Abstract
RNA interference (RNAi) is a naturally occurring posttranscriptional gene-silencing mechanism that has been adapted as a genetic tool for loss-of-function studies of a variety of organisms. It is more widely applicable than classical gene targeting and allows for the simultaneous inactivation of several homologous genes with a single transgene. Recently, RNAi has been used for conditional and conventional gene inactivation in mice. Unlike gene targeting, RNAi is a dynamic process, and its efficiency may vary both between cell types and throughout development. Here we demonstrate that RNAi can be used to target three separately encoded isoforms of the bcl-2 family gene bfl-1/A1 in a conditional manner in mice. The extent of gene inactivation varies between different cell types and is least efficient in mature lymphocytes. Our data suggest that RNAi is affected by factors beyond small interfering RNA-mRNA stoichiometry.
RNA interference (RNAi) is a highly conserved mechanism involved in posttranscriptional gene regulation which was first observed in Caenorhabditis elegans as a response to double-stranded (ds) RNA (6). During the initial steps of RNAi, the RNase III-like enzyme Dicer processes long ds RNAs and complex hairpin RNAs into small interfering RNAs (siRNAs) (2, 16, 18). siRNAs are 21- to 23-bp RNA duplexes with characteristic dinucleotide overhangs (46). These duplexes are unwound by an RNA helicase, and single-stranded siRNAs are then incorporated into the multicomponent RNA-induced silencing complex (RISC) (37). RISC functions as an siRNA-induced endonuclease and mediates the cleavage of target RNA that is perfectly complementary to the siRNA (10, 29).
Recent reports have demonstrated the successful application of RNAi as a tool for posttranscriptional gene silencing in mammalian cells (reviewed in reference 38). Transgene-driven and hence stable RNAi is generally achieved using RNA polymerase III (PolIII) promoters, which mediate transcription of short hairpin RNA (shRNA)-expressing genes. shRNAs are then processed into siRNAs by Dicer (4, 32, 40). While RNAi is used extensively to manipulate gene expression in cell culture, little is known about its efficiency in mice (reviewed in reference 25). Several reports have shown that transgenic RNAi can be used to inactivate endogenous genes in mice; however, these analyses were limited to one particular cell type (23, 36) or to early embryonic stages (19, 44). Since RNAi is a dynamic process that depends on multiple components (11, 22, 24), its efficiency is likely to vary between different cell types or developmental stages.
Here we used transgene-driven RNAi to interfere with the expression of the antiapoptotic bcl-2 family member bfl-1/A1 (A1) in adult mice and analyzed RNAi efficiency and its phenotypic consequences in several hematopoietic cell types and differentiation stages. A1 consists of three highly homologous, separately encoded isoforms, A1a, A1b, and A1d (13, 21). A1b and A1d are mainly expressed in lymphocytes (43, 45), whereas A1a is the predominant isoform in granulocytes (9). Expression profiling and overexpression studies implicate A1 in lymphocyte maintenance and activation (8, 43, 45). Recently, A1 has also been suggested to play a role in the positive selection of developing thymocytes (7). Inactivation of A1a in mice by gene targeting had only a minor impact on hematopoiesis and did not affect lymphocyte survival (9). While the extensive homology and potential redundancy between the A1 isoforms impeded classical gene inactivation, it allowed us to simultaneously inactivate all three isoforms using a single shRNA (shA1). Given the early lethality observed in mice that lack the bcl-2 family genes bcl-X (26) or mcl-1 (26, 35), it was desirable to achieve inactivation of A1 by RNAi in a conditional manner. We therefore designed a modified PolIII promoter that allows shRNA transcription only after Cre-mediated deletion of a loxP-flanked PolIII-specific transcriptional STOP cassette.
MATERIALS AND METHODS
Design of shRNAs.
Three siRNAs were designed for each target gene and tested in NIH 3T3 fibroblasts. Double-stranded synthetic siRNAs were transfected using Oligofectamine (Invitrogen) according to the manufacturer's instructions. Alternatively, shRNA expression vectors were transduced by retroviral gene transfer. The following is a list of effective siRNA target sequences for the indicated gene products: for A1 (siA1), GGGAAATGCTCTTTCTCCTCA; for Itch (si-itch 3), GACCTGAGAAGACGTTTGT; and for Nedd-4 (si-nedd4 2), AGAATACGCTTACTTCAGT.
Cloning and targeted insertion of the U6-STOP-shA1 cassette.
The modified loxP-containing U6 promoter was PCR amplified from the U6 promoter containing plasmid pU6 (a kind gift from Yang Shi; see reference 40) using primers XbaI-U6 (GACTCTAGATCCGACGCCGCCATCTCTAG) and U6lox-T-RI (TGCGAATTCAAAAATCGCAAAAACGTAATAACTTCGTATAAGTATGCTATACGAAGTTATAGTCTCAAAACACACAATTACTTAC). The 3′ primer replaces the sequence 3′ of the TATA box with a loxP site followed by two T stretches. The PolIII STOP sequence was PCR amplified from C57BL/6 genomic DNA using primers U6termRI (TGTGAATTCGTTCCTCAGAGGAACTGA) and U6term1B (TGTGGATCCCCCGGGCGTGGCTTGGTGGTACACCTC). A third fragment consisting of a mutant loxP site fused to shA1 was generated by oligonucleotide synthesis of two complementary oligomers, lox-shA1-s and lox-shA1-as (see Fig. 1A for sequence information). The three subfragments together form the U6-STOP-shA1 cassette, which was inserted into a modified pMP-8SKB targeting vector (3). The U6 promoter has the same transcriptional orientation as the hypoxanthine phosphoribosyltransferase (HPRT) gene. HM-1 embryonic stem (ES) cells (41) were transfected with the linearized targeting construct as described previously (33). Targeted ES cell clones were identified using hypoxanthine-aminopterin-thymidine (Sigma) selection. The U6-STOP-shA1 allele was identified by Southern blotting or by PCR using primers HPRT-SAH (TTCCTAATAACCCAGCCTTTG) and HPRT-hpro (GTGATGGCAGGAGATTTGTAA).
FIG. 1.
Targeted insertion of U6-STOP-shA1 cassette into the mouse HPRT locus. (A) Scheme of conditional shRNA expression construct U6-STOP-shA1 before and after Cre-mediated recombination. Since transcriptional initiation at +1, 26 bp downstream of the TATA box, is crucial for the precise generation of short RNAs by PolIII, and STOP deletion will leave one loxP site, the shA1-proximal loxP site was modified as shown. The first 3 bp of the loxP site (ATA) are part of the U6 TATA box (shown in capital letters), and the last 5 bp of the loxP site were replaced by the first 5 bp of the shRNA coding sequence (in grey letters), resulting in a mutant loxP site. U6lox stands for a modified U6 promoter that contains a loxP site downstream of the TATA box, STOP symbolizes a PolIII transcription termination cassette, and triangles represent loxP sites flanking the STOP cassette. (B) Targeting strategy for U6-STOP-shA1 insertion into the HPRT locus. Partial restriction endonuclease maps of the HPRT wild-type locus, the mutant HM1 locus lacking HPRT exons 1 and 2, and the targeted U6-STOP-shA1 locus are shown. Roman numerals indicate exons; hI, human exon 1. Dashed arrows depict fragment sizes as revealed with probe RSA. A Southern blot analysis to verify homologous recombination is shown. Genomic DNA from two targeted clones and HM1 ES cells was digested with StuI and hybridized to probe RSA. Expected fragments before and after homologous recombination are indicated. B, BamHI; S, StuI.
Generation and transfection of (A1)IRES-GFP expression vectors.
The coding sequence of the mouse A1d gene was PCR amplified from splenic cDNA using primers A1d-X (TGCTCGAGATGTCTGAGTACGAGTTCATGCATATC) and A1d-B (CTGGATCCTTACTTGAGGAGAAAGAGCATTTC). The PCR fragment was subcloned into pIRES2-EGFP (Clontech) to generate the A1-IRES-GFP fusion construct. A second, mutated A1 expression construct (mutA1-IRES-GFP) was generated by PCR mutagenesis using primers A1d-X andmutA1d-B (CTGGATCCTTATTTCAGCAGGAACAGCATCTCCCATATCTG). A1-IRES-GFP, mutA1-IRES-GFP, and IRES-GFP constructs [collectively referred to as (A1)IRES-GFP constructs] were subcloned into the neoR selectable marker containing expression vector pCXN2 (28). Twenty micrograms of each expression vector was transfected into U6-STOP-shA1 ES cells. Stable integrants were selected with 200 μg/ml G418 starting 2 days after transfection. G418-resistant ES cell colonies were analyzed for green fluorescent protein (GFP) expression in order to confirm expression of the reporter transgene.
Northern blotting.
To analyze mRNA expression, 20 μg of total RNA was run on a 1.2% agarose gel. Gels were transferred by Northern blotting and subsequently hybridized to radiolabeled DNA probes. The GFP probe was excised from pIRES2-EGFP (Clontech). The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe was excised from pTRI-GAPDH-mouse (Ambion).
To determine siRNA expression, 5- to 20-μg portions of total RNA were separated on a 10% polyacrylamide-8 M urea gel. Ethidium bromide (EtBr) staining was performed to confirm equal loading. Gels were blotted using wet transfer at 230 mA for 6 to 16 h. Radiolabeled oligonucleotide DNA complementary to siA1 or si-itch 3 was used as a probe.
RT-PCR analysis and RNase protection assay.
Total RNA was subjected to quantitative RT-PCR (QRT-PCR) analysis using SYBR green PCR core reagents (Applied Biosystems) as described by the manufacturer and the iCycler iQ real-time PCR detection system (Bio-Rad). A1 was amplified using primers A1-s (CATTAACTGGGGAAGGATTGTGAC) and A1-as (GCAGAAAAGTCAGCCAGCCAGATT), and ago2 was amplified using ago2-s (AGTTCTTGCTTCTCCTGCTCCG) and ago2-as (GATGCGATCTTTGCCTTCTCCC). L32 rRNA served as an internal reference and was amplified using the primer pair L32B-s (CAAGAGGGAGAGCAAGCCTA) and L32B-as (CGTCTCAGGCCTTCAGTGAG). PCR conditions were as follows: 95°C for 4 min 30 s, 40 cycles of 95°C for 30 s, 61°C for 30 s, and 72°C for 30 s. PCR was followed by melt curve data collection. Samples were analyzed in quadruplicate. Classical RT-PCR was performed using the following primer pairs: ago1-s (TGTTTCAGGCACCCCGCCGGCC) and ago1-as (AATTCTATCCTTCCCCTCCCC), ago2-s and ago2-as (same sequences as immediately above), ago3-s (CCCAGCCCCTATTCATCGTGCCC) and ago3-as (TCGATCTTTTCCACCTTCCCC), and ago4-s (GAGGCCGGAAGAGCCGTGACAGAG) and ago4-as (AAGGCCAGGACGCCGAGGTGG).
An RNase protection assay was performed using an BD RiboQuant RNase protection assay kit (BD Pharmingen) and an mAPO-2 multiprobe template set (BD Pharmingen) following the manufacturer's protocol. A 2.5-μg portion of total RNA was used for each sample.
Flow cytometry and cell sorting.
Single cell suspensions were stained with the respective monoclonal antibodies (mAbs) conjugated to fluorescein isothiocyanate, phycoerythrin, or CyChrome (BD Pharmingen or eBioscience). Stained cells were acquired on FACSCalibur, data were analyzed with CellQuest software, and cell sorting was performed on FACSVantage (all from Becton Dickinson, San Jose, CA). Dead cells were excluded based on their incorporation of Topro-3. Alternatively, splenic B and T cells were sorted by magnetic cell sorting (MACS; Miltenyi Biotec) in accordance with the manufacturer’s instructions.
Generation of bone marrow-derived macrophages (BMDM).
A single cell suspension from one femur was plated on a 10-cm tissue culture dish and cultured in RPMI medium, 10% fetal calf serum (FCS), 2 mM sodium pyruvate, and 2 mM l-glutamine supplemented with 10 ng/ml mouse recombinant granulocyte-macrophage colony-stimulating factor (Preprotech). Plates were washed several times to remove nonadhering cells, and adhering cells were harvested after 1 week with a cell scraper. Macrophage identity was confirmed by fluorescence-activated cell sorter (FACS) analysis for surface expression of CD11b and F4/80. Cells were >90% pure.
CD4 T-cell culture.
Splenic and lymph node T cells were isolated via mouse CD4 cell selection with Dynabeads (Dynal). T cells were stimulated with 0.25 μg/ml anti-CD3 (145-2C11; Pharmingen) and 2.5 μg/ml anti-CD28 (37.51; Pharmingen) under Th1 conditions using 10 μg/ml anti-interleukin-4 (anti-IL-4) (2B11) and 10 ng/ml recombinant IL-12 for 48 h in Dulbecco's modified Eagle medium (DMEM) and 10% FCS. Cells were expanded in DMEM and 10% FCS supplemented with 10 U/ml IL-2 for 5 days, and restimulation was performed after 7 days of culturing as described above with the inclusion of anti-gamma interferon (XMG1.2) at 1 μg/ml. NIH 3T3 cells were grown in DMEM supplemented with 10% FCS.
Transduction of ES cells with Cre-expressing adenovirus.
ES cells were trypsinized and resuspended at 105 cells/ml in ES cell medium. Cells (5 × 105) were plated on each well of an embryonic feeder cell-containing six-well plate. Infectious units (107 to 108) of Cre-expressing adenovirus (1) were added to each well, and the cells were incubated for 16 h at 37°C.
Retroviral transduction.
To obtain retroviral supernatants, Phoenix 293T cells that stably express Env and Gag-Pol genes (a kind gift from Garry P. Nolan of Stanford University) were CaPO4 transfected using 10 μg of pSuperRetro-H1 shRNA expression vectors (a kind gift from Reuven Agami of The Netherlands Cancer Institute). Phoenix supernatants were harvested 2 and 3 days after transfection and used directly for infection of NIH 3T3 and T cells with 5 and 8 μg/ml polybrene, respectively. CD4 T cells were infected with virus 48 h and 72 h after isolation and activation by spinning T cells with viral supernatants at 900 × g for 1 h. Infections were repeated once, and the transduced cells were selected with 5 μg/ml puromycin for 3 days.
Western blots.
Protein extracts were prepared by lysis of 1 × 106 T cells or 50,000 NIH 3T3 cells in 10 μl radioimmunoprecipitation assay buffer (20 mM Tris [pH 7.5], 250 mM NaCl, 10 mM MgCl2, 1% NP-40, 0.1% sodium dodecyl sulfate, and 0.5% sodium desoxycholate supplemented with 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 25 μg/ml aprotinin, 25 μg/ml leupeptin, 10 mM NaF, 8 mM β-glycerophosphate, and 0.1 mM sodium orthovanadate). Each lane of a 9% sodium dodecyl sulfate-polyacrylamide gel was loaded with 20 μl protein extract, the separated proteins were transferred onto nitrocellulose membrane (Protran; Schleicher & Schuell), and membrane-blocking and antibody incubations were performed using 5% nonfat milk in Tris-buffered saline (10 mM Tris-HCl, pH 8.0, 150 mM NaCl), anti-Itch antibodies (I84520; BD Transduction Labs) at a ratio of 1:1,000, and anti-Nedd4 antibodies (07-049; Upstate Biotechnologies) at a ratio of 1:20,000. Immunoblots were washed in TBST (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.05% Tween 20), and antibody binding was visualized using a Western Lightning Chemiluminescence Reagent Plus kit (Perkin-Elmer) according to instructions from the manufacturer.
RESULTS
Generation and targeting of a U6 promoter-based construct for Cre-induced shRNA expression.
In order to generate a system that permits the inducible expression of shRNAs and, consequently, conditional gene silencing through RNAi, it is imperative to control PolIII-mediated transcription. It has been demonstrated previously that transcription via PolII can be regulated by Cre-mediated recombination via a loxP-flanked transcription STOP cassette (20). To regulate PolIII-mediated transcription in a similar manner, we designed a PolIII-specific transcription termination cassette. Termination of PolIII-mediated transcription generally occurs when PolIII encounters four to five consecutive thymidines (referred to as a T stretch), with transcription being aborted after the second thymidine. Mutational analysis of PolIII-mediated transcription showed that the untranscribed sequence immediately downstream of the T stretch may also be involved in transcriptional control (5). We therefore generated a U6 STOP sequence that comprises two T stretches followed by 190 bp of downstream genomic DNA. The additional T stretch was inserted to enhance the efficiency of transcriptional termination. Insertion of the loxP-flanked STOP cassette between the U6 promoter and the shRNA gene required a 5-bp mutation at the 3′ end of the downstream loxP site in order to ensure proper shRNA transcription upon Cre-mediated deletion of the STOP sequence (see Fig. 1A for details). Based on previous results, this mutation is not expected to dramatically decrease recombination efficiency (30). Hence, this strategy should be applicable for any shRNA sequence. In the present study, the STOP cassette drives expression of the A1-specific shRNA, shA1, and is thus referred to as U6-STOP-shA1.
To generate a mouse strain that allows ubiquitous induction of shA1-mediated RNAi upon Cre-mediated recombination, the U6-STOP-shA1 cassette was targeted into the X-linked HPRT locus by homologous recombination in ES cells. Integration of the shRNA expression cassette into a defined genetic locus ensures reproducible and predictable siRNA expression and is thus preferable to random integration of shRNA expression cassettes by retro- or lentiviral gene transference. The targeting strategy is shown in Fig. 1B. The integrity of hypoxanthine-aminopterin-thymidine-resistant colonies was confirmed by Southern blotting using a StuI digest and probe RSA (3) (Fig. 1B). Two independent ES cell clones were injected into C57BL/6 blastocysts to generate U6-STOP-shA1 mice.
Efficient knockdown of A1 transgene in ES cells.
As a first functional test for the U6-STOP-shA1 cassette, we analyzed Cre-mediated induction of shRNA expression and subsequent knockdown of A1 in ES cells. Since endogenous A1 expression is barely detectable in ES cells, an A1-encoding transgene was introduced into targeted ES cells. To be able to detect changes in A1 protein levels by FACS analysis, A1d cDNA was fused to an internal ribosomal entry site (IRES) (14, 15) followed by enhanced GFP (EGFP) cDNA. Expression of this fusion construct yields a bicistronic mRNA encoding A1 and EGFP, such that siA1-mediated mRNA degradation will result in the loss of both A1 and EGFP expression. The sequence specificity of siA1 was confirmed using a second, mutated A1 expression construct (mutA1-IRES-GFP), in which the siA1 target sequence carries six silent point mutations (Fig. 2A). GFP+ clones of both (A1-)IRES-GFP transgenic ES cell lines and a third line that contained only IRES-GFP were transduced with a Cre-expressing adenovirus (1) in order to delete the loxP-flanked STOP cassette and induce shRNA expression. Untransduced cells served as a negative control. Seven days after transduction, ES cells were analyzed for GFP expression by FACS analysis. Only ES cell clones that were exposed to Cre and carried the perfectly complementary A1-IRES-GFP transgene showed downregulation of GFP expression (Fig. 2B). The fact that GFP downregulation occurred in only ∼60% of the cells suggested an incomplete deletion of the STOP cassette. This was confirmed by PCR analysis of genomic DNA isolated from total-cell lysate or from subpopulations that were sorted according to GFP expression levels. Deletion of the STOP cassette was incomplete in the bulk sample and was exclusively detected in GFPlow cells (Fig. 2C). Similar levels of Cre-mediated deletion (Fig. 2C) and concomitant siA1 generation (Fig. 2D and E) were detected in all Cre-treated ES cell lines. Detection of processed 21- to 23-nt siRNA by Northern blotting is generally restricted to RISC-incorporated siRNA molecules, since single-stranded siRNA molecules that are not RISC-protected are rapidly degraded (17, 37). Indeed, we failed to detect the processed siA1 antisense strand (Fig. 2E), indicating that our assay predominantly detects functional, RISC-protected siRNA.
FIG. 2.
Efficient and Cre-dependent A1 knockdown in ES cells. (A) Since endogenous A1 expression is not detectable in ES cells, the indicated (A1)IRES-GFP transgenes were introduced into U6-STOP-shA1 ES cells. mutA1 stands for mutated A1 cDNA. This cDNA contains six mutations in the siA1 target sequence (shown in the lower part of the panel; mutations are shown in grey letters). siA1 targets the 3′ end of the A1 coding sequence. pA, rabbit β-globin poly(A). (B) FACS analysis of GFP expression in the indicated ES cell lines transduced (open histograms) or untransduced (shaded histograms) with adenoviral Cre (Av-Cre). GFP knockdown is detected only in ES cells bearing A1-IRES-GFP with a wild-type shA1 target sequence and depends on Cre expression. (C) PCR analysis to detect Cre-mediated deletion of the STOP cassette. A schematic of the targeted HPRT locus is shown, and half arrows depict primers flanking the inserted U6-STOP-shA1 cassette. The arrow represents the human HPRT promoter, the grey box depicts human exon 1, and the white box depicts mouse exon 2; the map is not drawn to scale. PCR results are shown for transduced and untransduced ES cells transgenic for IRES-GFP (IRES), A1-IRES-GFP (A1), or mutA1-IRES-GFP (mutA1). A1-IRES-GFP transgenic ES cells were sorted according to GFP expression levels. DNA from GFPhigh cells and GFPlow cells was also subjected to PCR. The expected sizes for PCR fragments before (U6-STOP-shA1) and after (U6Δ-shA1) STOP deletion are indicated. The asterisks indicate hybrids between U6-STOP-shA1 and U6Δ-shA1 fragments. (D) Northern blot analysis of siA1 expression in transduced and untransduced ES cells carrying the indicated transgene. Total RNA (20 μg per lane) was loaded. Synthetic ds siRNA of identical sequence was loaded as indicated to estimate siRNA expression levels. An EtBr staining of the gel before Northern blotting served as a loading control. (E) Northern blot analysis of siA1 sense (siA1) and siA1 antisense(siA1-as) strand expression in transduced and untransduced mutA1-IRES-GFP transgenic U6-STOP-shA1 ES cells. Total RNA (20 μg per lane) was loaded. Synthetic ds siRNA served as an internal control. EtBr staining was performed as a loading control. (F) Northern blot analysis of (A1)IRES-GFP mRNA expression levels before and after transduction. Total RNA (20 μg per lane) was loaded. Cre-transduced A1-IRES-GFP transgenic ES cells were sorted according to GFP expression levels, and total RNA from 106 cells was loaded for GFPhigh and GFPlow samples. Targeted ES cells without IRES-GFP transgenes served as a negative control (−). Blots were hybridized to a GFP probe. To account for loading differences, blots were stripped and rehybridized to a GAPDH probe. The efficiency of siA1-mediated A1-IRES-GFP knockdown was determined using a phosphorimager.
To determine the extent of mRNA degradation, (A1)IRES-GFP mRNA levels were analyzed by Northern blotting using a probe specific for GFP (Fig. 2F). The sizes of the mRNA differed depending on the presence or absence of A1 cDNA. GFP mRNA levels were strongly reduced in total-cell lysate. When the cells were sorted according to GFP expression, A1-IRES-GFP mRNA was barely detectable in GFPlow cells, and image quantification showed a >10-fold reduction of mRNA when compared to GFPhigh cells. We did not observe mRNA reduction in untransduced A1-IRES-GFP transgenic ES cells or in IRES-GFP control samples. These data demonstrate that a single copy of the U6-STOP-shA1 cassette mediates efficient, sequence-specific, and re-dependent suppression of A1 in vitro.
Tightly regulated expression of U6-STOP-shA1 in mice.
To be able to address RNAi in a variety of hematopoietic tissues, a U6Δ-shA1 allele was generated through germ line deletion of the STOP cassette. U6Δ-shA1 mice are viable and phenotypically indistinguishable from wild-type (WT) littermates, thus permitting the analysis of A1 knockdown in multiple tissues. We first determined siA1 expression by Northern blot analysis in several lymphocyte subsets Fig. 3A). Based on the siA1 loading control and the fact that lymphocytes express 1 to 2 μg total RNA per 106 cells, we estimate that thymocytes express 500 to 1,000 copies of processed siA per cell. Expression levels in splenic B cells (Fig. 3A) and T cells (data not shown) are approximately threefold lower.
FIG. 3.
Tightly regulated and reproducible siA1 expression in vivo. (A) Northern blot analysis of siA1 expression in U6-STOP-shA1 × CD19-cre mice and U6Δ-shA1 mice. Ten or 5 μg (*) of total RNA from unsorted thymocytes or MACS-enriched, ∼95% pure splenic B cells was loaded per lane. Synthetic ds siRNA served as an internal control. An EtBr staining of the gel before Northern blotting is shown as a loading control. (B) Semiquantitative PCR to determine deletion efficiency in bone marrow (BM) and splenic B cells from U6-STOP-shA1 × CD19-cre mice (bottom panel). CD19+ B cells were MACS-enriched, and genomic DNA was PCR amplified as described in the legend for Fig. 2C. The top panel represents a titration using DNA from U6-STOP-shA1 and U6Δ-shA1 mice mixed at the indicated ratios.
To demonstrate Cre-regulated induction of siA1 in vivo, we bred U6-STOP-shA1 mice with CD19-cre transgenic mice (34), which express Cre exclusively in B lineage cells. siA1 expression was restricted to B cells (Fig. 3A, compare lanes 2, 4, and 5) and could be detected only in the presence of the CD19-cre transgene (see Fig. 6A). Based on a semiquantitative PCR assay, the efficiency of deletion of the STOP cassette in CD19-cre mice was estimated to be 80 to 90% in mature B cells (Fig. 3B). Taking into account that deletion was incomplete, siA1 expression levels are comparable to those observed in U6Δ-shA1 B cells (Fig. 3A; note that half the amount of RNA was loaded in lanes 5 and 6). These results indicate tightly regulated control of PolIII-mediated transcription by the PolIII STOP cassette in vivo. As a consequence of the defined genetic location of a single siA1 expression cassette, siA1 expression levels were reproducible for different animals (Fig. 3A, lanes 4 and 5).
FIG. 6.
Comparison of siRNA-mRNA stoichiometry between mature lympocytes, macrophages, and ES cells. (A) Analysis of siA1 expression and A1 mRNA knockdown in splenic B cells from U6-STOP-shA1 × CD19-cre mice and sorted GFPhigh or GFPlow A1-IRES-GFP transgenic U6-STOP-shA1 ES cells (Fig. 2). A1 mRNA was detected by QRT-PCR as described above. To detect siA1, 20 μg or 10 μg (*) of the respective samples was analyzed by Northern blotting. Synthetic ds siRNA served as an internal control. Each histogram bar corresponds to the sample on the Northern blot shown below it. (B) QRT-PCR analysis of A1 mRNA expression levels in resting, mature BMDΜ from U6Δ-shA1 mice (black) or wild-type controls (grey). (C) Comparison of A1 mRNA levels in wild-type BMDM and splenic B cells (CD19+) by QRT-PCR. (D) Northern blot analysis of siA1 expression levels in BMDM and splenic B cells. Total RNA (10 μg per lane) was loaded. Synthetic ds siRNA served as an internal control. An EtBr staining of the gel before blotting is shown as a loading control.
Efficiency of RNAi varies between cell types and developmental stages.
Having shown functional and RISC-stabilized siA1 expression in lymphocyte subsets, we next analyzed the efficiency of A1 mRNA knockdown and its potential impact on lymphocyte development. To quantify A1 mRNA, we designed a QRT-PCR strategy that detects all three isoforms of A1. A1 mRNA levels were normalized against rRNA L32 (Fig. 4A). U6Δ-shA1 mice reveal efficient RNAi in thymocytes (on average, 16% of A1 wild-type mRNA levels) but significantly less efficient RNAi in splenic T and B cells (33% and 47% of wild-type mRNA levels, respectively). Despite variations in knockdown efficiencies between mice, we observed the same pattern for every animal (Fig. 4B). Importantly, no significant differences were observed in the distribution of lymphocyte subsets between U6Δ-shA1 mice and wild-type littermates (data not shown), suggesting that the U6Δ-shA1 allele does not affect lymphocyte development. To address whether all A1 isoforms are equally affected by siA1-mediated RNAi, we analyzed the distribution of A1 isoforms in splenic B cells and thymocytes from U6Δ-shA1 mice and wild-type littermates by sequencing individual A1 mRNAs (Fig. 4C). Consistent with previous findings, both thymocytes and splenic B cells from wild-type mice expressed exclusively A1b and A1d (9), with A1b being the predominant isoform (45). The distribution of A1 isoforms was unchanged in response to siA1, indicating that all expressed A1 isoforms are degraded with equal efficiency.
FIG. 4.
Cell-type-specific differences in RNAi efficiency. (A) QRT-PCR analysis to determine expression of all A1 isoforms in thymocytes and FACS-sorted splenic B and T cells. Relative A1 expression levels are shown as the ratios of A1 mRNA to L32 mRNA for wild-type (light grey), U6Δ-shA1 (black), and U6-STOP-shA1 × CD19-cre mice (dark grey). (B) Downregulation of A1 mRNA in indicated cell types from indicated mouse strains. Black circles represent U6-STOP-shA1 × CD19-cre mice, and white symbols represent individual U6Δ-shA1 mice. Light grey bars show the average percentages of wild-type A1 mRNA levels. (C) Distribution of A1 isoforms A1a (black, not detected), A1b (grey), and A1d (white) in thymocytes and splenic B cells from U6Δ-shA1 mice or wild-type littermates. A1 cDNA from eachsample was PCR amplified using primers A1-s and A1-as (see Materials and Methods) and subcloned into a TOPO-TA cloning vector (Invitrogen). Individual clones were sequenced to distinguish between A1 isoforms.
In U6-STOP-shA1 × CD19-cre animals, knockdown is restricted to B-lineage cells but is less efficient than in splenic B cells from U6Δ-shA1 mice. This can be partially explained by the fact that 10 to 20% of splenic CD19-cre B cells still carry the STOP cassette and hence do not downregulate A1 (Fig. 3C). In addition, cells that have only recently deleted the STOP cassette may not yet have reached maximal knockdown efficiency. We conclude that siA1-mediated knockdown is inefficient in mature lymphocytes, particularly in B cells, and Fig. 3A shows that this phenomenon is not due to a lack of RISC-protected siRNA.
Cell-type-specific differences in RNAi are not solely attributable to the siRNA-to-mRNA ratio.
The fact that thymocytes undergo RNAi more efficiently than lymphocytes is consistent with the observation that thymocytes express more siA1 molecules per A1 mRNA than mature lymphocytes. To test whether this is the sole cause for cell-type-specific differences in RNAi efficiency, we analyzed the knockdown efficiency in U6Δ-shA1 thymocytes that were stimulated in vitro through T-cell receptor (TCR) cross-linking. It has been shown previously that TCR signaling induces A1 mRNA expression (45). Our data demonstrate that, in wild-type thymocytes, A1 expression increases threefold 6 h after TCR cross-linking, yielding A1 mRNA levels equivalent to what we detected in splenic B cells. In the presence of the U6Δ-shA1 transgene, however, A1 levels were reduced by 80% both in stimulated and unstimulated thymocytes (Fig. 5A). Given the significant increase in A1 mRNA expression relative to that of L32 mRNA and the decrease in siA1 expression relative to that of total RNA upon activation (Fig. 5B), the absolute number of A1 mRNA molecules that are degraded per siA1 molecule is at least threefold higher in activated thymocytes. This result therefore suggests that the efficiency of RNAi is not simply a function of siA1-A1 mRNA stoichiometry.
FIG. 5.
Differences in RNAi efficiency are not determined solely by the siRNA-to-mRNA ratio. Real-time PCR analysis of A1 mRNA levels (A) and Northern blot analysis for siA1 expression (B) in unstimulated (ex vivo) or anti-CD3/anti-CD28 stimulated (CD3/CD28) thymocytes from wild-type (light grey), U6Δ-shA1 (black), and U6-STOP-shA1 × CD19-cre mice (dark grey).
We next sought to extend this finding to other cell types. A1 mRNA levels and siA1 levels were both normalized to the RNA, allowing us to compare siA1-to-A1 mRNA ratios between the different cell types. When evaluating siA1-mediated knockdown in splenic B cells from U6-STOP-shA1 × CD19-cre mice and A1-IRES-GFP transgenic U6-STOP-shA1 ES cells (Fig. 2) side by side, we find similar amounts of siRNA and A1 mRNA per total RNA in both cell types. However, A1 levels are dramatically reduced only in ES cells (Fig. 6A). Furthermore, BMDM from U6Δ-shA1 mice undergo RNAi two- to threefold more efficiently than splenic B cells from the same animal (Fig. 4B and 6B), despite the fact that macrophages express >10-fold more A1 mRNA (Fig. 6C). Northern blot analysis shows that equal amounts of RNA from B cells and BMDM contain comparable numbers of RISC-protected siA1 molecules (Fig. 6D), resulting in a >10-fold-decreased siRNA-to-mRNA ratio in the latter. Taken together, these findings show that the efficiency of RNAi in vivo varies between tissues and indicate that it is not determined solely by the siRNA-to-target mRNA ratio.
Inefficient RNAi in mature T cells is not restricted to siA1.
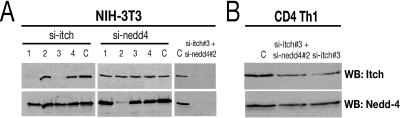
The inefficient A1 knockdown in mature lymphocytes is unlikely to be specifically due to the siRNA siA1, since siA1 mediates efficient RNAi in most other cell types tested. This suggests a cell-intrinsic defect of RNAi in mature lymphocytes. To address whether other siRNAs would show similarly inefficient target RNA degradation, we took advantage of retroviral gene transfer to integrate unrelated shRNA expression cassettes into the genome of ex vivo-isolated splenic CD4 T cells. We chose the E3 ligases Itch and Nedd-4 as shRNA target genes, since reduction in their expression levels is not expected to negatively affect T-cell proliferation or survival (reviewed in reference 27). Both Itch-specific (si-itch) and Nedd-4-specific (si-nedd4) siRNAs were tested in NIH 3T3 fibroblasts under the same retroviral infection conditions and were able to mediate >10-fold-greater knockdowns of Itch and Nedd-4, respectively (Fig. 7A). In CD4 T cells, however, target gene expression was reduced by only ∼50% (Fig. 7B), despite the presence of processed siRNA molecules (data not shown). These results are consistent with the notion that mature lymphocytes are generally impaired in their ability to undergo RNAi. However, it will be interesting to determine whether the efficiency of RNAi can be improved by increasing the number of viral integrants per cell as suggested in earlier experiments (36).
FIG. 7.
Inefficient RNAi in mature T cells is not limited to siA1. (A) NIH 3T3 cells were retrovirally transduced with sets of siRNA expression vectors directed against Itch (si-itch 1 to 4) or Nedd-4 (si-nedd4 1 to 4) or with a control vector encoding a scrambled, unspecific siRNA (C). Equal amounts of cell lysates were analyzed by Western blotting for Itch (top panel) and Nedd-4 expression (bottom panel). Nedd-4 serves as a loading control for si-itch samples and vice versa. The most efficient RNAi constructs (si-itch 3 and si-nedd4 2) were used for simultaneous infection in two samples as indicated. (B) Ex vivo-isolated CD4 T cells were transduced with si-itch 3 alone or si-itch 3 and si-nedd4 2 simultaneously as indicated. The control is as described for panel A. Cells were kept in Th1-conditioned medium and analyzed 1 week after retroviral infection. Equal amounts of cell lysates were analyzed by Western blotting (WB) for Itch (top panel) and Nedd-4 expression (bottom panel).
ago2 expression does not correlate with RNAi efficiency.
As mentioned earlier, RNAi is inefficient in mature lymphocytes, despite the presence of RISC-protected siRNA. Consistent with this notion, we find expression of the known RISC components argonaute 1 (ago1) to ago4 in mature lymphocytes (Fig. 8A). It has been recently suggested that ago2 may be the only argonaute family member that has endonuclease activity and thus acts as the catalytic engine for RISC-mediated cleavage of target mRNA (22, 24). We therefore reasoned that differential ago2 expression may account for cell-type-specific differences in RNAi. ago2 expression was analyzed by QRT-PCR in B cells, thymocytes, and macrophages (Fig. 8B). While B cells and thymocytes express similar levels of ago2, its expression is significantly reduced in macrophages. Hence, we were unable to observe a correlation between RISC endonuclease expression levels and RNAi efficiency.
FIG. 8.
Expression of argonaute genes in mature lymphocytes and BMDM. (A) RT-PCR for ago 1 to 4. β-Actin was amplified as a loading control. Total RNA from MACS-purified CD19+ and Thy1+ splenic lymphocytes was analyzed for expression of the indicated mRNAs. (B) QRT-PCR for ago2. Relative ago2 expression levels are shown as the ratio of ago2 to L32 for FACS-sorted splenic B cells, thymocytes, and cultured BMDM.
Sevenfold reduction of A1 expression does not affect distribution of thymocyte subsets.
To address whether reduced A1 expression affects thymocyte development, we analyzed the distribution of thymocyte subsets based on the expression of several surface markers. Thymic cellularity was comparable between WT and U6Δ-shA1 mice (Fig. 9A), and the distribution of thymocyte subsets was largely unaffected by the expression of siA1 (Fig. 9B and C). While we observe a slight reduction in the CD25−/CD44+ subset of CD4−/CD8− double negative thymocytes (termed double negative 1 [DN1]) in U6Δ-shA1 mice, the overall normal thymic phenotype of these mice suggests that this reduction has no significant effect on thymocyte development. Since A1 has been implicated to be important for pre-TCR-mediated positive selection of DN3 thymocytes, we examined A1 expression in sorted CD25+/CD44− (DN3) and CD25−/CD44− (DN4) thymocyte subsets from U6Δ-shA1 mice. Our data show that, as is the case with total thymocytes, A1 expression is reduced six- and sevenfold in DN4 and DN3 thymocytes, respectively (Fig. 9D). Importantly, the sizes of the DN3 and DN4 thymocyte subsets are not affected by A1 knockdown (Fig. 9C). We conclude that a sevenfold reduction of A1 expression does not interfere with pre-TCR-mediated survival and progression to the DN4 and CD4+/CD8+ double positive (DP) stages of thymocyte development. To assess whether this reduction is compensated for by the cells through changes in the expression pattern of other bcl-2 family members, we analyzed the expression of several pro- and antiapoptotic genes by RNase protection assay (Fig. 9E). The observed reduction of A1 mRNA levels is comparable to what has been detected by real-time PCR. However, mRNA levels of other bcl-2 family genes, including the antiapoptotic genes bcl-2 and bcl-X, were unaltered.
FIG. 9.
Thymocyte subsets show efficient knockdown of A1 mRNA but no changes in their distribution. (A) Thymocyte numbers from U6Δ-shA1 (n = 3) and wild-type (n = 4) mice. (B) FACS analysis of thymocytes from wild-type and U6Δ-shA1 mice. Thymocytes were stained for CD4 and CD8 (top panels). To stain for DN thymocyte subsets, cells positive for CD4, CD8, CD19, and NK1.1 were excluded from the analysis. Negative cells were analyzed for CD25 and CD44 expression (bottom panels). The percentage of each subset is indicated. This result is representative of three independent experiments. (C) Sizes of DN1 (CD44+/CD25−), DN2 (CD44+/CD25+), DN3 (CD44−/CD25+), and DN4 (CD44−/CD25−) subsets in U6Δ-shA1 mice relative to WT littermates. DN subsets were identified by FACS as described for panel B. The percentage represents the ratio of U6Δ-shA1 to WT DN subset. Results are based on three independent experiments. (D) QRT-PCR to determine A1 mRNA levels in sorted CD25+/CD44− DN3 and CD25−/C44− DN4 thymocyte subsets. Percentages of wild-type A1 mRNA levels are indicated. Unsorted thymocytes (Thymus) represent predominantly CD4+/CD8+ DP thymocytes. The scheme at the top of the panel depicts the pre-TCR-induced developmental progression from DN3 to DN4 via a proliferating and hence large DN3 subpopulation (DN3L). (E) RNase protection assay of total thymocytes to determine expression levels of several bcl-2 family genes. Portions of total RNA (2.5 μg) were loaded for each sample; identities of the protected bands are indicated.
DISCUSSION
In recent years, RNAi has become a widely used tool in reverse genetics. A major advantage of RNAi over classical gene targeting is its potential to simultaneously inactivate multiple, sufficiently homologous gene family members using a single siRNA. In the mammalian system, shRNA-expressing transgenes have been shown to mediate effective inactivation of gene expression in cell culture, and several genetic approaches have achieved interference with gene expression in mice. While up to 20-fold in downregulation of target mRNA levels has been observed in cell culture, little is known about the efficiency of RNAi in animals (reviewed in reference 25). Since the RNAi machinery depends on multiple components with only poorly characterized expression patterns (reviewed in reference 12), it is conceivable that the efficiency of RNAi varies between different cell types and differentiation stages. Addressing the efficiency of RNAi in a cell-type-specific fashion is therefore of particular interest for the use of RNAi as a general tool in mouse genetics.
Here, we employed RNAi to simultaneously inactivate all three isoforms of the bcl-2 family member A1 and analyzed the efficiency of A1 knockdown in several hematopoietic lineages. In view of the early lethality of mice that lack the bcl-2 family genes mcl-1 or bcl-X (26, 35), we designed a Cre/loxP-based approach to allow for conditional shRNA expression in vivo. This approach differs from two previously reported systems (42, 44) in that it only changes the PolIII promoter sequence downstream of the TATA box. We chose this part of the U6 promoter, since its site-directed mutagenesis did not affect the transcription efficiency of a U6 minigene (5). Our results demonstrate tightly regulated, Cre-inducible induction of shRNA both in ES cells and in vivo. While all expressed A1 isoforms appear to be degraded with equal efficiency (Fig. 4C), the extent of siA1-mediated A1 knockdown varies from 10-fold in ES cells to less than threefold in mature B cells. In accordance with published results, we observe efficient (∼sevenfold) knockdown in thymocytes (36, 39) and macrophages (36, 39).
Mature lymphocytes appear to be particularly resistant to siA1-mediated A1 mRNA degradation. One reason for this phenomenon could be counterselection of cells that have inactivated A1. However, a number of observations argue against this possibility: First, the majority of B cells from mice that carry the conditional U6-STOP-shA1 allele in combination with the B-cell-specific CD19-cre transgene have deleted the STOP cassette and express single-stranded and predominantly RISC-incorporated siA1 (Fig. 3) (17, 37). Expression of functional siA1 therefore does not interfere with the survival of mature B cells. Second, mice that express siA1 ubiquitously show no differences in lymphocyte subsets and numbers, indicating that siA1 expression does not cause a developmental block or cell loss at a particular stage in lymphocyte development. Hence, our data suggest that RNAi is inefficient in mature lymphocytes, despite the presence of processed, functional siRNA. We further demonstrate that this phenomenon is not limited to siA1, since retroviral infection of ex vivo-isolated splenic T cells with two independent shRNA constructs yield similar knockdown efficiencies (Fig. 7).
While the efficiency of RNAi varies between animals, we consistently observe that thymocytes undergo RNAi more efficiently than splenic T or B cells (Fig. 4A and B). This can be partially explained by the fact that thymocytes express more siA1 per A1 mRNA molecule and thus have a favorable siA1-to-A1 mRNA ratio. It is indeed likely that the amount of siRNA per target mRNA affects the efficiency of RNAi, since up to 10-fold knockdown has been reported in mature T cells with multiple copies of a particular shRNA expression cassette (36). However, the TCR-triggered selective increase in A1 mRNA but not siA1 expression levels does not alter the efficiency of A1 knockdown in thymocytes (Fig. 5A). Similarly, RNAi in mature B cells is three- to fivefold less efficient than in ES cells or macrophages, despite comparable siA1 expression levels and, in the case of bone marrow-derived macrophages, >10-fold-increased A1 mRNA levels (Fig. 6). These data indicate that additional factors besides the expression level of siA1 or A1 mRNA are responsible for differences in RNAi efficiency and that these factors appear to be differentially expressed in distinct cell types or developmental stages. The fluctuation in RNAi efficiency between individual animals which are not of an inbred background is consistent with this notion.
What could be responsible for cell-type-specific differences in RNAi? Variations in the composition of the RNAi machinery are a likely cause for differences in RNAi efficiency. This possibility is particularly appealing, since a single RISC component, ago2, appears to be sufficient to mediate RISC endonuclease activity (22, 24). We did not observe a correlation between elevated ago2 levels and increased RNAi efficiency in our system (Fig. 8) and hence conclude that ago2 expression levels are not the sole determinant of RNAi efficiency. The overall understanding of RISC-mediated mRNA degradation is progressing rapidly, and intensive screening efforts are expected to reveal novel components involved in posttranscriptional gene silencing. Our data suggest that some of these components may be differentially expressed both in distinct cell types and during cellular differentiation and thereby account for changes in the ability of a cell to undergo RNAi.
This report presents the first comparative analysis of RNAi efficiency in different cell types of the hematopoietic system in vivo. We observe on average a sevenfold reduction in target mRNA levels in thymocytes and macrophages, suggesting that RNAi can be effectively used to analyze gene function in these cell types. The observation that mature lymphocytes undergo RNAi rather inefficiently poses a limitation to the use of RNAi in mice. Increasing the siRNA expression level may be one way to bypass this limitation (36) but requires multiple copies of a particular shRNA expression cassette. It will therefore be important to identify methods to manipulate the RNAi machinery itself in order to obtain knockdown efficiencies that are sufficient for a broad range of loss-of-function studies.
With respect to the physiological role of A1, we demonstrate that thymocyte development is not significantly altered in mice expressing only 10 to 20% of the wild-type A1 mRNA levels. Given the fact that A1 has three close relatives (bcl2, bcl-X, and mcl-1), which may compensate for a reduction in A1 levels, this result is not surprising. The high level of bcl-X expression in thymocytes is consistent with this notion (Fig. 9E) and it will be interesting to analyze the impact of A1 knockdown on a bcl-X-deficient background. Our analysis did not cover all known antiapoptotic bcl-2 family members, and bcl-X may therefore not be the only gene able to compensate for a loss of A1. In this context, mcl-1 is particularly interesting, as it has been shown to be essential for the progression from DN to DP T-cell stages (31). Alternatively, A1 expression may be required for thymocyte development at a level below that observed in U6Δ-shA1 thymocytes. Given that RNAi does not allow for complete gene inactivation, this method may be insufficient to reduce A1 levels below this potential threshold. An alternative approach for the simultaneous inactivation of all A1 isoforms will be needed to fully address this issue. Similarly, we can only speculate that A1 is dispensable for the survival of bone marrow-derived macrophages, since the extent of A1 inactivation may be insufficient to affect macrophage survival or compensated for by other bcl-2 family members.
Acknowledgments
We thank N. Barteneva for cell sorting; V. Dreier, D. Ghitza, and S. Linehan for technical help; and S. Casola, S. Muljo, S. Rana, D. Schenten, and M. Schmidt-Supprian for helpful discussions and critical reading.
This work was supported by National Institutes of Health grant POI A156900. V.H. is a Cancer Research Institute postdoctoral fellow. I.A. was supported by the V Foundation for Cancer Research and the Cancer Research Institute.
REFERENCES
- 1.Bassing, C. H., K. F. Chua, J. Sekiguchi, H. Suh, S. R. Whitlow, J. C. Fleming, B. C. Monroe, D. N. Ciccone, C. Yan, K. Vlasakova, D. M. Livingston, D. O. Ferguson, R. Scully, and F. W. Alt. 2002. Increased ionizing radiation sensitivity and genomic instability in the absence of histone H2AX. Proc. Natl. Acad. Sci. USA 99:8173-8178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernstein, E., A. A. Caudy, S. M. Hammond, and G. J. Hannon. 2001. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409:363-366. [DOI] [PubMed] [Google Scholar]
- 3.Bronson, S. K., E. G. Plaehn, K. D. Kluckman, J. R. Hagaman, N. Maeda, and O. Smithies. 1996. Single-copy transgenic mice with chosen-site integration. Proc. Natl. Acad. Sci. USA 93:9067-9072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550-553. [DOI] [PubMed] [Google Scholar]
- 5.Das, G., D. Henning, D. Wright, and R. Reddy. 1988. Upstream regulatory elements are necessary and sufficient for transcription of a U6 RNA gene by RNA polymerase III. EMBO J. 7:503-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fire, A., S. Xu, M. K. Montgomery, S. A. Kostas, S. E. Driver, and C. C. Mello. 1998. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391:806-811. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez, J., A. Orlofsky, and M. B. Prystowsky. 2003. A1 is a growth-permissive antiapoptotic factor mediating postactivation survival in T cells. Blood 101:2679-2685. [DOI] [PubMed] [Google Scholar]
- 8.Grumont, R. J., I. J. Rourke, and S. Gerondakis. 1999. Rel-dependent induction of A1 transcription is required to protect B cells from antigen receptor ligation-induced apoptosis. Genes Dev. 13:400-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamasaki, A., F. Sendo, K. Nakayama, N. Ishida, I. Negishi, and S. Hatakeyama. 1998. Accelerated neutrophil apoptosis in mice lacking A1-a, a subtype of the bcl-2-related A1 gene. J. Exp. Med. 188:1985-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammond, S. M., E. Bernstein, D. Beach, and G. J. Hannon. 2000. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 404:293-296. [DOI] [PubMed] [Google Scholar]
- 11.Hammond, S. M., S. Boettcher, A. A. Caudy, R. Kobayashi, and G. J. Hannon. 2001. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science 293:1146-1150. [DOI] [PubMed] [Google Scholar]
- 12.Hannon, G. J. 2002. RNA interference. Nature 418:244-251. [DOI] [PubMed] [Google Scholar]
- 13.Hatakeyama, S., A. Hamasaki, I. Negishi, D. Y. Loh, F. Sendo, and K. Nakayama. 1998. Multiple gene duplication and expression of mouse bcl-2-related genes, A1. Int. Immunol. 10:631-637. [DOI] [PubMed] [Google Scholar]
- 14.Jackson, R. J., M. T. Howell, and A. Kaminski. 1990. The novel mechanism of initiation of picornavirus RNA translation. Trends Biochem. Sci. 15:477-483. [DOI] [PubMed] [Google Scholar]
- 15.Jang, S. K., and E. Wimmer. 1990. Cap-independent translation of encephalomyocarditis virus RNA: structural elements of the internal ribosomal entry site and involvement of a cellular 57-kD RNA-binding protein. Genes Dev. 4:1560-1572. [DOI] [PubMed] [Google Scholar]
- 16.Ketting, R. F., S. E. Fischer, E. Bernstein, T. Sijen, G. J. Hannon, and R. H. Plasterk. 2001. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev. 15:2654-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khvorova, A., A. Reynolds, and S. D. Jayasena. 2003. Functional siRNAs and miRNAs exhibit strand bias. Cell 115:209-216. [DOI] [PubMed] [Google Scholar]
- 18.Knight, S. W., and B. L. Bass. 2001. A role for the RNase III enzyme DCR-1 in RNA interference and germ line development in Caenorhabditis elegans. Science 293:2269-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunath, T., G. Gish, H. Lickert, N. Jones, T. Pawson, and J. Rossant. 2003. Transgenic RNA interference in ES cell-derived embryos recapitulates a genetic null phenotype. Nat. Biotechnol. 21:559-561. [DOI] [PubMed] [Google Scholar]
- 20.Lakso, M., B. Sauer, B. Mosinger, Jr., E. J. Lee, R. W. Manning, S. H. Yu, K. L. Mulder, and H. Westphal. 1992. Targeted oncogene activation by site-specific recombination in transgenic mice. Proc. Natl. Acad. Sci. USA 89:6232-6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin, E. Y., A. Orlofsky, M. S. Berger, and M. B. Prystowsky. 1993. Characterization of A1, a novel hemopoietic-specific early-response gene with sequence similarity to bcl-2. J. Immunol. 151:1979-1988. [PubMed] [Google Scholar]
- 22.Liu, J., M. A. Carmell, F. V. Rivas, C. G. Marsden, J. M. Thomson, J. J. Song, S. M. Hammond, L. Joshua-Tor, and G. J. Hannon. 2004. Argonaute2 is the catalytic engine of mammalian RNAi. Science 305:1437-1441. [Online.] [DOI] [PubMed] [Google Scholar]
- 23.McManus, M. T., B. B. Haines, C. P. Dillon, C. E. Whitehurst, L. Van Parijs, J. Chen, and P. A. Sharp. 2002. Small interfering RNA-mediated gene silencing in T lymphocytes. J. Immunol. 169:5754-5760. [DOI] [PubMed] [Google Scholar]
- 24.Meister, G., M. Landthaler, A. Patkaniowska, Y. Dorsett, G. Teng, and T. Tuschl. 2004. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol. Cell 15:185-197. [DOI] [PubMed] [Google Scholar]
- 25.Mittal, V. 2004. Improving the efficiency of RNA interference in mammals. Nat. Rev. Genet. 5:355-365. [DOI] [PubMed] [Google Scholar]
- 26.Motoyama, N., F. Wang, K. A. Roth, H. Sawa, K. Nakayama, I. Negishi, S. Senju, Q. Zhang, S. Fujii, et al. 1995. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science 267:1506-1510. [DOI] [PubMed] [Google Scholar]
- 27.Mueller, D. L. 2004. E3 ubiquitin ligases as T cell anergy factors. Nat. Immunol. 5:883-890. [DOI] [PubMed] [Google Scholar]
- 28.Niwa, H., K. Yamamura, and J. Miyazaki. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193-199. [DOI] [PubMed] [Google Scholar]
- 29.Nykanen, A., B. Haley, and P. D. Zamore. 2001. ATP requirements and small interfering RNA structure in the RNA interference pathway. Cell 107:309-321. [DOI] [PubMed] [Google Scholar]
- 30.Oberdoerffer, P., K. L. Otipoby, M. Maruyama, and K. Rajewsky. 2003. Unidirectional Cre-mediated genetic inversion in mice using the mutant loxP pair lox66/lox71. Nucleic Acids Res. 31:e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Opferman, J. T., A. Letai, C. Beard, M. D. Sorcinelli, C. C. Ong, and S. J. Korsmeyer. 2003. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 426:671-676. [DOI] [PubMed] [Google Scholar]
- 32.Paddison, P. J., A. A. Caudy, E. Bernstein, G. J. Hannon, and D. S. Conklin. 2002. Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev. 16:948-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pasparakis, M., and G. Kollias. 1995. Production of cytokine transgenic and knockout mice, p. 297-324. In F. R. Balkwill (ed.), Cytokines: a practical approach. Oxford University Press, Oxford, England.
- 34.Rickert, R. C., J. Roes, and K. Rajewsky. 1997. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 25:1317-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rinkenberger, J. L., S. Horning, B. Klocke, K. Roth, and S. J. Korsmeyer. 2000. Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev. 14:23-27. [PMC free article] [PubMed] [Google Scholar]
- 36.Rubinson, D. A., C. P. Dillon, A. V. Kwiatkowski, C. Sievers, L. Yang, J. Kopinja, D. L. Rooney, M. M. Ihrig, M. T. McManus, F. B. Gertler, M. L. Scott, and L. Van Parijs. 2003. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat. Genet. 33:401-406. [DOI] [PubMed] [Google Scholar]
- 37.Schwarz, D. S., G. Hutvagner, T. Du, Z. Xu, N. Aronin, and P. D. Zamore. 2003. Asymmetry in the assembly of the RNAi enzyme complex. Cell 115:199-208. [DOI] [PubMed] [Google Scholar]
- 38.Shi, Y. 2003. Mammalian RNAi for the masses. Trends Genet. 19:9-12. [DOI] [PubMed] [Google Scholar]
- 39.Song, E., S. K. Lee, J. Wang, N. Ince, N. Ouyang, J. Min, J. Chen, P. Shankar, and J. Lieberman. 2003. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat. Med. 9:347-351. [DOI] [PubMed] [Google Scholar]
- 40.Sui, G., C. Soohoo, E. B. Affar, F. Gay, Y. Shi, W. C. Forrester, and Y. Shi. 2002. A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc. Natl. Acad. Sci. USA 99:5515-5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson, S., A. R. Clarke, A. M. Pow, M. L. Hooper, and D. W. Melton. 1989. Germ line transmission and expression of a corrected HPRT gene produced by gene targeting in embryonic stem cells. Cell 56:313-321. [DOI] [PubMed] [Google Scholar]
- 42.Tiscornia, G., V. Tergaonkar, F. Galimi, and I. M. Verma. 2004. CRE recombinase-inducible RNA interference mediated by lentiviral vectors. Proc. Natl. Acad. Sci. USA 101:7347-7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trescol-Biemont, M. C., C. Verschelde, A. Cottalorda, and N. Bonnefoy-Berard. 2004. Regulation of A1/Bfl-1 expression in peripheral splenic B cells. Biochimie 86:287-294. [DOI] [PubMed] [Google Scholar]
- 44.Ventura, A., A. Meissner, C. P. Dillon, M. McManus, P. A. Sharp, L. Van Parijs, R. Jaenisch, and T. Jacks. 2004. Cre-lox-regulated conditional RNA interference from transgenes. Proc. Natl. Acad. Sci. USA 101:10380-10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verschelde, C., T. Walzer, P. Galia, M. C. Biemont, L. Quemeneur, J. P. Revillard, J. Marvel, and N. Bonnefoy-Berard. 2003. A1/Bfl-1 expression is restricted to TCR engagement in T lymphocytes. Cell Death Differ. 10:1059-1067. [DOI] [PubMed] [Google Scholar]
- 46.Zamore, P. D., T. Tuschl, P. A. Sharp, and D. P. Bartel. 2000. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell 101:25-33. [DOI] [PubMed] [Google Scholar]