Abstract
We report a case of tibial osteochondroma in a 25-year-old female who presented with a palpable calf mass. This mass was associated with a thick cartilaginous cap on cross-sectional imaging, suggesting chondrosarcoma. A CT-guided biopsy was performed, and histology, however, was consistent with osteochondroma. Orthopedic oncology recommended surgical excision due to the potential high sampling error with chondroid lesions. The patient underwent surgical resection, resulting in a final diagnosis of osteochondroma. No post-surgical complications occurred, and a 12-month follow-up showed no evidence of local recurrence. This case highlights the atypical imaging feature of a thick cartilaginous cap in a benign etiology without malignant transformation.
Keywords: Osteochondroma, Cartilaginous cap, Chondrosarcoma
Introduction
Osteochondroma is a common type of benign bone tumor that typically develops during childhood or adolescence. It is often asymptomatic, which makes the reported incidence underestimated. Symptoms could occur secondary to bursa formations, impingement on adjacent structures, and progressive pain due to rapid growth [1]. It typically affects the long bones of the appendicular skeleton, with the most common locations being the femur (30%), tibia (20%), and humerus (20%) [1,2]. It consists of a sessile or pedunculated bony outgrowth, arising from the external surface of the affected bone [3]. Imaging characteristics of osteochondroma include: (1) A stalk-like structure called a pedicle, which connects the tumor to the underlying bone. (2) Cartilage cap: The bony projection is covered by a cartilage cap, giving it a smooth surface. The thickness and size of the cartilage cap can however vary. (3) Cortical and medullary continuity: The tumor maintains a continuity with the surrounding bone cortex and medullary canal, indicating that it arises from the normal bone structure. (4) Growth pattern: Osteochondromas typically grow away from the adjacent joint, and their growth plates close when skeletal maturity is reached [3], [4], [5].
Osteochondromas can occur as solitary lesions (85%) or as part of an autosomal dominant hereditary condition called hereditary multiple osteochondromas (HMO), when multiple lesions occur. In HMO, multiple osteochondromas are present throughout the skeleton [6,7].
It's important to note that while most osteochondromas are benign, there is a less than 1% risk of malignant transformation for solitary osteochondromas, despite slightly higher risks, up to 5%, with multiple hereditary exostoses (HMOs) [1,2,5]. Rapid growth, increasing destruction on radiographs and thick cartilage cap greater than 2 cm are considered as a manifestation of malignant transformation [1], [2], [3],5]. Usually, imaging surveillances or follow-ups are typically recommended for asymptomatic individuals with osteochondromas [6,8]. Surgical resection is recommended for symptomatic patients with imaging features of malignant transformation [1,6]. Excision of osteochondromas is usually curative, with a less than 2% chance of recurrence for a complete removal [1]. Multiple recurrences of an incomplete removal or a recurrence of a complete removal are also signs of malignant transformation [1,3,9].
Herein, we reported a unique case of an osteochondroma with atypical imaging findings including lack of stalk-like pedicle on radiograph, and unusual thickened cartilage cap on MRI in a 25-year-old female. This case also highlights the dilemma of how best to manage a patient with palpable osteochondroma.
Case report
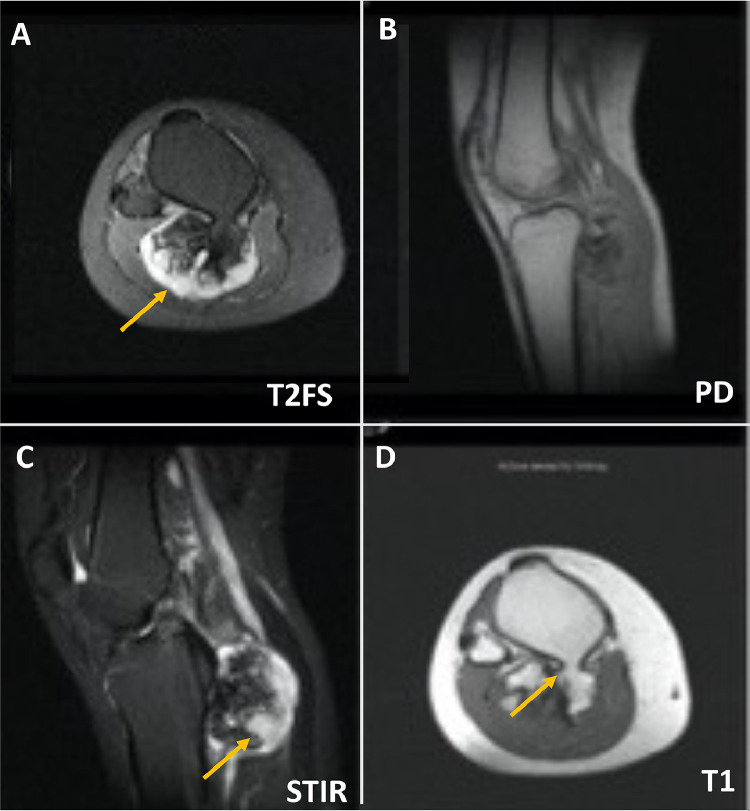
A 25-year-old female presented with a large hard palpable mass in the right posterior proximal tibia. Knee radiography showed a large, exophytic osseous lesion posterior to the proximal right tibia without definite stalk or corticomedullary continuation. It appears cauliflower-like without obvious medullary extension; therefore, it was favored to represent a parosteal osteosarcoma. Diagnostic considerations would also include juxtacortical chondroma, bizarre parosteal osteochondromatous lesion (BPOP), and osteochondroma. (Fig. 1A). Three-phase bone scan examination of the knees, with SPECT CT was subsequently performed to rule out other sites of disease. No significant abnormalities seen on the flow or blood pool images. On the delayed views, intense tracer uptake was identified in the large exostosis at the posterior proximal right tibia. The focal uptake was nonspecific. No other abnormalities were identified to suggest osseous metastases (Fig. 1B). To further characterise the lesion, multiple sequences of MRI were performed. Again, noted was a large exostosis arising from the posterior proximal tibial metaphysis which extended posteriorly; however, this clearly demonstrated cortical and medullary continuity and measured 5.6 cm medial - lateral by 3.9 cm anterior - posterior by 4.9 cm cranial-caudal. On T2 weighted images, there was a cartilaginous cap measuring up to 2 cm in maximal thickness. The popliteal neurovascular bundle was displaced laterally by the lesion and the gastrocnemius musculature was displaced slightly posteriorly. No edema was seen with the adjacent soft tissues. T1 weighted images demonstrated an apparent osseous pedicle connecting the lesion and tibial cortex. (Fig. 2A-D). The findings were most suggestive for an osteochondroma. Other diagnostic considerations including juxtacortical chondroma, and parosteal osteosarcoma were thought to be less likely but cannot be entirely excluded. The cartilaginous cap was however irregular and up to 2 cm in maximal thickness in this skeletally mature patient. Therefore, an element of malignant degeneration was difficult to exclude given the thickness of the cartilaginous cap. Consultation with orthopedic oncology was recommended. Based on the size of the cartilage cap, the surgeon agreed that a diagnosis of chondrosarcoma must be excluded. A CT- guided biopsy of the lesion in upper right tibia was obtained as requested by orthopedics (Fig. 3A). Specimens were obtained from the cartilaginous cap as well as the adjacent ossified region. Histology was consistent with osteochondroma.
Fig. 1.
(A) Frontal and lateral radiography showed a large, exophytic, ossific lesion posterior to the proximal right tibia as arrow indicated. (B). Three-phase examination of the knees, with SPECT CT was performed to rule out other sites of disease. No significant abnormalities were seen on the flow of blood pool images. On the delayed views, intense tracer uptake is identified in the large exostosis at the posterior proximal right tibia as arrow indicated.
Fig. 2.
(A-D) Multiple sequences of MRI remonstrated a large exostosis arising from the proximal tibial metaphysis which extends posteriorly. This measures 5.6 cm medial - lateral by 3.9 cm anterior - posterior by 4.9 cm craniocaudad. (A) T2 weighting demonstrates a cartilaginous cap which measures up to 2 cm in maximal thickness (as arrow indicated). (B). Marrow signal of tibia is otherwise preserved on PD sequence. The popliteal neurovascular bundle is displaced laterally by the lesion and the gastrocnemius musculature is displaced slightly posteriorly. (C) No edema is seen with the adjacent soft tissues on STIR sequence. (D) This confirmed cortical and medullary continuity best appreciated on T1 sequence, with an apparent osseous pedicle connecting the tumor and tibial cortex as arrow indicated. The findings are suggestive with an osteochondroma. Given the thick cartilaginous cap, an element of malignant degeneration is possible.
Fig. 3.
(A) A biopsy of the osteochondroma the upper right tibia has been obtained as requested by orthopedics. Specimens have been obtained from the cartilaginous cap. as well as the adjacent ossified region. No complication occurred. (B) Immediate follow-up radiography post-surgery confirm the complete removal of the tumor.
Although the patient was asymptomatic, orthopedic oncology recommended a surgical excision based on the potential high sampling error with chondroid lesions. Staging CT scans along with the bone scan were performed which did not show any evidence of metastatic disease. The patient signed a consent form and underwent a resection of posterior proximal tibial lesion. The procedure also involved dissection and protection of the short saphenous vein, popliteal artery and vein, posterior tibial artery and vein, and neurolysis of the right sural and tibial nerves. No complication occurred. Follow up radiography immediate post-surgery confirm the complete removal of the tumor (Fig. 3B).
Received in pathology was a fragment of bony tissue measuring 6.2 × 5.5 × 4.4 cm. The base of the bony tissue was flat without a definite stalk. The external surface was smooth and covered by periosteum. The cut surface contained a well-defined cartilage cap which had a glistening, slightly lobulated, gray-blue appearance (Fig. 4). The cartilage cap measured up to 1.8 cm in thickness (measured perpendicular to bone-cartilage interface). Histologically, the lesion was composed of three layers with perichondrium, cartilage, and bone (Fig. 5A). The cartilage cap demonstrated the zonation seen in endochondral ossification, that is, from superficial to deep, zone of reserve cells (Fig. 5B), zone of proliferation (Fig. 5C), zone of hypertrophy (Fig. 5D), zone of degeneration (Fig. 5E) and zone of ossification (Fig. 5F). There was no significant cytologic atypia. Mitoses were absent.
Fig. 4.
On gross examination, the cartilage cap measured up to 1.8 cm in thickness (measured perpendicular to the bone-cartilage interface) and had a glistening, slightly lobulated, gray-blue appearance.
Fig. 5.
(A) Low power of the lesion showed the three layers with perichondrium cartilage, and bone (0.7x). The cartilage cap demonstrated the zonation seen in endochondral ossification with zone of reserve cells (B, 4x), zone of proliferation (C, 4x), zone of hypertrophy (D, 4x) and zone of degeneration (E, 4x). The interface of bone and cartilage demonstrated the zone of ossification (F, 10x).
After the surgery, the patient quickly resumed his prior level of activity, and there was no evidence of the lesion recurring during the 12-month monitoring period.
Discussion
Malignant transformation of an osteochondroma is an extremely rare occurrence, but it is a recognized complication that can occur in a small percentage of cases [10]. The exact cause of malignant transformation is not fully understood, but certain risk factors may increase the likelihood, such as multiple hereditary exostoses [7]. When an osteochondroma undergoes malignant transformation, it becomes a form of sarcoma [9], [10], [11]. Osteosarcoma, spindle cell sarcoma, and dedifferentiated chondrosarcomas have been reported to develop in osteochondromas [8]. However, the most common malignancy is peripheral chondrosarcoma [1,4].
This case is unique since thick cartilage is present in a benign osteochondroma therefore highlights the limitations of imaging features to suggest potential risk of malignant transformation. Very few similar literatures exist in adult [1]. Javdan et al. [12] documented a case of a juxta-articular benign tibia osteochondroma featuring a substantial layer of hyaline cartilage. In pediatric population, a thickness cut off is greater than 3 cm worrisome for malignant transformation [13,14], while some literatures suggest the cutoff of 1.5 cm regardless of the ages [3,15]. Nevertheless, accurate measurements of cartilage cap thickness may be challenging in osteochondroma with associated bursa formation since the bursal fluid and the cartilage cap with high fluid content have similar CT densities and similar MRI intensities on most MRI sequences [16]. In our case, in the absence of bursa formation, atypical image findings including thick cartilaginous cap concerned the clinical specialist, therefore biopsy was performed.
The distinction between a benign osteochondroma and low grade 1 peripheral chondrosarcoma also known as secondary peripheral atypical cartilaginous tumor (ACT) could be also difficult to differentiate histologically; benign osteochondromas can show some atypical features such as binucleation, focal necrosis, nodularity, and degenerative changes. It has higher than usual sampling error or interobserver reliability with chondroid lesions [17]. The histological features would also require to interpret with caution [1].The diagnosis of a secondary peripheral atypical cartilaginous tumor (ACT) arising from osteochondroma sometimes require to incorporate with the clinical manifestations, such as the patient's age and symptoms, and radiology findings, including the thickness of the cartilage cap [1,9].
This case also emphasizes the clinical implications for different treatments of osteochondroma and highlights the dilemma of how best to manage a patient with palpable osteochondroma. The treatment approach for an osteochondroma also depends on the symptoms and potential complications it presents [1]. Benign osteochondromas have an excellent prognosis. In most cases, particularly in asymptomatic patients, osteochondromas do not require treatment. If the cartilage cap thickness measures 1.0-1.5 cm, the risk of malignant transformation is low. Imaging surveillance or follow-ups are typically sufficient [6,8]. If there is rapid growth of tumor and/or thick cartilage cap greater than 2 cm or other imaging manifestation of malignant transformation, then surgical resection is warranted [11]. In some instances, the cartilaginous cap can lead to complications, such as pain, limited range of motion, or pressure on surrounding structures (nerves or blood vessels). When significant pain or functional impairment are present, surgical removal may be considered. The treatment of a malignant osteochondroma or secondary chondrosarcoma typically involves surgical removal of the tumor, along with a margin of healthy tissue. The extent of surgery depends on the size, location, and stage of the tumor. In some cases, additional treatments such as radiation therapy or chemotherapy may be recommended to target any remaining cancer cells or to treat potential metastases [1].
The main limitation of this study stems from the potential variability in outcomes across different clinical settings. In the absence of surgical intervention, the benign tumor may demonstrate stability, reduction, or increase during follow-up examinations. Interestingly, Avramidis et al. [18] have noted instances of rapid growth in benign osteochondromas, while Heyworth et al. [19] have reported occurrences of slow regression in specific situations.
If one suspects malignant transformation of an osteochondroma or has concerns about specific condition, it is essential to consult with an orthopedic oncologist, who can evaluate the specific case and provide appropriate advice and treatment options. They will take into account factors such as the location, size, symptoms, and potential complications associated with the osteochondroma to guide decision-making and develop an individualized treatment plan.
Conclusion
In summary, we described a patient with a tibial osteochondroma associated with an unexpected thick cartilaginous cap on cross-sectional imaging. This case highlights the atypical imaging feature to benign etiology without malignant transformation. Furthermore, it underscores the importance of considering varied treatment approaches for osteochondromas. Seeking consultation with an orthopedic oncologist is recommended to evaluate the specific case and receive tailored advice and treatment options.
Patient consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Footnotes
Competing Interests: The authors declare that they have no competing interests. All authors have seen and approved the final version of the manuscript being submitted. We warrant that the article is the authors' original work, hasn't received prior publication and isn't under consideration for publication elsewhere.
References
- 1.Tepelenis K, Papathanakos G, Kitsouli A, Troupis T, Barbouti A, Vlachos K, et al. Osteochondromas: an updated review of epidemiology, pathogenesis, clinical presentation, radiological features and treatment options. In Vivo. 2021;35(2):681–691. doi: 10.21873/invivo.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pontes ICM, Leao RV, Lobo CFT, Paula VT, Yamachira VS, Baptista AM, et al. Imaging of solitary and multiple osteochondromas: from head to toe - a review. Clin Imaging. 2023;103 doi: 10.1016/j.clinimag.2023.109989. [DOI] [PubMed] [Google Scholar]
- 3.Murphey MD, Choi JJ, Kransdorf MJ, Flemming DJ, Gannon FH. Imaging of osteochondroma: variants and complications with radiologic-pathologic correlation. Radiographics. 2000;20(5):1407–1434. doi: 10.1148/radiographics.20.5.g00se171407. [DOI] [PubMed] [Google Scholar]
- 4.Brien EW, Mirra JM, Kerr R. Benign and malignant cartilage tumors of bone and joint: their anatomic and theoretical basis with an emphasis on radiology, pathology and clinical biology. I. The intramedullary cartilage tumors. Skeletal Radiol. 1997;26(6):325–353. doi: 10.1007/s002560050246. [DOI] [PubMed] [Google Scholar]
- 5.Woertler K, Lindner N, Gosheger G, Brinkschmidt C, Heindel W. Osteochondroma: MR imaging of tumor-related complications. Eur Radiol. 2000;10(5):832–840. doi: 10.1007/s003300051014. [DOI] [PubMed] [Google Scholar]
- 6.de Souza AM, Bispo Junior RZ. Osteochondroma: ignore or investigate? Rev Bras Ortop. 2014;49(6):555–564. doi: 10.1016/j.rboe.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DuBose CO. Multiple hereditary exostoses. Radiol Technol. 2016;87(3):305–321. quiz 22-5. [PubMed] [Google Scholar]
- 8.Dekker AP, Grimer RJ. Transformation of solitary osteochondroma to dedifferentiated chondrosarcoma arising in the distal radius: a case report. Musculoskelet Surg. 2013;97(1):89–92. doi: 10.1007/s12306-012-0226-z. [DOI] [PubMed] [Google Scholar]
- 9.Tong K, Liu H, Wang X, Zhong Z, Cao S, Zhong C, et al. Osteochondroma: review of 431 patients from one medical institution in South China. J Bone Oncol. 2017;8:23–29. doi: 10.1016/j.jbo.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hari A, Kavar B. Rare case of malignant transformation of a solitary spinal osteochondroma into recurrent metastatic chondrosarcoma. J Clin Neurosci. 2019;67:280–288. doi: 10.1016/j.jocn.2019.05.045. [DOI] [PubMed] [Google Scholar]
- 11.Sajid S, Yousaf A, Nabi U, Shahbaz A, Amin U. Sarcomatous transformation of recurrent scapular osteochondroma in a patient with the hereditary multiple osteochondromas: a case report and literature review. Cureus. 2019;11(12):e6308. doi: 10.7759/cureus.6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Javdan M, Hekmatnia A, Ghazavi A, Basiratnia R, Mehrzad M, Hekmatnia F, et al. A case report of osteochondroma with unusual clinical and imaging presentation. Adv Biomed Res. 2015;4:2. doi: 10.4103/2277-9175.148258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernard SA, Murphey MD, Flemming DJ, Kransdorf MJ. Improved differentiation of benign osteochondromas from secondary chondrosarcomas with standardized measurement of cartilage cap at CT and MR imaging. Radiology. 2010;255(3):857–865. doi: 10.1148/radiol.10082120. [DOI] [PubMed] [Google Scholar]
- 14.Richardson RR. Variants of exostosis of the bone in children. Semin Roentgenol. 2005;40(4):380–390. doi: 10.1053/j.ro.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Shah ZK, Peh WC, Wong Y, Shek TW, Davies AM. Sarcomatous transformation in diaphyseal aclasis. Australas Radiol. 2007;51(2):110–119. doi: 10.1111/j.1440-1673.2007.01679.x. [DOI] [PubMed] [Google Scholar]
- 16.Maras Ozdemir Z, Karakaplan M, Kahraman AS, Karadag N. Adventitious bursitis overlying an osteochondroma of the humerus facing the thoracic wall. Case Rep Radiol. 2013;2013 doi: 10.1155/2013/939372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eefting D, Schrage YM, Geirnaerdt MJ, Le Cessie S, Taminiau AH, Bovee JV, et al. Assessment of interobserver variability and histologic parameters to improve reliability in classification and grading of central cartilaginous tumors. Am J Surg Pathol. 2009;33(1):50–57. doi: 10.1097/PAS.0b013e31817eec2b. [DOI] [PubMed] [Google Scholar]
- 18.Avramidis K, Katounis C, Krikis P, Skoufogiannis P. A solitary, large calcaneal osteochondroma growing extensively after skeletal maturity: a case report and review of the literature. Cureus. 2023;15(7):e42570. doi: 10.7759/cureus.42570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heyworth PB, Rashid M. Regression of a solitary osteochondroma of the distal humerus in a toddler following trauma. Radiol Case Rep. 2019;14(2):187–189. doi: 10.1016/j.radcr.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]