Abstract
Borrelia burgdorferi, the Lyme disease agent, binds glycosaminoglycans (GAGs) such as heparin, heparan sulfate, and dermatan sulfate. Heparin or heparan sulfate fractions separated by size or charge were tested for their ability to inhibit attachment of B. burgdorferi to Vero cells. GAG chains of increasing length and/or charge showed increasing inhibitory potency, and detectable heparin inhibition of bacterial binding required a minimum of 16 residues. The ability of a given heparin fraction to inhibit binding to Vero cells was strongly predictive of its ability to inhibit hemagglutination, suggesting that hemagglutination reflects the capacity of B. burgdorferi to bind to GAGs.
Borrelia burgdorferi sensu lato is the spirochetal agent of Lyme borreliosis, a chronic, multisystemic illness (12, 24). The bacterium is acquired from an infected Ixodes tick, and after infecting the skin at the site of the bite, it can disseminate throughout the mammalian host. The ability of the spirochete to bind to extracellular matrix or to the surface of host cells is likely to promote tissue colonization, and the spirochete has been shown to recognize several classes of host molecules (3, 4, 7, 8), including the glycosaminoglycan (GAG) heparin (11, 16).
GAGs consist of long, linear, highly sulfated disaccharide repeats and are usually found covalently linked to a protein core as a component of proteoglycans (13, 23). Based on the extent and location of sulfation and on the composition of the disaccharide unit, GAGs can be separated into different classes, such as heparin, heparan sulfate, keratan sulfate, chondroitin-4-sulfate, chondroitin-6-sulfate, or dermatan sulfate. Even within a given class of GAG there is extensive heterogeneity due to epimerization of the sugar backbone, differences in chain length, and variations in the location of N-acetyl, N-sulfate, and O-sulfate groups. The complexity and heterogeneity of GAGs complicate the analysis of protein-GAG chain interactions.
B. burgdorferi has been shown to bind to heparin, heparan sulfate, and dermatan sulfate, as well as to decorin, a dermatan sulfate/chondroitin sulfate proteoglycan (8, 11, 16). B. burgdorferi strains that bind heparin have also been shown to agglutinate rabbit erythrocytes in a heparin-inhibitable manner, suggesting that the two activities may be linked (16). It is not clear whether the recognition of different proteoglycans reflects multiple binding pathways or a single, somewhat promiscuous pathway. All GAG chains share the feature of high negative charge, and charge has been demonstrated to be critical for B. burgdorferi binding (11, 16). Nevertheless, charge is not the sole determinant of binding, because not all GAG chains are recognized by the spirochete, and the spectrum of GAGs that are efficiently bound varies among different B. burgdorferi strains (18). Thus, GAG binding by this bacterium displays an element of specificity.
Characterization of the structural requirements for heparin recognition by B. burgdorferi would promote a better understanding of the nature of this binding specificity as well as the mechanisms of cell attachment and tissue colonization by this pathogen. In the present study, we have identified some of the features of GAG chain structure that are critical for cell attachment by B. burgdorferi. We have further shown that these features are also critical for inhibition of hemagglutination, suggesting that B. burgdorferi GAG binding and hemagglutination are closely linked.
GAG chain charge and chain length are critical for recognition by B. burgdorferi.
Mammalian cell attachment by strain N40, clone D10/E9 (an infectious B. burgdorferi sensu stricto isolate [3]), is inhibited by the presence of exogenous dextran sulfate, heparin, heparan sulfate, and dermatan sulfate (16, 17). This inhibition is due to an interaction between spirochetes and GAG, because treatment of bacteria with GAG inhibited cell binding even after excess GAG was removed by washing (data not shown). In contrast, treatment of cell monolayers with GAG followed by washing resulted in no inhibition of bacterial binding. Dextran and desulfated heparin failed to inhibit attachment of B. burgdorferi, suggesting that a negative charge is required for inhibitory activity (11, 16). To further explore the relationship between charge and binding inhibition, we analyzed several GAG preparations that differed in charge for the ability to inhibit B. burgdorferi N40 binding of Vero (monkey kidney) cells, an interaction that was previously shown to be mediated by proteoglycans (16). Attachment of radiolabeled B. burgdorferi N40 to Vero cells was determined in the presence of various concentrations of GAG, as described elsewhere (16, 17). As previously shown, completely desulfated, N-acetylated heparin (Seikagaku, Inc., Tokyo, Japan) had no detectable inhibitory activity, even when tested at 1 mg/ml (Table 1). N-desulfated, N-acetylated heparin, which retains O sulfation, demonstrated weak inhibitory activity.
TABLE 1.
Inhibition of cell attachment and hemagglutination by GAGs
| GAGa | Fraction | Eluting concn of LiCl (M)b | Estimated size (kDa) | IC50 (μg/ml) for cell attachmentc | MIC (μg/ml) for hemagglutinationd |
|---|---|---|---|---|---|
| Heparin | NAe | NA | NDf | 6.3 | 0.16 |
| Keratan sulfate | NA | NA | ND | >500 | >500 |
| Dermatan sulfate | NA | NA | ND | 32 | 100 |
| Chondroitin-6-sulfate | NA | NA | ND | >500 | >100 |
| Chondroitin-4-sulfate | NA | NA | ND | >500 | >100 |
| Heparan sulfateg | HS-D | 0.75 | 6 | 170 | >100 |
| HS-A | 0.55 | 17 | >500 | >100 | |
| Heparin | |||||
| N-desulfated, N-acetylated | NA | NA | ND | 1,000 | 100 |
| Completely desulfated, N-acetylated | NA | NA | ND | >1,000 | >100 |
| Fractionated by ion exchange | IE-4 | 1.73 | 21.4 | 1.5 | 0.16 |
| IE-3 | 1.64 | 19.7 | 3.5 | 20 | |
| IE-2 | 1.57 | 18.0 | 10.1 | >100 | |
| IE-1 | 1.50 | 15.3 | 30.0 | >100 | |
| Fractionated by gel filtration | CF1 | NA | 21.0 | 3.9 | 0.032 |
| CF2 | NA | 16.0 | 10.2 | 0.80 | |
| CF3 | NA | 12.0 | 11.2 | 4 | |
| CF4 | NA | 9.6 | 12.0 | 100 | |
| CF5 | NA | 7.0 | 51.0 | 100 |
Unmodified and unfractionated GAGs were purchased from Sigma Chemical Co. Fractionated and modified GAGs were prepared as described in the text.
GAG preparations separated by ion-exchange chromatography were eluted by the designated concentration of LiCl (21).
The IC50 indicates the estimated concentration of GAG at which bacterial binding is 50% of the level of binding in the absence of inhibitor.
The minimum concentration at which inhibition of hemagglutination could be detected for a given GAG preparation varied as much as fivefold from experiment to experiment. We postulate that this variation was due to small differences in temperature, the age of the erythrocyte preparation, or other, unknown factors. Nevertheless, the relative potency of each GAG fraction remained constant relative to that of dextran sulfate (500 kDa; Sigma Chemical Co.), which was included in all experiments to provide standardization. Shown are the results of two experiments; all of the above GAG preparations except the IE heparin series were tested simultaneously, and in that experiment, the MIC for dextran sulfate was 6.4 ng/ml. The IE series was tested on a separate occasion, and on that occasion, the MIC for dextran sulfate was 32 ng/ml (data not shown).
NA, not applicable.
ND, not determined.
Fractionated by ion exchange.
Fractions of heparan sulfate of different charge density, obtained from the laboratory of the late Isidore Danishefsky (with the approval of the interim chairman, Ira Schwartz), were also tested for inhibitory activity. Crude heparan sulfate, derived from porcine intestinal mucosa (Cohelfred Laboratories, Chicago, Ill.), was digested with chondroitinase ABC (Seikagaku Inc.) and pronase B (Sigma Chemical Co., St. Louis, Mo.), and the dialyzed product was purified on a DE-52 cellulose column (3.5 by 39 cm) and eluted with a linear gradient of LiCl (0.4 to 1.5 M) in ammonium acetate buffer, pH 4.0. Fractions were ethanol precipitated and analyzed on a Beckman amino acid analyzer, which revealed that they were free of galactosamine and protein (data not shown). The molecular weight was estimated by migration on a 20% polyacrylamide gel, as described elsewhere (15, 22). The more highly negatively charged fraction of heparan sulfate (HS-D) was a better inhibitor of attachment than was the less highly negatively charged fraction (HS-A) (Table 1).
Heparin (porcine; Sigma Chemical Co.) was also fractionated by ion-exchange chromatography, using a QAE Sephadex A-25 column as described elsewhere (21), and tested for the ability to inhibit bacterial binding. A direct correlation was observed between total charge and inhibitory potency (Table 1, IE series fractions). For example, the 50% inhibitory concentration (IC50) of fraction IE-4, which was eluted from the ion-exchange column in 1.73 M salt, was 20-fold lower than the IC50 of fraction IE-1, which was eluted in 1.50 M salt.
These heparin fractions differ not only in total charge but also in median molecular weight, as estimated by Bio-Gel P-100 gel filtration (21). Therefore, heparin fractionated by gel filtration (from the laboratory of the late Isidore Danishefsky) was also tested for inhibition of bacterial attachment (Table 1, CF series fractions). Heparin (beef lung; Upjohn) was separated on a 90- by 5-cm Bio-Gel P-100 column and eluted in 0.05 M Tris–0.5 M LiCl, pH 7.4, as described elsewhere (20). The median molecular weight of each fraction was estimated on the basis of sedimentation equilibrium (20) and confirmed by high-performance liquid chromatography (data not shown). As in the case of the heparin series that was fractionated by charge, a direct correlation between GAG length and potency was observed, indicating that both chain length and charge are important factors governing GAG recognition by B. burgdorferi.
In Europe, two other B. burgdorferi sensu lato species, B. garinii and B. afzelii, are also common agents of Lyme disease. To test whether chain length is an important feature of GAG recognition by other Lyme disease spirochetes, low- or high-molecular-weight heparin fractions were assayed for their ability to inhibit Vero cell binding by B. burgdorferi sensu stricto strain CA20-2A, B. garinii PBi, and B. afzelii VS461 (described in reference 5). For each strain, the 21-kDa fraction of heparin inhibited better than the 7-kDa fraction (Table 2).
TABLE 2.
Size-dependent heparin inhibition of Vero cell binding by diverse Lyme disease spirochetes
| Heparin fraction | IC50 (μg/ml)a for:
|
|||
|---|---|---|---|---|
|
B. burgdorferi
|
B. garinii PBi | B. afzelii VS461 | ||
| N40 | CA20-2A | |||
| Unfractionated | 8.0 | 1.9 | <0.5 | <0.5 |
| CF1 (21 kDa) | 3.9 | 1.0 | <0.5 | <0.5 |
| CF5 (7 kDa) | 51.0 | 12.0 | 3.2 | 2.9 |
The IC50 indicates the estimated concentration of GAG at which bacterial binding is 50% of the level of binding in the absence of heparin. Size-fractionated heparin preparations were prepared as described in the text. The specific lot of unfractionated heparin from which CF1 and CF5 were derived was unavailable for testing; thus, a different preparation of heparin was included for comparison.
A minimum chain length of 16 residues is required for heparin to inhibit cell binding by B. burgdorferi.
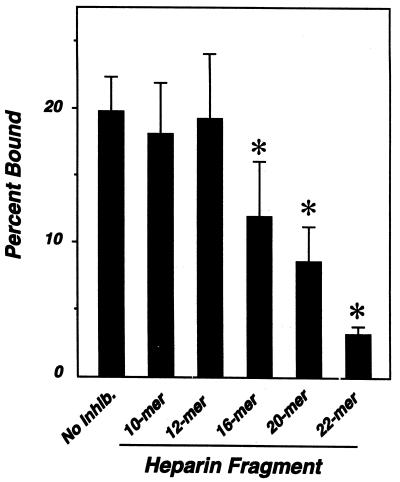
The correlation between sugar chain length and inhibition prompted us to test for a minimum size requirement for GAG recognition by B. burgdorferi. Heparin fragments containing 10, 12, 16, 20, or 22 monosaccharide units (gifts of Ulf Lindahl [Department of Medical and Physiological Chemistry, University of Uppsala, Uppsala, Sweden] and Jean Choay [Institut Choay, Paris, France] to the late Isidore Danishefsky [15, 19]) were tested for the ability to inhibit bacterial attachment to Vero cells. The 22-mer almost completely blocked attachment, while the 10- and 12-mers had no effect (Fig. 1). The 16-mer (P < 0.05 versus no-inhibitor control) and 20-mer (P < 0.005) inhibited at intermediate levels.
FIG. 1.
Identification of the minimum heparin fragment required for inhibition of attachment of B. burgdorferi to mammalian cells. Radiolabeled B. burgdorferi N40 was preincubated with 500 μg of heparin fragment per ml prior to infection of Vero cells, and bound bacteria were quantitated. Each bar represents the average ± the standard deviation of four determinations. Statistically significant differences were determined by using Microsoft Excel to calculate two-tailed t values between the no-inhibitor control (No Inhib.) and heparin fractions; the asterisks indicate P values of <0.05.
Inhibition of cell binding closely correlates with inhibition of hemagglutination.
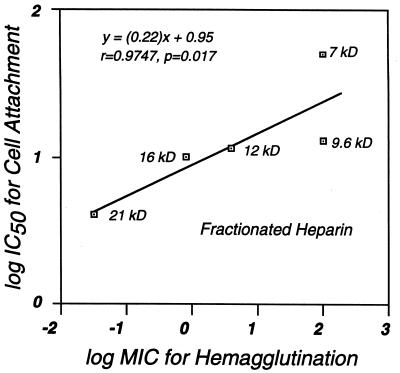
It has been postulated that the GAG-binding activity of B. burgdorferi is reflected by the agglutination of rabbit erythrocytes, because hemagglutination is inhibited by heparin and those strains that exhibit low-level hemagglutination activity also exhibit low-level GAG-binding activity (16). A further prediction of this hypothesis is that the ability of a given GAG preparation to inhibit cell attachment should correlate with its ability to inhibit hemagglutination. Used in these assays was a detergent extract of B. burgdorferi N40, prepared in bulk and frozen in aliquots at −70°C, that was found to give a more predictable hemagglutination titer than intact spirochetes (the aggregation of which introduced variability in the hemagglutination titer [data not shown]). To generate this extract, a suspension containing 1011 B. burgdorferi N40 cells per ml was lysed by sonication, and the insoluble fraction was extracted in a solution containing 2% deoxycholate, 20 mM HEPES, 4 mM EDTA, and 0.01 trypsin-inhibitory units of aprotinin (Sigma Chemical Co.) per ml for 40 min at 4°C. The hemagglutination activity of the detergent-soluble fraction and the effect of each of the GAG preparations on this activity were determined as described previously (16). When we tested different classes of GAGs for the ability to inhibit hemagglutination, keratan sulfate, chondroitin-4-sulfate, and chondroitin-6-sulfate had no effect whereas heparin and dermatan sulfate were inhibitory, the former at a much lower concentration than the latter (Table 1). Completely desulfated, N-acetylated heparin was devoid of hemagglutination blocking activity, and N-desulfated, N-acetylated heparin retained weak inhibitory activity. Fractions containing longer, more highly charged heparin chains were better inhibitors than fractions containing shorter, less highly charged fractions (IE and CF series fractions, Table 1). All of these results paralleled inhibition of Vero cell binding by the same GAG preparations, and within the CF heparin fractionation series, the minimum concentration for inhibition of hemagglutination correlated with the IC50 for cell attachment (Fig. 2).
FIG. 2.
The relative ability of heparin fractions to inhibit cell binding by B. burgdorferi correlates with the ability to inhibit hemagglutination. The IC50 for Vero cell binding and the minimum inhibitory concentration (MIC) for hemagglutination of each of the CF heparin fractions were determined as described in Table 1. The nonparametric (Spearman) correlation coefficient r and the two-tailed P value were calculated by using InStat version 2.01.
Discussion.
We undertook this study to better define the requirements for GAG chain recognition by B. burgdorferi, and we found that both the charge and the chain length of GAGs are critical factors for bacterial binding. The length and/or charge of the GAG chain correlated with inhibition of cell binding by B. burgdorferi, and heparin chains with less than 16 residues lacked detectable inhibitory activity. Given the intimate relationship between the size and total charge of GAGs, it is difficult to assess the relative importance of these two features for bacterial recognition.
The size of the heparin chain has been shown to be important in heparin recognition by other pathogenic microorganisms, such as Chlamydia trachomatis and Plasmodium falciparum (1, 2), and by many heparin-binding proteins as well (14). The correlation between GAG length and inhibitory activity for B. burgdorferi binding could indicate that the bacterial receptor for GAGs recognizes longer GAGs better. Alternatively, the effective inhibition of bacterial binding to mammalian cells may involve steric hindrance or electrostatic repulsion between the bacterium and the host cell, features that are likely to be provided better by longer heparin fragments. This issue could be addressed by direct measurement of heparin-spirochete interaction, but this would require either labeling of each GAG preparation or the use of significantly larger quantities of GAG (11, 16).
The results presented here suggest that the B. burgdorferi heparin-binding component(s) is also responsible for this bacterium’s hemagglutinating activity. Those GAG preparations that were potent inhibitors of cell binding were also potent inhibitors of hemagglutination, and those preparations that did not inhibit binding did not inhibit hemagglutination. A few GAG preparations, such as heparan sulfate HS-A and heparin fractions IE-1 and IE-2, inhibited cell binding but did not inhibit hemagglutination, suggesting that inhibition of cell attachment is a more sensitive method for detecting the GAG-binding activity of B. burgdorferi.
B. burgdorferi has been demonstrated to bind to both heparin, heparan sulfate, and dermatan sulfate GAGs (11, 16, 17) as well as to specific proteoglycans, such as decorin (8). It is not known whether binding to multiple GAGs or proteoglycans reflects a single or multiple mechanisms of cell attachment. Two decorin-binding proteins have been identified (6, 9, 10), but binding of B. burgdorferi to decorin was not inhibited by heparin, suggesting that binding of heparin and binding of decorin by this bacterium are distinct activities (8). The evidence presented here suggesting that GAG binding by B. burgdorferi is strongly linked to hemagglutination provides a strategy for evaluating candidate GAG-binding proteins and for the purification and identification of the B. burgdorferi GAG-binding hemagglutinin(s).
Acknowledgments
We thank Ira Schwartz for facilitating the initiation of this study, Ulf Lindahl and the late Jean Choay for their gifts of defined heparin fragments to the late Isidore Danishefsky, and Jenifer Coburn and Trudy Morrison for helpful discussion and careful review of the manuscript.
This work was supported by NIH grant R01-AI 37601 to J.M.L. and by New York State Affiliate American Heart Association grant-in-aid award 91-011G to L.R. B.L. was supported in part by NIH grant HL-16955 to Isidore Danishefsky. J.M.L. is an Established Investigator of the American Heart Association.
Footnotes
This paper is dedicated to the memory of Isidore Danishefsky.
REFERENCES
- 1.Chen J C, Zhang J P, Stephens R S. Structural requirements of heparin binding to Chlamydia trachomatis. J Biol Chem. 1996;271:11134–11140. [PubMed] [Google Scholar]
- 2.Clark D L, Su S, Davidson E A. Saccharide anions as inhibitors of the malaria parasite. Glycoconj J. 1997;14:473–479. doi: 10.1023/a:1018551518610. [DOI] [PubMed] [Google Scholar]
- 3.Coburn J, Leong J, Erban J. Integrin αIIbβ3 mediates binding of the Lyme disease agent, Borrelia burgdorferi, to human platelets. Proc Natl Acad Sci USA. 1993;90:7058–7063. doi: 10.1073/pnas.90.15.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coburn J, Magoun L, Bodary S C, Leong J M. Integrins αvβ3 and α5β1 mediate attachment of Lyme disease spirochetes to human cells. Infect Immun. 1998;66:1946–1952. doi: 10.1128/iai.66.5.1946-1952.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coburn J, Barthold S W, Leong J M. Diverse Lyme disease spirochetes bind integrin αIIbβ3 on human platelets. Infect Immun. 1994;62:5559–5567. doi: 10.1128/iai.62.12.5559-5567.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng S, Hodzic E, Stevenson B, Barthold S W. Humoral immunity to Borrelia burgdorferi N40 decorin binding proteins during infection of laboratory mice. Infect Immun. 1998;66:2827–2835. doi: 10.1128/iai.66.6.2827-2835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia Monco J C, Fernandez Villar B, Rogers R C, Szczepanski A, Wheeler C M, Benach J L. Borrelia burgdorferi and other related spirochetes bind to galactocerebroside. Neurology. 1992;42:1341–1348. doi: 10.1212/wnl.42.7.1341. [DOI] [PubMed] [Google Scholar]
- 8.Guo B P, Norris S J, Rosenberg L C, Höök M. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect Immun. 1995;63:3467–3472. doi: 10.1128/iai.63.9.3467-3472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagman K E, Lahdenne P, Popova T G, Porcella S F, Akins D R, Radolf J D, Norgard M V. Decorin-binding protein of Borrelia burgdorferi is encoded within a two-gene operon and is protective in the murine model of Lyme borreliosis. Infect Immun. 1998;66:2674–2683. doi: 10.1128/iai.66.6.2674-2683.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanson M S, Cassatt D R, Guo B P, Patel N K, McCarthy M P, Dorward D W, Höök M. Active and passive immunity against Borrelia burgdorferi decorin binding protein A (DbpA) protects against infection. Infect Immun. 1998;66:2143–2153. doi: 10.1128/iai.66.5.2143-2153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isaacs R. Borrelia burgdorferi bind to epithelial cell proteoglycan. J Clin Investig. 1994;93:809–819. doi: 10.1172/JCI117035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalish R A. Lyme disease. Rheum Dis Clin N Am. 1993;19:399–426. [PubMed] [Google Scholar]
- 13.Kjellen L, Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- 14.Klebe R J, Mock P J. Effect of glycosaminoglycans on fibronectin-mediated cell attachment. J Cell Physiol. 1982;112:5–9. doi: 10.1002/jcp.1041120103. [DOI] [PubMed] [Google Scholar]
- 15.Lahiri B, Lai P S, Pousada M, Stanton D, Danishefsky I. Depolymerization of heparin by complexed ferrous ions. Arch Biochem Biophys. 1992;293:54–60. doi: 10.1016/0003-9861(92)90364-3. [DOI] [PubMed] [Google Scholar]
- 16.Leong J M, Morrissey P E, Ortega-Barria E, Pereira M E A, Coburn J. Hemagglutination and proteoglycan binding by the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:874–883. doi: 10.1128/iai.63.3.874-883.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leong J M, Wang H, Magoun L, Field J A, Morrissey P E, Robbins D, Tatro J B, Coburn J, Parveen N. Different classes of proteoglycans contribute to the attachment of Borrelia burgdorferi to cultured endothelial and brain cells. Infect Immun. 1998;66:994–999. doi: 10.1128/iai.66.3.994-999.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parveen, N., D. Robbins, and J. M. Leong. Strain-to-strain variation in glycosaminoglycan binding and cell type-specific binding among Lyme disease spirochetes. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 19.Pejler G, Lindahl U, Larm O, Scholander E, Sandgren E, Lundblad A. Monoclonal antibodies specific for oligosaccharides prepared by partial nitrous acid deamination of heparin. J Biol Chem. 1988;263:5197–5201. [PubMed] [Google Scholar]
- 20.Radoff S, Danishefsky I. Isolation and properties of high molecular weight heparin. Thromb Res. 1981;22:353–365. doi: 10.1016/0049-3848(81)90128-6. [DOI] [PubMed] [Google Scholar]
- 21.Reantragoon S, Arrigo L M, Dweck H S, Rosenfeld L. Suppression of endothelin-1 production in cultured human umbilical vein endothelial cells by heparin fractions separated by strong anion exchange chromatography. Arch Biochem Biophys. 1996;327:234–238. doi: 10.1006/abbi.1996.0115. [DOI] [PubMed] [Google Scholar]
- 22.Rice K G, Rottink M K, Lindhardt R J. Fractionation of heparin oligosaccharides by gradient polyacrylamide gel electrophoresis. Biochem J. 1987;244:512–522. doi: 10.1042/bj2440515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silbert J E, Bernfield M, Kokenyesi R. Proteoglycans: a special class of glycoproteins. In: Montreuil J, Vliegenthart J F G, Schachter J, editors. Glycoproteins II. Oxford, United Kingdom: Elsevier; 1997. pp. 1–31. [Google Scholar]
- 24.Steere A C. Lyme disease. New Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]