Abstract
Early heart development in Drosophila and vertebrates involves the specification of cardiac precursor cells within paired progenitor fields, followed by their movement into a linear heart tube structure. The latter process requires coordinated cell interactions, migration, and differentiation as the primitive heart develops toward status as a functional organ. In the Drosophila embryo, cardioblasts emerge from bilateral dorsal mesoderm primordia, followed by alignment as rows of cells that meet at the midline and morph into a dorsal vessel. Genes that function in coordinating cardioblast organization, migration, and assembly are integral to heart development, and their encoded proteins need to be understood as to their roles in this vital morphogenetic process. Here we prove the Toll transmembrane protein is expressed in a secondary phase of heart formation, at lateral cardioblast surfaces as they align, migrate to the midline, and form the linear tube. The Toll dorsal vessel enhancer has been characterized, with its activity controlled by Dorsocross and Tinman transcription factors. Consistent with the observed protein expression pattern, phenotype analyses demonstrate Toll function is essential for normal dorsal vessel formation. Such findings implicate Toll as a critical cell adhesion molecule in the alignment and migration of cardioblasts during dorsal vessel morphogenesis.
Similarities exist between heart development in Drosophila melanogaster and vertebrates, particularly during early stages of organ formation (6, 8, 13, 47, 57). Cardiogenesis in these animals initiates with the appearance of cardiac precursor cells within paired progenitor fields, followed by their movement into a linear tubular structure. Cardiac cell specification occurs due to instructive communication between different tissue and cell types and the resulting activation and function of distinct combinations of transcriptional regulators.
In Drosophila, cardioblasts emerge from bilateral dorsal mesoderm primordia because of inductive signals received from the dorsal ectoderm via the Decapentaplegic and Wingless signaling pathways (14, 52). Genes encoding the homeodomain protein Tinman (Tin) and GATA factor Pannier (Pnr) are induced, and their combined activities promote cardioblast specification (1, 3, 5, 17, 19, 29, 47, 53). Such cardioblasts eventually populate the dorsal vessel, which is the linear cardiac organ of the fly. Comparably in vertebrates, a primitive heart tube develops at the ventral midline, being composed of cells derived from the bilaterally arranged cardiac crescent and anterior heart-forming field (8, 10, 28, 57). As in the fly, conserved signaling pathways and transcriptional regulators function to generate cardiac cells that assemble within the linear heart formed early in vertebrate development. Requisite signaling molecules include BMP and Wnt family members, while key transcription factors consist of Nkx, GATA, Iroquois, and T-Box class proteins.
While mechanistic insights have been gained on the generation of cardiac precursors in these animals, less is known about genes controlling subsequent events of heart development. The Drosophila dorsal vessel has emerged as an amenable tissue to study genetic and cellular aspects of heart tube formation. Postspecification, cardioblasts align as two parallel rows of cells, migrate synchronously with the overlying ectoderm, establish an apical-basal epithelial polarity, and meet and organize at the dorsal midline as a linear tube enclosing a lumen (22, 46). A few contributors to this phase of heart development have been identified thus far. Haag and colleagues (22) investigated the functions of certain cell adhesion molecules in dorsal vessel morphogenesis and showed an immunoglobulin-like protein, encoded by the faint sausage (fas) gene, was required for the correct alignment of cardioblasts prior to their migration. Also, the fly E-cadherin homolog was needed for normal adhesion of opposing cardioblast rows and lumen formation. The same study confirmed the findings of Yarnitzky and Volk (54) by observing normal Laminin A function was essential for the maintenance of dorsal vessel ultrastructure.
Proteins involved in generating or maintaining the polarity of cardioblasts have likewise been implicated in dorsal vessel formation. The α subunit of the heterotrimeric Go protein is somehow needed to promote cell polarity (15), while the basal expression of the extracellular matrix protein Pericardin appears essential for cross talk between cardioblasts and ectoderm cells as they move coordinately during dorsal closure (11). Mutations in either of these genes result in an abnormal organization of cardioblasts within a broken and dysfunctional tissue. Recent studies with zebra fish have likewise demonstrated myocardial precursors form polarized epithelia, and their integrity during migration to the midline depends on defined cell-substratum interactions (48).
Once formed, the dorsal vessel functions as the contractile organ for circulation in postembryonic Drosophila, with an intrinsic morphological and functional anterior-posterior (A-P) polarity (13, 37, 47). That is, the linear tube is composed of an anterior aorta region with a small lumen and lacking inflow tracts and a posterior heart region with an expanded lumen and three pairs of valvelike ostia. Also, the distinctive anteriormost region of the aorta is complexed with ring and lymph glands, with the latter being the site of hematopoiesis during larval development.
This intricate cardiac structure is a result of intersecting genetic networks that produce cardioblasts of nonequivalent identities and differentiation states. The homeotic gene abdominal-A functions autonomously to determine heart cell identities in the posterior segments of the dorsal vessel (38, 39, 43). Additionally, distinct cardioblast subgroups are found within aorta and heart segments, based on the expression of the homeodomain proteins Tin and Ladybird, orphan nuclear receptor Seven-Up (Svp), and T-box protein Dorsocross (Doc) (3, 5, 16, 26, 36, 50). Likely due to the combinatorial functions of these cardiac transcription factors and homeotic selector proteins, terminal differentiation genes become activated and specialized cardioblast differentiation occurs, as in the formation of inflow tracts solely within the heart chamber (38, 39, 40, 43). This A-P polarity is reminiscent of the primitive heart tube of vertebrates, wherein cells contribute to eventual outflow tract, ventricular, atrial, and inflow tract regions (37, 57). Additionally, the movement of hemolymph through the dorsal vessel occurs in a posterior to anterior direction, comparable to the direction of blood flow through the vertebrate heart.
Of the three discernible aspects of cardioblast progression during dorsal vessel formation—specification early on, behavior during morphogenesis, and late specialization—arguably the least understood is cardioblast function during organ development. Clearly, additional players need to be discovered and characterized within this secondary phase of cardiogenesis. The Toll signaling pathway has been studied extensively in terms of its central role in determining dorsal-ventral cell fates in the blastoderm embryo (42) and the pathway is integral to the genetic control of innate immune recognition and response in Drosophila (9, 34). Toll encodes a transmembrane protein with leucine-rich repeats within its extracellular domain (24, 25). This structural feature, along with the broad zygotic expression observed on many cell surfaces, is consistent not only with ligand binding to Toll during signal transduction but also a function in promoting cell-cell interactions and adhesion.
Intriguingly, a transcriptional enhancer possessing dorsal vessel activity was fortuitously discovered upstream of the Toll transcription unit (51). To investigate a potential role for this versatile protein in heart development, we analyzed Toll expression, regulation, and requirement during dorsal vessel formation. In this report, we demonstrate the protein is expressed on lateral surfaces of all cardioblasts during cardiogenesis and Toll mutations result in defects in dorsal vessel structure. We also characterize the dorsal vessel enhancer and show its transcriptional regulators include the Doc and Tin factors. Such findings identify Toll as an integral part of heart development in Drosophila. Additionally, newly generated Toll dorsal vessel enhancer-GFP strains provide sensitive cell-specific markers for heart development in Drosophila.
MATERIALS AND METHODS
Drosophila strains.
Transgenic lines carrying Toll-clacZ, Toll-cGFP, or Toll-nGFP DNA were established postinjection of yw67c23 embryos using standard transformation techniques (17). UAS-Doc2 and UAS-tin transgenic flies have been described previously by Reim et al. (45) and Yin and Frasch (55), respectively. The Toll mutant stocks Tlr3 ca1/TM3, Sb1 (temperature sensitive) and ru1 h1 th1 st1 kniri-1 rnroe-1 pp e1 Tlr4/TM3, Ser1 (2) were obtained from the Bloomington Stock Center (Indiana University).
mRNA and protein expression analyses.
A Toll cDNA clone was obtained from ResGen Invitrogen Corporation (Carlsbad, CA), and a corresponding digoxigenin-labeled cRNA probe was generated using the DIG RNA Labeling Kit (SP6/T7) from Roche Applied Science (Indianapolis, IN). Toll mRNA was detected in embryos by in situ hybridization using protocols described by Gajewski et al. (17). Toll protein was detected in embryos by immunohistochemical staining using a rabbit anti-Toll antibody (24) at a 1:500 dilution, goat anti-rabbit biotinylated secondary antibody (Vector Laboratories, Burlington, CA) at a 1:500 dilution, and the Vectastain ABC Kit (Vector Laboratories) as described by Gajewski et al. (17). Images of stained or green fluorescent protein (GFP)-expressing whole-mount embryos were captured with a Zeiss Axioplan 2 microscope using AXIOVISION V.3.1 software.
For confocal microscope analyses, primary reagents included the anti-Toll antibody rabbit anti-D-MEF2 antibody (35) used at a 1:1,000 dilution and mouse anti-A.v. GFP antibody (BD Biosciences Clontech, Palo Alto, CA) used at a 1:200 dilution. Secondary antibodies were fluorescein isothiocyanate or Cy3 AffiniPure goat Fab fragments from Jackson ImmunoResearch Laboratories (West Grove, PA). After incubating embryos with secondary antibodies at a 1:600 dilution in blocking solution for 4 h at 4°C, the embryos were washed in PBT (17), mounted with VECTASHIELD mounting medium (Vector Laboratories), and examined with an Olympus FV500 confocal microscope using FLUOVIEW version 4.3 software.
Molecular analyses of the Toll heart enhancer.
For Toll enhancer analyses, DNAs were generated by PCR amplification using the BAC clone RPCI-98 24.O.14, obtained from the BACPAC Resource Center (Children's Hospital, Oakland Research Institute). DNAs were cloned into the P element vector Chab (cytoplasmic β-galactosidase reporter), pGreen H-Pelican (cytoplasmic enhanced GFP reporter), or pH-Stinger (nuclear enhanced GFP reporter) (4). To generate the Toll 305 DNA, PCR primers were Toll 305-A (forward), 5′-ACCGAAATCCAAAAGATATTTCAAG-3′, and Toll 305-B (reverse), 5′-GATACAAATGGAGCGCAGCG-3′. Truncated versions of this DNA used the Toll 305-B reverse primer and the following forward primers: Toll 258-A, 5′-GGCCCAGGAAATGAAGAGATA-3′; Toll 264-A, 5′-GGTGTGGGCCCAGGAAATG-3′; Toll 270-A, 5′-TGGTGTGGTGTGGGCCCAG-3′; Toll 276-A, 5′-GTGGTGTGGTGTGGTGTGG-3′; Toll 282-A, 5′-AGACACGTGGTGTGGTGTGG-3′; Toll 287-A, 5′-TTTCAAGACACGTGGTGTGG-3′. DNAs were generated by PCR amplification using deoxynucleoside triphosphate solution, Taq DNA polymerase, and PCR buffer provided by Roche Applied Science. PCR products were cloned into the pCRII-TOPO vector using the TA cloning kit from Invitrogen (San Diego, CA) and then inserted into P element vectors. To generate the Toll 305 mTin and Toll 258 mTin fragments, we used the QuickChange site-directed mutagenesis kit from Stratagene (La Jolla, CA). Polyacrylamide gel electrophoresis-purified mutant oligonucleotides were mTin-A, 5′-GCACTTCAGTGATTATGTTTgtAGacCCTGGGCTTCTGGCAATG-3′, and mTin-B, 5′-CATTGCCACAAGCCCAGGgtCTacAAACATAATCACTGAAGTGC-3′, where lowercase letters correspond to the introduced base changes. For all PCR-generated Toll clones, DNAs were sequenced to assure their accuracy prior to further use.
For electrophoretic mobility shift assays, GST-Tin fusion protein was synthesized using the TnT Quick Coupled Transcription/Translation System (Promega, Madison, WI). These assays were performed as described by Ranganayakulu et al. (44). Complementary oligonucleotides containing the consensus Tin binding site of the Toll 305 enhancer were used. The primer sequences for wild-type probe and competitor DNAs were Tin-A, 5′-GATTATGTTTCAAGTGCCTGGGCTTC-3′, and Tin-B, 5′-GAAGCCCAGGCACTTGAAACATAATC-3′ (Tin binding site underlined). Oligonucleotides with an altered sequence, previously shown to disrupt Tin DNA binding (19), served as the mutant competitor DNA. These included Tin-A mutant, 5′-GATTATGTT TgAAGTcCCTGGGCTTC-3′, and Tin-B mutant, 5′-GAAGCCCAGGgACTTcAAACATAATC-3′. The double base changes within the Tin binding sites are in lowercase.
For DNA binding assays with Doc, GST-Doc fusion proteins were expressed in Escherichia coli and purified. Doc1, Doc2, and Doc3 are clustered and highly related T-box class genes with greater than 95% amino acid identities in their T-box domains (45). As all three Doc genes are expressed in Tin-negative and Svp-positive cardioblasts (36, 45), two of the proteins were used in Toll enhancer binding studies. Expression constructs were generated by subcloning of Doc1 and Doc2 cDNAs into pGEX-4T-1. pGEX-Doc1 was obtained by ligation of a filled-in BstBI/NotI fragment of the Doc1-a1.1 cDNA and SmaI/NotI-digested pGEX-4T-1. pGEX-Doc2 containing the entire Doc2 coding sequence and pGEX-Doc2T containing the Doc2 T-box as described by Reim et al. (45) were obtained by cloning of PCR-generated EcoRI fragments derived from cDNA Doc2-c6.2. DNase I footprinting assays were performed as described by Yin et al. (56), with the following minor modifications. DNA probes for strand-specific labeling were generated by PCR using pairs of 5′-phosphorylated and unphosphorylated primers complementary to the ends of the cloned Toll 305 dorsal vessel enhancer. DNA probes were labeled via [γ-32P]ATP and T4 DNA polynucleotide kinase reaction. Each 50-μl binding reaction mixture contained various amounts of GST fusion protein, 18,000 cpm of labeled DNA probe (1 to 3 fmol), 100 mM KCl, 25 mM HEPES (pH 7.5), 10 mM MgCl2, 1 mM EDTA, 1 mM dithiothreitol, 10% glycerol, 0.05% NP-40, 0.02 mg/ml poly(dI-dC), and 0.2 mg/ml bovine serum albumin. After incubation on ice for 100 min, 50 μl of fresh DNase I mix (0.05 to 0.1 U of DNase I, 10 mM MgCl2, 10 mM CaCl2) was added and DNA digestion was stopped after 2 min by adding 100 μl of stop buffer (1% sodium dodecyl sulfate, 20% EDTA, 200 mM NaCl, 0.1 mg/ml yeast tRNA). DNA fragments were purified and separated on polyacrylamide DNA sequencing gels.
Ectopic activation of Toll by Doc and Tin.
A UAS-Doc2;UAS-tin stock was established by standard crosses for combined expression of Doc2 and tin in the embryonic mesoderm. Ectopic expression was induced by crossing 2xPE-twi-GAL4;Toll 305-clacZ females with homozygous UAS construct-carrying males. Embryos from these crosses were allowed to develop at 28°C, and then fixed and processed by double fluorescent immunohistochemical stainings as described by Knirr et al. (30). Rabbit anti-D-MEF2 antibody (7) was used at a 1:750 dilution, and monoclonal anti-β-galactosidase antibody 40-1a, obtained from the Developmental Studies Hybridoma Bank (University of Iowa), was used at a 1:60 dilution. Primary antibodies were detected with Cy3 AffiniPure goat Fab fragments (1:200 dilution) and horse anti-mouse biotinylated secondary antibodies (1:500 dilution) in combination with the Vectastain ABC Kit and Fluorescein Tyramide Reagent (Perkin-Elmer).
Toll mutant phenotype analyses.
Males from Tlr3 or Tlr4 balanced stocks were crossed to virgin females from the homozygous Toll 305-cGFP strain (second chromosome insertion) to generate Tl heterozygous animals lacking the balancer chromosome and containing the GFP dorsal vessel marker. Toll-cGFP/+;Tlr3/+ virgin females were mated with Toll-cGFP/+;Tlr4/+ males, and embryos were collected on grape plates over a 2-h period. Embryos were aged for 4 h at 25°C and either maintained at 25°C (control) or moved to the 29°C restrictive temperature. Embryos were allowed to advance to stage 15 to 16 of development and analyzed for normal or abnormal dorsal vessel formation based on GFP marker expression. Additionally, embryos were processed and stained for D-MEF2 expression in body wall and heart muscles. Reproducible dorsal vessel phenotypes were not observed in control embryo populations in five experiments, that is, in non-heat-shifted embryos derived from the mating of Tlr3/+ females and Tlr4/+ males. In contrast, moderate to severe dorsal vessel phenotypes were observed in 10 to 20% of the total egg lay from the cross of Tlr3/+ and Tlr4/+ animals, when temperature shifted in five test experiments. These embryos would correspond to ∼40 to 80% of the expected mutant population. Tlr3 served as the temperature-sensitive allele in these experiments (20).
RESULTS
Toll expression during dorsal vessel formation.
At the time dorsal-ventral polarity is established during early Drosophila development, Toll is associated with the plasma membrane around the entire syncytial blastoderm embryo (24). Thereafter, Toll exhibits zygotic expression on several cell surfaces, including a specific dorsal cell type in late-stage embryos. These were identified at first as leading-edge cells of the two-epidermal sheets moving toward the dorsal midline (24). We reevaluated Toll expression in dorsal aspects of the embryo and, to the contrary, concluded the gene is expressed in cardioblasts of the developing and formed dorsal vessel.
Initially, Toll mRNA accumulation was analyzed by in situ hybridization, with gene transcripts first detected in dorsal cell populations in stage 12 embryos and later in two converging rows of cells during the process of dorsal closure (Fig. 1A and B). The likelihood of the Toll-positive cells being cardioblasts was strongly implied by the pattern of mRNA accumulation in stage 16 embryos. Toll expression was detected in roughly 50 cell pairs, and the organization of said cells was reminiscent of cardioblasts within structurally identifiable aorta and heart regions of the assembled dorsal vessel (Fig. 1C). The pattern of Toll protein expression was also investigated, with results comparable to those obtained in the RNA analysis. The transmembrane protein was detected in dorsal cells in late stage 12/early stage 13 embryos. Thereafter, it showed a clear presence on lateral surfaces of all cells aligned within two contiguous rows as they migrate toward the dorsal midline (Fig. 1D and E). By stage 16, the Toll-positive cells populate the core of the dorsal vessel, again within defined aorta and heart subregions (Fig. 1F). Toll was found exclusively on cardioblast surfaces, while organ-associated pericardial, lymph gland, and ring gland cells failed to express the protein. High-resolution analysis by confocal microscopy demonstrated Toll presence at lateral points of contact between all cardioblasts of the mature dorsal vessel (discussed below).
FIG. 1.
Toll mRNA and protein expression in cardioblasts of the forming and mature dorsal vessel. (A and D) Embryos at stage 14; (B and E) embryos at stage 15; (C and F) embryos at stage 16. Abbreviations: a, aorta region of the dorsal vessel; cb, cardioblasts; h, heart region of the dorsal vessel.
Delimitation of the Toll dorsal vessel enhancer.
Toll zygotic transcription is complex based on the numerous cell and tissue types that express the gene (24). Through efforts to identify a regulatory sequence controlling Toll expression in central nervous system (CNS) midline glial cells, Wharton and Crews (51) located three regions upstream of the gene that possessed transcriptional enhancer activity. Relevant to our demonstration of Toll expression in the dorsal vessel, a 6.5-kb DNA was fortuitously found to direct lacZ reporter expression in all cardioblasts, and in pharyngeal and body wall muscles as well. Due to our interest in understanding how this expression might be regulated, we proceeded to delimit the Toll cardioblast enhancer within the defined upstream region.
At first, our analysis involved testing Toll 5′-flanking DNAs for the ability to drive lacZ expression in embryos of transgenic strains. A 7.1-kb region located between −9.3 and −2.2 upstream of the gene showed strong enhancer function in all cardioblasts of the dorsal vessel (Fig. 2). The DNA was subdivided into five overlapping segments, and only the most distal 1.7-kb DNA maintained cardioblast activity. Subsequently, five fragments spanning this 1.7-kb interval were tested for enhancer function and we were able to map dorsal vessel activity to a 305-bp sequence located between −8.3 and −8.0 relative to the Toll transcription start site. Consistent with the timing of Toll mRNA and protein accumulation in cardioblasts, the 305-bp enhancer becomes active during stage 12 and maintains its activity through all subsequent events of dorsal vessel morphogenesis. It is noteworthy that this small DNA also functions in amnioserosa cells from stage 11 through stage 15. The relevance of such activity will be addressed shortly.
FIG. 2.
Mapping the location of the Toll dorsal vessel transcriptional enhancer. A condensed organization of the Toll gene is shown at the top. The gene is present at position 97D2-3 of the D. melanogaster cytogenetic map. Various DNA regions located upstream of the Toll transcription start site were tested for enhancer function in dorsal vessel (DV) cardioblasts. DNAs possessing enhancer activity were scored as positive (+), while those lacking activity were scored as negative (−). At least four independently derived transgenic lines were assayed for each of the DNAs tested.
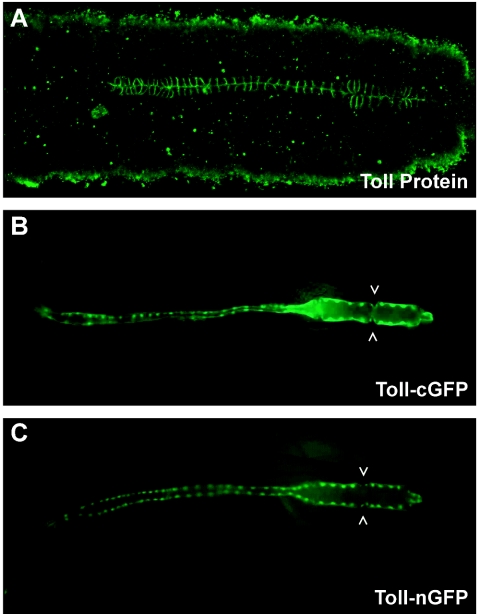
The use of additional P-element enhancer-test vectors, which contain different versions of the GFP reporter gene, significantly increased the sensitivity of the Toll regulatory sequence analysis. Figure 3 compares the expression of Toll protein on lateral surfaces of all cardioblasts at stage 16 (Fig. 3A), with the location of cytoplasmic GFP (cGFP; Fig. 3B) or nuclear GFP (nGFP; Fig. 3C) expressed under the control of the Toll 305-bp dorsal vessel enhancer at the same stage. The nGFP marker allows one to count with precision the nuclei of all 104 cardioblasts that make up the linear cardiac organ. Furthermore, the cGFP reporter allows the study, in living animals, of the formation and function of three pairs of inflow tracts within the heart chamber. To summarize, the 305-bp transcriptional enhancer faithfully recapitulates the pattern of Toll gene expression in cardioblasts during dorsal vessel formation.
FIG. 3.
The Toll 305-bp enhancer functions in all cardioblasts of the dorsal vessel. (A) Toll protein expression at lateral surfaces of all cardioblasts in the formed dorsal vessel at embryonic stage 16. Enhancer activity was detected through the analysis of stage 16 embryos from transgenic strains expressing the Toll DNA fused to marker genes encoding cytoplasmic green fluorescent protein (Toll-cGFP) (B) or nuclear green fluorescent protein (Toll-nGFP) (C). Open arrowheads indicate a pair of valvelike ostia in panel B and small nuclei present in Svp/Doc cells in panel C.
Molecular analysis of Doc and Tin regulation of the Toll dorsal vessel enhancer.
We envisioned that Toll dorsal vessel expression could be controlled by either a single transcription factor with activity in all cardioblasts or a combination of regulators that functioned in complementing groups of cells. That is, it was conceivable the Toll enhancer served as a direct target of D-MEF2 or Hand transcriptional activation, for example, as both proteins are expressed in all cardioblasts of the cardiac tube (7, 31, 35, 41). Alternatively, while Tin is expressed in a majority of cardioblasts of the dorsal vessel, the Svp and Doc transcription factors are present in neighboring Tin-negative cells (16, 36, 45). In combination, two of these cardiac regulators could activate Toll expression in the complete repertoire of cells.
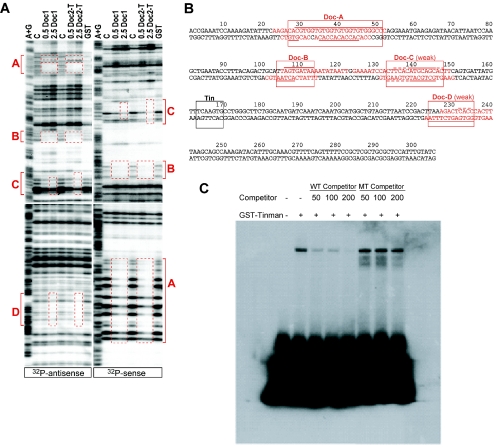
Testing variations of the Toll 305-bp DNA pointed to the dorsal vessel enhancer being regulated by at least two transcription factors. Deletion of 47 bp from the distal end of the fragment resulted in a Toll 258 DNA with enhancer activity in a subset of cardioblasts, those being Tin-positive cells (Fig. 4C). Because of this truncation, enhancer function was selectively lost from the Svp/Doc-expressing cells. To identify the factor responsible for Toll expression in non-Tin cardioblasts, we considered the Svp and Doc proteins expressed in these cells. Genetic studies have shown Svp acts as a negative regulator of tin transcription in Svp/Doc-positive cells; thus, it seemed unlikely this orphan nuclear receptor functioned as an activator of Toll gene expression. We therefore turned to the Doc T-box protein(s) as a candidate regulator. DNase I protection assays were used to demonstrate the in vitro binding of Doc1 and Doc2 to the Toll 305-bp dorsal vessel enhancer; four regions were protected by recombinant Doc molecules (Fig. 5A). Areas A and B are strong interaction sites, as protection was observed even at low protein concentrations. The extensive footprint at site A contains repetitive GTG T-box core elements (Fig. 5B); hence, it is likely to bind multiple Doc molecules. In contrast, the C and D footprint areas correspond to more weakly protected sites. The use of either full-length proteins, or solely the T-box domains of Doc1 and Doc 2, resulted in essentially the same DNase I protection patterns on the Toll enhancer DNA (Fig. 5A and data not shown).
FIG. 4.
Functional dissection of the Toll 305-bp dorsal vessel enhancer. (Left) The Toll 305-bp DNA is schematized at the top, with Doc binding sites indicated by boxes (Doc-A through Doc-D) and the Tin binding site highlighted as an oval. 5′-truncated and/or site-mutated versions of the Toll enhancer are schematized below the wild-type DNA, with constructs named according to their size in nucleotides and the included mutation. For example, Toll 258 represents a DNA lacking 47 bp of the 5′ sequence, including most of the Doc-A binding region; Toll 258 mTin is the same DNA with a mutated Tin binding site. Activities of the various DNAs in Tin or Svp/Doc subsets of cardioblasts are indicated at the right as positive (+), negative (−), or positive but variable (+/−) in enhancer function. At least four independently derived transgenic lines were assayed for each of the DNAs tested. (Right) Expression patterns of Toll enhancer DNAs in transgenic animals. Stage 16 embryos were assayed for enhancer activity using cytoplasmic β-galactosidase (clacZ) and cGFP markers. (A) Toll 305-clacZ expression in all cardioblasts. (B) Mutation of the Tin-binding site in Toll 305 mTin results in variable and irregular activity of the enhancer. Arrows point out the lack of reporter expression in Tin cardioblasts. (C) Toll 258-clacZ expression solely in Tin cardioblasts. Open arrowheads highlight enhancer inactivity in Svp/Doc cells. (D) Mutation of the Tin-binding site in Toll 258 mTin results in enhancer nonfunction in Tin cardioblasts. (E) Toll 264-cGFP expression solely in Tin cardioblasts. Open arrowheads indicate enhancer inactivity in Svp/Doc cells. (F, G, and H) Toll 270, Toll 276, and Toll 282 DNAs drive cGFP expression strongly in Tin cardioblasts and variably in Svp/Doc cardioblasts. Solid arrowheads point out the variable enhancer function in Svp/Doc cells. (I) Toll 287-cGFP expression in all cardioblasts of the dorsal vessel.
FIG. 5.
DNA binding studies on the Toll dorsal vessel enhancer. (A) In vitro binding of Doc proteins to the Toll dorsal vessel enhancer as determined by DNase I footprint analysis. The antisense (left side) and sense (right side) strands of the 305-bp DNA were 32P labeled and incubated with either 0.5 or 2.5 μg of recombinant GST-Doc fusion protein (full-length Doc1 or T-box fragment of Doc2), 5 μg of GST control protein, or control buffer only. Four areas were found to be protected by recombinant Doc proteins (red boxes labeled A, B, C, and D). Sites A and B show significant DNase I protection at both low and high concentrations of Doc proteins; the C and D sites require the higher protein concentration for protection. Site D is poorly protected on the sense strand (not shown). Abbreviations: A+G, adenosine/guanosine ladder produced via Maxam-Gilbert sequencing; C, control buffer; Doc2-T, T-box fragment of Doc2. (B) Sequence of the Toll 305-bp dorsal vessel enhancer. Binding sites of two relevant cardiac transcription factors are indicated by boxes. Tin: demonstrated Tin binding site matching the known Tin consensus element. Doc-A through Doc-D: Doc binding sites corresponding to areas protected in DNase I footprint assays. The Doc-A and Doc-B regions are bound with higher affinity than the Doc-C and Doc-D sites. Lines above and below the enhancer DNA indicate the protected sequences for the sense and antisense strands, respectively. Dashed lines denote additional protection observed only at high concentrations of Doc2-T protein. Red and nonunderlined nucleotides adjacent to protected sites indicate the range between the outermost band affected by footprinting and the next unaffected band that is detectable in the assay. (C) Electrophoretic mobility shift assay demonstrates that Tin selectively binds to a consensus recognition element present in the Toll 305-bp enhancer. GST-Tin fusion protein was used in the binding assay with a 32P-end-labeled double-stranded oligonucleotide that included the Tin binding sequence present at coordinates 163 to 169 of the enhancer DNA. Specificity of Tin binding to the probe was tested by competition with wild-type (WT) and Tin site-mutated (MT) double-stranded oligonucleotides, used at increasing (50, 100, or 200) molar excess concentrations.
To further investigate a role for Doc in Toll enhancer regulation, additional Toll DNA-cGFP constructs were analyzed for the requirement of the Doc-binding sequences. As the Toll 258 DNA is functionally silent in Svp/Doc cardioblasts, a series of enhancer-test DNAs was generated by ordered addition of the GTG T-box core elements found in the Doc-A footprint region. These ranged from Toll 264, which adds on only two GTG repeats, to Toll 287, which contains the complete Doc-A sequence (Fig. 4). The analysis of enhancer function in embryos from the various transgenic strains demonstrated that the sequential addition of Doc binding sites resulted in either no change in enhancer activity relative to Toll 258 (Toll 264 DNA, Fig. 4E), a variable increased activity in Svp/Doc cardioblasts (Toll 270, Toll 276, and Toll 282 DNAs, Fig. 4F, G, and H), or a complete reconstitution of enhancer function in both cardioblast populations (Toll 287 DNA, Fig. 4I). We thus concluded that a Doc T-box family member was the likely activator of Toll gene expression in Svp/Doc cells.
Tin is known to bind the sequence TCAAGTG, and this recognition element serves as an essential protein-binding site within enhancers of all known Tin target genes (18, 19, 32, 53). The Toll dorsal vessel enhancer contains a single TCAAGTG sequence at nucleotides 163 to 169 (Fig. 4 and 5B). Tin can bind specifically to a double-stranded oligonucleotide containing this consensus site in an electrophoretic mobility shift assay, but not to an oligonucleotide containing a mutant version of this sequence (Fig. 5C). To determine the functional importance of the Tin recognition site, the sequence was altered within the Toll 305 DNA and the Toll 305 mTin-lacZ transgene was tested for enhancer function. An irregular pattern of variable expression in Tin and Svp/Doc cardioblasts was observed (Fig. 4B), demonstrating the importance of this perfect Tin consensus sequence for normal enhancer activity. A Toll 258 mTin-lacZ transgene was also tested for enhancer function, and this mutated DNA failed to drive reporter gene expression in any cardioblast population (Fig. 4D). Thus, in the absence of the distal 47 bp of DNA, the Tin recognition sequence is absolutely required for enhancer function in the Tin-positive cells (Fig. 4D). We therefore concluded the Tin homeodomain protein was also a probable activator of the Toll dorsal vessel enhancer.
Ectopic activation of the Toll dorsal vessel enhancer by Doc and Tin.
The requirement of the Doc and Tin proteins for Toll expression in heart cells cannot be directly tested since embryos mutant for these genes fail to specify cardioblasts (3, 5; I.R. and M.F., unpublished observations). We therefore turned to forced-expression experiments to determine if ectopic expression of the cardiac factors could induce transcription of the Toll-clacZ transgene. Initially, UAS-tin and UAS-Doc2 were expressed individually throughout the mesoderm and mesectoderm under the control of the twi-GAL4 driver. We observed that forced Tin expression caused a slightly expanded activity of the Toll 305 enhancer in the dorsal mesoderm (Fig. 6B, F, and J) while also inducing de novo activity in CNS midline cells (Fig. 6F). Likewise, forced Doc2 expression resulted in a widespread expansion of enhancer function within the mesoderm, particularly in dorsal areas (Fig. 6C, G, and K). Expressing both UAS-tin and UAS-Doc2 under the control of twi-GAL4 culminated in an even more pronounced induction of enhancer activity throughout the mesoderm and in midline cells (Fig. 6D, H, and L). Such findings support a model wherein the Doc and Tin factors serve as direct transcriptional regulators of the Toll dorsal vessel enhancer.
FIG. 6.
Forced-expression studies of tin and Doc2 with Toll 305-clacZ in the Drosophila mesoderm. (A, E, and I) Noninduced control embryos at stage 11 or 14. Panels A and I show lateral views of embryos; panel E shows a ventral embryo view. (B, F, and J) twi-GAL4;UAS-tin embryos at the indicated stages. Panels B and J show lateral views; panel F is a ventral view. (C, G, and K) twi-GAL4; UAS-Doc2 embryos at the indicated stages. Panels C and K show lateral views; panel G is a ventral view. (D, H, and L) twi-GAL4;UAS-tin;UAS-Doc2 embryos at the indicated stages. Panels D and L show lateral views; panel H is a ventral view. Green indicates β-galactosidase expression under the control of the Toll 305 enhancer, while red indicates D-MEF2 expression in the mesoderm. Arrows point to areas of induced expression of the β-galactosidase marker in CNS midline cells and throughout the mesoderm. (Bottom) Analysis of Toll enhancer function in amnioserosa cells expressing Toll 305-cGFP (M), Toll 258-cGFP (N), or Toll 287-cGFP (O).
The use of the Toll 305-clacZ transgene in these studies revealed an activity of the enhancer in the amnioserosa, as well as in the forming dorsal vessel, in embryos at stage 11 through stage 15 (Fig. 6E, I, and M). As Doc proteins are known to be expressed and function in amnioserosa cells (45), we investigated whether a Toll enhancer mutation that caused inactivity in Svp/Doc cardioblasts also led to a loss of function in the amnioserosa. Indeed, removal of the distal 47 bp (which includes the Doc-A footprint region) to generate Toll 258 resulted in a loss of enhancer activity in both cell types (Fig. 4C and 6N). Conversely, addition of the Doc-A sequence in Toll 287 restored enhancer activity in Svp/Doc cardioblasts and amnioserosa cells (Fig. 4I and 6O). Such results are consistent with Doc proteins working through the Doc-A binding region to activate the Toll 305 enhancer in cells in which they are normally expressed.
Toll function is required for normal morphogenesis of the dorsal vessel.
The maternal requirement for the Toll gene in establishing dorsal-ventral cell polarity in the blastoderm embryo has been well documented (42). Moreover, Toll is widely expressed zygotically and a late embryonic function for the protein has been demonstrated in motorneuron and somatic muscle development (23). Due to the proven expression and regulation of the Toll gene in cardioblasts, we initiated a phenotypic analysis of mutant embryos to search for potential defects in dorsal vessel formation.
We used two recessive alleles of Toll in this analysis, with one having a temperature-sensitive requirement. The first marker used to assess the status of the dorsal vessel was the Toll-cGFP transgene, which is expressed in all cardioblasts of the cardiac tube (Fig. 7A). After switching Toll mutants to the 29°C restrictive temperature, late-stage embryos were routinely observed with gaps in the aorta and/or heart regions (Fig. 7B and C). D-MEF2 is expressed in all contractile cells of the dorsal vessel (Fig. 7D), and the visualization of this protein allowed an analysis of cardioblast number and organization. Multiple assays with D-MEF2 showed correct numbers of cardioblasts were specified early on in the Toll mutant background. However, abnormalities in cell alignment and migration were observed, resulting in most but not all cardioblasts making their way into the forming dorsal vessel (Fig. 7E and F). The phenotype of cardioblasts missing from, or disorganized within, disrupted heart tubes of Toll embryos was further confirmed with cardioblast-expressed laminin A, MSP-300, and α-spectrin protein probes (33, 49, 54; data not shown). Thus, consistent with the presence of Toll on lateral surfaces of all cardioblasts, mutations in the gene result in visible defects in dorsal vessel formation and structure.
FIG. 7.
Dorsal vessel phenotypes resultant from mutations in the Toll gene. (A) Toll 305-cGFP expression in a stage 16 wild-type (WT) embryo. (B and C) Toll 305-cGFP expression in two different Tlr3/Tlr4 embryos at stage 16, developed at a nonpermissive temperature. (D) D-MEF2 expression in a stage 15 wild-type embryo. (E and F) D-MEF2 expression in two different Tlr3/Tlr4 embryos at stage 15, developed at a nonpermissive temperature. Open arrowheads emphasize cellular gaps in the dorsal vessel, while filled arrowheads or lines highlight the abnormal positioning of cardioblasts in Toll mutant embryos.
DISCUSSION
Based on our interest in characterizing new genes required for dorsal vessel formation, we studied the expression, regulation, and requirement of the Toll gene in cardiac cells. As Toll encodes a transmembrane protein with leucine-rich repeats in its extracellular domain, a prediction was made that Toll could function as a homophilic cell adhesion molecule, in addition to its well-characterized role as a signal-transducing receptor (24). In support of this hypothesis, induced expression of the protein in the nonadhesive Schneider 2 cell line caused cellular aggregation, with Toll accumulating at sites of cell-cell interaction (27). Such a localization property is characteristic of cellular adhesion molecules. Our protein analyses indicate that Toll is expressed in a unique pattern during heart development, that being on lateral surfaces of all cardioblasts as they align and migrate to the dorsal midline (Fig. 1 and 3). Given this highly specialized localization, and the aforementioned structural and functional features of the protein, it is likely that Toll contributes prominently to the molecular environment that aligns and stabilizes cardioblasts on their path toward assembly within the dorsal vessel.
The observation of structurally defective dorsal vessels within Toll mutant embryos (Fig. 7) is consistent with the pattern of Toll expression in cardioblasts. D-MEF2 serves as a marker for all cardioblasts, from their early appearance through their organization within the mature organ. Based on D-MEF2 staining, it appears appropriate numbers of cardioblasts are specified in mutant embryos, but deviations are observed from the normal process of cardioblast alignment and synchronous migration as two contiguous rows of 52 cells. Several other markers for the formed dorsal vessel identified random gaps in the linear organ due to missing and/or abnormally located cardioblasts. Such cardiac phenotypes are reminiscent of those presented by faint sausage (fas) mutant embryos, as mutations of the immunoglobulin-like cell adhesion molecule also lead to cardioblast alignment problems (22). Whether Toll and Fas work in combination for the proper alignment and migration of these cells remains to be investigated. Additionally, while structural and phenotypic properties are consistent with its role as a cardioblast adhesion molecule, we cannot rule out a function for Toll in mediating signaling events between neighboring cardiac cells. So far, no indicators exist for the latter possibility, as we have been unable to demonstrate expression of potential Toll transcriptional effectors (Dorsal and Dif) in cells of the dorsal vessel. Either way, these molecular and genetic findings identify Toll as a vital player in dorsal vessel formation.
The regulation of Toll expression in cardioblasts was pursued due to an interest in further defining the transcriptional network controlling heart development in Drosophila. Our studies demonstrated Toll heart expression is controlled by a 305-bp DNA located 8.0 kb upstream of the transcription start site. This regulatory module contains multiple binding sites for Doc T-box proteins and a single recognition site for the Tin homeodomain protein (Fig. 5). The evidence is strong for the transcriptional enhancer being regulated by both of these cardiogenic factors. Doc and Tin are expressed in adjacent but nonoverlapping sets of cardioblasts within segments of the dorsal vessel; together, they make up the complete population of inner cardiac cells. A deletion of the distal part of the Toll 305-bp enhancer that removes the strong Doc-A footprint sequence, which likely binds multiple Doc molecules through T-box domain recognition of GTG motifs (12), eliminates enhancer function in Svp/Doc cells while maintaining activity in Tin cells (Fig. 4 and 5). Systematically adding back T-box core binding elements to partially, then fully, reestablish the Doc-A binding site restores enhancer function in the Svp/Doc population.
As for Tin, mutation of its recognition element in the Toll 305 DNA leads to decreased and variable enhancer activity in both Tin and Svp/Doc cardioblasts (Fig. 4B). This result suggests that Tin is required not only for the activation of Toll expression in the four cardioblasts per hemisegment that are Tin positive after stage 12 but also for its initiation in all six cardioblasts in each hemisegment during early stage 12. The residual activity of the mutated Toll 305 DNA may reflect some degree of Tin regulation through cryptic, low-affinity binding sites present in the enhancer. Indeed, perusal of the Toll sequence identifies three candidate Tin elements that match the binding consensus at six of seven nucleotide pairs, and other Tin-regulated enhancers of genes such as D-mef2 (18), tin (53), β3-tubulin (32), and pnr (19) also employ more than one Tin binding site.
In contrast to the Toll 305 enhancer element, mutation of the exact Tin site in the Toll 258 DNA completely silenced the enhancer in the normally Tin-active cells. This result strongly implies that Tin, and at least one other factor working through the distal 47 bp of DNA, are required for activating the Toll gene. Candidates for such factors are the Doc T-box proteins, which are initially expressed in all cardioblast progenitors during mid stage 12 (I.R. and M.F., unpublished observations), as well as products of the T-box genes H15 (21) and Midline (Mid), which are expressed in all cardioblasts from mid stage 12 onward (I.R. and M.F., unpublished observations). Mid can bind to the same regions of Toll DNA as Doc (P. Lo and M. Frasch, unpublished observations), although the relevance of such interactions remains to be investigated. A combinatorial requirement for T-box proteins and Tin during the initiation and/or maintenance of Toll expression is further supported by the observation that derivatives of the enhancer containing only the Doc-A sequences fail to show activity in Svp/Doc cells (J.W. and R.A.S., unpublished observations). Together, these molecular data point to a mechanism wherein T-box proteins, in combination with Tin, initially activate the Toll gene in all cardioblast progenitors. After stage 12, Doc and Tin (perhaps in cooperation with H15 and/or Mid) activate Toll in two complementary subsets of cardioblasts of the dorsal vessel.
Unfortunately, a genetic requirement for these two factors in the regulation of the Toll enhancer cannot be proven at this time since Doc (I.R. and M.F., unpublished observations) and tin (3, 5) mutant embryos fail to produce cardioblasts. Such an analysis could be attempted with the generation of specialized Doc or tin genetic backgrounds that allow for cardioblast specification early on, while lacking protein functions in later stages of dorsal vessel formation. However, forced-expression studies have demonstrated that individual expression of Tin or Doc2 leads to expanded enhancer activity, while simultaneous expression of the cardiac factors results in a robust activation of Toll transcription (Fig. 6). These findings convincingly support the model of Doc and Tin being positive transcriptional regulators of the Toll dorsal vessel enhancer.
In addition to the demonstration of Doc and Tin as activators of Toll expression in the dorsal vessel, our regulatory analysis has generated important reagents that should facilitate the discovery of novel cardiac-functioning genes of Drosophila. That is, the Toll-cGFP and Toll-nGFP transgenes serve as sensitive markers for assessing distinct aspects of dorsal vessel morphogenesis in living animals. In stage 16 to 17 embryos and thereafter, Toll-cGFP expression can be used to monitor the formation and function of the three pairs of valvelike ostia within the heart region of the dorsal vessel (Fig. 3B). Likewise, Toll-nGFP can be used to determine the exact number and diversification status of cardioblasts, as larger nuclei are present within Tin-determined cells while smaller nuclei are found in Svp/Doc-determined cells (40; Fig. 3C). Such sensitive and easy-to-use reagents will be valuable in genomewide screens to discover new genes involved in Drosophila heart development.
Acknowledgments
We are grateful to H. Nguyen and S. Wasserman for providing D-MEF2 and Toll antibodies. We also thank H. Huang for technical advice and L. McCord for assistance with figures.
This research was supported by grants to R.A.S. from the Muscular Dystrophy Association and the National Institutes of Health (HL59151) and a grant to M.F. from the National Institutes of Health (HD30832). K.G. was supported in part by a Scientist Development Grant Award from the American Heart Association. I.R. was an American Heart Association/Harriet and Robert Heilbrunn Research Fellow in Cardiovascular Genetic Research.
REFERENCES
- 1.Alvarez, A. D., W. Shi, B. A. Wilson, and J. B. Skeath. 2003. pannier and pointedP2 act sequentially to regulate Drosophila heart development. Development 130:3015-3026. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, K. V., G. Jurgens, and C. Nusslein-Volhard. 1985. Establishment of dorsal-ventral polarity in the Drosophila embryo: Genetic studies on the role of the Toll gene product. Cell 42:779-789. [DOI] [PubMed] [Google Scholar]
- 3.Azpiazu, N., and M. Frasch. 1993. tinman and bagpipe: two homeo box genes that determine cell fates in the dorsal mesoderm of Drosophila. Genes Dev. 7:1325-1340. [DOI] [PubMed] [Google Scholar]
- 4.Barolo, S., L. A. Carver, and J. W. Posakony. 2000. GFP and β-Galactosidase transformation vectors for promoter/enhancer analysis in Drosophila. BioTechniques 29:726-732. [DOI] [PubMed] [Google Scholar]
- 5.Bodmer, R. 1993. The gene tinman is required for specification of the heart and visceral muscles in Drosophila. Development 118:719-729. [DOI] [PubMed] [Google Scholar]
- 6.Bodmer, R., and M. Frasch. 1999. Genetic determination of Drosophila heart development, p. 65-90. In R. P. Harvey and N. Rosenthal (ed.), Heart development. Academic Press, San Diego, Calif.
- 7.Bour, B. A., M. A. O'Brien, W. L. Lockwood, E. S. Goldstein, R. Bodmer, P. H. Taghert, S. M. Abmayr, and H. T. Nguyen. 1995. Drosophila MEF2, a transcription factor that is essential for myogenesis. Genes Dev. 9:730-741. [DOI] [PubMed] [Google Scholar]
- 8.Brand, T. 2003. Heart development: molecular insights into cardiac specification and early morphogenesis. Dev. Biol. 258:1-19. [DOI] [PubMed] [Google Scholar]
- 9.Brennan, C. A., and K. V. Anderson. 2004. Drosophila: The genetics of innate immune recognition and response. Annu. Rev. Immunol. 22:457-483. [DOI] [PubMed] [Google Scholar]
- 10.Cai, C. L., X. Liang, Y. Shi, P. H. Chu, S. L. Pfaff, J. Chen, and S. Evans. 2003. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev. Cell 5:877-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chartier, A., S. Zaffran, M. Astier, M. Semeriva, and D. Gratecos. 2002. Pericardin, a Drosophila type IV collagen-like protein is involved in the morphogenesis and maintenance of the heart epithelium during dorsal ectoderm closure. Development 129:3241-3253. [DOI] [PubMed] [Google Scholar]
- 12.Conlon, F. L., L. Fairclough, B. M. J. Price, E. S. Casey, and J. C. Smith. 2001. Determinants of T box protein specificity. Development 128:3749-3758. [DOI] [PubMed] [Google Scholar]
- 13.Cripps, R. M., and E. N. Olson. 2002. Control of cardiac development by an evolutionarily conserved transcriptional network. Dev. Biol. 246:14-28. [DOI] [PubMed] [Google Scholar]
- 14.Frasch, M. 1995. Induction of visceral and cardiac mesoderm by ectodermal Dpp in the early Drosophila embryo. Nature 374:464-467. [DOI] [PubMed] [Google Scholar]
- 15.Fremion, F., M. Astier, S. Zaffran, A. Guillen, V. Homburger, and M. Semeriva. 1999. The heterotrimeric protein Go is required for the formation of heart epithelium in Drosophila. J. Cell Biol. 145:1063-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gajewski, K., C. Y. Choi, Y. Kim, and R. A. Schulz. 2000. Genetically distinct cardial cells within the Drosophila heart. Genesis 28:36-43. [DOI] [PubMed] [Google Scholar]
- 17.Gajewski, K., N. Fossett, J. D. Molkentin, and R. A. Schulz. 1999. The zinc finger proteins Pannier and GATA4 function as cardiogenic factors in Drosophila. Development 126:5679-5688. [DOI] [PubMed] [Google Scholar]
- 18.Gajewski, K., Y. Kim, Y. M. Lee, E. N. Olson, and R. A. Schulz. 1997. D-mef2 is a target for Tinman activation during Drosophila heart development. EMBO J. 16:515-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gajewski, K., Q. Zhang, C. Y. Choi, N. Fossett, A. Dang, Y. H. Kim, Y. Kim, and R. A. Schulz. 2001. Pannier is a transcriptional target and partner of Tinman during Drosophila cardiogenesis. Dev. Biol. 233:425-436. [DOI] [PubMed] [Google Scholar]
- 20.Gerttula, S., Y. Jin, and K. V. Anderson. 1988. Zygotic expression and activity of the Drosophila Toll gene, a gene required maternally for embryonic dorsal-ventral pattern formation. Genetics 119:123-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffin, K. J. P., J. Stoller, M. Gibson, S. Chen, D. Yelon, D. Y. R. Stainier, and D. Kimmelman. 2000. A conserved role for H15-related T-box transcription factors in zebrafish and Drosophila heart formation. Dev. Biol. 218:235-247. [DOI] [PubMed] [Google Scholar]
- 22.Haag, T. A., N. P. Haag, A. C. Lekven, and V. Hartenstein. 1999. The role of cell adhesion molecules in Drosophila heart morphogenesis: faint sausage, shotgun/DE-cadherin, and laminin A are required for discrete stages in heart development. Dev. Biol. 208:56-69. [DOI] [PubMed] [Google Scholar]
- 23.Halfon, M. S., C. Hashimoto, and H. Keshishian. 1995. The Drosophila Toll gene functions zygotically and is necessary for proper motorneuron and muscle development. Dev. Biol. 169:151-167. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto, C., S. Gerttula, and K. V. Anderson. 1991. Plasma membrane localization of the Toll protein in the syncytial Drosophila embryo: importance of transmembrane signaling for dorsal-ventral pattern formation. Development 111:1021-1028. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto, C., K. L. Hudson, and K. V. Anderson. 1988. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell 52:269-279. [DOI] [PubMed] [Google Scholar]
- 26.Jagla, K., M. Frasch, T. Jagla, G. Dretzen, F. Bellard, and M. Bellard. 1997. ladybird, a new component of the cardiogenic pathway in Drosophila required for diversification of heart precursors. Development 124:3471-3479. [DOI] [PubMed] [Google Scholar]
- 27.Keith, F. J., and N. J. Gay. 1990. The Drosophila membrane receptor Toll can function to promote cellular adhesion. EMBO J. 9:4299-4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly, R. G., and M. E. Buckingham. 2002. The anterior heart-forming field: voyage to the arterial pole of the heart. Trends Genet. 18:210-216. [DOI] [PubMed] [Google Scholar]
- 29.Klinedinst, S. L., and R. Bodmer. 2003. Gata factor Pannier is required to establish competence for heart progenitor formation. Development 130:3027-3038. [DOI] [PubMed] [Google Scholar]
- 30.Knirr, S., N. Azpiazu, and M. Frasch. 1999. The role of the NK-homeobox gene slouch (S59) in somatic muscle patterning. Development 126:4525-4535. [DOI] [PubMed] [Google Scholar]
- 31.Kolsch, V., and A. Paululat. 2002. The highly conserved cardiogenic bHLH factor Hand is specifically expressed in circular visceral muscle progenitor cells and in all cell types of the dorsal vessel during Drosophila embryogenesis. Dev. Genes Evol. 212:473-485. [DOI] [PubMed] [Google Scholar]
- 32.Kremser, T., K. Gajewski, R. A. Schulz, and R. Renkawitz-Pohl. 1999. Tinman regulates the transcription of the β3-tubulin gene (βTub60D) in the dorsal vessel of Drosophila. Dev. Biol. 216:327-339. [DOI] [PubMed] [Google Scholar]
- 33.Lee, J. K., R. S. Coyne, R. R. Dubreuil, L. S. Goldstein, and D. Branton. 1993. Cell shape and interaction defects in α-spectrin mutants of Drosophila melanogaster. J. Cell Biol. 123:1797-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemaitre, B., E. Nicolas, L. Michaut, J. M. Reichhart, and J. A. Hoffmann. 1996. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86:973-983. [DOI] [PubMed] [Google Scholar]
- 35.Lilly, B., B. Zhao, G. Ranganayakulu, B. M. Paterson, R. A. Schulz, and E. N. Olson. 1995. Requirement of MADS domain transcription factor D-MEF2 for muscle formation in Drosophila. Science 267:688-693. [DOI] [PubMed] [Google Scholar]
- 36.Lo, P. C. H., and M. Frasch. 2001. A role for the COUP-TF-related gene seven-up in the diversification of cardioblast identities in the dorsal vessel of Drosophila. Mech. Dev. 104:49-60. [DOI] [PubMed] [Google Scholar]
- 37.Lo, P. C. H., and M. Frasch. 2003. Establishing A-P polarity in the embryonic heart tube: a conserved function of Hox genes in Drosophila and vertebrates? Trends Cardiovasc. Med. 13:182-187. [DOI] [PubMed] [Google Scholar]
- 38.Lo, P. C. H., J. B. Skeath, K. Gajewski, R. A. Schulz, and M. Frasch. 2002. Homeotic genes autonomously specify the anteroposterior subdivision of the Drosophila dorsal vessel into aorta and heart. Dev. Biol. 251:307-319. [DOI] [PubMed] [Google Scholar]
- 39.Lovato, T. L., T. P. Nguyen, M. R. Molina, and R. M. Cripps. 2002. The Hox gene abdominal-A specifies heart cell fate in the Drosophila dorsal vessel. Development 129:5019-5027. [DOI] [PubMed] [Google Scholar]
- 40.Molina, M. R., and R. M. Cripps. 2001. Ostia, the inflow tracts of the Drosophila heart, develop from a genetically distinct subset of cardial cells. Mech. Dev. 109:51-59. [DOI] [PubMed] [Google Scholar]
- 41.Moore, A. W., S. Barbel, L. Y. Jan, and Y. N. Jan. 2000. A genomewide survey of basic helix-loop-helix factors in Drosophila. Proc. Natl. Acad. Sci. USA 97:10436-10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morisato, D., and K. V. Anderson. 1995. Signaling pathways that establish the dorsal-ventral pattern of the Drosophila embryo. Annu. Rev. Genet. 29:371-399. [DOI] [PubMed] [Google Scholar]
- 43.Ponzielli, R., M. Astier, A. Chartier, A. Gallet, P. Therond, and M. Semeriva. 2002. Heart tube patterning in Drosophila requires integration of axial and segmental information provided by the Bithorax Complex genes and hedgehog signaling. Development 129:4509-4521. [DOI] [PubMed] [Google Scholar]
- 44.Ranganayakulu, G., B. Zhao, A. Dokidis, J. D. Molkentin, E. N. Olson, and R. A. Schulz. 1995. A series of mutations in the D-MEF2 transcription factor reveal multiple functions in larval and adult myogenesis in Drosophila. Dev. Biol. 171:169-181. [DOI] [PubMed] [Google Scholar]
- 45.Reim, I., H. H. Lee, and M. Frasch. 2003. The T-box-encoding Dorsocross genes function in amnioserosa development and the patterning of the dorsolateral germ band downstream of Dpp. Development 130:3187-3204. [DOI] [PubMed] [Google Scholar]
- 46.Rugendorff, A., A. Younossi-Hartenstein, and V. Hartenstein. 1994. Embryonic origin and differentiation of the Drosophila heart. Roux's Arch. Dev. Biol. 203:266-280. [DOI] [PubMed] [Google Scholar]
- 47.Sorrentino, R. P., K. Gajewski, and R. A. Schulz. 2005. GATA factors in Drosophila heart and blood cell development. Semin. Cell Dev. Biol. 16:107-116. [DOI] [PubMed] [Google Scholar]
- 48.Trinh, L. A., and D. Y. R. Stainier. 2004. Fibronectin regulates epithelial organization during myocardial migration in zebrafish. Dev. Cell 6:371-382. [DOI] [PubMed] [Google Scholar]
- 49.Volk, T. 1992. A new member of the spectrin superfamily may participate in the formation of embryonic muscle attachments in Drosophila. Development 116:721-730. [DOI] [PubMed] [Google Scholar]
- 50.Ward, E. J., and J. B. Skeath. 2000. Characterization of a novel subset of cardiac cells and their progenitors in the Drosophila embryo. Development 127:4959-4969. [DOI] [PubMed] [Google Scholar]
- 51.Wharton, K. A., Jr., and S. T. Crews. 1993. CNS midline enhancers of the Drosophila slit and Toll genes. Mech. Dev. 40:141-154. [DOI] [PubMed] [Google Scholar]
- 52.Wu, X., K. Golden, and R. Bodmer. 1995. Heart development in Drosophila requires the segment polarity gene wingless. Dev. Biol. 169:619-628. [DOI] [PubMed] [Google Scholar]
- 53.Xu, X., Z. Yin, J. B. Hudson, E. L. Ferguson, and M. Frasch. 1998. Smad proteins act in combination with synergistic and antagonistic regulators to target Dpp responses to the Drosophila mesoderm. Genes Dev. 12:2354-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yarnitzky, T., and T. Volk. 1995. Laminin is required for heart, somatic muscles, and gut development in the Drosophila embryo. Dev. Biol. 169:609-618. [DOI] [PubMed] [Google Scholar]
- 55.Yin, Z., and M. Frasch. 1998. Regulation and function of tinman during dorsal mesoderm induction and heart specification in Drosophila. Dev. Genet. 22:187-200. [DOI] [PubMed] [Google Scholar]
- 56.Yin, Z., X. L. Xu, and M. Frasch. 1997. Regulation of the Twist target gene tinman by modular cis-regulatory elements during early mesoderm development. Development 124:4971-4982. [DOI] [PubMed] [Google Scholar]
- 57.Zaffran, S., and M. Frasch. 2002. Early signals in cardiac development. Circ. Res. 91:457-469. [DOI] [PubMed] [Google Scholar]