Abstract
The gastrointestinal hormone peptide YY is a potent inhibitor of food intake and is expressed early during differentiation of intestinal and pancreatic endocrine cells. In order to better understand the role of peptide YY in energy homeostasis and development, we created mice with a targeted deletion of the peptide YY gene. All intestinal and pancreatic endocrine cells developed normally in the absence of peptide YY with the exception of pancreatic polypeptide (PP) cells, indicating that peptide YY expression was not required for terminal differentiation. We used recombination-based cell lineage trace to determine if peptide YY cells were progenitors for gastrointestinal endocrine cells. Peptide YY+ cells gave rise to all L-type enteroendocrine cells and to islet ∂ and PP cells. In the pancreas, approximately 40% of pancreatic α and rare β cells arose from peptide YY+ cells, suggesting that most β cells and surprisingly the majority of α cells are not descendants of peptide YY+/glucagon-positive/insulin-positive cells that appear during early pancreagenesis. Despite the anorectic effects of exogenous peptide YY3-36 following intraperitoneal administration, mice lacking peptide YY showed normal growth, food intake, energy expenditure, and responsiveness to peptide YY3-36. These observations suggest that targeted disruption of the peptide YY gene does not perturb terminal endocrine cell differentiation or the control of food intake and energy homeostasis.
Peptide YY is a 36-amino-acid hormone (45) synthesized primarily in L-type enteroendocrine cells in the colon and ileum and in cells along the periphery of pancreatic islets. Like that of other gastrointestinal hormones, release of peptide YY appears to be stimulated by the presence of enteral secretagogues, especially dietary fat, present in the distal intestine. The mechanisms that control this release are not completely understood (14). Peptide YY functions as a ligand for a number of receptors of the closely related neuropeptide Y. Peptide YY appears to have widespread inhibitory effects on gastrointestinal function, including inhibition of gastric emptying, gastric motility, and pancreatic exocrine secretion (25, 31). The putative actions of peptide YY do not appear to result from direct effects on neuropeptide Y receptors in the gastrointestinal tract but instead by binding to receptors in the dorsal vagal complex of the brain stem and inhibiting vagal tone (16, 32).
Recent work has suggested that peptide YY might function as a major regulator of food intake, consistent with observations that peptide YY is released in proportion to caloric intake (33). Peptide YY3-36, which is generated by enzymatic cleavage of the full-length peptide by dipeptidyl peptidase IV (26), inhibited food intake for up to 12 h when administered to either rodents and humans (3). Following a meal, approximately 63% of circulating peptide YY in humans was shown to be peptide YY3-36 (13). Unlike the full-length peptide, peptide YY3-36, functions as a ligand for the Y2 receptor (22) and appeared to regulate food intake in mice via Y2 receptors on neurons in the hypothalamus that inhibit the orixegenic neuropeptide Y system in the arcuate nucleus (3).
We previously showed that peptide YY was expressed in murine pancreatic and colonic endocrine cells when they first appeared during embryonic development. This observation raised the possibility that cells expressing peptide YY were endocrine precursor cells (49, 50). Several studies suggested that pancreatic polypeptide or possibly neuropeptide Y were expressed in immature islets in the developing murine pancreas (17, 46). Subsequently, peptide YY was identified as the pancreatic polypeptide (PP) family member expressed during the earliest stage of endocrine differentiation at embryonic day 9.5 (E9.5), when the pancreas is budding from the foregut (21, 49). These peptide YY-positive cells were also immunopositive for glucagon and, in many cases, insulin immunoreactivity. Peptide YY was coexpressed in somatostatin and pancreatic polypeptide cells when they first appeared, later in development. This early coexpression was not maintained in adult mice, where peptide YY was expressed in only a fraction of glucagon-producing alpha cells and was not expressed in insulin-producing β cells. These observations led to the suggestion that peptide YY was expressed in a common islet progenitor cell and that its expression was extinguished during the maturation of alpha and β cells (49).
Peptide YY is also the first hormone expressed in the developing murine colon, appearing at day 15.5 of gestation. PP was coexpressed with other hormones (glucagon-like peptide 1, neurotensin, cholecystokinin, substance P, serotonin, secretin, and gastrin) when they first appeared later during fetal development, between days 16.5 and 18.5. This coexpression was sustained in most cell types into adulthood, with the exception of serotonin and substance P-expressing cells, which no longer coexpressed peptide YY in the adult distal intestine (50). These experimental findings raised the possibility that all enteroendocrine cells in the colon arose from a common peptide YY-expressing precursor cell.
In the present work we generated mice lacking a functional peptide YY gene to further understand the importance of peptide YY in energy homeostasis and endocrine differentiation in the gastrointestinal tract. Peptide YY null mice exhibited normal growth and food intake, illustrating the functional redundancy of factors controlling energy homeostasis. In addition, our results indicate that terminal differentiation of endocrine cells in the pancreas and intestine is largely unaffected by the absence of peptide YY. Using recombination based cell lineage tracing, we also identified L-type enteroendocrine cells in the distal intestinal tract, as well as pancreatic ∂ and PP cells, which arose from peptide YY-expressing cells. In contrast, most pancreatic β cells, and jejunal and duodenal endocrine cells did not appear to differentiate from cells that previously expressed peptide YY.
MATERIALS AND METHODS
Animal care.
All animal protocols were approved by the Tufts-New England Medical Center Institutional Care and Use Committee in compliance with federal and institutional policies.
Targeting construct.
A 16-kb fragment containing the Pyy gene, isolated from a mouse genomic phage library, was subcloned into pGEM-5Zf (+) (Pyy-NotI) and mapped using a combination of PCR, restriction enzyme digests, and Southern analysis. The targeting vector was assembled as follows. A 3.5-kb EcoRI fragment containing Pyy was subcloned into PGEM-7 (Pyy-RI). A BamHI-SalI (blunt) fragment containing the bacterial lacZ gene (pBS2.LacZ) with a nuclear localization signal was subcloned into StuI sites within Pyy-RI resulting in a deletion of most of exons 2 to 4 (spanning amino acids 7 to 93), creating an in-frame fusion. A HindIII/Xho fragment from this Pyy-lacZ construct was then subcloned back into Pyy-NotI, creating Pyy-NotI-lacZ. A SalI fragment containing a phosphoglycerate kinase-neomycin cassette (PGK-Neo) from PNTK (A) LP2 (MAM) was inserted into a SalI site downstream of lacZ. A blunt NotI-BamHI fragment containing herpes simplex virus thymidine kinase (also from PNTK[A]LP2) was then inserted into a SpeI site at the 5′ end of the Pyy-NotI-lacZ-PGK-Neo construct to create the final targeting vector.
ES cell electroporation and blastocyst injections.
The targeting vector described above was used to replace the coding sequence of Pyy by homologous recombination in TL-1 embryonic stem (ES) cells. The targeting vector was introduced into ES cells by electroporation and selected for growth in the presence of G418. Surviving clones were screened for the targeted allele by Southern analysis using a probe specific for Pyy sequence adjacent to the targeted disruption. Two correctly targeted clones were identified from 494 colonies that were picked. Both clones were microinjected into blastocysts derived from matings of C57BL/6 animals. Chimeras generated from clone 5H10 were bred to C57BL/6 females and resulted in germ line transmission. To remove the loxP-flanked neo cassette, mice that were heterozygous for the targeted allele (PyylacZ+neo) were crossed with EIIa-cre transgenic mice (FVB/N-Tg[EIIa-cre]C5379Lmgd) that were obtained from Jackson Laboratories.
Genotyping.
Genotyping was performed by PCR of genomic DNA obtained from tail tissue by using a mix of three primers: 5′ Pyy sense (TCAGTAGCTGTCGAGCCTTC), lacZ antisense (AAAGCGCCATTCGCCATTC), and 3′ Pyy antisense (CGA-GCA-GGA-TTA-GCA-GCA-TT).
BAC transgene construction by homologous recombination in Escherichia coli.
We introduced sequences encoding human growth hormone (hGH) (24) spanning from the ATG to the poly(A) site into a suicide vector (derived from pKD4) (10) upstream of a frt flanked aminoglycoside phosphotransferase gene encoding kanamycin resistance (KANA) to make pKD4-hGH-KANA. We also introduced sequences encoding Cre recombinase, a nuclear localization signal, and simian virus 40 polyadenylation sequences (a gift from K. Kaestner, University of Pennsylvania) into pKD4 to make pKD4-nlsCre-KANA. PKD4-hGH-KANA and PKD4-nlsCreKANA were used as a PCR template to amplify fragments containing hghKANA and nlsCreKANA and 5′ and 3′ ends homologous to sequences flanking mouse Pyy exon 1 primers (PyyhGH sense, GGG-AAG-CTC-TGA-GCA-GAG-GCC-ACG-GAG-TTCAGTAGCTGTCGATCCCAAGGCCCAACTCCC; PyyKANA antisense, GAG-CAG-GAG-GAG-ATG-GAA-GGT-GGG-AGA-AGC-TCG-AAG-GCT-CCC-TCC-TTA-GTT-CCT-ATT-CCG-A; and PyyCre sense, GGG-AAG-CTC-TGA-GCA-GAGGCC-ACG-GAG-TTC-AGT-AGC-TGTCGA-CCA-TGC-CCA-AGA-AGA-AGAG; italic regions are homologous to sequence flanking Pyy exon 1).
Pyy bacterial artificial chromosome (BAC) clone 168M3 was obtained from screening a genomic mouse library (Incyte Genomics). End sequence was obtained and we determined that the Pyy BAC clone contained genomic sequence from −117 kb to +130 kb using initially Southern analysis and subsequently by comparison with the mouse genome. Bacteria containing the BAC and expressing the λ phage red recombination system (8, 10) were made competent and transformed with the amplified fragments. Clones in which the amplified fragment was inserted via homologous recombination were selected using resistance to chloramphenicol and kanamycin. Selected colonies were screened for correct recombination by amplification using Pyy and hGH or Cre-specific primers. The FRT-flanked KANA gene cassettes were excised from PyyhGHKANA and Pyy-nlsCreKANA BAC clones by transiently expressing FLP recombinase (8, 10). Southern blots, field inversion gel electrophoresis, and partial DNA sequencing confirmed the structure of the transgene and integrity of the BAC flanking sequence.
Production of transgenic mice.
The PyyhGH and Pyynls-Cre BACs were linearized with NotI and purified for pronuclear microinjection at a concentration of 1 ng/μl (20). Potential founder mice were genotyped by tail DNA amplification using primers specific for hGH and Cre coding sequence (hGH primers: sense, CCT-CAG-GGT-TTG-GGG-TTC-TGA-A; antisense, TCC-TGG-TAG-GTG-TCA-AAG-GCC-A) (Cre primers: sense, CTA-ATC-GCC-ATC-TTC-CAG-CAG; antisense, ATG-TCC-AAT-TTA-CTG-ACC-GTA). Transgenic pedigrees were maintained on a CD1 background. The Pyy-hGH line and one of two Pyy-Cre lines showed essentially identical patterns of expression and were used for all studies. A second Pyy-Cre line showed incomplete Cre expression in PYY-expressing enteroendocrine cells and misexpression of Cre in PYY-islet cells and was not used for further studies. Pyy-Cre transgenic mice were crossed to homozygous R26R mice (B6.129S4-Gt 26SortmSor) from Jackson Laboratories (43), to produce Pyy-Cre × R26R heterozygous mice.
Immunohistochemistry.
Tissue samples were fixed as described previously (36, 38) and processed for either paraffin or frozen sections. 5-Bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-Gal) histochemistry on tissues was carried out as described previously (41). For X-Gal histochemistry to detect lacZ expression from the ROSA26 indicator strain, tissues were fixed in 1.0% gluteraldehyde, 5 mM EGTA, in rinse buffer (phosphate-buffered saline plus 2 mM MgCl2, 0.01% sodium deoxycholate, 0.1% Triton X) and stained with rinse buffer containing X-Gal solution for 12 to 24 h.
Slides were incubated with the following primary antibodies: rabbit anti-β-galactosidase at 1:2,500 (Cappel), rabbit anti-Cre at 1:10,000 (Novagen), rabbit anti-chromogranin A at 1:3,000 (ImmunoStar), and sheep anti-hGH at 1:50,000 (Cortex Biochem). Previously described primary antibodies included: guinea pig anti-insulin at 1:5,000, rabbit anti-glucagon at 1:3,000, rabbit anti-somatostatin at 1:3,000, guinea pig anti-PYY 1:5,000, rabbit anti-GLP1 at 1:10,000 and rabbit anti-serotonin 1:40,000 (24, 50). Rabbit anti-human PP 1:2,000, obtained from Ron Chance (Eli Lilly), did not cross-react with PYY or immunostain PYY in the colon. Most antibodies were detected by immunoperoxidase using the avidin-biotin complex method and detected with diaminobenzidine. For double immunofluorescence experiments antibodies were detected with Cy3 or Cy2-conjugated secondary antibodies (Jackson Immunoresearch), or by tyramide signal amplification (TSA, Molecular probes). Colocalization studies with two rabbit primary antibodies were performed using a monovalent Fab fragment strategy (29) or by tyramide amplification (5). In all cases controls showed that the anti-rabbit immunoglobulin G antibodies were unable to detect the first rabbit primary.
Morphometric analysis.
Multiple sections from pancreas, small intestine, and colon from at least 3 animals from each transgenic line, and from Pyy+/+, Pyy+/− and Pyy−/− mice were analyzed for each immunohistochemical experiment. For calculating the number of chromogranin A cells per unit length of intestine, at least 3 different animals were analyzed for each genotype and a minimum of 25 mm of intestinal length was analyzed per animal.
PYY and pancreatic polypeptide reverse transcriptase-PCR.
RNA was isolated from whole pancreas or colon of Pyy−/−, Pyy+/−, and Pyy+/+ mice as described previously (51); 2.5 μg of total pancreatic RNA was reverse transcribed using random decamers into cDNA (Retroscript kit; Ambion). cDNA (1.5 μl) was used in a 25-μl PCR mixture containing primers specific to Pyy (sense, CGTCACGGTCGCAATGCTGCTA; antisense, AACACATCTCGCAGGAGGCCTTGG) or PP (sense, TCTCGTATCCACTTGGGTGG; antisense, AAGTCCATTGGGCAGAGCTC) and to 18S rRNA (Quantum RNA 18S internal standards; Ambion). The reactions were amplified in 30 cycles (annealing temperature was 59°C). The lengths of the PCR products were as follows: PP product, 300 bp; and Pyy product, 303 bp. Amplification of 18S rRNA (489 bp) served as an internal control.
Null animals were injected with either 150 nmol/kg of PYY1-36 or saline three times a day for 5 days. The pancreas of the treated animals were examined by immunohistochemistry and reverse transcription-PCR, as described above, to assess PP expression.
Feeding studies.
For analysis of food intake, mice were fasted overnight (16 to 18 h). The following day mice were weighed and then placed into individual cages containing preweighed rodent chow, with free access to water. For basal food intake analysis, the chow was reweighed at 2, 4, 8, and 24 h, and total food intake (g/g body weight) was calculated. Food intake in response to PYY administration was determined as above except that mice were given an intraperitoneal injection (100 μl) of PYY3-36 (20 μg/100 g body weight) or vehicle (phosphate-buffered saline) prior to placement into individual cages containing preweighed food.
Indirect calorimetry.
Oxygen consumption (VO2), carbon dioxide generation (VCO2), and respiratory exchange ratio (ratio of VO2 to VCO2) were determined by indirect calorimetry using an Oxymax System (Columbus Instruments, Columbus, OH). For basal studies, mice were placed into individual metabolic chambers with free access to food and water. VO2 and VCO2 were measured and respiratory exchange ratio was determined at 15-minute intervals from 11:00 a.m. to 7:45 a.m. the following day (approximately 21 h). VO2, VCO2, and respiratory exchange ratio measurements in response to PYY administration were determined as above except that mice were given an intraperitoneal injection (100 μl) of PYY3-36 (20 μg/100 g body weight) or vehicle (phosphate-buffered saline) prior to placement into metabolic chambers for a period of 21 h.
Statistics.
Data were analyzed using Prism version 3.03 software (GraphPAD Software Inc., San Diego, CA), and statistical significance was determined by analysis of variance and Bonferonni's posttest.
RESULTS
Targeted replacement of the mouse Pyy gene.
We generated Pyy null mice to further elucidate the role of this peptide in the regulation of energy homeostasis and in gastrointestinal endocrine differentiation. A targeting vector was designed to replace most of exons 2 to 4 of the Pyy gene with the bacterial lacZ gene containing a nuclear localization signal to enable us to identify cells that transcribe the targeted allele in the absence of detectable PYY. The replacement of exons 2 to 4 of the Pyy gene with the lacZ gene resulted in the deletion of amino acids 7 to 93 of the prepropeptide YY and the creation of an in-frame fusion of β-galactosidase to the first 6 residues of the signal peptide. Chimeras obtained from two correctly targeted ES cell clones were bred to C57BL/6 mice with resultant germ line transmission (Fig. 1A). We then bred heterozygous mice with a Cre recombinase expressing strain (23) to excise the PGK-neor cassette to avoid any potential effects on the expression of lacZ (40). Mice with the desired Pyy−/−, PGK-neo−/− alleles were identified by Southern blotting (Fig. 1B). All subsequent studies used Pyy−/− and Pyy+/− mice with the neo cassette excised. Wild-type littermates served as controls.
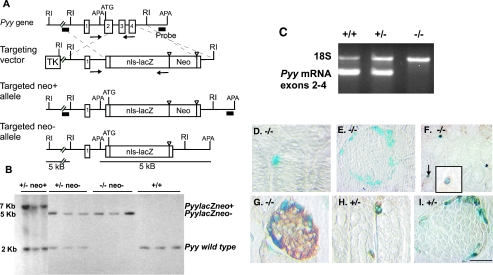
FIG. 1.
Targeted replacement of the mouse Pyy gene. A. Maps of the wild-type Pyy gene containing four exons, the targeting vector, and the disrupted alleles (before and after excision of PGK-neor). The lacZ gene was engineered to replace the Pyy gene. The 5′ and 3′ external probes used for Southern blotting are depicted by a black bars. (RI = EcoRI; APA = ApaI), and the PCR primers used for genotyping are shown as arrows. B. Southern analysis of ApaI digested DNA from Pyy+/− intercrosses using the 3′ probe shown in panel A. Sizes of the wild-type, targeted neo− and targeted neo+ alleles are 2, 5, and 7 kb, respectively. DNA correctly targeted at the 5′ end revealed a 5-kb EcoRI fragment with the 5′ probe (not shown). C. Reverse transcription-PCR of RNA from the colon of control, heterozygous, and null mice showing the presence of Pyy transcripts in Pyy+/+ and Pyy+/− mice but not in Pyy−/− mice. 18S rRNA transcripts served as a positive control for reverse transcription-PCR in the null mice. D and E. The colon (D) and pancreas (E) of Pyy−/− mice stained for β-galactosidase activity and PYY immunoreactivity. Note blue nuclear staining for β-galactosidase activity arising from expression of the targeted allele and the absence of brown staining for PYY. F. Colocalization of GLP-1 immunoreactivity (brown, cytoplasmic staining) with nuclear β-galactosidase activity (blue) in the colon of Pyy−/− mice. G. Chromogranin A immunostained cells (brown) at the islet periphery colocalize with β-galactosidase in Pyy−/− mice. H and I. All β-galactosidase stained cells in the colon (H) and islet mantle (I) in Pyy+/− mice coexpress PYY (brown). Scale bar (in panel I): D, 110 μm; E and F 55 μm; G and H, 84 μm; I, 50 μm.
We were unable to detect Pyy transcripts by reverse transcriptase-PCR in the distal intestinal tract, the major site of PYY expression, indicating that we had disrupted the Pyy gene in null mice (Fig. 1C). We further confirmed that the Pyy locus was targeted correctly in the Pyy−/− animals by the absence of PYY immunostained cells in the colon and pancreas (Fig. 1D and E) in contrast to Pyy+/− (Fig. 1H and I) and Pyy+/+ animals (Fig. 1C and not shown). In Pyy−/− animals, nuclear β-galactosidase activity was readily observed in individual cells in the colon and around the periphery of the islets, indicating that the targeted allele continued to be expressed in the absence of functional peptide (Fig. 1D and E). These β-galactosidase-expressing cells appear to be endocrine cells, as they stained for GLP-1 in the colon (Fig. 1F) and for chromogranin A in the pancreas (Fig. 1G). In Pyy+/− heterozygous mice, all cells expressing β-galactosidase in the colon and pancreas also stained for PYY, indicating that the inserted lacZ gene recapitulates PYY expression (Fig. 1H and I). PYY expression in Pyy+/− mice was indistinguishable from that of wild-type littermates (not shown). Taken together, these data indicate that the Pyy−/− animals do not express PYY and that expression of the Pyy-lacZ gene serves as a specific marker for Pyy promoter-driven expression.
Pyy−/− mice show normal weight gain.
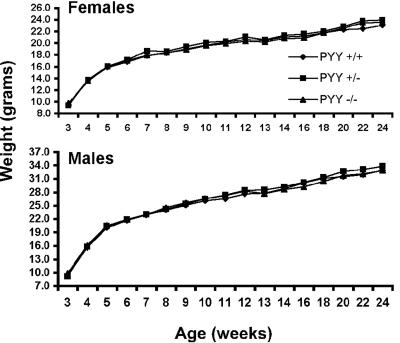
To determine whether the complete absence of PYY caused differences in energy homeostasis or other gross physiological defects we analyzed the growth and reproduction of Pyy−/− animals. Genotype analysis of weanlings from heterozygous intercrosses had the following allelic frequencies: 26% wild-type, 51% heterozygous, and 23% homozygous null, indicating that Pyy−/− mice were viable. Null mice also reproduced normally and had normal blood glucose levels in the fed state (data not shown). Weight gain was assessed from weaning until 6 months of age in male and female Pyy+/+, Pyy+/− and Pyy−/− mice (Fig. 2) and no significant differences in growth (weight gain) were observed between Pyy+/+, Pyy+/− or Pyy−/− littermates.
FIG. 2.
Weight gain in Pyy+/+, Pyy+/−, and Pyy−/− mice. Pyy+/+, Pyy+/−, and Pyy−/− littermates were weighed weekly from weaning until 6 months of age. Average weights are shown (n ≥ 11); analysis of variance of weights at all time points showed no significant difference between Pyy+/+, Pyy+/−, and Pyy−/− mice.
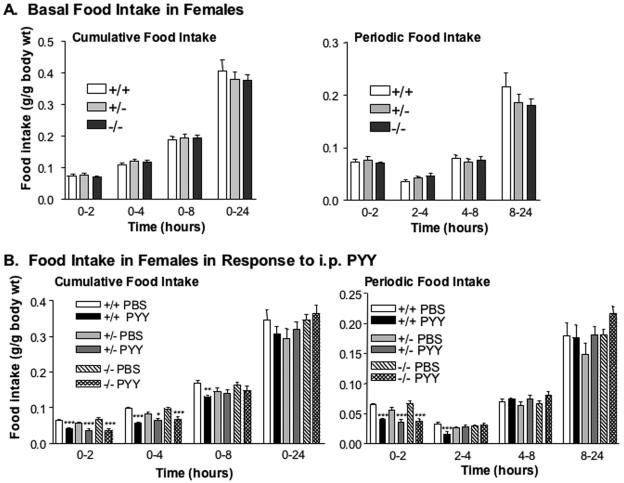
PYY3-36 has been shown to inhibit food intake following central or peripheral administration (3), however, the effects of exogenous PYY3-36 on food intake remain controversial (47). Accordingly, we examined whether the disruption of Pyy was associated with perturbations in food intake in ad libitum-fed mice. Pyy−/− mice showed no significant difference in food intake compared to their Pyy+/− and Pyy+/+ littermates during the immediate two hours following refeeding after an overnight fast. Likewise, cumulative food intake over the 24 h after fasting was similar between null mice and their heterozygous or wild-type littermates (Fig. 3 A).
FIG. 3.
Basal feeding and food intake in response to PYY administration in Pyy+/+, Pyy+/− and Pyy−/− females. A. Basal cumulative and periodic food intake in Pyy+/+, Pyy+/−, and Pyy−/− female mice. B. Cumulative and periodic food intake following intraperitoneal administration of PYY3-36 (20 μg/100 g body weight) or vehicle (phosphate-buffered saline) in Pyy+/+, Pyy+/−, and Pyy−/− female mice. For panels A and B, data are represented as means ± standard error of the mean; n = 4 to 8 mice/group. *, P < 0.05; **, P < 0.01; ***, P < 0.001 for phosphate-buffered saline- versus PYY-treated mice.
Since circulating PYY exists as both the full-length PYY1-36, an agonist for the Y1 receptor with potential orexigenic activity, and the N-terminally cleaved PYY3-36, it is theoretically possible that the ratio of these two forms could regulate food intake. Since the two forms are present at similar concentrations in plasma, the inability to demonstrate changes in food intake may have resulted from the absence of both forms in Pyy−/− null mice (13). If this were true, one might predict that Pyy−/− null mice would show increased sensitivity to the anorectic effects of exogenous PYY3-36. We subsequently examined food intake in mice following peripheral administration of PYY3-36. Intraperitoneal administration of PYY3-36 produced a significant inhibition of food intake in Pyy+/+, Pyy+/−, and Pyy−/− mice (Fig. 3B). There was no significant difference in the reduction of food intake between normal or Pyy null mice given PYY3-36. The anorexic effects of PYY3-36 were most pronounced between 0 to 2 and 2 to 4 h following PYY3-36 administration. By 24 h, no reduction in cumulative food intake was observed in either Pyy+/+ or Pyy−/− mice (Fig. 3B). Thus, Pyy−/− mice did not show increased sensitivity to PYY3-36 in the absence of the Pyy gene product.
The failure to observe increased growth or changes in food intake in Pyy null mice could be attributed to compensatory changes in energy expenditure in these animals. Analysis of energy expenditure revealed no significant differences in O2 consumption and respiratory quotient between Pyy−/−, Pyy+/−, and Pyy+/+ mice (Fig. 4A). Furthermore, both Pyy+/+ and Pyy−/− mice exhibited a comparable transient reduction in oxygen consumption and a progressive increase in the respiratory exchange ratio following intraperitoneal administration of PYY3-36 (Fig. 4B). Taken together, these findings demonstrate that deletion of the Pyy gene is not associated with significant perturbations in the regulation of feeding behavior, energy expenditure, or responsiveness to the anorectic effects of exogenous PYY3-36.
FIG. 4.
Oxygen consumption (VO2) and respiratory exchange ratio (RER) in Pyy+/+, Pyy+/− and Pyy−/− females. A. Basal VO2 and respiratory exchange ratio in Pyy+/+, Pyy+/−, and Pyy−/− female mice, n = 5 or 6 mice/group. B. VO2 and respiratory exchange ratio following intraperitoneal administration of PYY3-36 (20 μg/100 g body weight) in Pyy+/+, Pyy+/−, and Pyy−/− female mice, n = 4 to 6 mice/group.
Differentiation of gastrointestinal endocrine cells does not require peptide YY.
PYY expression has been observed as an early marker of endocrine differentiation in the developing murine gastrointestinal tract (49, 50). Scattered cells expressing the secretory granule marker chromogranin A (12) were easily identified in the distal intestine of Pyy −/− mice (Fig. 5A). The number of chromogranin A-positive cells in the ileum and colon was similar in Pyy+/+ and Pyy−/− animals, indicating that the overall size of the enteroendocrine population was unaffected in Pyy−/− mice (Fig. 5B). As noted earlier, β-galactosidase activity was expressed in individual epithelial cells of the colon of Pyy null mice, suggesting the presence of gut endocrine cells that normally express PYY (Fig. 1F). All β-galactosidase-positive gut endocrine cells in Pyy null mice stained for GLP-1, indicating that L-type enteroendocrine cells continue to differentiate in the absence of PYY (Fig. 1F). Serotonin-positive cells were also present in the colon of Pyy−/− animals but did not show staining for β-galactosidase (Fig. 5C), confirming that this lineage is distinct from the L-cell lineage (39).
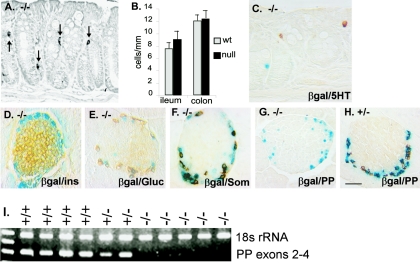
FIG. 5.
Endocrine differentiation in Pyy−/− mice. A. Colon from a Pyy−/− mouse showing normal distribution of chromogranin A (CGA)-immunopositive cells (arrows). B. Pyy−/− mice show similar numbers of enteroendocrine cells staining for chromogranin A in the ileum and colon compared to wild-type mice. Results are expressed as mean number of stained cells counted per unit intestinal mucosal length ± standard error of the mean. C to H. Distribution of different endocrine cell types in the colon and pancreas of Pyy−/− and Pyy+/− mice. Tissues were examined for β-galactosidase activity by X-Gal staining (blue nuclear staining) and for hormones by immunoperoxidase staining (brown cytoplasmic staining). C. Serotonin-expressing cells were present in normal numbers and did not colocalize with β-galactosidase in Pyy−/− colon. D. Normal islet architecture with insulin-expressing β cells in the islet core in Pyy−/− mice. E and F. A subset of glucagon (E) and somatostatin (F) cells colocalized with β-galactosidase at the islet periphery in Pyy−/− mice similar to their coexpression with PYY in normal mice. G. PP-immunopositive cells were absent from islets of Pyy−/− mice. H. Cells staining for PP (brown) were readily identified in the pancreas of Pyy+/− mice and colocalized with staining for β-galactosidase activity (blue). I. PP transcripts were not detected by reverse transcription-PCR of pancreatic RNA extracted from Pyy−/− mice. In contrast, PP transcripts were detected in RNA from Pyy+/− and Pyy+/+ mice. Scale bar (in panel H): A, 17 μm; C, 22 μm; D to H, 25 μm.
We examined pancreatic islets in Pyy−/− mice to determine whether expression of peptide YY in early islet precursor cells was required for normal islet differentiation. Islet architecture in Pyy−/− mice was indistinguishable from normal mice with insulin-producing β cells in the central core and normal numbers of glucagon-expressing α cells and somatostatin-producing ∂ cells distributed around the islet mantle (Fig. 5D to F).
However, we were unable to identify PP cells in the islets of Pyy−/− mice (Fig. 5G). Examination of Pyy+/− mice showed that all PP-stained cells expressed β-galactosidase from the targeted allele (Fig. 5H). However, none of the β-galactosidase-stained cells in null mice stained for PP, suggesting that PP is not expressed in the absence of PYY (Fig. 5G). The absence of PP gene expression was confirmed by reverse transcription-PCR amplification of pancreatic RNA (Fig. 5I). We were unable to detect PP transcripts in Pyy null mice and yet had little difficulty demonstrating PP expression in Pyy+/+ and Pyy+/− animals. The absence of PP gene expression was not restored to Pyy null mice treated with PYY1-36 for 5 days, indicating that the failure to express PP did not result from the loss of PYY-generated signaling (not shown).
Delineation of sequences in the peptide YY gene required to direct spatially regulated expression in transgenic mice.
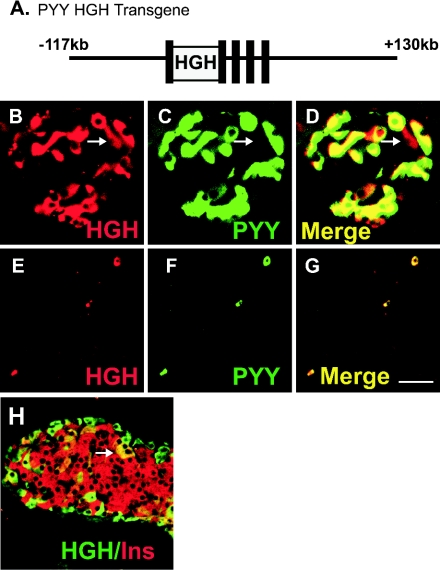
Three lines of transgenic mice were initially generated to express a human growth hormone reporter gene (hGH) under the control of different lengths of the Pyy gene to establish a model for lineage analysis. None of the three initial transgenes, the largest of which contained 12 kb of 5′-flanking sequence, all exons and introns of the Pyy gene, and 2 kb of 3′-flanking sequence, were expressed in more than an occasional PYY cell, suggesting that they did not include sequences necessary to recapitulate tissue- and cell type-specific expression. In order to ensure that sufficient sequences were included to direct cell-specific expression in gastrointestinal endocrine cells, we created a transgene using a murine genomic Pyy bacterial artificial chromosome (BAC) containing 117 kb of 5′-flanking sequence and 130 kb of 3′-flanking sequence. We introduced the hGH reporter gene into exon 1 of the murine Pyy gene within the BAC by homologous recombination in E. coli using the phage λ red recombination system (8, 10, 52) (Fig. 6 A).
FIG. 6.
Expression of a Pyy-hGH transgene is directed to PYY-expressing cells. A. Structure of the Pyy-hGH BAC transgene. The hGH gene was inserted into the first exon of the Pyy gene within the Pyy bacterial artificial chromosome (BAC) using homologous recombination in E. coli. B to D. Double immunofluorescent staining in the pancreas of Pyy-hGH mice for hGH (B, red), and PYY (C, green). Cells expressing both hGH and PYY appear yellow in the merged image (D). The arrow denotes a very rare PYY-negative/hGH-positive cell. E to G. Double immunofluorescent staining in the colon of Pyy-hGH mice for hGH (E, red), and PYY (F, green). The merged image (G) shows that hGH staining is only seen in PYY-expressing cells, which appear yellow. H. Double immunofluorescent staining in the pancreas of Pyy-hGH mice for hGH (green), and insulin (red). PYY expression is seen in a single rare β cell (arrow). Scale bar (in panel G): B to D, 98 μm; E to G, 50 μm; H, 85 μm.
We identified cells expressing hGH in isolated mucosal cells in the ileum and colon and around the periphery of pancreatic islets of Pyy-hGH mice (Fig. 6). Double immunofluorescent staining for PYY and hGH showed that all PYY+ cells were immunopositive for hGH in the pancreas and intestine of adult and fetal mice, indicating that the transgene contained sufficient sequences to direct hGH expression to all PYY-expressing cells in the intestine and pancreas (Fig. 6B to G). All hGH-immunopositive cells in the intestine also contained PYY immunopositivity, whereas we identified rare hGH-positive cells in the interior of the pancreatic islets that did not express PYY (Fig. 6B to D, arrow). These rare hGH-positive/PYY-negative cells appeared to be a small subpopulation of insulin-expressing β cells (Fig. 6H) and may represent cells that express PYY below the detection limit of immunohistochemistry or cells where the transgene expression was not appropriately restricted. In developing animals, examination of the pancreas at E13.5 and the intestine at E17.5 revealed expression of hGH in all PYY cells, and hGH was restricted only to PYY-expressing cells (not shown). Thus, the Pyy BAC includes sufficient regulatory sequences to direct developmentally regulated, cell-specific transgene expression to PYY cells but may lack some information necessary to fully restrict expression to the correct cell types in pancreatic islets.
Relationship between peptide YY-expressing cells in the pancreas and other islet lineages.
To determine if PYY-positive pancreatic endocrine cells represent precursors of adult islet lineages we examined the descendants of PYY-expressing cells using the Cre/loxP system for recombination-based lineage tracing. Cre encoding sequences containing a nuclear localization signal were inserted into the untranslated first exon of the murine Pyy gene within the BAC clone described in the preceding section. All PYY cells expressed Cre and almost all Cre staining was restricted to PYY cells, except for an occasional islet cell, indicating that specificity of transgene expression was nearly identical to the Pyy-hGH line (Fig. 7 B and 8D).
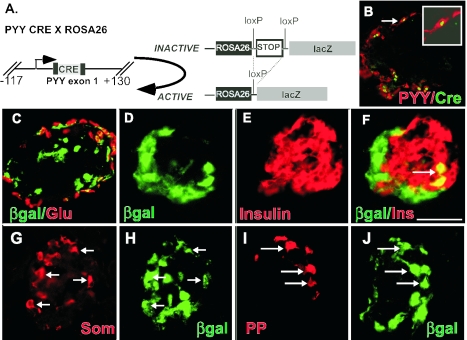
FIG. 7.
Most pancreatic β cells and a significant fraction of glucagon-expressing α cells do not arise from PYY-expressing precursors. A. Descendants of PYY cells were marked by crossing Pyy Cre transgenic mice to the ROSA26 Cre indicator strain, which results in activation of lacZ expression in all PYY cells and all progeny of PYY-expressing cells. B. Double immunofluorescent staining for Cre (green) and PYY (red) in the pancreas of Pyy CRE mice. The arrow denotes a double-positive cell that is magnified in the inset. Cre is localized to the nucleus, and PYY stains the cytoplasm. Cre expression was restricted to PYY-expressing cells. C. Double immunofluorescent staining in the pancreas of Pyy Cre × RosA26 mice for β-galactosidase (green) and glucagon (red). Approximately 40% of α cells coexpressed β-galactosidase and appeared yellow, indicating that the majority do not arise from PYY-expressing cells. D to F. Double immunofluorescent staining in the pancreas of Pyy CRE × RosA26 mice for β-galactosidase (D, green) and insulin (E, red). An arrow denotes a rare double-positive cell in panel F. G and H. Most somatostatin-expressing ∂ cells arise from cells that express PYY, as shown by double immunofluorescent staining for somatostatin (G, red) and β-galactosidase (H, green) in Pyy Cre × RosA26 mice. I and J. All pancreatic polypeptide cells (I, red) appear to immunostain for β-galactosidase (J, green) in Pyy Cre × RosA26 mice, indicating that they are all related to PYY-expressing cells. For panels G to J, arrows indicate double-positive cells. Scale bar (in panel F): B, 28 μm; C to J, 50 μm.
Pyy-Cre transgenic mice were crossed with the modified murine reporter line, Rosa26 (R26R) (43) (Fig. 7A). The ROSA26 locus directs generalized constitutive expression in most tissues, including the gastrointestinal tract, throughout development. The indicator strain was created by inserting lacZ into the ROSA26 locus and modified by introducing floxed poly(A) stop sequences upstream of lacZ, thus preventing lacZ expression except in cells expressing Cre recombinase. Transcription readthrough and expression of lacZ is permanently activated by Cre-mediated recombination between the loxP sites to excise the stop sequences. As a result, cells and their descendants that transiently express PYY will be permanently marked by β-galactosidase expression independent of their eventual cell fate.
In the adult pancreas, PYY cells tend to be distributed at the periphery of pancreatic islets with other non-β-cell lineages, whereas insulin-expressing cells are located in the central islet core (11). We analyzed the descendants of PYY-expressing cells by staining for β-galactosidase in Pyy-Cre × ROSA26 mice. β-Galactosidase was expressed mainly around the periphery of the islet in Pyy-Cre × ROSA26 mice. To determine which islet lineages arise from or coexpress PYY, we stained adult islets for β-galactosidase and each of the islet hormones.
Multiple immunofluorescent staining for glucagon and β-galactosidase revealed that approximately 40% of α cells coexpressed β-galactosidase, suggesting that a significant fraction of α cells do not arise from PYY-positive precursor cells (Fig. 7C). Most insulin-stained cells did not coexpress β-galactosidase in multiple labeling experiments. However, we identified occasional insulin-positive/β-galactosidase-positive cells in the central islet core, indicating that only a very small fraction of β cells may arise from PYY-positive cells (Fig. 7D to F). These cells in the islet core did not stain for either PYY or Cre, suggesting that they may be descendants of cells that expressed PYY earlier in development prior to switching off expression in adult mice or cells that transiently misexpressed Cre. As expected, all PYY cells coexpressed β-galactosidase as did all somatostatin-expressing ∂ cells and PP cells, consistent with their known coexpression of PYY (Fig. 7G to J).
Relationship between PYY-expressing cells and other enteroendocrine lineages.
To determine which endocrine cells within the distal intestine arise from PYY cells, we examined cells expressing the endocrine cell marker chromogranin A for βgal expression. Many chromogranin A-positive endocrine cells did not express β-galactosidase (data not shown), indicating that not all endocrine cells arose from PYY precursors. PYY cells in the ileum and colon of Pyy-Cre X ROSA26 mice expressed β-galactosidase as expected (Fig. 8 A to C). L-type enteroendocrine cells coexpress several hormones in addition to PYY, including GLP-1, GLP-2, neurotensin, and occasionally CCK and secretin.
FIG. 8.
PYY-expressing cells give rise to L-type enteroendocrine cells in the colon but not to serotonin-expressing enteroendocrine cells. Descendants of PYY cells in the colon of Pyy-Cre × ROSA26 mice were identified by double immunostaining for β-galactosidase and the indicated hormone. A. Double immunofluorescent staining in the colon of Pyy-Cre mice for PYY (green cytoplasmic staining) and Cre (red, nuclear staining) shows that Cre expression was restricted to PYY-expressing cells. B to D. Double immunofluorescent staining in the colon of Pyy-Cre × Rosa26 mice for β-galactosidase (B, green) and PYY (C, red). Double-stained cells appear yellow in the merged image (D). As expected, all L-type enteroendocrine cells express PYY at some stage of development. E. Serotonin-expressing enteroendocrine cells do not arise from PYY-expressing precursors. Double immunofluorescent staining in the colon of β-galactosidase (green) and serotonin (5HT, red) shows that serotonin cells do not express β-galactosidase. Scale bar (in panel E): A, 82 μm; B to D, 58 μm; E, 50 μm.
In the ileum and colon, cells expressing serotonin and substance P comprise the other major lineage besides L cells. Serotonin-expressing cells in the distal intestine did not show staining for β-galactosidase indicating that serotonin cells are not derived from PYY-expressing precursors (Fig. 8E). In addition, we were unable to identify β-galactosidase expression in serotonin cells in the developing intestine at E18.5 (data not shown), indicating that the previously observed colocalization of PYY and serotonin in the early mouse intestine (50) occurs transiently in a small number of cells that do not represent precursor cells. The absence of peptide YY-expressing cells in the proximal small intestine raised the question of whether PYY expression is an early event in small intestine endocrine differentiation. These earlier observations, however, could not exclude transient, low-level expression of this peptide. Examination of the small intestine of Pyy-Cre × ROSA26 mice showed β-galactosidase expression in very rare cells, indicating that most enteroendocrine cells of the proximal small intestine do not express PYY earlier in development. Therefore, PYY-expressing cells give rise to L-type enteroendocrine cells but not to other enteroendocrine lineages.
DISCUSSION
The physiologic function of peptide YY is not well understood. Peptide YY was first believed to inhibit secretory functions in several organs of the gastrointestinal tract by mechanisms that required intact vagal innervation. Subsequent studies showed that peptide YY expression appeared to be an early event in the differentiation of pancreatic and distal intestinal endocrine cells. More recently, PYY3-36, which is generated by cleavage of its N-terminal two amino acids by the protease dipeptidyl peptidase IV, was identified as a potent appetite suppressant when peripherally administered to rodents and humans, and its anorectic effects last up to 24 h (3). However, there is considerable controversy regarding the anorectic effects of PYY3-36. A number of investigators were unable to duplicate the effects of PYY3-36 on food intake (47). In contrast, several independent research groups have confirmed the original observation that PYY3-36 does inhibit food intake (1, 6, 7, 9, 15, 27, 34, 37). In the present work, we also show that PYY3-36 inhibits food intake in mice. We have further examined the function of PYY in both energy homeostasis and gastrointestinal endocrine differentiation using both Pyy−/− mice and recombination-based cell lineage tracing.
Exogenous administration of PYY3-36 decreases food intake and weight gain in mice, rats, and humans (3). PYY3-36 is thought to regulate food intake by activating the Y2 receptor, which is highly expressed on orexigenic neurons in the arcuate nucleus. Activation of the Y2 receptor by PYY3-36 is thought to inhibit the activity of the stimulatory neurons, which express neuropeptide Y and agouti-related peptide (AgRP), causing inhibition of food intake (42). Given the anorectic actions of exogenous PYY3-36 in mice, we predicted that Pyy null mice would show increased food intake and obesity.
The absence of PP in the Pyy−/− mice was not anticipated. The pancreas represents the major site of PP expression in rodents, making it likely that the Pyy null mice are also deficient in PP expression. Overexpression of PP in transgenic mice as well as its administration to rodents and humans inhibited food intake, suggesting that PP may also function as an anorectic hormone (2, 4, 48). Despite the absence of both PYY and PP, Pyy−/− mice grow, reproduce, feed, and gain weight similarly to wild-type animals, indicating that PYY and PP are not critical endogenous mediators of anorectic responses or that other regulatory peptides compensate for loss of PYY and PP function in the regulation of food intake.
Many peptide hormones have overlapping functions that serve to modulate physiological processes, especially the modulation of energy balance. For example, exogenous administration of the peptide ghrelin, which is secreted from endocrine cells in the stomach prior to meals, stimulates the neuropeptide Y and agouti-related peptide neurons and increases feeding (28). However, no defects in growth or appetite are observed in ghrelin−/− mice (44). To further illustrate the redundancy in the regulation of energy balance, NPY−/−, AgRP−/−, and NPY−/−/AgRP−/− mice exhibit no defects in food intake, weight gain, or other measures of energy homeostasis (35). Since these peptides are expressed in neurons believed to be central to the control of feeding, the lack of a phenotype in their absence illustrates additional examples of genetic redundancy in the hormonal control of feeding and satiety.
Since the full-length PYY is an agonist for orexigenic Y1 receptors whereas PYY3-36 selectively activates anorectic Y2 receptors, it is possible that the ratio of the two forms determines food intake. If this were true, the anorectic response to PYY3-36 should be potentiated in null mice. However, PYY3-36 inhibited food intake similarly in Pyy−/− and Pyy+/+ mice despite the complete absence of the Pyy gene product in the null animals. Thus, PYY3-36 may override the stimulatory effects of PYY1-36 on Y1 receptors. Perturbations of behavior related to energy homeostasis resulting from exogenous administration of peptides may reflect pharmacologic effects rather than the underlying physiologic role of the endogenous peptide. Our findings are consistent with studies of mice lacking ghrelin or neuropeptide Y, which also fail to exhibit defects in basal feeding behavior despite the regulatory roles of these peptides for food intake following exogenous pharmacological administration of the respective peptides.
During fetal development of the pancreas and colon, PYY is coexpressed in all endocrine cell types when they first appear (49, 50), suggesting that PYY expression is an early event marking the onset of endocrine differentiation in these tissues. Examination of Pyy null mice shows that endocrine differentiation in the colon and pancreas does not, for the most part, require expression of PYY, with the exception of PP cells of the pancreas. Our findings suggest that the deleted Pyy locus is required for proper expression of the PP gene, which is approximately 10 kb downstream. Expression of the inserted reporter lacZ gene suggests that the Pyy locus is “transcriptionally competent” in Pyy−/− mice. Therefore, it is possible that deleted exons 2 to 4 of Pyy contain a regulatory element required for PP expression. Another possibility is that the inserted lacZ sequences disrupted the context of the transcriptional control elements involved in PP gene expression. At present we cannot distinguish between these two mechanisms. An additional possibility, that PP expression depends on the presence of PYY, seems less likely, since exogenous PYY did not restore PP staining.
Here, we describe recombination-based cell lineage tracing using the Cre/loxP system to determine whether PYY-expressing cells are progenitor cells for other endocrine cell types in the adult pancreas, ileum, and colon (43). This system allowed us to follow the fate of all cells that expressed PYY, including cells that transiently express PYY at some stage during development. Demonstration that we can recapitulate the expression of PYY in transgenic mice using an easily detected reporter (hGH) was critical to validating subsequent recombination-based lineage tracing.
Insulin-positive/glucagon-positive/PYY-positive cells are the first endocrine cells seen at the earliest stage of pancreatic bud formation as early as E9.5. These early triple-positive cells had been hypothesized to be potential precursors of mature α and β cells in the pancreas with subsequent loss of PYY coexpression later in development (21, 49). A number of observations do not support the notion that β cells arise from the triple-positive cells. The major expansion of the β cell population occurring between E15.5 and E18.5 appears to arise from cells expressing the transcription factors Pdx-1 and Nkx6.1, which are not expressed in PYY-positive/glucagon-positive/insulin-positive cells seen earlier in development (30). Cell lineage tracing described here, which enabled us to trace the cell fate of PYY-positive cells even after they stop expressing the hormone, clearly shows that most β cells do not arise from cells that expressed PYY earlier in development and that the triple-positive cells do not represent the precursors for most β cells.
Others have suggested that all pancreatic β cells arise from a PP family member (18). Since the Cre transgene contained the linked PP gene with extensive flanking sequence, it seems unlikely that β cells arise from PP cells, as suggested earlier (18, 19). Based on our experience, which indicated that very large fragments of the Pyy gene were required to direct cell type-specific expression, it is possible that the observed activation of insulin gene expression by a PP-Cre transgene under control of 0.6 kb of flanking sequence may have resulted from Cre misexpression.
An unexpected conclusion from the cell lineage tracing was the identification of least two distinct subpopulations of glucagon-expressing α cells. Since nearly all L-type enteroendocrine cells and half of the glucagon-expressing cells in the islets of adult mice coexpressed PYY, we had assumed that a substantial proportion of all α cells arose from PYY-expressing cells. Cell lineage analysis suggests that approximately half of α cells do not arise from PYY-positive cells or from the triple-positive cells seen during pancreatic bud formation. Thus, the origin of PYY-negative/glucagon-positive α cells remains to be elucidated, as these cells do not appear to arise from cells that transiently express PYY at levels sufficient to induce recombination early in development. Our results also imply that the very first endocrine cells that appear during pancreagenesis may represent transit cells rather than the major precursor pool for adult islets.
Cell fate analysis of PYY cells in the intestine indicates that enteroendocrine cells in the proximal small intestine do not arise from PYY cells. In the ileum and colon, L-type enteroendocrine cells appear to be the only cell type that arises directly from PYY cells, whereas the other major endocrine cell population of the hindgut, serotonin and substance P-expressing cells, does not. These findings are consistent with previous experiments suggesting that these endocrine cell subpopulations are distinct (39). Thus, coexpression of PYY in small numbers of colonic serotonin cells late in gestation is probably a transient developmental event and PYY-positive cells are not precursors for serotonin-expressing cells (50).
Our results suggest that the expression of hormones like PYY occurs at a later time point during the differentiation of gastrointestinal endocrine cells than was originally believed and that PYY-expressing cells do not represent precursor cells for most pancreatic and intestinal endocrine cell lineages. Furthermore, despite the anorectic effects of exogenous PYY3-36 on inhibition of food intake in rodents and humans, genetic deletion of Pyy does not produce disturbances of feeding behavior, somatic growth, or energy expenditure.
Acknowledgments
This work was supported in part by NIH grants DK43673, DK52870, and DK67166 to A.B.L. and the GRASP Digestive Disease Center grants P30-DK34928, T32-CA65441 and DK42502 to M.A.M. D. Drucker is supported by a Canada Research Chair in Regulatory Peptides, and the studies were supported in part by operating grants from the Canadian Institutes of Health Research. Use of the Transgenic/ES Cell Shared Resource was supported by DK20593.
We thank the staff of the Transgenic/ES Cell Shared Resource for their expert technical assistance.
REFERENCES
- 1.Adams, S. H., W. B. Won, S. E. Schonhoff, A. B. Leiter, and J. R. Paterniti, Jr. 2004. Effects of peptide YY[3-36] on short-term food intake in mice are not affected by prevailing plasma ghrelin levels. Endocrinology 145:4967-4975. [DOI] [PubMed] [Google Scholar]
- 2.Asakawa, A., A. Inui, N. Ueno, M. Fujimiya, M. A. Fujino, and M. Kasuga. 1999. Mouse pancreatic polypeptide modulates food intake, while not influencing anxiety in mice. Peptides 20:1445-1448. [DOI] [PubMed] [Google Scholar]
- 3.Batterham, R. L., M. A. Cowley, C. J. Small, H. Herzog, M. A. Cohen, C. L. Dakin, A. M. Wren, A. E. Brynes, M. J. Low, M. A. Ghatei, R. D. Cone, and S. R. Bloom. 2002. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature 418:650-654. [DOI] [PubMed] [Google Scholar]
- 4.Batterham, R. L., C. W. Le Roux, M. A. Cohen, A. J. Park, S. M. Ellis, M. Patterson, G. S. Frost, M. A. Ghatei, and S. R. Bloom. 2003. Pancreatic polypeptide reduces appetite and food intake in humans. J. Clin. Endocrinol. Metab. 88:3989-3992. [DOI] [PubMed] [Google Scholar]
- 5.Brouns, I., L. Van Nassauw, J. Van Genechten, M. Majewski, D. W. Scheuermann, J. P. Timmermans, and D. Adriaensen. 2002. Triple immunofluorescence staining with antibodies raised in the same species to study the complex innervation pattern of intrapulmonary chemoreceptors. J. Histochem. Cytochem. 50:575-582. [DOI] [PubMed] [Google Scholar]
- 6.Challis, B. G., A. P. Coll, G. S. Yeo, S. B. Pinnock, S. L. Dickson, R. R. Thresher, J. Dixon, D. Zahn, J. J. Rochford, A. White, R. L. Oliver, G. Millington, S. A. Aparicio, W. H. Colledge, A. P. Russ, M. B. Carlton, and S. O'Rahilly. 2004. Mice lacking pro-opiomelanocortin are sensitive to high-fat feeding but respond normally to the acute anorectic effects of peptide-YY(3-36). Proc. Natl. Acad. Sci. USA 101:4695-4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chelikani, P. K., A. C. Haver, and R. D. Reidelberger. 2005. Intravenous infusion of peptide YY(3-36) potently inhibits food intake in rats. Endocrinology. 146:879-888. [DOI] [PubMed] [Google Scholar]
- 8.Cotta-De-Almeida, V., S. Schonhoff, T. Shibata, A. Leiter, and S. B. Snapper. 2003. A new method for rapidly generating gene-targeting vectors by engineering BACs through homologous recombination in bacteria. Genome Res. 13:2190-2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox, J. E., and A. Randich. 2004. Enhancement of feeding suppression by PYY(3-36) in rats with area postrema ablations. Peptides 25:985-989. [DOI] [PubMed] [Google Scholar]
- 10.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edlund, H. 2002. Pancreatic organogenesis—developmental mechanisms and implications for therapy. Nat. Rev. Genet. 3:524-532. [DOI] [PubMed] [Google Scholar]
- 12.Facer, P., A. E. Bishop, G. A. Cole, M. Aitchison, C. H. Kendall, G. van Aswegen, R. J. A. Penketh, C. H. Rodek, P. McKeever, and J. M. Polak. 1989. Developmental profile of chromagranin, hormonal peptides, and 5-hydroxytryptamine in gastrointestinal endocrine cells. Gastroenterology 97:48-57. [DOI] [PubMed] [Google Scholar]
- 13.Grandt, D., M. Schimiczek, C. Beglinger, P. Layer, H. Goebell, V. E. Eysselein, and J. R. Reeve, Jr. 1994. Two molecular forms of peptide YY (PYY) are abundant in human blood: characterization of a radioimmunoassay recognizing PYY 1-6 and PYY 3-36. Regul. Peptides 51:151-159. [DOI] [PubMed] [Google Scholar]
- 14.Greeley, G. H. J., T. Hashimoto, M. Izukura, G. Gomez, J. Jeng, F. L. Hill, F. Lluis, and J. C. Thompson. 1989. A comparison of intraduodenally and intracolonically administered nutrients on the release of peptide-YY in the dog. Endocrinology 125:1761-1765. [DOI] [PubMed] [Google Scholar]
- 15.Halatchev, I. G., K. L. Ellacott, W. Fan, and R. D. Cone. 2004. Peptide YY3-36 inhibits food intake in mice through a melanocortin-4 receptor-independent mechanism. Endocrinology 145:2585-2590. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez, E. J., D. C. Whitcomb, S. R. Vigna, and I. L. Taylor. 1994. Saturable binding of circulating peptide YY in the dorsal vagal complex of rats. Am. J. Physiol. 266:G511-516. [DOI] [PubMed] [Google Scholar]
- 17.Herrera, P., J. Huarte, F. Sanvito, P. Meda, L. Orci, and J. Vassalli. 1991. Embryogenesis of the murine pancreas; early expression of pancreatic polypeptide gene. Development 113:1257-1265. [DOI] [PubMed] [Google Scholar]
- 18.Herrera, P. L. 2000. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development 127:2317-2322. [DOI] [PubMed] [Google Scholar]
- 19.Herrera, P. L., J. Huarte, R. Zufferey, A. Nichols, B. Mermillod, J. Philippe, P. Muniesa, F. Sanvito, L. Orci, and J. D. Vassalli. 1994. Ablation of islet endocrine cells by targeted expression of hormone-promoter-driven toxigenes. Proc. Natl. Acad. Sci. USA 91:12999-13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hogan, B., F. Costantini, and E. Lacy. 1994. Manipulating the mouse embryo, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 21.Jackerott, M., A. Oster, and L. I. Larsson. 1996. PYY in developing murine islet cells: comparisons to development of islet hormones, NPY, and BrdU incorporation. J. Histochem. Cytochem. 44:809-817. [DOI] [PubMed] [Google Scholar]
- 22.Keire, D. A., P. Mannon, M. Kobayashi, J. H. Walsh, T. E. Solomon, and J. R. Reeve, Jr. 2000. Primary structures of PYY, [Pro(34)]PYY, and PYY-(3-36) confer different conformations and receptor selectivity. Am. J. Physiol. Gastrointest. Liver Physiol. 279:G126-G131. [DOI] [PubMed] [Google Scholar]
- 23.Lakso, M., J. G. Pichel, J. R. Gorman, B. Sauer, Y. Okamoto, E. Lee, F. W. Alt, and H. Westphal. 1996. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc. Natl. Acad. Sci. USA 93:5860-5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez, M. J., B. H. Upchurch, G. Rindi, and A. B. Leiter. 1995. Studies in transgenic mice reveal potential relationships between secretin-producing cells and other endocrine cell types. J. Biol. Chem. 270:885-891. [DOI] [PubMed] [Google Scholar]
- 25.Lundberg, J. M., K. Tatemoto, L. Terenius, P. M. Hellstrom, V. Mutt, T. Hokfelt, and B. Hamberger. 1982. Localization of peptide YY (PYY) in gastrointestinal endocrine cells and effects on intestinal blood flow and motility. Proc. Natl. Acad. Sci. USA 79:4471-4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mentlein, R., P. Dahms, D. Grandt, and R. Kruger. 1993. Proteolytic processing of neuropeptide Y and peptide YY by dipeptidyl peptidase IV. Regul. Peptides 49:133-144. [DOI] [PubMed] [Google Scholar]
- 27.Moran, T. H., U. Smedh, K. P. Kinzig, K. A. Scott, S. Knipp, and E. E. Ladenheim. 2005. Peptide YY(3-36) inhibits gastric emptying and produces acute reductions in food intake in rhesus monkeys. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288:R384-R388. [DOI] [PubMed] [Google Scholar]
- 28.Nakazato, M., N. Murakami, Y. Date, M. Kojima, H. Matsuo, K. Kangawa, and S. Matsukura. 2001. A role for ghrelin in the central regulation of feeding. Nature 409:194-198. [DOI] [PubMed] [Google Scholar]
- 29.Negoescu, A., F. Labat-Moleur, P. Lorimier, L. Lamarcq, C. Guillermet, E. Chambaz, and E. Brambilla. 1994. F(ab) secondary antibodies: a general method for double immunolabeling with primary antisera from the same species. Efficiency control by chemiluminescence. J. Histochem. Cytochem. 42:433-437. [DOI] [PubMed] [Google Scholar]
- 30.Oster, A., J. Jensen, P. Serup, P. Galante, O. D. Madsen, and L. I. Larsson. 1998. Rat endocrine pancreatic development in relation to two homeobox gene products (Pdx-1 and Nkx 6.1). J. Histochem. Cytochem. 46:707-715. [DOI] [PubMed] [Google Scholar]
- 31.Pappas, T. N., H. T. Debas, A. M. Chang, and I. L. Taylor. 1986. Peptide YY release by fatty acids is sufficient to inhibit gastric emptying in dogs. Gastroenterology 91:1386-1389. [DOI] [PubMed] [Google Scholar]
- 32.Pappas, T. N., H. T. Debas, and I. L. Taylor. 1986. Enterogastrone-like effect of peptide YY is vagally mediated in the dog. J. Clin. Investig. 77:49-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pedersen-Bjergaard, U., U. Host, H. Kelbaek, S. Schifter, J. F. Rehfeld, J. Faber, and N. J. Christensen. 1996. Influence of meal composition on postprandial peripheral plasma concentrations of vasoactive peptides in man. Scand. J. Clin. Lab. Investig. 56:497-503. [DOI] [PubMed] [Google Scholar]
- 34.Pittner, R. A., C. X. Moore, S. P. Bhavsar, B. R. Gedulin, P. A. Smith, C. M. Jodka, D. G. Parkes, J. R. Paterniti, V. P. Srivastava, and A. A. Young. 2004. Effects of PYY[3-36] in rodent models of diabetes and obesity. Int. J. Obes. Relat. Metab. Disord. 28:963-971. [DOI] [PubMed] [Google Scholar]
- 35.Qian, S., H. Chen, D. Weingarth, M. E. Trumbauer, D. E. Novi, X. Guan, H. Yu, Z. Shen, Y. Feng, E. Frazier, A. Chen, R. E. Camacho, L. P. Shearman, S. Gopal-Truter, D. J. MacNeil, L. H. Van der Ploeg, and D. J. Marsh. 2002. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol. Cell. Biol. 22:5027-5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ratineau, C., A. Ronco, and A. B. Leiter. 2000. Role of the amino-terminal domain of simian virus 40 early region in inducing tumors in secretin-expressing cells in transgenic mice. Gastroenterology 119:1305-1311. [DOI] [PubMed] [Google Scholar]
- 37.Riediger, T., C. Bothe, C. Becskei, and T. A. Lutz. 2004. Peptide YY directly inhibits ghrelin-activated neurons of the arcuate nucleus and reverses fasting-induced c-Fos expression. Neuroendocrinology. 79:317-326. [DOI] [PubMed] [Google Scholar]
- 38.Rindi, G., C. Ratineau, A. Ronco, M. E. Candusso, M. Tsai, and A. B. Leiter. 1999. Targeted ablation of secretin-producing cells in transgenic mice reveals a common differentiation pathway with multiple enteroendocrine cell lineages in the small intestine. Development 126:4149-4156. [DOI] [PubMed] [Google Scholar]
- 39.Roth, K. A., S. Kim, and J. I. Gordon. 1992. Immunocytochemical studies suggest two pathways for enteroendocrine cell differentiation in the colon. Am. J. Physiol. 263:G174-180. [DOI] [PubMed] [Google Scholar]
- 40.Scacheri, P. C., J. S. Crabtree, E. A. Novotny, L. Garrett-Beal, A. Chen, K. A. Edgemon, S. J. Marx, A. M. Spiegel, S. C. Chandrasekharappa, and F. S. Collins. 2001. Bidirectional transcriptional activity of PGK-neomycin and unexpected embryonic lethality in heterozygote chimeric knockout mice. Genesis 30:259-263. [DOI] [PubMed] [Google Scholar]
- 41.Schonhoff, S. E., M. Giel-Moloney, and A. B. Leiter. 2004. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev. Biol. 270:443-454. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz, M. W., and G. J. Morton. 2002. Obesity: keeping hunger at bay. Nature 418:595-597. [DOI] [PubMed] [Google Scholar]
- 43.Soriano, P. 1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21:70-71. [DOI] [PubMed] [Google Scholar]
- 44.Sun, Y., S. Ahmed, and R. G. Smith. 2003. Deletion of ghrelin impairs neither growth nor appetite. Mol. Cell. Biol. 23:7973-7981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tatemoto, K. 1982. Isolation and characterization of peptide YY (PYY), a candidate gut hormone that inhibits pancreatic exocrine secretion. Proc. Natl. Acad. Sci. USA 79:2514-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teitelman, G., S. Alpert, J. M. Polak, A. Martinez, and D. Hanahan. 1993. Precursor cells of mouse endocrine pancreas coexpress insulin, glucagon and the neuronal proteins tyrosine hydroxylase and neuropeptide Y, but not pancreatic polypeptide. Development 118:1031-1039. [DOI] [PubMed] [Google Scholar]
- 47.Tschop, M., T. R. Castaneda, H. G. Joost, C. Thone-Reineke, S. Ortmann, S. Klaus, M. M. Hagan, P. C. Chandler, K. D. Oswald, S. C. Benoit, R. J. Seeley, K. P. Kinzig, T. H. Moran, A. G. Beck-sickinger, N. Koglin, R. J. Rodgers, J. E. Blundell, Y. Ishii, A. H. Beattie, P. Holch, D. B. Allison, K. Raun, K. Madsen, B. S. Wulff, C. E. Stidsen, M. Birringer, O. J. Kreuzer, M. Schindler, K. Arndt, K. Rudolf, M. Mark, X. Y. Deng, D. C. Withcomb, H. Halem, J. Taylor, J. Dong, R. Datta, M. Culler, S. Craney, D. Flora, D. Smiley, and M. L. Heiman. 2004. Physiology: does gut hormone PYY3-36 decrease food intake in rodents? Nature 430:165. [DOI] [PubMed] [Google Scholar]
- 48.Ueno, N., A. Inui, M. Iwamoto, T. Kaga, A. Asakawa, M. Okita, M. Fujimiya, Y. Nakajima, Y. Ohmoto, M. Ohnaka, Y. Nakaya, J. I. Miyazaki, and M. Kasuga. 1999. Decreased food intake and body weight in pancreatic polypeptide-overexpressing mice. Gastroenterology 117:1427-1432. [DOI] [PubMed] [Google Scholar]
- 49.Upchurch, B. H., G. W. Aponte, and A. B. Leiter. 1994. Expression of peptide YY in all four islet cell types in the developing mouse pancreas suggests a common peptide YY-producing progenitor. Development 120:245-252. [DOI] [PubMed] [Google Scholar]
- 50.Upchurch, B. H., B. P. Fung, G. Rindi, A. Ronco, and A. B. Leiter. 1996. Peptide YY expression is an early event in colonic endocrine cell differentiation: evidence from normal and transgenic mice. Development 122:1157-1163. [DOI] [PubMed] [Google Scholar]
- 51.Wheeler, M. B., J. Nishitani, A. M. J. Buchan, A. S. Kopin, W. Y. Chey, T. Chang, and A. B. Leiter. 1992. Identification of a transcriptional enhancer important for enteroendocrine and pancreatic islet cell-specific expression of the secretin gene. Mol. Cell. Biol. 12:3531-3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu, D., H. M. Ellis, E. C. Lee, N. A. Jenkins, N. G. Copeland, and D. L. Court. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA 97:5978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]