Abstract
The pathophysiology of enteropathogenic Escherichia coli (EPEC) diarrhea remains uncertain. In vitro, EPEC stimulates a rapid increase in short-circuit current (Isc) across Caco-2 cell monolayers coincident with intimate attaching and effacing (A/E) bacterial adhesion. This study has examined the roles of specific EPEC virulence proteins in this Isc response. EPEC genes encoding EspA, EspB, and EspD, essential for signal transduction in host cells and A/E activity, were also required for modulation of Caco-2 electrolyte transport.
Enteropathogenic Escherichia coli (EPEC) infections among infants and young children are common in many developing countries (24); however, the pathophysiology of resultant diarrhea remains uncertain. EPEC produces no recognized enterotoxins (22, 25, 28); rather, diarrhea results from direct interaction of bacteria with the small intestinal epithelium. EPEC adheres to enterocytes and transduces signals (13) which produce an attaching and effacing (A/E) lesion in the brush border membrane characterized by a loss of microvilli and intimate adhesion of bacteria to the apical cell membrane, beneath which host cell cytoskeletal elements accumulate (18, 19, 34).
To study the effects of EPEC interaction on host cell electrolyte transport, we developed an in vitro model of EPEC infection in which monolayers of the human intestinal cell line Caco-2 are rapidly infected with EPEC before being mounted into Ussing chambers (2). Using this technique, we demonstrated a rapid stimulation of short-circuit current (Isc) across Caco-2 cell monolayers caused by EPEC which indicated modulation of transepithelial electrolyte transport (2). Moreover, the peak of this Isc response coincided with development of A/E lesions and was partially (∼35%) chloride dependent, consistent with EPEC-induced stimulation of chloride secretion.
The A/E phenotype is determined by a 35-kbp region of the EPEC chromosome designated the locus of enterocyte effacement (LEE) (7, 26). At the right end of this pathogenicity island are genes encoding three EPEC secreted proteins, EspA, EspD, and EspB, which are essential for signal transduction to host cells and A/E activity (4, 8, 14, 21); these proteins are exported via a type III secretion apparatus encoded by esc and sep genes at the left end of the LEE (7, 11). EPEC-induced signal transduction results in a number of intracellular changes, including a cascade of inositol phosphates, phosphorylation of host cell proteins, and most significantly, tyrosine phosphorylation of a large protein (formerly Hp90) in the host cell membrane (6, 9, 29). This protein, recently shown to be bacterial in origin and renamed Tir (15), acts as the receptor for intimin, a 94-kDa outer membrane protein product of the LEE eae gene (12). Intimin binding is essential for intimate adhesion of EPEC to host cells and promotes accretion of cytoskeletal elements beneath attached bacteria to produce the mature A/E lesion (30).
The present study was designed to investigate the roles of specific EPEC virulence proteins in the generation of Isc in Caco-2 cell monolayers; we utilized isogenic EPEC mutants deficient in expression of EspA, EspB, or EspD, in functional type III secretion (escN mutant), or in the expression of intimin (eae mutant). The origins and characteristics of EPEC strains used in this study are listed in Table 1. Bacteria were grown in bicarbonate-buffered Dulbecco’s modified Eagle’s medium (DMEM; Sigma Chemical Co., Poole, United Kingdom) in an atmosphere of 5% CO2 in air at 37°C for 4 to 5 h to stimulate expression of LEE-encoded virulence proteins (activated EPEC) (10, 17). As described elsewhere (2), bacteria (108 to ∼109 CFU) were centrifuged onto Caco-2 cell monolayers, which had been grown on Transwell polycarbonate microporous cell culture inserts, to facilitate synchronous initial attachment. Infected monolayers were immediately mounted into a modified Ussing chamber apparatus and maintained at 37°C in DMEM (gassed with 5% CO2-95% O2) for 60 min; during this time Isc and transepithelial electrical resistance (TEER) of monolayers were measured continuously (2). Uninfected Caco-2 cell monolayers, subject to all the procedures involved in the infection method except exposure to bacteria, maintained both a constant Isc and a stable TEER throughout the 60-min study period.
TABLE 1.
Characteristics of EPEC strains used in this study
| Strain | Description | Signal transduction | Intimin expression | A/E lesions | Reference(s) |
|---|---|---|---|---|---|
| E2348/69 | EPEC (O127:H6); infant diarrhea outbreak (United Kingdom) | + | + | + | 33 |
| UMD872 | E2348/69 ΔespA1::aphA-3 | − | + | − | 14 |
| UMD872pMSD2 | UMD872 containing plasmid bearing espA | + | + | + | 14 |
| UMD864 | E2348/69 ΔespB1 | − | + | − | 4, 13 |
| UMD864pMSD3 | UMD864 containing plasmid bearing espB | + | + | + | 4, 13 |
| UMD870 | E2348/69 ΔespD1::aphA-3 | − | + | − | 21 |
| UMD870pLCL123 | UMD870 containing plasmid bearing espD | + | + | + | 21 |
| CVD452 | E2348/69 ΔescN::aphA-3 | − | + | − | 11 |
| CVD206 | E2348/69 Δeae | + | − | − | 3, 29 |
| CVD206pCVD438 | CVD206 containing plasmid bearing eae | + | + | + | 3, 12 |
To investigate the respective roles of Esp proteins, functional type III secretion, and intimin-mediated adhesion in EPEC-induced electrical responses, Caco-2 cell monolayers were infected with EPEC wild-type strain E2348/69; with espA, espB, espD, escN, or eae mutants of E2348/69; or with their complementary transformants in which the deficient gene was reintroduced on a plasmid. Light microscopy studies revealed that for all strains, initial infection consistently produced adhesion of discrete bacterial colonies to at least 75% of cells (data not shown).
EPEC stimulates Isc in Caco-2 cell monolayers.
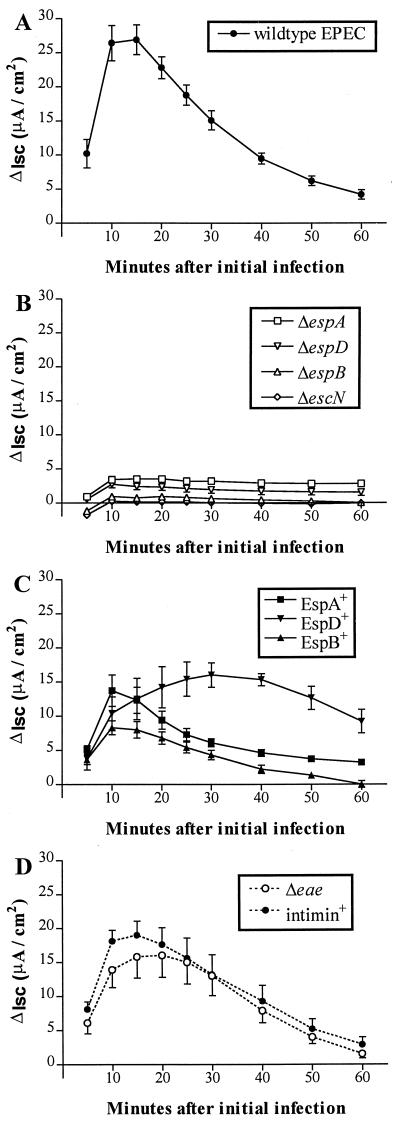
Consistent with our previous observations (2), infection of Caco-2 cells with the wild-type EPEC strain, E2348/69, stimulated a rapid increase in Isc, which peaked 10 to 15 min after initial infection (mean change in Isc ± standard error of the mean [SEM] at 10 min, 26.4 [2.6] μA/cm2 above the basal Isc of uninfected monolayers; n = 8; Student’s t test, P < 0.001) before falling gradually (Fig. 1A). The peak of this Isc response was not associated with any significant change in TEER (ΔTEER) of monolayers; however, between 10 and 60 min after initial infection, E2348/69 induced a 31% loss in TEER (mean ΔTEER [SEM] between 10 and 60 min postinfection, −81 [8] Ω · cm2; P < 0.001; n = 8) similar to that described previously (2).
FIG. 1.
Isc change (ΔIsc; μA/cm2) in Caco-2 cell monolayers during infection with wild-type EPEC E2348/69 (A); signal transduction-defective mutant UMD872 (ΔespA), UMD864 (ΔespB), UMD870 (ΔespD), or CVD452 (ΔescN) (B); complementary plasmid transformant UMD872pMSD2 (EspA+), UMD864pMSD3 (EspB+), or UMD870pLCL123 (EspD+) (C); and intimin-deficient mutant CVD206 (Δeae) or its plasmid transformant CVD206pCVD438 (intimin positive) (D). Each point represent the mean change in Isc ± SEM (error bars) of 5 to 10 monolayers compared with the basal Isc of uninfected cells (n = 25).
EspA, EspB, and EspD are required for stimulation of Caco-2 cell Isc.
Changes in Isc following infection of Caco-2 cell monolayers with E2348/69 gene deletion mutants or their plasmid transformants are shown in Fig. 1. The most striking finding was an absence of the characteristic EPEC-induced Isc response (Fig. 1A) when Caco-2 cell monolayers were infected with signal transduction-defective mutant UMD872 (ΔespA), UMD864 (ΔespB), UMD870 (ΔespD), or CVD452 (ΔescN) (Fig. 1B). Moreover, the Isc response was qualitatively restored, albeit at a reduced peak magnitude, when cells were infected with complementary plasmid transformants UMD872pMSD2 (EspA+) or UMD864pMSD3 (EspB+) (Fig. 1C). Infection with the EspD+ transformant strain UMD870pLCL123 induced a more gradual increase in Isc than that stimulated by the parental strain; this response peaked after ∼30 min (Fig. 1C). Therefore, the EPEC genes required for expression and functional type III secretion of three separate signalling proteins (i.e., EspA, EspB, and EspD) all appear to be prerequisite for eliciting an increase in Isc in Caco-2 cell monolayers. In contrast, infection with the intimin-deficient strain CVD206 (Δeae) or its transformant CVD206pCVD438 (intimin positive) induced Isc responses which were qualitatively similar to, although somewhat attenuated compared to, that seen with E2348/69 infection (Fig. 1D), thereby revealing that modulation of transepithelial electrolyte transport is independent of intimate EPEC adherence to host cells.
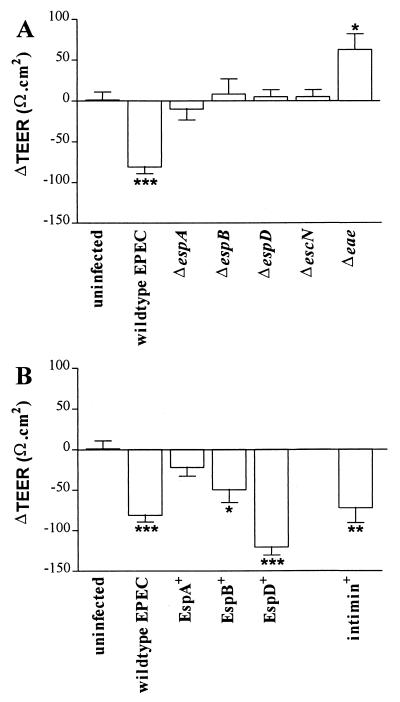
EPEC signal transduction and intimin expression are necessary for inducing loss of TEER.
The reduction in TEER of Caco-2 cell monolayers, seen during infection with strain E2348/69, was not apparent during infection with signal transduction-deficient strain UMD872 (ΔespA), UMD864 (ΔespB), UMD870 (ΔespD), or CVD452 (ΔescN) (Fig. 2A). However, marked reductions in TEER were induced by transformant strains UMD864pMSD3 (EspB+) and UMD870pLCL123 (EspD+) (Fig. 2B); infection with strain UMD872pMSD2 (EspA+) did not significantly reduce TEER. In contrast, infection with the intimin-deficient strain CVD206 (Δeae) produced a significant increase in TEER over 60 min (Fig. 2A), while infection with its intimin-positive transformant CVD206pCVD438 caused a reduction in TEER comparable to that induced by E2348/69 (Fig. 2B). A detrimental effect of EPEC infection on TEER has been described for several cultured epithelial cell lines, and although this effect was initially attributed to a transcellular defect (1), it is now considered to result primarily from the disruption of intercellular tight junctions, which reduces barrier function of the cell monolayer (27, 31). In Caco-2 cells, we found that both EPEC signal transduction and intimin expression were prerequisite for inducing loss of TEER. A similar observation was previously made by Canil et al. (1), who also noted an increase in TEER during infection with the intimin-deficient strain CVD206. Recently, specific signalling events induced in host cells by EPEC have been found to be dependent on intimin binding (16). Therefore, the detrimental effect of EPEC on the TEER of Caco-2 cell monolayers may occur after intimin binding as a result of late signalling events which alter the integrity of tight junctions. The increase in TEER observed during infection with the intimin-deficient strain CVD206 requires further investigation.
FIG. 2.
Change in TEER (ΔTEER; Ω · cm2) in uninfected Caco-2 cell monolayers between 10 and 60 min and after infection with wild-type EPEC E2348/69 or gene-deficient mutant UMD872 (ΔespA), UMD864 (ΔespB), UMD870 (ΔespD), CVD452 (ΔescN), or CVD206 (Δeae) (A) or complementary plasmid transformants UMD872pMSD2 (EspA+), UMD864pMSD3 (EspB+), UMD870pLCL123 (EspD+), or CVD206pCVD438 (intimin positive) (B). Each bar represents the mean ± SEM (error bar) for 5 to 10 infected monolayers. ∗, P < 0.05, ∗∗, P < 0.01, and ∗∗∗, P < 0.001 versus uninfected monolayers (n = 25).
In the absence of conventional enterotoxin production, the A/E phenotype is central to EPEC pathogenicity; however, this interaction of bacteria with host cells is complex and the precise mechanism(s) of EPEC induced diarrhea remains to be determined. Although loss of functional brush border membrane caused by A/E lesion formation and increased paracellular permeability induced by EPEC (27, 31) may contribute to the generation of an osmotic diarrhea, in vitro evidence indicates that EPEC is also able to directly modulate electrolyte transport in host cells. In HeLa and Caco-2 epithelial cell lines, Stein et al. (32) found that EPEC infection caused a reduction in resting membrane potential, indicating an altered distribution of ions across cell membranes. Significantly, this effect was dependent on signal transduction to host cells (sep and espB mutants) but was independent of intimate attachment to host cells (eae mutant) (32). More recently, we have described an EPEC-induced stimulation of Isc in Caco-2 cell monolayers which provided direct evidence of altered transepithelial electrolyte transport (2); in the present study we examined the roles of various EPEC virulence proteins in the generation of this Isc response. We have found that EPEC stimulation of Isc requires functional secretion of all three Esp proteins (EspA, EspB, and EspD) needed for signal transduction and A/E activity. This observation accords well with recent reports which have sought to explain the dynamics of the EPEC-host cell interaction. Type III secretion mechanisms, akin to that found in EPEC (11), are a feature of many gram-negative pathogens and are typically responsible for the export of virulence proteins which interact directly with host cells (23). The EspA protein of EPEC was recently shown to be a constituent of an organelle on the bacterial surface which creates a bridge to host cells essential for translocation of EspB (20), and translocation of Tir also requires EspA (15). The role of EspD in this export pathway is as yet unclear; however, it is apparent that all three secreted proteins are required for signalling which leads to A/E lesion formation and the stimulation of Isc. Previously, we demonstrated that the peak of the Isc response stimulated by wild-type EPEC coincided with development of A/E lesions, as assessed by transmission electron microscopy (2), suggesting that these EPEC phenotypes share similar kinetics. Moreover, in Caco-2 cell monolayers grown on glass coverslips, A/E lesions are identifiable by fluorescent staining of actin accumulated beneath adherent bacteria (19) 15 min after initial rapid infection with activated EPEC strain E2348/69 (data not shown); this lesion development coincides with peak Isc stimulation seen in Ussing chamber studies. This finding is also true for those A/E phenotypic strains used in the present study which demonstrated Isc kinetics typical of the wild type, irrespective of the magnitude of their peak Isc increase. In contrast, this result is not the case for the EspD+ transformant strain UMD870pLCL123. This strain, which stimulated a more gradual increase in Isc than does the wild type, also induces lesion formation at a lower rate such that the A/E phenotype is undetectable at 15 min and only evident after 60 min of infection (data not shown). Significantly, it has been found that the extracellular production of individual Esp proteins may be adversely affected by the absence of other esp genes, and in gene-deficient mutants this effect may not be fully redressed by the presence of a plasmid bearing the missing gene (21). We believe that this phenomenon may account for the failure of the transformant strains to elicit an Isc response equivalent to that caused by the wild-type EPEC strain and, in particular, is responsible for the uncharacteristic response seen for infection with strain UMD870pLCL123 (EspD+). Interestingly, this strain produces abnormally long EspA filaments (20), a consequence of which may be abnormal protein translocation and signal transduction.
That EPEC-induced modulation of transepithelial electrolyte transport is a consequence either of the cascade of signalling events which are central to A/E activity (e.g., flux of inositol phosphates or activation of protein kinases) or of the cytoskeletal rearrangements which occur during lesion formation remains unclear. However, the ability of strain CVD206 (Δeae) to cause a characteristic Isc increase in Caco-2 cell monolayers indicates that this stimulation is independent of intimate adherence. Although it lacks intimin, strain CVD206 retains the ability to transduce signals to host cells and cause some cytoskeletal disorganization (3, 29). The residual virulence of this strain in vivo (5) suggests that these elements of EPEC pathogenesis alone are indeed sufficient to cause diarrhea in the absence of intimate adherence.
Acknowledgments
This work was supported by a grant from Action Research (to S.K.) and by Public Health Service awards AI32074 (to M.S.D) and AI21657 (to J.B.K.) from the National Institutes of Health.
REFERENCES
- 1.Canil C, Rosenshine I, Ruschkowski S, Donnenberg M S, Kaper J B, Finlay B B. Enteropathogenic Escherichia coli decreases the transepithelial electrical resistance of polarized epithelial monolayers. Infect Immun. 1993;61:2755–2762. doi: 10.1128/iai.61.7.2755-2762.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collington G K, Booth I W, Knutton S. Rapid modulation of electrolyte transport in Caco-2 cell monolayers by enteropathogenic Escherichia coli (EPEC) infection. Gut. 1998;42:200–207. doi: 10.1136/gut.42.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donnenberg M S, Yu J, Kaper J B. A second chromosomal gene necessary for intimate attachment of enteropathogenic Escherichia coli to epithelial cells. J Bacteriol. 1993;175:4670–4680. doi: 10.1128/jb.175.15.4670-4680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donnenberg M S, Tacket C O, James S P, Losonsky G, Nataro J P, Wasserman S S, Kaper J B, Levine M M. Role of the eaeA gene in experimental enteropathogenic Escherichia coli infection. J Clin Invest. 1993;92:1412–1417. doi: 10.1172/JCI116717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dytoc M, Fedorko L, Sherman P M. Signal transduction in human epithelial cells infected with attaching and effacing Escherichia coli in vitro. Gastroenterology. 1994;106:1150–1161. doi: 10.1016/0016-5085(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 7.Elliott S J, Wainwright L A, McDaniel T K, Jarvis K G, Deng Y K, Lai L-C, MacNamara B P, Donnenberg M S, Kaper J B. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic E. coli E2348/69. Mol Microbiol. 1998;28:1–4. doi: 10.1046/j.1365-2958.1998.00783.x. [DOI] [PubMed] [Google Scholar]
- 8.Foubister V, Rosenshine I, Donnenberg M S, Finlay B B. The eaeB gene of enteropathogenic Escherichia coli is necessary for signal transduction in epithelial cells. Infect Immun. 1994;62:3038–3040. doi: 10.1128/iai.62.7.3038-3040.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foubister V, Rosenshine I, Finlay B B. A diarrheal pathogen, enteropathogenic Escherichia coli (EPEC), triggers a flux of inositol phosphates in infected epithelial cells. J Exp Med. 1994;179:993–998. doi: 10.1084/jem.179.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haigh R, Baldwin T, Knutton S, Williams P H. Carbon dioxide regulated secretion of the EaeB protein of enteropathogenic Escherichia coli. FEMS Microbiol Lett. 1995;129:63–67. doi: 10.1016/0378-1097(95)00136-S. [DOI] [PubMed] [Google Scholar]
- 11.Jarvis K G, Girón J A, Jerse A E, McDaniel T K, Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc Natl Acad Sci USA. 1995;92:7996–8000. doi: 10.1073/pnas.92.17.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jerse A E, Kaper J B. The eae gene of enteropathogenic Escherichia coli encodes a 94-kilodalton membrane protein, the expression of which is influenced by the EAF plasmid. Infect Immun. 1991;59:4302–4309. doi: 10.1128/iai.59.12.4302-4309.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenny B, Finlay B B. Protein secretion by enteropathogenic Escherichia coli is essential for transducing signals in epithelial cells. Proc Natl Acad Sci USA. 1995;92:7991–7995. doi: 10.1073/pnas.92.17.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kenny B, Lai L-C, Finlay B B, Donnenberg M S. EspA, a protein secreted by enteropathogenic Escherichia coli (EPEC) is required to induce signals in epithelial cells. Mol Microbiol. 1996;20:313–323. doi: 10.1111/j.1365-2958.1996.tb02619.x. [DOI] [PubMed] [Google Scholar]
- 15.Kenny B, DeVinney R, Stein M, Reinscheid D J, Frey E A, Finlay B B. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 16.Kenny B, Finlay B B. Intimin-dependent binding of enteropathogenic Escherichia coli to host cells triggers novel signaling events, including tyrosine phosphorylation of phospholipase C-γ1. Infect Immun. 1997;65:2528–2536. doi: 10.1128/iai.65.7.2528-2536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenny B, Abe A, Stein M, Finlay B B. Enteropathogenic Escherichia coli protein secretion is induced in response to conditions similar to those in the gastrointestinal tract. Infect Immun. 1997;65:2606–2612. doi: 10.1128/iai.65.7.2606-2612.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knutton S, Lloyd D R, McNeish A S. Adhesion of enteropathogenic Escherichia coli to human intestinal enterocytes and cultured human intestinal mucosa. Infect Immun. 1987;55:69–77. doi: 10.1128/iai.55.1.69-77.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knutton S, Baldwin T, Williams P H, McNeish A S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;57:1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knutton S, Rosenshine I, Pallen M J, Nisan I, Neves B C, Bain C, Wolff C, Dougan G, Frankel G. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 1998;17:2166–2176. doi: 10.1093/emboj/17.8.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai L-C, Wainwright L A, Stone K D, Donnenberg M S. A third secreted protein that is encoded by the enteropathogenic Escherichia coli pathogenicity island is required for transduction of signals and for attaching and effacing activities in host cells. Infect Immun. 1997;65:2211–2217. doi: 10.1128/iai.65.6.2211-2217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Law D, Wilkie K M, Freeman R. Examination of enteropathogenic Escherichia coli for an adenyl cyclase stimulating factor. J Med Microbiol. 1987;23:335–338. doi: 10.1099/00222615-23-4-335. [DOI] [PubMed] [Google Scholar]
- 23.Lee C A. Type III secretion systems: machines to deliver bacterial proteins into eukaryotic cells? Trends Microbiol. 1997;5:148–156. doi: 10.1016/S0966-842X(97)01029-9. [DOI] [PubMed] [Google Scholar]
- 24.Levine M M. Escherichia coli that cause diarrhoea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohaemorrhagic, and enteroadherent. J Infect Dis. 1987;155:377–389. doi: 10.1093/infdis/155.3.377. [DOI] [PubMed] [Google Scholar]
- 25.Long-Krug S A, Weikel C S, Tiemens K T, Hewlett E L, Levine M M, Guerrant R L. Does enteropathogenic Escherichia coli produce heat-labile enterotoxin, heat-stable enterotoxins a or b, or cholera toxin A subunits? Infect Immun. 1984;46:612–614. doi: 10.1128/iai.46.2.612-614.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDaniel T K, Jarvis K G, Donnenberg M S, Kaper J B. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci USA. 1995;92:1664–1668. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Philpott D J, McKay D M, Sherman P M, Perdue M H. Infection of T84 cells with enteropathogenic Escherichia coli alters barrier and transport functions. Am J Physiol. 1996;270:G634–G645. doi: 10.1152/ajpgi.1996.270.4.G634. [DOI] [PubMed] [Google Scholar]
- 28.Robins-Browne R M, Levine M M, Rowe B, Gabriel E M. Failure to detect conventional enterotoxins in classical enteropathogenic (serotyped) Escherichia coli strains of proven pathogenicity. Infect Immun. 1982;38:798–801. doi: 10.1128/iai.38.2.798-801.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenshine I, Donnenberg M S, Kaper J B, Finlay B B. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 1992;11:3551–3560. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenshine I, Ruschkowski S, Stein M, Reinscheid D J, Mills S D, Finlay B B. A pathogenic bacterium triggers epithelial signals to form a functional bacterial receptor that mediates actin pseudopod formation. EMBO J. 1996;15:2613–2624. [PMC free article] [PubMed] [Google Scholar]
- 31.Spitz J, Yuhan R, Koutsouris A, Blatt C, Alverdy J, Hecht G. Enteropathogenic Escherichia coli adherence to intestinal epithelial monolayers diminishes barrier function. Am J Physiol. 1995;268:G375–G379. doi: 10.1152/ajpgi.1995.268.2.G374. [DOI] [PubMed] [Google Scholar]
- 32.Stein M A, Mathers D A, Yan H, Baimbridge K G, Finlay B B. Enteropathogenic Escherichia coli markedly decreases the resting membrane potential of Caco-2 and HeLa human epithelial cells. Infect Immun. 1996;64:4820–4825. doi: 10.1128/iai.64.11.4820-4825.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor J. Infectious infantile enteritis, yesterday and today. Proc R Soc Med. 1970;63:1297–1301. doi: 10.1177/003591577006301233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ulshen M H, Rollo J L. Pathogenesis of Escherichia coli gastroenteritis in man—another mechanism. N Engl J Med. 1980;302:99–101. doi: 10.1056/NEJM198001103020207. [DOI] [PubMed] [Google Scholar]