Abstract
We report the case of a woman in her 40s who presented with sensory disturbances in all 4 limbs and left facial palsy. MRI revealed asymmetric enlargement of the dorsal root ganglia, which was enhanced by gadolinium—a chest CT scan identified enlarged supraclavicular, mediastinal, and hilar lymph nodes. A biopsy of a hilar lymph node showed noncaseating epithelioid granulomas, confirming a sarcoidosis diagnosis. Prednisolone treatment led to symptomatic improvements. In sarcoidosis of the peripheral nervous system, there might be observable enlargement of the dorsal root ganglion alongside enhanced gadolinium contrast. Obtaining a biopsy from the dorsal root ganglion poses challenges, and radiologists should be mindful of this specific imaging characteristic.
Keywords: Neurosarcoidosis, Peripheral nervous system, Dorsal root ganglion enlargement, MRI
Introduction
Sarcoidosis is a systemic disease pathologically characterized by noncaseating epithelioid granulomas. Chest lymph node enlargement and pulmonary involvement are most common (86%-95%), followed by skin involvement (24.2%-60.6%) [1], [2]–3]. Neurosarcoidosis, characterized by nervous system involvement, is present in approximately 4%-8.8% of cases [1–3]. Among patients with neurosarcoidosis, peripheral nerve involvement is less common (10.3%) than brain parenchyma (55.6%), spinal cord (26.5%), and cranial nerve (36.8%) involvement [4]. The most prevalent peripheral neuropathy comprises sensory disturbances (98%), with most patients (89%) exhibiting an acute or subacute onset [5]. Sarcoid peripheral neuropathy nerve biopsies reveal perineurial noncaseating epithelioid granulomas and granulomatous vasculitis [6]. Whereas various imaging characteristics have been reported for central and cranial nerve sarcoidosis, peripheral nervous system involvement reports are less common.
Case report
We report the case of a 44-year-old woman who presented with a 2-month history of diminishing visual acuity in her left eye and erythema on both thighs. There were no notable findings in her medical or lifestyle history. Funduscopy revealed vitreous opacities and phlebitis in the left eye, leading to a uveitis diagnosis. A chest X-ray showed enlarged bilateral hilar lymph nodes. The laboratory analysis revealed increased serum ACE activity (33.4 IU/L (normal range: 8.3-21.4 IU/L)), increased serum lysozyme levels (12.9 µg/mL (normal range: 5.0-10.2 µg/mL)), and increased serum sIL-2R (1442 U/mL (normal range: 121-613 U/mL)), leading to the suspicion of sarcoidosis. Initially, she solely presented with ocular and skin lesions, and these manifestations improved with the application of steroid eye drops and ointment. However, 3 weeks later, numbness developed on the front surface of her left thigh, spreading to her left cheek, both upper limbs (with right-side predominance), her left back, and both feet. She also developed dexterity issues in her right hand and left facial paralysis. MR neurography showed unilateral enlargement of the right C7,8, left L3, and right L5 dorsal root ganglia (Fig. 1). Gadolinium MRI showed an enhanced contrast effect in the same area (Fig. 2). FDG-PET/CT showed increased accumulation in the same area and the left dorsal root ganglion of Th11 (Fig. 3). Gadolinium MRI showed an enhanced left facial nerve and enhanced nodules along the cauda equina. Chest CT showed enlarged supraclavicular, mediastinal, and hilar lymph nodes. Endoscopic ultrasound-guided fine-needle aspiration revealed noncaseating epithelioid granulomas from the hilar lymph node. Moreover, a skin punch biopsy from an erythema annulare in the right plantar region and the right nasal septum revealed granulomas consistent with sarcoidosis. Methylprednisolone pulse therapy, at a dose of 1 g per day, resulted in a trend in sensory disturbance improvements in the limbs. However, the persistence of left facial paralysis led to the subsequent prescription of 50 mg/day of prednisolone as a post-therapy measure. The patient remains on a steroid-tapering regimen.
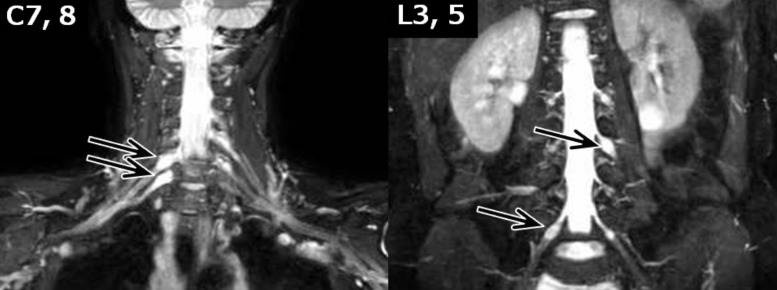
Fig. 1.
MR neurography. The MR neurography (3D-spin echo T2-weighted imaging with fat suppression) shows unilateral enlargement of the dorsal root ganglia of right C7, right C8, left L3, and right L5 (arrows).
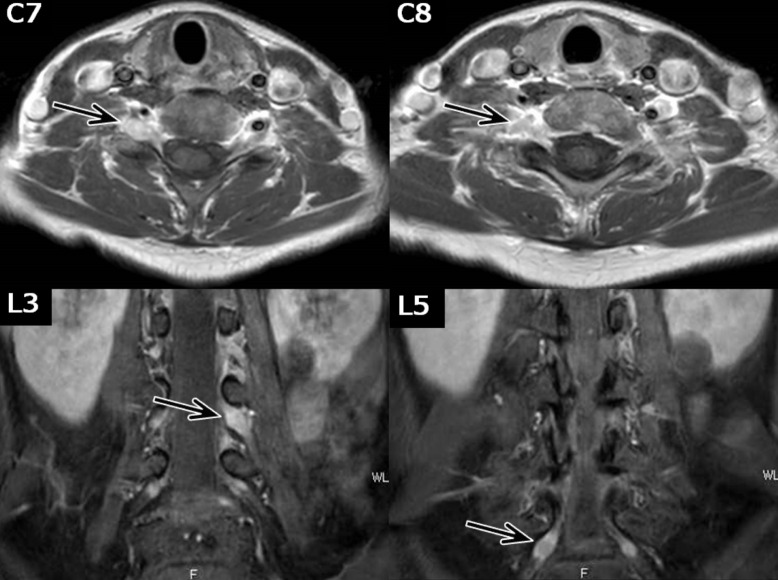
Fig. 2.
Gadolinium-enhanced MRI T1-weighted images. The dorsal root ganglia, with enlargement on MR neurography, showed an enhanced contrast effect (arrow).
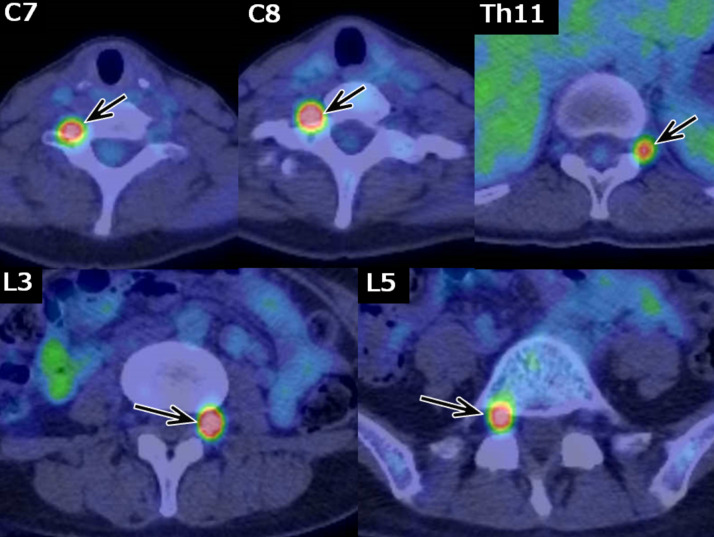
Fig. 3.
FDG-PET. Increased FDG accumulation was observed in the dorsal root ganglia (arrows), corresponding to the dorsal root ganglia that showed enlargement and an enhanced contrast effect on MRI. The left Th11 dorsal root ganglion (upper right, arrow) showed no obvious abnormalities on MRI but hyperaccumulation on FDG-PET.
Discussion
Imaging reports of sarcoidosis of the peripheral nervous system are scarce; however, this case demonstrated characteristic findings on MRI and FDG-PET/CT. Specifically, MRI revealed asymmetric enlargement and an enhanced contrast effect in the dorsal root ganglia, and FDG-PET/CT showed increased accumulation in the corresponding area. These imaging results were correlated with the clinical neurological symptoms observed. Despite being less frequently reported, cases with enlarged and contrast-enhanced dorsal root ganglia, similar to in the present case, have been reported [7,8], demonstrating consistency with prior reports. Neurosarcoidosis is not the sole disease that targets the dorsal root ganglion. There have been instances of amyloidosis, where the dorsal root ganglion initially shows enlargement, and pathological examinations uncover amyloid deposits in the same region [9]. Further, dorsal root ganglion cells are damaged during chemotherapy-induced peripheral neuropathy, leading to sensory neuropathy [10]. The absence of a blood–nerve barrier in the dorsal root ganglion is presumed to be a contributing factor in both cases [9,10]. For the same reason, sarcoid granulomas might be deposited in the dorsal root ganglia during neurosarcoidosis.
Conclusion
We described a sarcoidosis case characterized by dorsal root ganglion enlargement, an enhanced gadolinium contrast effect, and increased FDG accumulation. The absence of a blood–nerve barrier in the dorsal root ganglion suggests that it could be prone to the deposition of granulomas. Neurosarcoidosis should be considered as a differential diagnosis when dorsal root ganglion enlargement and gadolinium enhancement are observed.
Patient consent
Written informed consent for patient information to be published in this article was obtained.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Mañá J, Rubio-Rivas M, Villalba N, Marcoval J, Iriarte A, Molina-Molina M, et al. Multidisciplinary approach and long-term follow-up in a series of 640 consecutive patients with sarcoidosis: cohort study of a 40-year clinical experience at a tertiary referral center in Barcelona, Spain. Medicine (Baltimore) 2017;96(29):e7595. doi: 10.1097/MD.0000000000007595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James DG, Neville E, Siltzbach LE. A worldwide review of sarcoidosis. Ann N Y Acad Sci. 1976;278:321–334. doi: 10.1111/j.1749-6632.1976.tb47043.x. [DOI] [PubMed] [Google Scholar]
- 3.Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H, Bresnitz EA, et al. Case control etiologic study of sarcoidosis (ACCESS) research group. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1885–1889. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 4.Joubert B, Chapelon-Abric C, Biard L, Saadoun D, Demeret S, Dormont D, et al. Association of prognostic factors and immunosuppressive treatment with long-term outcomes in neurosarcoidosis. JAMA Neurol. 2017;74(11):1336–1344. doi: 10.1001/jamaneurol.2017.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nozaki K, Judson MA. Neurosarcoidosis: clinical manifestations, diagnosis, and treatment. Presse Med. 2012;41(6 Pt 2):e331–e348. doi: 10.1016/j.lpm.2011.12.017. Epub May 15, 2012. [DOI] [PubMed] [Google Scholar]
- 6.Said G, Lacroix C, Planté-Bordeneuve V, Le Page L, Pico F, Presles O, et al. Nerve granulomas and vasculitis in sarcoid peripheral neuropathy: a clinicopathological study of 11 patients. Brain. 2002;125(Pt 2):264–275. doi: 10.1093/brain/awf027. [DOI] [PubMed] [Google Scholar]
- 7.Hamodat H, Tran A. Neurosarcoidosis resulting in thoracic radiculopathy: a case report. J Med Case Rep. 2019;13(1):130. doi: 10.1186/s13256-019-2065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishibashi M, Kimura N, Takahashi Y, Kimura Y, Hazama Y, Kumamoto T. A case of neurosarcoidosis with swelling and gadolinium enhancement of spinal nerve roots on magnetic resonance imaging. Rinsho Shinkeigaku. 2011;51(7):483–486. doi: 10.5692/clinicalneurol.51.483. Japanese. [DOI] [PubMed] [Google Scholar]
- 9.Murakami T, Watanabe H, Yamamoto A, Sunada Y. Magnetic resonance imaging of dorsal root ganglion in a pre-symptomatic subject with familial amyloid polyneuropathy transthyretin E61K. J Neurol Sci. 2022;440 doi: 10.1016/j.jns.2022.120329. Epub June 24, 2022. [DOI] [PubMed] [Google Scholar]
- 10.Argyriou AA, Park SB, Islam B, Tamburin S, Velasco R, Alberti P, et al. Toxic neuropathy consortium (TNC). Neurophysiological, nerve imaging and other techniques to assess chemotherapy-induced peripheral neurotoxicity in the clinical and research settings. J Neurol Neurosurg Psychiatry. 2019;90(12):1361–1369. doi: 10.1136/jnnp-2019-320969. Epub June 29, 2019. [DOI] [PubMed] [Google Scholar]