not observed, though, indicating that sic1Δ met30-6 mutants did not complete S phase (Fig. 5B).
FIG. 5.
Deletion of SIC1 does not suppress the G1 arrest of met30 mutants. (A) Serial dilutions of cells with the indicated genotypes were spotted onto yeast extract-peptone-dextrose plates and incubated at the temperatures specified. (B) sic1Δ and sic1Δ met30-6 mutants were synchronized with α-factor and released from the cell cycle block as described in the legend to Fig. 2A. Samples were analyzed by flow cytometry.
Overexpression of Clb5 induces DNA replication in met30 mutants.
Previous experiments (30) and experiments presented above (Fig. 1, 2, 4, and 5) demonstrated a major defect of met30 mutants in initiation of S phase, while progression through S phase appeared to be largely Met30 independent (Fig. 2B). Two kinases are required for activation of DNA replication, namely Clb5/Cdc28 and Cdc7/Dbf4 (3, 8, 37). We first asked whether Cdc7/Dbf4 is affected by loss of Met30 function. To this end we analyzed expression of endogenous Myc-tagged Dbf4 in met30-6 mutants that were synchronized in G1, shifted to the restrictive temperature, and released from the G1 arrest. Dbf4 protein accumulated to levels very similar to that of wild-type cells (data not shown), indicating that Dbf4 is not limiting for initiation of S phase in met30 mutants. Consistent with this conclusion, introduction of the mcm5-bob1 allele (17), which makes S phase independent of Cdc7/Dbf4, did not suppress the G1/S transition defect of met30-6 mutants in a pheromone arrest/release experiment (data not shown). Accordingly, cell spotting assays detected no suppression of met30-6 mutants by the mcm5-bob1 allele even when testing was carried out only slightly above the restrictive temperature (data not shown). These results essentially excluded Cdc7/Dbf4 deficiency as the cause for the G1/S transition defect of met30 mutants.
We next tested whether the second S-phase-promoting kinase, Clb5/Cdc28, might be limiting in met30 mutants. We examined expression of CLB5 in a pheromone block/release experiment similar to that shown in Fig. 2A by real-time RT-PCR. There was a significantly lower level of CLB5 expression in met30-6 mutants than in wild-type cells (Fig. 6A). We then analyzed the effect of overexpression of Clb5 from the GAL1 promoter on S-phase initiation in met30 mutants. Cells were synchronized in G1 with mating pheromone, shifted to the restrictive temperature for met30-6 mutants, and released from the G1 block at restrictive temperature in galactose-containing medium to induce Clb5 overexpression. Flow cytometric analysis showed a significant increase in DNA content in met30-6 mutants beginning at 2 h after the release from the pheromone arrest (Fig. 6B). The DNA content continued to increase over the time course, but the bulk of the cells never reached the “2C stage,” which would indicate completion of S phase (Fig. 6B). The increase in DNA content demonstrated by flow cytometry indicated that overexpression of Clb5 might induce DNA replication in met30-6 mutants. However, the shift of the DNA profile towards 2C DNA content could also be a reflection of mitochondrial DNA amplification that often accompanies an increase in cell size seen in cell cycle mutants. We therefore asked whether the ratio of chromosomal DNA to mitochondrial DNA in met30-6 GAL1-CLB5 cells changed over the time course. To this end we used real-time PCR to amplify one nuclear locus (ACT1) and two mitochondrial loci ([COX1] and [COX3]). We arbitrarily set the ratio of mitochondrial to nuclear copy number to 1 for pheromone-arrested cells (Fig. 6C, 0 h). An increase in this ratio would imply disproportional amplification of mitochondrial DNA. Six hours after release of met30-6 mutants overexpressing Clb5, the ratio of mitochondrial DNA to nuclear DNA did not increase (Fig. 6C), suggesting that the shift in the DNA profile reflected nuclear DNA replication induced by Clb5 overexpression.
FIG. 6.
Clb5 overexpression induces DNA replication in met30 mutants. (A) Wild-type cells and met30-6 mutants were synchronized in G1 phase and released from the cell cycle block as described in the legend to Fig. 2A. Samples were taken at the time points indicated and analyzed by quantitative reverse transcriptase PCR using SYBR green. CLB5 expression levels were normalized to ACT1 levels. (B) Wild-type cells and met30-6 mutants harboring a GAL1-CLB5-9myc allele integrated at the ARS1 locus were grown in yeast extract-peptone-raffinose at 25°C to an optical density at 600 nm of 0.2, arrested with α-factor until the fraction of budded cells was less than 10%. The culture was shifted to 37°C, and incubation in the presence of α-factor was continued for 90 min. For the release, the cells were washed in prewarmed (37°C) yeast extract-peptone-raffinose and incubated in fresh prewarmed yeast extract-peptone-galactose (2% galactose, final concentration) at 37°C. Samples were taken at the time intervals indicated and analyzed by flow cytometry. Expression of Clb5-9myc was confirmed by immunoblotting using anti-Myc antibodies (data not shown). (C) Total DNA was isolated from wild-type cells and met30-6 cells expressing GAL1-CLB5-9myc for 6 h after release from the α-factor block, and the nuclear locus ACT1 as well as two mitochondrial loci ([COX1] and [COX3]) were quantified by real-time PCR using SYBR green. (D) Serial dilutions of cells with the genotypes indicated were spotted onto yeast extract-peptone-galactose plates and incubated at the temperatures specified.
These experiments indicate that overexpression of Clb5 can induce DNA replication in met30-6 mutants. However, the induced DNA replication is very slow and aberrant. Clb5 overexpression did not induce budding in met30-6 mutants. Identical results were observed when we analyzed met30-6 sic1Δ double mutants overexpressing Clb5 from the GAL1 promoter (data not shown), indicating that inhibition of Clb5/Cdc28 kinase is not responsible for slow S-phase progression in these experiments. Consistent with these results, overexpression of Clb5 in met30-6 or met30-6 sic1Δ mutants could not suppress the proliferation defect of these mutants even when analyzed slightly above the restrictive temperature (Fig. 6D).
Efficient binding of Cdc45 and Mcm proteins to origins of replication requires Met30 function.
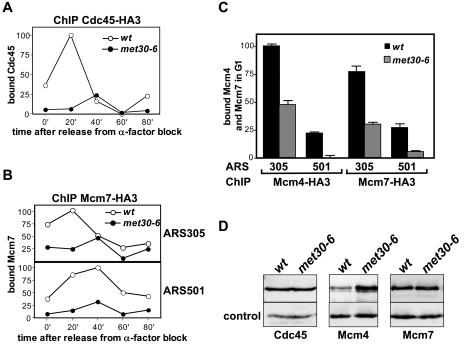
To further address the question of which steps of S-phase initiation are dependent on Met30, we analyzed formation of replication complexes on origins in wild-type cells and met30 mutants. Prereplication complexes (preRCs) consisting of Orc and Mcm proteins and Cdc6 are formed on origins during G1 phase. preRCs are maintained throughout G1 phase until phosphorylation events catalyzed by Clb5/Cdc28 and Dbf4/Cdc7 induce release of Cdc6 from origins and recruitment of other replication factors, such as Cdc45, to initiate DNA replication (3, 8). We first monitored Cdc45 recruitment to ARS305 in a pheromone block/release experiment by ChIP (Fig. 7A). Wild-type cells and met30-6 mutants were synchronized in G1, shifted to 37°C to inactivate met30-6, and then released from the pheromone block at 37°C. Cdc45 binding was analyzed as cells progressed through the cell cycle by ChIP assays followed by real-time PCR detection of ARS305. As expected for wild-type cells, Cdc45 was detected at ARS305 soon after cells were released from G1 arrest and disappeared again as replication progressed (Fig. 7A). In contrast, recruitment of Cdc45 to ARS305 was severely repressed in met30 mutants (Fig. 7A). This is consistent with the defect of met30 mutants in Clb5 expression (Fig. 6A), because binding of Cdc45 to preRCs requires Clb5/Cdc28 (3, 8). The defect in Cdc45 recruitment observed in met30 mutants could be an indirect consequence of low Clb5 expression (Fig. 6A). In contrast to Cdc45 recruitment, binding of preRC components to origins does not require CDK activity but rather is prevented by active B-type cylin/Cdk activity (3, 8). We therefore asked whether association of preRCs with replication origins is also dependent on Met30 function. In a similar experiment, as described above (Fig. 7A), we examined binding of Mcm7 to the early-replicating ARS305 and the late-replicating ARS501. Significantly less Mcm7 was bound to ARS305 and ARS501 throughout the time course in met30 mutants than in wild-type cells, suggesting that Met30 is required for preRC association with origins in G1 phase (Fig. 7B). This was further supported when we analyzed binding of another preRC component, Mcm4. Similar to Mcm7, we found significantly less Mcm4 associated with ARS305 and ARS501 in G1-synchronized met30 mutants than in wild-type cells (Fig. 7C). To test whether lower levels of Cdc45 or Mcm4/7 in met30 mutants are causing the decrease in origin occupancy, we analyzed all samples by immunoblotting (Fig. 7D and data not shown). Mcm4 levels were higher in met30 mutants than in wild-type cells (Fig. 7D). Cdc45 and Mcm7 protein levels were similar in wild-type cells and met30 mutants (Fig. 7D), indicating that origin association and not expression of Cdc45 or Mcm4/7 depends on Met30.
FIG. 7.
Met30 is required for efficient origin binding of replication factors. (A) Wild-type cells (PY1202) and met30-6 mutants (PY1203) expressing Cdc45-HA3 from its chromosomal locus were grown at 25°C to an optical density at 600 nm of 0.5, arrested with α-factor until the fraction of budded cells was less than 10%. The culture was shifted to 37°C, and incubation in the presence of α-factor was continued for 90 min. Cells were released from the G1 arrest at 37°C, and samples were taken at the time intervals indicated and analyzed by chromatin immunoprecipitation followed by detection of ARS305 by real-time PCR using SYBR green. Wild-type (wt) cells and met30-6 mutants (PY283) expressing untagged Cdc45 were processed in parallel, and values obtained in the PCR detection were subtracted as background in the graphs shown. (B) Wild-type cells (PY1204) and met30-6 mutants (PY1207) expressing Mcm7-HA3 from its chromosomal locus were grown and analyzed as for panel A. (C) Wild-type cells and met30-6 mutants expressing Mcm4-HA3 (PY1058 and PY1059) or Mcm7-HA3 from their chromosomal loci were grown and arrested with α-factor as described for panel A. Cells were shifted to 37°C for 90 min in the presence of α-factor, and samples were taken and analyzed as for panel A. (D) Total cell lysates prepared from α-factor-arrested samples after 90 min at 37°C (time point 0′ in panels A, B, and C) were analyzed by immunoblotting with anti-HA (12CA5) antibodies to detect Cdc45-HA3, Mcm4-HA3, and Mcm7-HA3. anti-PSTAIRE antibodies were used for detection of Cdc28 as a loading control.
These results suggest that Met30 function is important for efficient association of intact preRCs with replication origins in G1 and help to explain the defect of met30 mutants in S-phase initiation.
DISCUSSION
We have used temperature-sensitive alleles of met30 to characterize cell cycle steps dependent on Met30. Cell cycle arrest/release experiments demonstrated that Met30 is required for initiation of S phase but not for progression through S phase (Fig. 2). In addition, we detected a requirement for Met30 in M phase. In agreement with a previous study (30), we found a severe defect in expression of the G1 cyclins Cln1 and Cln2 in met30 mutants (Fig. 3). Activation of CLN1 and CLN2 transcription requires the G1 cyclin Cln3 to remove the inhibitor Whi5 from CLN1,2 promoters (5, 6). Cln3 was induced to wild-type levels in met30 mutants (Fig. 3A), suggesting that Met30 is perhaps important for Whi5 inactivation. However, deletion of WHI5 could not increase CLN2 expression levels in met30 mutants (data not shown), indicating that Met30 regulates G1-specific transcription downstream of Whi5. We also analyzed protein levels of Swi4 and Swi6, the two components of SBF, the transcription factor that activates CLN1,2 transcription (27, 29). Swi6 levels were normal (Fig. 3A). Although Swi4 levels were somewhat lower in met30 mutants at the later time points (Fig. 3A), initial levels were comparable to that in wild-type cells, yet CLN1,2 expression was not induced (Fig. 3).
Expression of the S-phase cyclin Clb5 depends on MBF, a transcription factor composed of Swi6 and Mbp1 (22). Like CLN1,2 expression, CLB5 expression was severely affected by inactivation of Met30 (Fig. 6A), indicating that Met30 is required for SBF- and MBF-dependent transcription.
The G1/S transition defect of met30 mutants cannot be explained by the lack of the G1 cyclins Cln1,2, because overexpression of Cln2 could not overcome the cell cycle arrest in G1 (Fig. 4). Previous experiments using a conditional met30 mutant (met4::GAL1-MET4 met30Δ) showed that Cln2 was undetectable even when expressed from the GAL1 or ADH1 promoter (30). Those results led the authors to propose a potent G1-cyclin RNA degradation mechanism that is induced by Met4 activation (30). Expression of Cln2 from the GAL1 promoter in temperature-sensitive met30-6 or met30-9 mutants was not affected by inactivation of Met30 (Fig, 4A, data not shown). It is unclear whether these differences are due to different genetic backgrounds or a consequence of Met4 overproduction. Regardless, our results demonstrate that the defect in G1 cyclin expression cannot explain the G1 cell cycle block of met30 mutants (Fig. 4).
Although overexpression of Cln2 led to high levels of Cln2 protein and Cln2/Cdc28 kinase activity in met30 mutants, these cells failed to initiate DNA replication or budding (Fig. 4; also data not shown). Budding requires polarization of the actin cytoskeleton to the future bud site, which is regulated by G1 cyclin/Cdc28 activity (24). Since budding in met30 mutants is not induced even when Cln2/Cdc28 kinase activity is overproduced, Met30 seems to function downstream of G1 kinase activation.
Overexpression of the S-phase cyclin Clb5 induced slow DNA replication in met30 mutants (Fig. 6). In contrast, high levels of the second S-phase-promoting factor, Dbf4 (data not shown), could not suppress the S-phase block of met30 mutants (data not shown), nor could the mcm5-bob1 allele (17), which makes S phase independent of Dbf4/Cdc7 (data not shown). DNA replication induced by Clb5 overexpression was very slow and did not lead to complete duplication of the genome over a period of 6 h (Fig. 6). Since the hydroxyurea synchrony release experiments indicated that Met30 function was not required for progression through S phase (Fig. 2B), the slow S phase observed in met30-6 GAL1-CLB5 cells is more likely a reflection of inefficient firing of replication origins rather than stalled or slow-moving replication forks. This was further supported by analyses of association of preRCs with replication origins. We found significantly less Mcm4 and Mcm7 associated with early- or late-firing origins in met30 mutants (Fig. 7). This cannot be an indirect consequence of the CLN1,2 or CLB5 expression defects of met30 mutants, because preRCs are formed and maintained under conditions of low CDK activity (wt in Fig. 7) (3, 8). These results suggest that Met30 is required to maintain intact prereplication complexes in late G1.
Our data and those of previous studies (30) indicate that the F-box protein Met30 is required for several cell cycle-related steps, including, G1-specific transcription, initiation of DNA replication, actin polarization, and M-phase progression. These requirements for Met30 are most likely not caused by a general metabolic defect of met30 mutants, because, first, met30 mutants continue to increase mass during the cell cycle arrest (30; also data not shown); second, translation in met30 mutants was reduced only to 80% 2.5 h after induction of the cell cycle arrest (30); third, met30 mutants progressed through S phase with wild-type kinetics (Fig. 2B) (30); and fourth, expression of Cln3, which is hypersensitive to nutrient limitation (14, 31), was not affected by inactivation of Met30 (Fig. 3A).
It is important to bear in mind that the lethality of met30 mutants is suppressed by deletion of MET4 or MET32 (20, 30). Both Met4 and Met32 are transcription factors. It is therefore likely that the various cell cycle defects associated with loss of Met30 function are caused by inappropriate gene induction or repression. Microarray experiments and genetic approaches in our laboratory have so far failed to associate individual genes with the cell cycle arrest phenotype of met30 mutants, indicating that concomitant misregulation of several genes contributes to the arrest phenotype. More-sensitive genetic screens are currently being conducted to identify these genes.
Acknowledgments
We are grateful to Oscar Aparicio, Fred Cross, Robert Sclafani, David Stuart, and Curt Wittenberg for materials used in this study. We express thanks to Steve Reed for continued support and helpful discussions and to Steve Haase and Curt Wittenberg for valuable suggestions and comments on the manuscript. We thank the members of the Kaiser and Nomura labs for helpful discussions.
This work was supported by grants from NIH (GM66164) and the University of California Cancer Research Coordinating Committee to P. Kaiser.
REFERENCES
- 1.Andrews, B., and V. Measday. 1998. The cyclin family of budding yeast: abundant use of a good idea. Trends Genet. 14:66-72. [DOI] [PubMed] [Google Scholar]
- 2.Basco, R. D., M. D. Segal, and S. I. Reed. 1995. Negative regulation of G1 and G2 by S-phase cyclins of Saccharomyces cerevisiae. Mol. Cell. Biol. 15:5030-5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell, S. P., and A. Dutta. 2002. DNA replication in eukaryotic cells. Annu. Rev. Biochem. 71:333-374. [DOI] [PubMed] [Google Scholar]
- 4.Breeden, L. L. 2003. Periodic transcription: a cycle within a cycle. Curr. Biol. 13:R31-R38. [DOI] [PubMed] [Google Scholar]
- 5.Costanzo, M., J. L. Nishikawa, X. Tang, J. S. Millman, O. Schub, K. Breitkreuz, D. Dewar, I. Rupes, B. Andrews, and M. Tyers. 2004. CDK activity antagonizes Whi5, an inhibitor of G1/S transcription in yeast. Cell 117:899-913. [DOI] [PubMed] [Google Scholar]
- 6.de Bruin, R. A., W. H. McDonald, T. I. Kalashnikova, J. Yates III, and C. Wittenberg. 2004. Cln3 activates G1-specific transcription via phosphorylation of the SBF bound repressor Whi5. Cell 117:887-898. [DOI] [PubMed] [Google Scholar]
- 7.Deshaies, R. J. 1999. SCF and Cullin/Ring H2-based ubiquitin ligases. Annu. Rev. Cell Dev. Biol. 15:435-467. [DOI] [PubMed] [Google Scholar]
- 8.Diffley, J. F. 2004. Regulation of early events in chromosome replication. Curr. Biol. 14:R778-R786. [DOI] [PubMed] [Google Scholar]
- 9.Dirick, L., T. Bohm, and K. Nasmyth. 1995. Roles and regulation of Cln-Cdc28 kinases at the start of the cell cycle of Saccharomyces cerevisiae. EMBO J. 14:4803-4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein, C. B., and F. R. Cross. 1994. Genes that can bypass the CLN requirement for Saccharomyces cerevisiae cell cycle START. Mol. Cell. Biol. 14:2041-2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldmann, R. M. R., C. C. Correll, K. B. Kaplan, and R. J. Deshaies. 1997. A complex of Cdc4p, Skp1p, and Cdc53p/Cullin catalyzes ubiquitination of the phosphorylated CDK inhibitor Sic1p. Cell 91:221-230. [DOI] [PubMed] [Google Scholar]
- 12.Flick, K., I. Ouni, J. A. Wohlschlegel, C. Capati, W. H. McDonald, J. R. Yates, and P. Kaiser. 2004. Proteolysis-independent regulation of the transcription factor Met4 by a single Lys 48-linked ubiquitin chain. Nat. Cell Biol. 6:634-641. [DOI] [PubMed] [Google Scholar]
- 13.Flick, K., and C. Wittenberg. 2005. Multiple pathways for suppression of mutants affecting G1-specific transcription in Saccharomyces cerevisiae. Genetics 169:37-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallego, C., E. Gari, N. Colomina, E. Herrero, and M. Aldea. 1997. The Cln3 cyclin is down-regulated by translational repression and degradation during the G1 arrest caused by nitrogen deprivation in budding yeast. EMBO J. 16:7196-7206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guthrie, C., and G. R. Fink. 1991. Guide to yeast genetics and molecular biology, vol. 194. Academic Press, Inc., San Diego, Calif.
- 16.Haase, S. B., and S. I. Reed. 2002. Improved flow cytometric analysis of the budding yeast cell cycle. Cell Cycle 1:132-136. [PubMed] [Google Scholar]
- 17.Hardy, C. F., O. Dryga, S. Seematter, P. M. Pahl, and R. A. Sclafani. 1997. mcm5/cdc46-bob1 bypasses the requirement for the S phase activator Cdc7p. Proc. Natl. Acad. Sci. USA 94:3151-3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hershko, A., and A. Ciechanover. 1998. The ubiquitin system. Annu. Rev. Biochem. 67:425-479. [DOI] [PubMed] [Google Scholar]
- 19.Hochstrasser, M. 1996. Ubiquitin-dependent protein degradation. Annu. Rev. Genet. 30:405-439. [DOI] [PubMed] [Google Scholar]
- 20.Kaiser, P., K. Flick, C. Wittenberg, and S. I. Reed. 2000. Regulation of transcription by ubiquitination without proteolysis: Cdc34/SCF(Met30)-mediated inactivation of the transcription factor Met4. Cell 102:303-314. [DOI] [PubMed] [Google Scholar]
- 21.Kaiser, P., R. A. Sia, E. G. Bardes, D. J. Lew, and S. I. Reed. 1998. Cdc34 and the F-box protein Met30 are required for degradation of the Cdk-inhibitory kinase Swe1. Genes Dev. 12:2587-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koch, C., T. Moll, M. Neuberg, H. Ahorn, and K. Nasmyth. 1993. A role for the transcription factors Mbp1 and Swi4 in progression from G1 to S phase. Science 261:1551-1557. [DOI] [PubMed] [Google Scholar]
- 23.Kuras, L., A. Rouillon, T. Lee, R. Barbey, M. Tyers, and D. Thomas. 2002. Dual regulation of the met4 transcription factor by ubiquitin-dependent degradation and inhibition of promoter recruitment. Mol. Cell 10:69-80. [DOI] [PubMed] [Google Scholar]
- 24.Lew, D. J., and S. I. Reed. 1995. Cell cycle control of morphogenesis in budding yeast. Curr. Opin. Genet. Dev. 5:17-23. [DOI] [PubMed] [Google Scholar]
- 25.McMillan, J. N., C. L. Theesfeld, J. C. Harrison, E. S. Bardes, and D. J. Lew. 2002. Determinants of Swe1p degradation in Saccharomyces cerevisiae. Mol. Biol. Cell 13:3560-3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendenhall, M. D., and A. E. Hodge. 1998. Regulation of Cdc28 cyclin-dependent protein kinase activity during the cell cycle of the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 62:1191-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nasmyth, K., and L. Dirick. 1991. The role of SWI4 and SWI6 in the activity of G1 cyclins in yeast. Cell 66:995-1013. [DOI] [PubMed] [Google Scholar]
- 28.Nugroho, T., and M. D. Mendenhall. 1994. An inhibitor of yeast cyclin-dependent protein kinase plays an important role in ensuring the genomic integrity of daughter cells. Mol. Cell. Biol. 14:3320-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogas, J., B. J. Andrews, and I. Herskowitz. 1991. Transcriptional activation of CLN1, CLN2, and a putative new G1 cyclin (HCS26) by SWI4, a positive regulator of G1-specific transcription. Cell 66:1015-1026. [DOI] [PubMed] [Google Scholar]
- 30.Patton, E. E., C. Peyraud, A. Rouillon, K. Y. Surdin, M. Tyers, and D. Thomas. 2000. SCF(Met30)-mediated control of the transcriptional activator Met4 is required for the G(1)-S transition. EMBO J. 19:1613-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polymenis, M., and E. V. Schmidt. 1997. Coupling of cell division to cell growth by translational control of the G1 cyclin CLN3 in yeast. Genes Dev. 11:2522-2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reed, S. I. 2003. Ratchets and clocks: the cell cycle, ubiquitylation and protein turnover. Nat. Rev. Mol. Cell Biol. 4:855-864. [DOI] [PubMed] [Google Scholar]
- 33.Reed, S. I., J. A. Hadwiger, and A. T. Lorincz. 1985. Protein kinase activity associated with the product of the yeast cell division cycle gene CDC28. Proc. Natl. Acad. Sci. USA 82:4055-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schaefer, J. B., and L. L. Breeden. 2004. RB from a bud's eye view. Cell 117:849-850. [DOI] [PubMed] [Google Scholar]
- 35.Schneider, B. L., Q. H. Yang, and A. B. Futcher. 1996. Linkage of replication to start by Cdk inhibitor Sic1. Science 272:560-562. [DOI] [PubMed] [Google Scholar]
- 36.Schwob, E., T. Bohm, M. D. Mendenhall, and K. Nasmyth. 1994. The B-type cyclin kinase inhibitor p40SIC1 controls the G1 to S transition in S. cerevisiae. Cell 79:233-244. [DOI] [PubMed] [Google Scholar]
- 37.Sclafani, R. A. 2000. Cdc7p-Dbf4p becomes famous in the cell cycle. J. Cell Sci. 113:2111-2117. [DOI] [PubMed] [Google Scholar]
- 38.Skowyra, D., K. L. Craig, M. Tyers, S. J. Elledge, and J. W. Harper. 1997. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell 91:209-219. [DOI] [PubMed] [Google Scholar]
- 39.Stuart, D., and C. Wittenberg. 1994. Cell cycle-dependent transcription of CLN2 is conferred by multiple distinct cis-acting regulatory elements. Mol. Cell. Biol. 14:4788-4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stuart, D., and C. Wittenberg. 1995. CLN3, not positive feedback, determines the timing of CLN2 transcription in cycling cells. Genes Dev. 9:2780-2794. [DOI] [PubMed] [Google Scholar]
- 41.Thomas, D., and Y. Surdin-Kerjan. 1997. Metabolism of sulfur amino acids in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 61:503-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tyers, M. 1996. The cyclin-dependent kinase inhibitor p40SIC1 imposes the requirement for Cln G1 cyclin function at Start. Proc. Natl. Acad. Sci. USA 93:7772-7776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tyers, M., and P. Jorgensen. 2000. Proteolysis and the cell cycle: with this RING I do thee destroy. Curr. Opin. Genet. Dev. 10:54-64. [DOI] [PubMed] [Google Scholar]
- 44.Tyers, M., G. Tokiwa, and B. Futcher. 1993. Comparison of the Saccharomyces cerevisiae G1 cyclins: Cln3 may be an upstream activator of Cln1, Cln2 and other cyclins. EMBO J. 12:1955-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verma, R., R. S. Annan, M. J. Huddleston, S. A. Carr, G. Reynard, and R. J. Deshaies. 1997. Phosphorylation of Sic1p by G1 Cdk required for its degradation and entry into S phase. Science 278:455-460. [DOI] [PubMed] [Google Scholar]
- 46.Verma, R., R. M. Feldman, and R. J. Deshaies. 1997. SIC1 is ubiquitinated in vitro by a pathway that requires CDC4, CDC34, and cyclin/CDK activities. Mol. Biol. Cell 8:1427-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wittenberg, C., and K. Flick. 2003. Cell cycle regulation during G1 phase in yeast: decisions, decisions, decisions, p. 14-39. In J. Boonstra (ed.), G1 phase progression. Landes Biosciences, Georgetown, Tex.