Abstract
Gap junctions are composed of connexins and are critical for the maintenance of the differentiated state. Consistently, connexin expression is impaired in most cancer cells, and forced expression of connexins following cDNA transfection reverses the tumor phenotype. We have found that the restoration of density inhibition of human pancreatic cancer cells by the antiproliferative somatostatin receptor 2 (sst2) is due to overexpression of endogenous connexins Cx26 and Cx43 and consequent formation of functional gap junctions. Immunoblotting along with protein metabolic labeling and mRNA monitoring revealed that connexin expression is enhanced at the level of translation but is not sensitive to the inhibition of cap-dependent translation initiation. Furthermore, we identified a new internal ribosome entry site (IRES) in the Cx26 mRNA. The activity of Cx26 IRES and that of the previously described Cx43 IRES are enhanced in density-inhibited cells. These data indicate that the restoration of functional gap junctions is likely a critical event in the antiproliferative action of the sst2 receptor. We further suggest that the existence of IRESes in connexin mRNAs permits connexin expression in density-inhibited or differentiated cells, where cap-dependent translation is generally reduced.
Gap junction intercellular communication plays an important function in tissue integrity. To form a gap junction channel, each cell of an adjacent pair synthesizes a hemichannel composed of six connexin subunits called a connexon. A complete channel is then formed when a connexon of one cell docks with that of the adjacent cell. Connexins belong to a large family of at least 20 protein isoforms in humans whose expression is tissue specific (48, 60). Normal differentiated and contact-inhibited cells express at least one connexin isoform that assembles, alone or in combination, into gap junctions. Consistently, the lack of growth control and the inability to differentiate or apoptose in most cancer cells are related to altered connexin expression and consequent dysfunctional gap junction intercellular communication.
The alteration of connexin expression is of importance, as their forced expression, following cDNA transfection, can promote cell density inhibition and reverse the tumor phenotype (34). The loss of connexin function in cancer cells or in cells treated with tumor promoters results from various defects, including alteration of transcription (51, 62), protein trafficking (16, 47), stability (26, 45), or phosphorylation (36, 52). Another proposed mechanism that could be altered is the translation of connexin mRNAs (40).
All nucleus-encoded eukaryotic mRNAs are modified at their 5′ end with a structure termed the cap (m7GpppN, where N is any nucleotide). Cap-dependent translation is mediated by the eukaryotic translation initiation factor (eIF) 4F, a protein complex composed of three subunits: eIF4E, which recognizes the cap; eIF4A, an RNA helicase; and eIF4G, a scaffolding protein that simultaneously binds eIF4E and eIF4A (13). Through eIF3, eIF4G also binds the 40S ribosomal subunit (19) and therefore can bridge the ribosome to the mRNA 5′ end by its simultaneous interaction with eIF4E. eIF4E activity is modulated by its reversible association with the binding protein 4E-BP1 (30, 41). Hypophosphorylated 4E-BP1 competes with eIF4G for a common binding site on eIF4E, while hyperphosphorylated 4E-BP1 does not (18, 31). The binding of hypophosphorylated 4E-BP1 to eIF4E disrupts the eIF4F complex and results in the inhibition of cap-dependent translation (41).
Although cap-dependent translation initiation is thought to be the prevalent mode of ribosome binding to mRNAs in eukaryotes, a growing list of cellular mRNAs exhibit an inherent ability to bypass the requirement for the cap structure. Translation of these mRNAs is independent of eIF4E and thus insensitive to the hypophosphorylated forms of 4E-BP1. Ribosomes access these mRNAs by binding directly to an internal ribosome entry site (IRES). IRESes were first identified in picornavirus RNAs, which do not possess a 5′ cap structure (21, 42). A restricted but increasing number of cellular IRESes have since been discovered (57). It is thought that one function of cellular IRESes is to permit the control of translation even when cap-dependent translation is impaired. That the c-sis IRES is activated during differentiation supports this presumption (50).
The mRNAs coding for the connexin 32 (Cx32) and Cx43 proteins can be translated by a cap-independent, IRES-mediated mechanism (20, 49). Since gap junctions play a vital function in the maintenance of the differentiated state, it has been hypothesized that the existence of IRESes in connexin mRNAs would ensure connexin expression even in contact-inhibited cells, where cap-mediated translation is impaired (59).
We have addressed this hypothesis in pancreatic cancer cells whose tumor phenotype has been reversed by stable transfection of somatostatin receptor 2 (sst2). Among the five somatostatin receptor subtypes, sst2 plays a critical role in tumor inhibition. However, since it is no longer expressed in most human pancreatic adenocarcinomas (4), sst2 cannot transmit the antitumoral signal of somatostatin. Conversely, reintroducing sst2 is sufficient to inhibit cell proliferation and to attenuate malignancy even in the absence of exogenous ligand. No exogenous ligand is required because stable expression of sst2 induces the synthesis and secretion of somatostatin, which permanently activates the receptor (8, 46).
Given the important role of gap junction intercellular communication in the inhibition of cell proliferation (34), we propose that the antiproliferative action of the sst2 receptor could be mediated, at least in part, by connexins. Here we show that in sst2-expressing cells, the restoration of density inhibition is dependent on the formation of functional gap junctions composed of Cx26 and Cx43. We further show that, in addition to Cx32 and Cx43, Cx26 mRNA also contains an IRES in its 5′ untranslated region (UTR). The expression of both Cx26 and Cx43 is ensured mainly by a posttranscriptional mechanism through cap-independent, IRES-mediated mRNA translation.
MATERIALS AND METHODS
Materials.
Dulbecco's modified Eagle's medium with 1 g/liter glucose (DMEM) and other culture reagents were supplied by GIBCO except fetal calf serum (FCS, Sigma) and Plasmocin (InvivoGen). ExGen 500 transfection reagent was from Euromedex. 18β-Glycyrrhetinic acid (β-GA) was purchased from Aldrich. Rhodamine-dextran (10,000 Da), biocytin, and Alexa Fluor 488-conjugated avidin were obtained from Molecular Probes. The rabbit anti-sst2, which was generated against a peptide corresponding to a sequence of the carboxyl-terminal tail of the human sst2, was generously provided by S. Schulz (Department of Pharmacology and Toxicology, Otto-von-Guericke University, Magdeburg, Germany) (56). The anti-Cx43 rabbit polyclonal antibody was purchased from Santa Cruz Biotechnology. Monoclonal anti-Cx43 (clone CX-1B1) (7, 39) and anti-Cx32 (clone CX-2C2) antibodies were from Zymed. The anti-Cx26 rabbit polyclonal antibody used for immunofluorescence, immunoblotting, and immunoprecipitation experiments and the anti-Cx26 monoclonal antibody (clone CX-12H10) used for immunofluorescence experiments were purchased from Zymed. Monoclonal anti-cyclin D1 was purchased from Cell Signaling Technology. Polyclonal antibody against 4E-BP1 was kindly provided by N. Sonenberg (Department of Biochemistry and McGill Cancer Center, McGill University) (43). Monoclonal anti-β-tubulin was from Sigma. Rapamycin was purchased from Calbiochem. l-[35S]methionine (1,000 Ci/mmol; 10 mCi/ml) was purchased from Amersham Biosciences. All other materials were obtained from Sigma unless otherwise stated.
Cell culture and growth assay.
Human pancreatic tumor BxPC-3 cells stably expressing human sst2 or the mock vector (8, 46) were routinely grown in DMEM supplemented with 10% FCS, 2 mM L-glutamine, 400 μg/ml geneticin, 2.5 μg/ml Fungizone, 5 U/ml streptomycin/penicillin, and 2.5 μg/ml Plasmocin. Cells were maintained in a water-saturated atmosphere of 5% CO2 at 37°C. For the growth assay, cells were plated in six-well dishes (105 cells/dish) and cultured for 6 days. Cell proliferation was measured every 2 days by counting with a Coulter counter model Z1 (Coulter Electronics) as described previously (3). To inhibit gap junction intercellular communication, β-GA was added to the culture medium at the time of plating. Cells were fed every day with fresh medium containing either 25 μM β-GA or vehicle (dimethyl sulfoxide). To analyze the effect of β-GA on BxPC-3 cell proliferation, cells were plated at 105 cells/dish to reach confluence after 6 days of growth.
Detection of sst2.
Cells grown on glass coverslips for 48 h were fixed with 3% paraformaldehyde for 20 min at +4°C and permeabilized for 1 min with 0.1% Triton X-100. After blocking for nonspecific antibody binding sites with 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS), cells were incubated for 1 h at room temperature with anti-sst2 antibody (1:500) diluted in PBS-BSA. After washing, cells were incubated for 1 h with fluorescein isothiocyanate-conjugated anti-rabbit immunoglobulin G (1:300) (Nordic Laboratories) diluted in PBS-BSA. Coverslips were mounted with fluorescent mounting medium (DAKO Corporation). The preparations were examined with a confocal laser scanning microscope (LSM 410, Carl Zeiss) using an argon laser adjusted to 488 nm.
Detection of connexins.
Cells grown on glass coverslips for 48 h were fixed with −20°C absolute methanol for 20 min. Unspecific protein binding was prevented by 1 h incubation with PBS containing 1% FCS. Cells were then incubated for 1 h at room temperature with primary antibodies (1:100) diluted in PBS containing 0.1% FCS (PBS-FCS). After washing, cells were incubated for 1 h at room temperature with Cy3-conjugated anti-mouse immunoglobulin G (1:250) or with Alexa Fluor 488-conjugated anti-rabbit immunoglobulin G (1:250) (Molecular Probes) diluted in PBS-FCS. Cells were then rinsed and stained with 0.4 μg/ml 4′,6′-diamidino-2-phenylindole. The coverslips were mounted onto glass slides with fluorescent mounting medium (DAKO Corporation). Coverslips were rinsed three times for 5 min in PBS-FCS between the steps. Immunofluorescence images were captured using a Nikon Eclipse E400 microscope and Biocom VisioLab 2000 software. Fluorescent controls were incubated without primary or secondary antibodies. No significant cross-labeling or unspecific labeling was observed. For double immunofluorescence, cells were incubated simultaneously with mouse monoclonal anti-Cx26 and rabbit polyclonal anti-Cx43 antibodies (or vice versa), followed by incubation with Alexa Fluor 488- and Cy3-conjugated secondary antibodies.
Immunoblotting.
Cells were plated in 100-mm-diameter culture dishes (106 cells/dish) and grown for 4 days unless otherwise noted. After a rapid wash with ice-cold PBS, cells were lysed at 4°C in lysis buffer (50 mM HEPES, pH 7.5, 150 mM NaCl, 100 mM NaF, 10 mM EDTA, 10 mM Na4P2O7, containing 1% Triton X-100, 1% sodium deoxycholate, 2 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 2 μg/ml aprotinin, and 20 μM leupeptin), sonicated for 30 seconds on ice, then clarified by centrifugation at 13,000 rpm for 15 min at 4°C. The protein content was determined in the supernatant using the Bradford method (Bio-Rad). Equal amounts of protein were dissolved in 4× sample buffer (250 mM Tris-HCl, pH 6.8, 8% sodium dodecyl sulfate [SDS], 20% 2-mercaptoethanol, 50% glycerol, bromophenol blue) and left at room temperature for 30 min. Total cell lysates were separated on SDS-polyacrylamide gel electrophoresis (SDS-PAGE) followed by electrophoretic transfer onto nitrocellulose membranes (Pall Life Sciences). Membranes were then subjected to immunoblotting using horseradish peroxidase-conjugated secondary antibodies (Pierce) as described previously (25). Peroxidase activity was revealed using the enhanced chemiluminescence (ECL) system (Pierce). Quantitative analyses were carried out by using a Biocom apparatus.
Scrape-loading and dye transfer assay for gap junction intercellular communication.
BxPC-3 cells were assessed for gap junction-mediated intercellular communication using a protocol adapted from that of Le and Musil (27, 28). Cells were plated on glass coverslips in 35-mm-diameter dishes (6 × 105 cells/dish) and grown for 4 days to confluence. The cells were rinsed three times with PBS containing 1% BSA, and the glass coverslip was removed from the dish; 3 μl of a solution of PBS containing 0.5% rhodamine-dextran and 1% biocytin was directly applied to the center of the coverslip, after which a scalpel was used to create a longitudinal scratch through the cell monolayer. Cells were incubated in the dye mix for exactly 3 min, and then the coverslip was put back in the dish. Cells were quickly rinsed twice with PBS and immediately fixed for 15 min with 2% paraformaldehyde (pH 7.0) in PBS. Fixed cells were then permeabilized for 15 min with PBS containing 0.1% Triton X-100 and 0.2% BSA, followed by incubation with Alexa Fluor 488-conjugated avidin (1:200) diluted in 0.2% BSA-PBS for 30 min at room temperature. The coverslips were mounted onto glass slides with fluorescent mounting medium. Coverslips were rinsed twice in 1% BSA-PBS between the different steps. Rhodamine-dextran and (after reaction with Alexa Fluor 488-conjugated avidin) biocytin were visualized by fluorescence microscopy using, respectively, G-2A and fluorescein isothiocyanate filter sets. Fluorescent controls were incubated without biocytin or without Alexa Fluor 488-conjugated avidin. Only a small amount of unspecific labeling was obtained with Alexa Fluor 488-conjugated avidin.
In vivo labeling and measurement of protein synthesis.
Cells were plated in 60-mm-diameter culture dishes (4 × 105 cells/dish) and grown for 2 days. Cells were starved for methionine for 30 min at 37°C in methionine-free DMEM and supplemented with 10% FCS and 2 mM glutamine. The medium was then replaced with fresh methionine-free medium containing [35S]methionine (10 μCi/ml). The radioactive medium was removed after a 30-min pulse, and cells were rinsed twice with ice-cold PBS. Cells were then lysed at 4°C in lysis buffer as described above, and protein content was quantified. Equal amounts were either immunoprecipitated using polyclonal antibodies directed against Cx26 (Zymed) or Cx43 (Santa Cruz) or directly dissolved in 4× sample buffer as described (25). Immunocomplexes or total cell lysates were submitted to SDS-PAGE, transferred onto nitrocellulose membranes, and analyzed by autoradiography using a PhosphorImager (Molecular Dynamics).
Real-time quantitative PCR.
Cells were plated in 100-mm-diameter culture dishes (106 cells/dish) and grown for 4 days. Total RNA was extracted with TRIzol reagent (Invitrogen) according to the manufacturer's instructions. After a DNase I treatment (BD Biosciences-Clontech), total RNA was reverse transcribed by using random hexamers and Superscript II reverse transcriptase (Invitrogen). Resulting cDNAs were used to perform quantitative analysis of Cx26 and Cx43 mRNA expression by real-time PCR using a Gene Amp 5700 Sequence Detection System and SYBR green as a dye (Applied Biosystems). The sequences of primers (MWG Biotech) were as follows: Cx26-forward primer, 5′-CTGTGTTGTGTGCATTCGTCTTT-3′; reverse primer, 5′-CAGCGTGCCCCAATCC-3′; Cx43-forward primer, 5′-GACAAGGTTCAAGCCTACTCAACTG-3′; reverse primer, 5′-TGTCCCCAGCAGCAGGAT-3′; and glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-forward primer, 5′-GTCGGAGTCAACGGATTTGG-3′; reverse primer, 5′-AAAAGCAGCCCTGGTGACC-3′. PCR efficiencies were measured by preparing a standard curve for each primer pair (efficiency >90%). Target gene expression was normalized by using GAPDH housekeeping gene expression as an internal control. Samples incubated without reverse transcriptase were used as negative template controls.
Plasmid constructs.
The plasmid encoding human Cx32 (pORF/hCx32) and the corresponding plasmid backbone were from InvivoGen. pRL-CMV was from Promega. The Renilla luciferase- and firefly luciferase-based dicistronic vectors pSL2-Cx43 (containing the Cx43 mRNA 5′ UTR) and pSL2-EMCV (containing the encephalomyocarditis virus IRES) were kindly provided by R. Werner (Department of Biochemistry and Molecular Biology, University of Miami School of Medicine, Miami, FL) (49). pSL2-Cx26 was obtained by substituting the Cx43 5′ UTR for the PCR-amplified Cx26 5′ UTR (forward primer: 5′-AAGAATTCAGGTGTTGCGGCCCCGCAGCG-3′, and reverse primer: 5′-TTTTCTCGAGCTTCTACTCTGGGCGGTTG-3′) in the EcoRI/XhoI-linearized pSL2-Cx43 vector. The second stem loop (SL2, see the Results section for details), which was lost during such substitution, was reintroduced in the EcoRI restriction site. The promoterless pGL3-Cx43 dicistronic plasmid, which permits the detection of cryptic promoter activities that could exist in the Cx43 5′ UTR, was generated by subcloning the NheI/NarI fragment of the pSL2-Cx43 vector (which contains the entire Renilla luciferase and Cx43 5′ UTR entire sequences and part of firefly luciferase) into a Nhe1/NarI-linearized pGL3basic vector (Promega). The pGL3-Cx26 vector was generated by substituting the Cx43 5′ UTR and firefly luciferase entire sequences for the Cx26 5′ UTR and firefly luciferase entire sequences into the pGL3-Cx43 vector using EcoRI and XbaI restriction sites.
Transient transfection and dual luciferase assays.
For transient transfections, BxPC-3 cells were grown up to 50% confluence in 100-mm-diameter dishes in DMEM containing 10% FCS and transfected with 6 μg of the desired cDNAs using polyethyleneimine (ExGen 500 transfection reagent; Euromedex) according to the manufacturer's protocol. Cells transfected with the appropriate empty vectors were used as a control. Transfected cells were then incubated for an additional 48 h before lysis with 1× passive lysis buffer (Promega). After measuring the protein content in the supernatant, 20 μg of soluble cell lysate (in a total volume of 50 μl) was assayed for Renilla and firefly luciferase activity with the dual luciferase reporter assay system (Promega) following the manufacturer's instructions. A Luminoskan Ascent microplate luminometer with dual injectors (Thermo Labsystems) was used.
Statistical analysis.
Statistical analysis was performed by using Student's t test. P values < 0.05 indicate a statistically significant difference.
RESULTS
Description of cell lines.
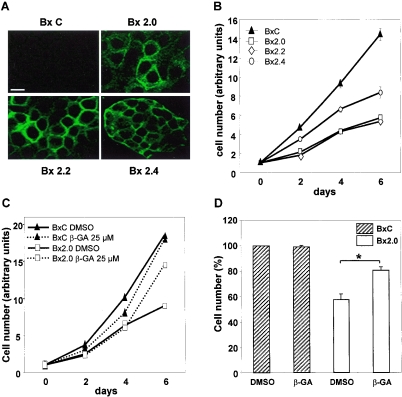
To evaluate the contribution of translational mechanisms in the expression of connexins and consequent formation of gap junctions, we have utilized the previously described mock- (BxC) and stably sst2-transfected (Bx2.0) pools of human ductal pancreatic cancer cells BxPC3 (8, 46). Pools were preferred to clonal populations because they are less likely to have additional alterations due to clonal expansion. To further strengthen our observations, most of the experiments were conducted in two additional sst2-expressing pools (Bx2.2 and Bx2.4). An immunostaining of cells visualized by confocal microscopy was first performed to verify that these two additional pools also express sst2. While sst2 was not detected in BxC cells, both Bx2.2 and Bx2.4 pools expressed sst2 to a level similar to that of the already characterized Bx2.0 cells (Fig. 1A). For clarity, the term Bx2 is used when a comment applies indifferently to any sst2-expressing pool (Bx2.0, Bx2.2, or Bx2.4).
FIG. 1.
sst2-dependent inhibition of BxPC-3 cell proliferation is reversed by an inhibitor of gap junction formation. (A) sst2 expression in BxPC-3 pools. BxC and Bx2 pools of cells were fixed and processed for indirect immunofluorescence coupled to confocal microscopy using a specific polyclonal anti-sst2 antibody as described in Materials and Methods. Pictures show optical sections of cells median areas. Bar, 10 μm. (B) Proliferation rates of BxC and sst2-expressing Bx2 cells. Pools of cells were seeded at equivalent concentrations and replicates were counted every two days as described in Materials and Methods. Results represent the means ± standard error of the mean of three independent experiments performed in triplicates. (C) sst2-mediated inhibition of proliferation is prevented by β-GA. BxC or Bx2.0 cells were seeded at equivalent concentrations in the presence of β-GA or vehicle (DMSO) alone, and replicates were counted every two days. Results represent one experiment performed in triplicates. (D) Results obtained following 6 days of culture and shown in C were repeated and quantified. Values represent the means ± standard error of the mean of three separate experiments performed in triplicates. *, P < 0.05.
Implication of gap junctions in sst2-mediated density inhibition.
The proliferation rate of Bx2 cells was lower than that of BxC cells (Fig. 1B). Depending on the pool analyzed, however, proliferation was not uniformly inhibited. Bx2.4 cells grew faster than Bx2.0 or Bx2.2 cells. A more striking observation was that all Bx2 cells were contact inhibited after 6 days of culture, while BxC cells continued to proliferate. These observations suggest that sst2 elicited antiproliferative mechanisms that could target both the rate of proliferation during the log phase and density inhibition once cells attained confluence.
One cellular function that is related to density inhibition is gap junction intercellular communication (14). To test for the involvement of gap junction intercellular communication in density inhibition of sst2-expressing cells, proliferation was monitored in the presence of 25 μM of 18β-glycyrrhetinic acid (β-GA). The dose of 25 μM was chosen because higher doses (50 or 100 μM) exhibited toxic effects (data not shown). β-GA is a chemical that reversibly precludes connexon packing in gap junction plaques and thus blocks gap junction intercellular communication (15). Interestingly, while the log-phase growth was not perturbed in any cell line, density inhibition of Bx2.0 cells was abrogated by β-GA (Fig. 1C). Similar results were obtained with Bx2.2 and Bx2.4 cells (data not shown). A statistical analysis showed that the action of β-GA was significant at 6 days of cell culture (Fig. 1D). These results indicate that sst2 provokes density inhibition through a mechanism dependent on gap junction intercellular communication.
Functional gap junction intercellular communications are composed of Cx26 and Cx43 in sst2-expressing cells.
Since β-GA precludes density inhibition exclusively in Bx2 cell lines, we hypothesized that sst2-expressing cells possess functional gap junctions while BxC cells do not. To verify this hypothesis, a gap junction-dependent scrape-loading/dye transfer assay was performed. The technique employs scraping to wound cells. Wounded cells are then loaded with a low-molecular-weight fluorescent dye, biocytin (molecular weight 372.48), that allows the monitoring of its transfer into contiguous intact cells (10). The involvement of gap junctions in biocytin transfer is verified by the concurrent loading of rhodamine-dextran (molecular weight 10,000), a marker dye conjugate which cannot cross the narrow gap junction channel and therefore stays in wounded cells.
Figure 2A shows that biocytin stained mainly loaded (wounded) BxC cells, but largely diffused (albeit to a different extent) from loaded to contiguous cells in the three sst2-expressing cell lines. In contrast, rhodamine-dextran, used as a negative control, remained confined in wounded cells. To ensure that the reversion of cell proliferation inhibition observed in the presence of β-GA (Fig. 1C) actually correlated with altered gap junction intercellular communication, the scrape-loading/dye transfer assay was also performed using cells pretreated with β-GA (Fig. 2B). β-GA severely impaired the transfer of biocytin in Bx2.0 cells. These data demonstrate that reintroducing sst2 in the human pancreatic cancer cell line BxPC-3 restores the formation of functional gap junctions which block cell proliferation.
FIG. 2.
sst2-expressing cells possess functional gap junctions. (A) Confluent BxC or Bx2 pools of cells were subjected to a scrape loading/dye transfer assay as described in Materials and Methods. (B) A scrape loading/dye transfer assay was performed with BxC or Bx2.0 cells in the presence of β-GA or vehicle (DMSO) alone, as described in Materials and Methods.
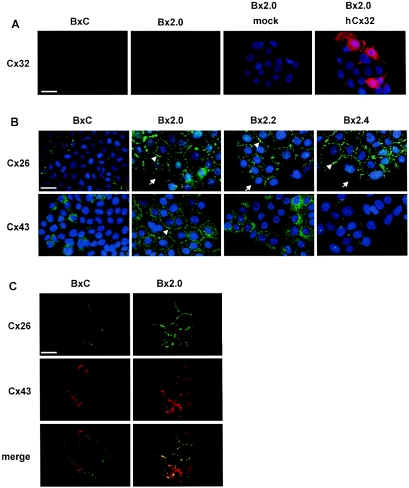
To identify which connexin(s) could compose gap junctions in Bx2 cells, we performed indirect immunofluorescence analyses using antibodies that specifically recognize each of the three connexins normally expressed in the pancreas (Cx26, Cx32, and Cx43). Cx26 and Cx32 have been shown to be specifically expressed in the exocrine pancreas (32, 33). However, Cx32 was detected neither in BxC nor in Bx2 cells (Fig. 3A). This was not due to a defect in antibody efficacy, as Cx32 was easily detectable following transient transfection of the human Cx32 cDNA into Bx2.0 cells (Fig. 3A). In contrast, Cx26 was barely detectable in BxC but strongly overexpressed in all Bx2 cells (Fig. 3B top).
FIG. 3.
Gap junctions are composed of Cx26 and Cx43. (A) Cx32 is expressed neither in BxC nor in Bx2.0. Cells were fixed and processed for indirect immunofluorescence using a specific polyclonal anti-Cx32 antibody as described in Materials and Methods. Where indicated, cells were transiently transfected with a vector carrying the human Cx32 cDNA (hCx32) or with the empty vector (mock) prior indirect immunofluorescence and DAPI staining. Bar, 20 μM. (B) Enhancement of Cx26 and Cx43 expression in Bx2 cells. BxC and Bx2 pools of cells were fixed and processed for indirect immunofluorescence using specific polyclonal anti-Cx26 or anti-Cx43 antibodies, and for DAPI staining as described in Materials and Methods. Bar, 20 μM. (C) Colocalization of Cx26 and Cx43. BxC and Bx2.0 cells were fixed and sequentially processed for indirect immunofluorescence using a polyclonal anti-Cx26 antibody and a monoclonal anti-Cx43 antibody, as described in Materials and Methods. Bar, 20 μM.
Strikingly, immunostaining in all Bx2 cells was concentrated to bright spots at cell-cell contacts (arrowheads), but absent where cell membranes did not contact each other (arrows), indicating that Cx26 is correctly addressed to the membrane. We also found that BxC cells express Cx43 (Fig. 3B, bottom). This was surprising since Cx43 has been considered specific to the endocrine pancreas. However, other mono- and polyclonal Cx43 antibodies gave similar results in both immunostaining (not shown) and Western blotting (see below) and thus confirmed that Cx43 is actually expressed in the exocrine pancreatic cancer BxPC-3 cells. Compared to control cells, both Bx2.0 and Bx2.2 possessed higher levels of Cx43 but with different subcellular repartitions. Cx43 was predominantly addressed to the membrane in Bx2.0 (arrowheads) but remained mostly cytoplasmic in Bx2.2. In Bx2.4, Cx43 did not appear to be overexpressed. However, while Cx43 was concentrated to spots at cell-cell contacts in Bx2.4 cells, the protein spread out in the cytoplasm in BxC. Because such different immunostaining did not permit accurate quantification, Cx43 expression was evaluated by Western blotting (see below).
The variable efficiencies observed for dye transfer in the three different Bx2 cell lines (see Fig. 2A) were consistent with the data obtained from connexin immunostaining (Fig. 3A and 3B). Indeed, efficient dye transfer occurred only when both Cx26 and Cx43 were expressed and localized to the membrane (Bx2.0). Dye transfer was much less efficient when Cx43 was either confined to the cytoplasm (Bx2.2) or poorly expressed (Bx2.4). Thus, for efficient dye transfer, at least two connexins must be coexpressed in BxPC-3 cells. One possible explanation is that gap junctions must be composed of both Cx26 and Cx43. This is supported by the fact that immunostaining of both Cx26 and Cx43 illuminated mostly identical spots at Bx2.0 cell-cell contacts (Fig. 3C).
Cx26 and Cx43 overexpression is mainly translational.
We then searched which step in Cx26 and Cx43 expression could be up-regulated in Bx2 cells. Because immunostaining does not permit accurate quantification, we first performed Western blotting analyzes. Immunoblotting using anti-Cx26 antibodies revealed that the amount of Cx26 protein in Bx2 cells was more than 10-fold higher compared to levels observed for BxC cells (Fig. 4A, top). Similarly, a polyclonal antibody that recognizes both unphosphorylated (Cx43) and phosphorylated (pCx43) forms revealed a 4- to 7-fold increase in Cx43 expression (Fig. 4A, middle). The amount of pCx43 paralleled that of Cx43, suggesting that Cx43 phosphorylation was modestly affected in sst2-expressing cells. This was further supported by data obtained using an antibody that specifically recognizes Cx43 only when the protein kinase C-targeted Ser368 is not phosphorylated (Fig. 4A, bottom). Next, the measure of [35S]methionine incorporation into immunoprecipitated Cx26 or Cx43 protein following a pulse (30 min) labeling of cells revealed that increased rates of Cx26 and Cx43 synthesis (Fig. 4B, bottom) paralleled the overexpression detected by immunoblot (Fig. 4A). Increases in the rate of Cx26 and Cx43 synthesis were specific because [35S]methionine incorporation into total protein was equivalent for both Bx2.0 and BxC cells (Fig. 4B, top).
FIG. 4.
Enhancement of Cx26 and Cx43 expression is mainly translational. (A) Protein expression. BxC or Bx2 cell extracts were processed for Western blotting using a specific anti-Cx26 antibody (top), an anti-Cx43 antibody that recognizes both phosphorylated (pCx43) and unphosphorylated forms of Cx43 (middle), or a monoclonal anti-Cx43 antibody that recognizes Cx43 only when Ser368 is not phosphorylated (bottom). Membranes were also probed with an anti-β-tubulin antibody. Blots are representative of three separate experiments. Numbers are the mean ± standard error of the mean of densitometric quantifications of the three experiments normalized to β-tubulin, and are expressed as a ratio relative to the value obtained for BxC cells, which was set at 1. (B) Protein biosynthesis. Following pulse-labeling of cells, [35S]methionine incorporation into total protein (top) or into immunoprecipitated Cx26 or Cx43 protein (bottom) was visualized by autoradiography of SDS-PAGE-separated and electrotransferred proteins, as described in Materials and Methods. (C) mRNA expression. The amount of Cx26 and Cx43 mRNAs was quantified by real-time quantitative PCR as described in Materials and Methods. Results are the means ± standard error of the mean of three separate experiments and are expressed as a ratio relative to the value obtained for BxC cells, which was set at 1.
To discriminate between transcriptional and posttranscriptional events, a quantitative real-time PCR was then performed. Only a moderate (1.5- to 3-fold) augmentation in the steady-state level of Cx26 mRNA could be detected in all Bx2 pools, while Cx43 mRNA did not display more than a 2-fold increase or was even unaffected in the Bx2.4 pool (Fig. 4C). These results suggest that in sst2-expressing cells, the rate of Cx26 and Cx43 synthesis is increased mainly through a translational mechanism.
Enhancement of Cx26 and Cx43 expression is insensitive to rapamycin.
To gain insight into the mechanism by which Cx26 and Cx43 mRNAs are translated in Bx2 cells, the effect of rapamycin was examined. Rapamycin prevents 4E-BP1 phosphorylation through inhibition of FRAP/mTOR kinase activity and consequently blocks cap-dependent translation initiation (2). We thus first analyzed the effect of rapamycin on 4E-BP1 phosphorylation status, which should parallel its effect on cap-dependent translation. In the absence of rapamycin, 4E-BP1 was partially hyperphosphorylated in BxC, as evidenced by the presence of the slow-migrating phosphorylated forms (Fig. 5A, top left, lane 1). In Bx2.0 cells, an additional fast-migrating form was detected (Fig. 5A, lane 2), suggesting that 4E-BP1 was more hypophosphorylated. As expected, 4E-BP1 phosphorylation was inhibited by rapamycin in both BxC and Bx2.0 cells, as the hypophosphorylated forms became most prominent (Fig. 5A, compare lanes 3 and 4 to lanes 1 and 2). Interestingly, the enhancement of both Cx26 and Cx43 translation in Bx2.0 cells was not prevented by rapamycin (Fig. 5A, bottom left) but was rather slightly more pronounced (see quantification, Fig. 5A, right).
FIG. 5.
Enhancement of Cx26 and Cx43 expression is not sensitive to rapamycin. (A) Left. Rapamycin has no effect on enhanced connexin expression. Extracts obtained from BxC or Bx2.0 cells treated or not with rapamycin were processed for Western blotting using a polyclonal anti-4E-BP1 or a monoclonal anti-β-tubulin antibody (top), and with anti-Cx43 or anti-Cx26 antibodies (bottom), as described in Materials and Methods. Right. The data obtained for Cx26 and Cx43 were subjected to a densitometric quantification normalized to β-tubulin. Histograms represent the ratios between the values obtained for Bx2.0 and BxC. The ratios calculated for rapamycin-untreated cells were set at 100%. (B) Rapamycin blocks cyclin D1 expression. Cells treated with rapamycin or vehicle (DMSO) alone were fixed and sequentially processed for indirect immunofluorescence using a monoclonal anti-cyclin D1 antibody (top) and either a polyclonal anti-Cx26 or a polyclonal anti-Cx43 antibody (bottom), as described in Materials and Methods. Cells were also stained with DAPI and the pictures obtained were superposed with that obtained for cyclin D1 (middle). Bar, 20 μM.
To ensure that 4E-BP1 hypophosphorylation actually inhibited cap-dependent translation, the expression of cyclin D1, whose expression has been shown to be very sensitive to rapamycin in pancreatic cancer cells (17), was also evaluated. Cells were incubated in the presence of rapamycin, and cyclin D1 was visualized by immunofluorescence. Rapamycin treatment strongly inhibited cyclin D1 expression (Fig. 5B, top and middle), while in the same cells, expression of Cx26 or Cx43 (Fig. 5B, bottom) was poorly affected. Thus, enhancement of connexin expression is not sensitive to rapamycin-mediated hypophosphorylation of 4E-BP1, suggesting that stimulation of Cx26 and Cx43 mRNAs translation in Bx2 cells occurs through a cap-independent translation mechanism.
The Cx26 5′ UTR possesses an IRES.
The presence of IRESes in the 5′ UTR of Cx32 and Cx43 has been described (20, 49). It is thought that the existence of IRESes in connexin mRNAs would ensure efficient connexin expression and consequent formation of functional gap junctions in differentiated or density-inhibited cells (59). Because sst2-expressing pancreatic tumor cells attain confluence and overexpress connexins through a translational mechanism insensitive to rapamycin, we hypothesized that the enhancement of Cx26 and Cx43 expression could occur through IRES-mediated translation initiation.
We first tested the possibility that, as for Cx43, Cx26 mRNA also possesses an IRES. To this end, the 5′ UTR of Cx26 mRNA was inserted between the two reporters (Renilla and firefly luciferases) of a bicistronic cytomegalovirus-based expression vector (pSL2-Cx26; Fig. 6). pSL2 was created to characterize the Cx32 and Cx43 IRESes (20, 49). The vector carries two stem-loop structures. The first stem-loop (SL1) was designed to limit cap-dependent translation of the first cistron, and the second one (SL2) was designed to attenuate leaky scanning or reinitiation from the first cistron. Following transfection into BxC pancreatic tumor cells, the measure of reporter activities showed that the presence of Cx26 5′UTR in the intercistronic space of a bicistronic vector permitted the expression of the second cistron (Fig. 7A, left, compare pSL2-Cx26 to pSL2), while expression of the first cap-dependent cistron remained unchanged (right, compare pSL2-Cx26 to pSL2).
FIG. 6.
Description of dicistronic constructs. The thick lines represent any 5′ UTR. The open and hatched boxes represent Renilla luciferase and firefly luciferase coding sequences, respectively, and the filled arrows represent the cytomegalovirus promoter. SL1 and SL2 represent the two stem-loop structures that frame Renilla luciferase sequence.
FIG. 7.
Characterization of the Cx26 IRES. (A) The Cx26 5′ UTR contains an IRES. Dicistronic vectors were transfected in BxC cells, and Renilla luciferase and firefly luciferase activities were assayed as described in Materials and Methods. Results are the means ± standard error of the mean of three independent experiments performed in doublets, and expressed as a ratio relative to the activity measured for the pSL2 containing no 5′ UTR, which was set at 1. (B) Relative strength of Cx26 IRES. Dicistronic constructs were transiently transfected in BxC cells, and Renilla luciferase (R-luc) and firefly luciferase (F-luc) activities were assayed as described in Materials and Methods. The ratio firefly luciferase/Renilla luciferase is shown. Results are the means ± standard error of the mean of three independent experiments performed in doublets and expressed as a ratio relative to the value obtained for the pSL2 construct containing no 5′ UTR, which was set at 1.
To exclude the possibility that the Cx26 5′ UTR possesses an intrinsic promoter activity that could bias data interpretation, a bicistronic vector carrying the Cx26 5′ UTR but with no cytomegalovirus promoter (Fig. 6, pGL3-Cx26) was also tested. Expression of the downstream cistron was not higher than the background obtained in the absence of Cx26 5′ UTR (Fig. 7A, compare pGL3-Cx26 to pSL2), indicating that the Cx26 5′ UTR did not contain a cryptic promoter. The lack of downstream cistron expression observed following pGL3-Cx26 vector transfection was not due to a defect in transfection efficiency as expression from a cotransfected vector carrying the Renilla luciferase reporter in a monocistronic context (pRL-CMV) was very high (compare pGL3-Cx26 plus pRL-CMV to pGL3-Cx26). These results indicate that the Cx26 5′ UTR possesses an IRES.
We then evaluated the relative strength of Cx26 IRES in pancreatic cancer cells. Following transfection into BxC cells, the data show that the Cx26 IRES is much less active than that of Cx43 but is around ≈2-fold stronger than the encephalomyocarditis virus IRES which was used as a positive control (Fig. 7B).
Cx26 and Cx43 IRESes are up-regulated in Bx2 cells.
The enhancement of connexin expression in Bx2 cells even in the presence of the inhibitor of cap-dependent translation rapamycin could be explained by the existence of IRESes in Cx26 and Cx43 mRNAs. To test this, IRES activity was measured in the three Bx2 pools and in BxC cells following transfection of bicistronic vectors. The activity of both Cx26 and Cx43 IRESes was enhanced in all Bx2 pools compared to BxC cells (Fig. 8). These data indicate that Cx26 and Cx43 IRESes are up-regulated in sst2-expressing tumoral pancreatic cells. They also argue for a role of IRES-dependent translation in the enhancement and maintenance of Cx26 and Cx43 expression in density-inhibited cells.
FIG. 8.
Enhancement of Cx26 and Cx43 IRES activities in sst2-expressing cells. BxC or Bx2 pools of cells were transiently transfected with bicistronic vectors, and Renilla luciferase and firefly luciferase activities were assayed as described in Materials and Methods. The ratio firefly luciferase/Renilla luciferase is shown. Results are the means ± standard error of the mean of three independent experiments performed in doublets, and expressed as a ratio relative to the value obtained for BxC cells, which was set at 1.
DISCUSSION
Using pancreatic cancer cells whose tumor phenotype has been reversed by stable transfection of the sst2 somatostatin receptor, we show that cap-independent, IRES-mediated translation initiation is an important component of Cx26 and Cx43 expression in nonproliferating cells. The data also strongly suggest that gap junction intercellular communication plays a critical role in the antiproliferative effect of sst2.
Cx26 and Cx32 have been detected in the exocrine pancreas (32, 33), but their presence in ductal cells is not clearly established. Cx26 is poorly expressed in BxPC3 cells (61; this study) but becomes much more abundant following expression of sst2. This suggests that Cx26 may be expressed in normal ductal cells but is down-regulated in cancer cells and that one important antiproliferative effect of sst2 could be the restoration of Cx26 expression. In contrast, Cx32 could be detected neither in BxC cells nor in Bx2 cells. Accordingly, Cx32 is now considered an acinar cell-specific connexin (63). On the other hand, what appeared at first surprising is that BxPC3 cells express relatively high levels of Cx43. Indeed, in normal tissues, Cx43 has been detected mainly in pancreatic islets (32, 33). However, other tumor cell lines (including PANC-1 and AsPC-1) (22, 35) and immortalized but not transformed human pancreatic ductal cell line H6c7 (53) also express Cx43. Because all these cell lines exhibit a ductal phenotype, it is likely that Cx43 is expressed in ductal cells. Another possibility is that Cx43 becomes apparent only during the process of immortalization or transformation, as is the case in the liver of rats treated with tumor-promoting compounds (23). Further studies are needed to clarify this point.
While the data that we obtained with the exocrine-specific Cx26 and Cx32 are unequivocal, Cx43 is unevenly expressed in the different Bx2 cells. Cx43 is overexpressed and correctly addressed to the membrane in Bx2.0 cells, overexpressed but retained in the cytoplasm of Bx2.2 cells, and overexpressed to a lower level in Bx2.4 cells (Fig. 3B). However, that Bx2.0 and Bx2.2 cells express larger amounts of Cx43 than Bx2.4 cells do could account for their lower saturation phase (Fig. 1B). Indeed, it has been shown that even when retained in the cytoplasm, Cx43 can inhibit cell proliferation (37, 44). Thus, it appears that de novo expression of endogenous Cx26 is sufficient to provoke BxPC3 cell density inhibition, but such inhibition is exacerbated when both Cx26 and Cx43 are overexpressed.
It was confirmed recently that Cx26 and Cx43 cannot pack together in a heteromeric connexon (12). Also, a connexon composed exclusively of Cx26 in one cell cannot dock with a connexon of a contiguous cell composed exclusively of Cx43 to form an heterotypic intercellular channel (9). Yet we did observe colocalization of Cx26 and Cx43 at cell-cell contacts in Bx2.0 (Fig. 3C), which correlates with a much more efficient dye transfer (Fig. 2A). It is then likely that in Bx2.0 cells, Cx26 and Cx43 form distinct homomeric intercellular channels that assemble into larger plaques at cell-cell contacts and hence facilitate gap junction intercellular communication (11, 12).
From the data shown in Fig. 4A, it seems that the ratio between Cx43 and phosphorylated Cx43 is nearly unchanged in sst2-expressing BxPC3 cells versus control cells. However, while Cx43 has often been shown to migrate as a triplet corresponding to different phosphorylated forms (36, 38), the polyclonal antibody that we utilized revealed only a doublet, suggesting that the antibody did not recognize all phosphorylated forms. It is therefore premature to draw conclusions about Cx43 phosphorylation status, particularly if one considers that Cx43 phosphorylation has been correlated to changes in Cx43 subcellular repartition and that, depending on the sst2-expressing pool, Cx43 is either correctly addressed to the membrane or concentrated into the cytoplasm. Also, one important protein kinase that phosphorylates Cx43 on Ser368 is the calcium-dependent protein kinase C (1, 26), and sst2 has been shown to regulate the release of intracellular calcium pools (24). Thus, further analyzes are required to evaluate the real impact of sst2 expression on Cx43 phosphorylation in the pancreatic tumor cell line BxPC3.
An important conclusion resulting from this paper is that the Cx26 and Cx43 IRESes are the target of translational control. In most connexin mRNAs of numerous species, the entire protein sequence is coded by the second exon, while the first exon constitutes the 5′ UTR (60). The conservation of such a noncoding exon at the mRNA 5′ end suggests that it has been selected throughout evolution to serve an important function. Since the recent discovery of cap-independent IRESes in the 5′ UTRs of Cx32 and Cx43 mRNAs (20, 49), it was hypothesized that such an important function could be the regulation of connexin expression at the translational level and that other connexin mRNAs should also contain translational regulatory sequences in the first exon (59).
The novel IRES we have identified in the Cx26 5′ UTR further supports this idea. One important role of IRESes in connexin mRNAs could be to ensure connexin expression, particularly in nonproliferating or differentiated cells, where cap-mediated translation rates are low. The data obtained in this paper strongly argue in favor of this hypothesis, since IRES-dependent translation initiation appears to be a critical component of Cx26 and Cx43 expression in contact-inhibited pancreatic cancer cells. One intriguing observation, however, is that we could not detect any difference in global rates of protein synthesis between the exponentially growing BxC cells and the density-inhibited Bx2.0 cells (Fig. 4B). Further analysis of 4E-BP1 using phosphorylation-specific antibodies (Rania Azar, personal communication) showed that 4E-BP1 is underphosphorylated in Bx2.0 cells and hence should attenuate cap-dependent translation. Nevertheless, it has been suggested that 4E-BP1 controls protein synthesis through qualitative rather than quantitative changes that result in a dynamic interplay between cap-dependent and cap-independent processes (29). It is thus likely that in Bx2 cells, the synthesis of most proteins is not affected by rapamycin, while translation of a subset of mRNAs carrying regulatory sequences in their 5′ UTR (including Cx26 and Cx43 mRNAs) is specifically targeted.
Thus, our data suggest that IRES-dependent synthesis is an important mechanism of the endogenous expression of Cx26 and Cx43. We further propose that the restoration of IRES-dependent synthesis of Cx26 and Cx43 and consequent formation of functional gap junctions are critical components of the antiproliferative effect of the sst2 receptor. This feature could be exploited in the treatment of pancreatic adenocarcinomas. Indeed, we and others have shown that gene therapy using sst2 as a tumor suppressor gene exerts antitumoral effects on experimental pancreatic cancers (6, 58). On the other hand, through the mediation of a strong by-stander effect, gap junctions are known to render tumors much more sensitive to common chemotherapeutic compounds (5, 54, 55). Then, in addition to its own antitumoral effect, sst2 may also sensitize pancreatic cancers to conventional chemotherapy through a gap junction-mediated by-stander effect.
Acknowledgments
We thank Rudolph Werner for pSL2 vectors, Marc Mesnil for critical reading of the manuscript, and Dina Arvanitis for English proofreading.
This work was supported by grants from the Association pour la Recherche contre le Cancer (ARC-4505) and Ligue contre le Cancer (Comités de Haute-Garonne et de Tarn et Garonne) to Stéphane Pyronnet. Hicham Lahlou was the recipient of two consecutive doctoral fellowships from ARC and the Ligue contre le Cancer (Comité de Haute-Garonne).
REFERENCES
- 1.Bao, X., L. Reuss, and G. A. Altenberg. 2004. Regulation of purified and reconstituted connexin 43 hemichannels by protein kinase C-mediated phosphorylation of serine 368. J. Biol. Chem. 279:20058-20066. [DOI] [PubMed] [Google Scholar]
- 2.Beretta, L., A. C. Gingras, Y. V. Svitkin, M. N. Hall, and N. Sonenberg. 1996. Rapamycin blocks the phosphorylation of 4E-BP1 and inhibits cap-dependent initiation of translation. EMBO J. 15:658-664. [PMC free article] [PubMed] [Google Scholar]
- 3.Buscail, L., J. P. Esteve, N. Saint-Laurent, V. Bertrand, T. Reisine, A. M. O'Carroll, G. I. Bell, A. V. Schally, N. Vaysse, and C. Susini. 1995. Inhibition of cell proliferation by the somatostatin analogue RC-160 is mediated by somatostatin receptor subtypes SSTR2 and SSTR5 through different mechanisms. Proc. Natl. Acad. Sci. USA 92:1580-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buscail, L., N. Saint-Laurent, E. Chastre, J. C. Vaillant, C. Gespach, G. Capella, H. Kalthoff, F. Lluis, N. Vaysse, and C. Susini. 1996. Loss of sst2 somatostatin receptor gene expression in human pancreatic and colorectal cancer. Cancer Res. 56:1823-1827. [PubMed] [Google Scholar]
- 5.Carystinos, G. D., M. A. Alaoui-Jamali, J. Phipps, L. Yen, and G. Batist. 2001. Upregulation of gap junctional intercellular communication and connexin 43 expression by cyclic-AMP and all-trans-retinoic acid is associated with glutathione depletion and chemosensitivity in neuroblastoma cells. Cancer Chemother. Pharmacol. 47:126-132. [DOI] [PubMed] [Google Scholar]
- 6.Celinski, S. A., W. E. Fisher, F. Amaya, Y. Q. Wu, Q. Yao, K. A. Youker, and M. Li. 2003. Somatostatin receptor gene transfer inhibits established pancreatic cancer xenografts. J. Surg. Res. 115:41-47. [DOI] [PubMed] [Google Scholar]
- 7.Cruciani, V., and S. O. Mikalsen. 1999. Stimulated phosphorylation of intracellular connexin43. Exp. Cell Res. 251:285-298. [DOI] [PubMed] [Google Scholar]
- 8.Delesque, N., L. Buscail, J. P. Esteve, N. Saint-Laurent, C. Muller, G. Weckbecker, C. Bruns, N. Vaysse, C. Susini, and I. Rauly. 1997. sst2 somatostatin receptor expression reverses tumorigenicity of human pancreatic cancer cells. Cancer Res. 57:956-962. [PubMed] [Google Scholar]
- 9.Elfgang, C., R. Eckert, H. Lichtenberg-Frate, A. Butterweck, O. Traub, R. A. Klein, D. F. Hulser, and K. Willecke. 1995. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J. Cell Biol. 129:805-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.el-Fouly, M. H., J. E. Trosko, and C. C. Chang. 1987. Scrape-loading and dye transfer. A rapid and simple technique to study gap junctional intercellular communication. Exp. Cell Res. 168:422-430. [DOI] [PubMed] [Google Scholar]
- 11.Falk, M. M. 2000. Connexin-specific distribution within gap junctions revealed in living cells. J. Cell Sci. 113:4109-4120. [DOI] [PubMed] [Google Scholar]
- 12.Gemel, J., V. Valiunas, P. R. Brink, and E. C. Beyer. 2004. Connexin43 and connexin26 form gap junctions, but not heteromeric channels in co-expressing cells. J. Cell Sci. 117:2469-2480. [DOI] [PubMed] [Google Scholar]
- 13.Gingras, A. C., B. Raught, and N. Sonenberg. 1999. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 68:913-963. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg, G. S., J. F. Bechberger, Y. Tajima, M. Merritt, Y. Omori, M. A. Gawinowicz, R. Narayanan, Y. Tan, Y. Sanai, H. Yamasaki, C. C. Naus, H. Tsuda, and B. J. Nicholson. 2000. Connexin43 suppresses MFG-E8 while inducing contact growth inhibition of glioma cells. Cancer Res. 60:6018-6026. [PubMed] [Google Scholar]
- 15.Goldberg, G. S., A. P. Moreno, J. F. Bechberger, S. S. Hearn, R. R. Shivers, D. J. MacPhee, Y. C. Zhang, and C. C. Naus. 1996. Evidence that disruption of connexon particle arrangements in gap junction plaques is associated with inhibition of gap junctional communication by a glycyrrhetinic acid derivative. Exp. Cell Res. 222:48-53. [DOI] [PubMed] [Google Scholar]
- 16.Govindarajan, R., S. Zhao, X. H. Song, R. J. Guo, M. Wheelock, K. R. Johnson, and P. P. Mehta. 2002. Impaired trafficking of connexins in androgen-independent human prostate cancer cell lines and its mitigation by alpha-catenin. J. Biol. Chem. 277:50087-50097. [DOI] [PubMed] [Google Scholar]
- 17.Grewe, M., F. Gansauge, R. M. Schmid, G. Adler, and T. Seufferlein. 1999. Regulation of cell growth and cyclin D1 expression by the constitutively active FRAP-p70s6K pathway in human pancreatic cancer cells. Cancer Res. 59:3581-3587. [PubMed] [Google Scholar]
- 18.Haghighat, A., S. Mader, A. Pause, and N. Sonenberg. 1995. Repression of cap-dependent translation by 4E-binding protein 1: competition with p220 for binding to eukaryotic initiation factor-4E. EMBO J. 14:5701-5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hershey, J. W. 1991. Translational control in mammalian cells. Annu. Rev. Biochem. 60:717-755. [DOI] [PubMed] [Google Scholar]
- 20.Hudder, A., and R. Werner. 2000. Analysis of a Charcot-Marie-Tooth disease mutation reveals an essential internal ribosome entry site element in the connexin-32 gene. J. Biol. Chem. 275:34586-34591. [DOI] [PubMed] [Google Scholar]
- 21.Jang, S. K., H. G. Krausslich, M. J. Nicklin, G. M. Duke, A. C. Palmenberg, and E. Wimmer. 1988. A segment of the 5′ nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J. Virol. 62:2636-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawasaki, Y., A. Tsuchida, T. Sasaki, S. Yamasaki, Y. Kuwada, M. Murakami, and K. Chayama. 2002. Irsogladine malate up-regulates gap junctional intercellular communication between pancreatic cancer cells via PKA pathway. Pancreas 25:373-377. [DOI] [PubMed] [Google Scholar]
- 23.Krutovskikh, V. A., M. Mesnil, G. Mazzoleni, and H. Yamasaki. 1995. Inhibition of rat liver gap junction intercellular communication by tumor-promoting agents in vivo. Association with aberrant localization of connexin proteins. Lab. Investig. 72:571-577. [PubMed] [Google Scholar]
- 24.Lahlou, H., J. Guillermet, M. Hortala, F. Vernejoul, S. Pyronnet, C. Bousquet, and C. Susini. 2004. Molecular signaling of somatostatin receptors. Ann. N. Y. Acad. Sci. 1014:121-131. [DOI] [PubMed] [Google Scholar]
- 25.Lahlou, H., N. Saint-Laurent, J. P. Esteve, A. Eychene, L. Pradayrol, S. Pyronnet, and C. Susini. 2003. sst2 somatostatin receptor inhibits cell proliferation through Ras-, Rap1-, and B-Raf-dependent ERK2 activation. J. Biol. Chem. 278:39356-39371. [DOI] [PubMed] [Google Scholar]
- 26.Lampe, P. D., E. M. TenBroek, J. M. Burt, W. E. Kurata, R. G. Johnson, and A. F. Lau. 2000. Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J. Cell Biol. 149:1503-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le, A. C., and L. S. Musil. 1998. Normal differentiation of cultured lens cells after inhibition of gap junction-mediated intercellular communication. Dev. Biol. 204:80-96. [DOI] [PubMed] [Google Scholar]
- 28.Le, A. C., and L. S. Musil. 2001. A novel role for FGF and extracellular signal-regulated kinase in gap junction-mediated intercellular communication in the lens. J. Cell Biol. 154:197-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, S., N. Sonenberg, A. C. Gingras, M. Peterson, S. Avdulov, V. A. Polunovsky, and P. B. Bitterman. 2002. Translational control of cell fate: availability of phosphorylation sites on translational repressor 4E-BP1 governs its proapoptotic potency. Mol. Cell. Biol. 22:2853-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin, T. A., X. Kong, T. A. Haystead, A. Pause, G. Belsham, N. Sonenberg, and J. C. Lawrence, Jr. 1994. PHAS-I as a link between mitogen-activated protein kinase and translation initiation. Science. 266:653-656. [DOI] [PubMed] [Google Scholar]
- 31.Mader, S., H. Lee, A. Pause, and N. Sonenberg. 1995. The translation initiation factor eIF-4E binds to a common motif shared by the translation factor eIF-4 gamma and the translational repressors 4E-binding proteins. Mol. Cell. Biol. 15:4990-4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meda, P. 1996. Gap junction involvement in secretion: the pancreas experience. Clin. Exp. Pharmacol. Physiol. 23:1053-1057. [DOI] [PubMed] [Google Scholar]
- 33.Meda, P., M. S. Pepper, O. Traub, K. Willecke, D. Gros, E. Beyer, B. Nicholson, D. Paul, and L. Orci. 1993. Differential expression of gap junction connexins in endocrine and exocrine glands. Endocrinology. 133:2371-2378. [DOI] [PubMed] [Google Scholar]
- 34.Mesnil, M. 2002. Connexins and cancer. Biol. Cell 94:493-500. [DOI] [PubMed] [Google Scholar]
- 35.Mesnil, M., D. Rideout, N. M. Kumar, and N. B. Gilula. 1994. Non-communicating human and murine carcinoma cells produce alpha 1 gap junction mRNA. Carcinogenesis. 15:1541-1547. [DOI] [PubMed] [Google Scholar]
- 36.Mograbi, B., E. Corcelle, N. Defamie, M. Samson, M. Nebout, D. Segretain, P. Fenichel, and G. Pointis. 2003. Aberrant connexin 43 endocytosis by the carcinogen lindane involves activation of the ERK/mitogen-activated protein kinase pathway. Carcinogenesis 24:1415-1423. [DOI] [PubMed] [Google Scholar]
- 37.Moorby, C., and M. Patel. 2001. Dual functions for connexins: Cx43 regulates growth independently of gap junction formation. Exp. Cell Res. 271:238-248. [DOI] [PubMed] [Google Scholar]
- 38.Musil, L. S., and D. A. Goodenough. 1991. Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J. Cell Biol. 115:1357-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagy, J. I., W. E. Li, C. Roy, B. W. Doble, J. S. Gilchrist, E. Kardami, and E. L. Hertzberg. 1997. Selective monoclonal antibody recognition and cellular localization of an unphosphorylated form of connexin43. Exp. Cell Res. 236:127-136. [DOI] [PubMed] [Google Scholar]
- 40.Neveu, M. J., J. R. Hully, K. L. Babcock, E. L. Hertzberg, B. J. Nicholson, D. L. Paul, and H. C. Pitot. 1994. Multiple mechanisms are responsible for altered expression of gap junction genes during oncogenesis in rat liver. J. Cell Sci. 107:83-95. [DOI] [PubMed] [Google Scholar]
- 41.Pause, A., G. J. Belsham, A. C. Gingras, O. Donze, T. A. Lin, J. C. Lawrence, Jr., and N. Sonenberg. 1994. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5′-cap function. Nature 371:762-767. [DOI] [PubMed] [Google Scholar]
- 42.Pelletier, J., and N. Sonenberg. 1988. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature 334:320-325. [DOI] [PubMed] [Google Scholar]
- 43.Pyronnet, S., J. Dostie, and N. Sonenberg. 2001. Suppression of cap-dependent translation in mitosis. Genes Dev. 15:2083-2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qin, H., Q. Shao, H. Curtis, J. Galipeau, D. J. Belliveau, T. Wang, M. A. Alaoui-Jamali, and D. W. Laird. 2002. Retroviral delivery of connexin genes to human breast tumor cells inhibits in vivo tumor growth by a mechanism that is independent of significant gap junctional intercellular communication. J. Biol. Chem. 277:29132-29138. [DOI] [PubMed] [Google Scholar]
- 45.Qin, H., Q. Shao, S. A. Igdoura, M. A. Alaoui-Jamali, and D. W. Laird. 2003. Lysosomal and proteasomal degradation play distinct roles in the life cycle of Cx43 in gap junctional intercellular communication-deficient and -competent breast tumor cells. J. Biol. Chem. 278:30005-30014. [DOI] [PubMed] [Google Scholar]
- 46.Rauly, I., N. Saint-Laurent, N. Delesque, L. Buscail, J. P. Esteve, N. Vaysse, and C. Susini. 1996. Induction of a negative autocrine loop by expression of sst2 somatostatin receptor in NIH 3T3 cells. J. Clin. Investig. 97:1874-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roger, C., B. Mograbi, D. Chevallier, J. F. Michiels, H. Tanaka, D. Segretain, G. Pointis, and P. Fenichel. 2004. Disrupted traffic of connexin 43 in human testicular seminoma cells: overexpression of Cx43 induces membrane location and cell proliferation decrease. J. Pathol. 202:241-246. [DOI] [PubMed] [Google Scholar]
- 48.Saez, J. C., V. M. Berthoud, M. C. Branes, A. D. Martinez, and E. C. Beyer. 2003. Plasma membrane channels formed by connexins: their regulation and functions. Physiol. Rev. 83:1359-1400. [DOI] [PubMed] [Google Scholar]
- 49.Schiavi, A., A. Hudder, and R. Werner. 1999. Connexin43 mRNA contains a functional internal ribosome entry site. FEBS Lett. 464:118-122. [DOI] [PubMed] [Google Scholar]
- 50.Sella, O., G. Gerlitz, S. Y. Le, and O. Elroy-Stein. 1999. Differentiation-induced internal translation of c-sis mRNA: analysis of the cis elements and their differentiation-linked binding to the hnRNP C protein. Mol. Cell. Biol. 19:5429-5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singal, R., Z. J. Tu, J. M. Vanwert, G. D. Ginder, and D. T. Kiang. 2000. Modulation of the connexin26 tumor suppressor gene expression through methylation in human mammary epithelial cell lines. Anticancer Res. 20:59-64. [PubMed] [Google Scholar]
- 52.Spinella, F., L. Rosano, V. Di Castro, M. R. Nicotra, P. G. Natali, and A. Bagnato. 2003. Endothelin-1 decreases gap junctional intercellular communication by inducing phosphorylation of connexin 43 in human ovarian carcinoma cells. J. Biol. Chem. 278:41294-41301. [DOI] [PubMed] [Google Scholar]
- 53.Tai, M. H., L. K. Olson, B. V. Madhukar, K. D. Linning, L. Van Camp, M. S. Tsao, and J. E. Trosko. 2003. Characterization of gap junctional intercellular communication in immortalized human pancreatic ductal epithelial cells with stem cell characteristics. Pancreas. 26:e18-26. [DOI] [PubMed] [Google Scholar]
- 54.Tanaka, M., and H. B. Grossman. 2001. Connexin 26 gene therapy of human bladder cancer: induction of growth suppression, apoptosis, and synergy with cisplatin. Hum. Gene Ther. 12:2225-2236. [DOI] [PubMed] [Google Scholar]
- 55.Tanaka, M., and H. B. Grossman. 2004. Connexin 26 induces growth suppression, apoptosis and increased efficacy of doxorubicin in prostate cancer cells. Oncol. Rep. 11:537-541. [PubMed] [Google Scholar]
- 56.Tulipano, G., R. Stumm, M. Pfeiffer, H. J. Kreienkamp, V. Hollt, and S. Schulz. 2004. Differential beta-arrestin trafficking and endosomal sorting of somatostatin receptor subtypes. J. Biol. Chem. 279:21374-21382. [DOI] [PubMed] [Google Scholar]
- 57.Vagner, S., B. Galy, and S. Pyronnet. 2001. Irresistible IRES. Attracting the translation machinery to internal ribosome entry sites. EMBO Rep. 2:893-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vernejoul, F., P. Faure, N. Benali, D. Calise, G. Tiraby, L. Pradayrol, C. Susini, and L. Buscail. 2002. Antitumor effect of in vivo somatostatin receptor subtype 2 gene transfer in primary and metastatic pancreatic cancer models. Cancer Res. 62:6124-6131. [PubMed] [Google Scholar]
- 59.Werner, R. 2000. IRES elements in connexin genes: a hypothesis explaining the need for connexins to be regulated at the translational level. IUBMB Life 50:173-176. [DOI] [PubMed] [Google Scholar]
- 60.Willecke, K., J. Eiberger, J. Degen, D. Eckardt, A. Romualdi, M. Guldenagel, U. Deutsch, and G. Sohl. 2002. Structural and functional diversity of connexin genes in the mouse and human genome. Biol. Chem. 383:725-737. [DOI] [PubMed] [Google Scholar]
- 61.Yang, L., Y. Chiang, H. J. Lenz, K. D. Danenberg, C. P. Spears, E. M. Gordon, W. F. Anderson, and D. Parekh. 1998. Intercellular communication mediates the bystander effect during herpes simplex thymidine kinase/ganciclovir-based gene therapy of human gastrointestinal tumor cells. Hum. Gene Ther. 9:719-728. [DOI] [PubMed] [Google Scholar]
- 62.Yano, T., F. Ito, K. Kobayashi, Y. Yonezawa, K. Suzuki, R. Asano, K. Hagiwara, H. Nakazawa, H. Toma, and H. Yamasaki. 2004. Hypermethylation of the CpG island of connexin 32, a candiate tumor suppressor gene in renal cell carcinomas from hemodialysis patients. Cancer Lett. 208:137-142. [DOI] [PubMed] [Google Scholar]
- 63.Zhu, L., T. Tran, J. M. Rukstalis, P. Sun, B. Damsz, and S. F. Konieczny. 2004. Inhibition of Mist1 homodimer formation induces pancreatic acinar-to-ductal metaplasia. Mol. Cell. Biol. 24:2673-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]