Abstract
Human peripheral blood mononuclear cells (PBMC) were stimulated with three nonpathogenic Lactobacillus strains and with one pathogenic Streptococcus pyogenes strain, and cytokine gene expression and protein production were analyzed. All bacteria strongly induced interleukin-1β (IL-1β), IL-6, and tumor necrosis factor alpha mRNA expression and protein production. S. pyogenes was the most potent inducer of secretion of IL-12 and gamma interferon (IFN-γ), and two of three Lactobacillus strains induced IL-12 and IFN-γ production. All strains induced IL-18 protein production. IL-10 and IL-4 production was induced weakly and not at all, respectively. Our data show that nonpathogenic lactobacilli and pathogenic streptococci can induce Th1 type cytokines IL-12, IL-18, and IFN-γ in human PBMC.
Proinflammatory cytokines tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and IL-6 as well as interferons (IFNs) are among the first cytokines produced in response to pathogenic bacteria (39). Cytokines produced later during microbial infection direct responses toward either cell-mediated T-helper type 1 (Th1) or humoral Th2 type immunity (2, 32). Th1 type cytokines include macrophage-derived IL-12 and T-cell-derived gamma IFN (IFN-γ). IL-12 stimulates IFN-γ production in T and NK cells (10) and enhances the development of naive CD4+ T cells into Th1 type cells (2, 40). IFN-γ enhances IL-12 production by phagocytic cells (21) and downregulates Th2 type cellular proliferation and activation. Recently described IL-18 and IFN-α/β have been shown to contribute to enhanced IFN-γ gene expression in T cells (28, 34) and are thus likely to enhance Th1 type cellular responses (20, 31). IL-18 produced by activated macrophage-like cells acts synergistically with IFN-α and IL-12 in enhancing IFN-γ gene expression in T cells (23, 28, 34, 41). IL-4 directly enhances the development of Th2 type cells from naive T cells (2, 36). IL-10 produced by macrophages and lymphocytes can inhibit the production of TNF-α, IL-1β, and IL-6 (12) as well as IFN-γ and IL-12 (9) and thus enhance Th2 type immune responses.
Streptococcus pyogenes, also known as group A streptococci (GAS), is an important human pathogen causing a wide range of infections (37). Nonpathogenic strains of lactic acid bacteria (LAB) such as Lactobacillus are part of normal human microflora (22), and some strains are used in the dairy industry. LAB have also been suggested to have beneficial effects on human health (14). Intact streptococci and their cell wall components have previously been shown to induce at least TNF-α, IL-1α, IL-6, and IL-12 (4, 6, 8, 18, 25, 30). Similarly, LAB have been shown to induce IFN-γ, IL-6, IL-10, and TNF-α in human lymphocytes (1, 24). In this study we analyzed the ability of several live nonpathogenic LAB strains and a pathogenic GAS strain to induce cytokine production in peripheral blood mononuclear cells (PBMC) with special interest in Th1 type cytokines.
Lactobacillus rhamnosus E509 was obtained from Arla R&D (Stockholm, Sweden), Lactobacillus rhamnosus GG E522 (ATCC 53103) from Valio Ltd. R&D (Helsinki, Finland), and Lactobacillus bulgaricus E585 from Chr. Hansen A/S (Horsholm, Denmark). Streptococcus pyogenes serotype T1 IH32030 isolated from a child with bacteremia was from the collection of the National Public Health Institute (Helsinki, Finland). All strains were stored in skim milk at −70°C and passaged three times as previously described (24) prior to their use in stimulation experiments. Lactobacilli were grown in MRS medium and streptococci were grown in TY medium supplemented with 0.2% glucose (19). For stimulation experiments, bacteria were grown to logarithmic growth phase and the number of bacterial cells was determined by counting in a Petroff-Hausser counting chamber.
Freshly collected buffy coats from healthy blood donors were kindly provided by the Finnish Red Cross Blood Transfusion Service (Helsinki, Finland). PBMC were isolated and cultured as previously described (24). To minimize interindividual variation, all experiments were performed with cells obtained from 4 to 10 blood donors. PBMC were stimulated with live bacteria in a 1:1 ratio with 6 × 106 cells/ml or with 100 ng of purified lipopolysaccharide (LPS) per ml from Escherichia coli HB101, which was kindly provided by Ilkka Helander from National Public Health Institute (Helsinki, Finland). Cell culture supernatants and cells were harvested at various times after stimulation and pooled.
Total cellular RNA was isolated by using guanidium isothiocyanate and CsCl centrifugation and analyzed by the Northern blot method as previously described (7, 16, 34). The following cDNA probes for hybridizations were used: human TNF-α, IL-1β, IL-6 (obtained from the American Type Culture Collection), IL-10 (kindly provided by DNAX, Palo Alto, Calif.), IL-12 p40, IL-12 p35 (kindly provided by Ueli Gubler), IL-18 (41), and IFN-γ (35).
TNF-α, IL-6, and IL-10 levels in cell culture supernatants were determined by enzyme-linked immunosorbent assay (ELISA) methods described previously (24) with sensitivities of 20 pg/ml for TNF-α and IL-6 and 10 pg/ml for IL-10. An ELISA for IL-1β with a sensitivity of 10 pg/ml was set up with monoclonal mouse anti-human IL-1β antibody clones 508A3H12 and 508A4A2 and biotinylated mouse anti-human IL-1β antibody clone 508A3H12. Recombinant human IL-1β was used as a standard. All IL-1β-specific reagents were purchased from Medgenix Diagnostics (Fleurus, Belgium). Monoclonal mouse anti-human IL-4 antibody clone 3010.211, biotinylated goat anti-human IL-4 antibody, and recombinant human IL-4 purchased from R&D Systems (Abingdon, United Kingdom) were used to set up an ELISA for IL-4 with a sensitivity of 20 pg/ml. IL-12 p70 protein levels were determined with IL-12 Quantikine HS Kit (R&D Systems) with a detection limit of 0.5 pg/ml. IL-18 ELISA with a sensitivity of 10 pg/ml was performed as previously described (38). Monoclonal mouse antibody pairs and recombinant IL-18 ELISA standard were from Hayashibara Biochemical Laboratories Inc., Fujisaki Institute (Okayama, Japan). An ELISA for IFN-γ with a sensitivity of 40 pg/ml was set up with monoclonal mouse anti-human IFN-γ antibody clone 43-11 and biotinylated mouse anti-human IFN-γ antibody clone 45-15 (ImmunoKontact, Frankfurt, Germany). Recombinant human IFN-γ standard was purchased from R&D Systems. IFN-α/β assay was done as previously described (33) with a detection limit of 3 IU/ml. IFN-α titers in samples were assayed by vesicular stomatitis virus plaque reduction in HEp-2 cells (5).
Cytokine production profiles induced by different bacteria are shown in Fig. 1. Considerable IL-1β, IL-6, and TNF-α secretion was already seen after 6 h of stimulation of PBMC with bacteria, and cytokine levels continued to increase up to 16 h. Bacterial strains appeared to have very similar abilities to induce secretion of IL-1β, IL-6, and TNF-α. After 6 h of stimulation, the corresponding cytokine mRNA levels were also high (Fig. 2) but appeared to decrease after 16 h of stimulation except for the L. bulgaricus E585 strain. While no measurable IL-4 was induced by any of the bacterial strains (data not shown), all strains induced IL-10 protein secretion and mRNA expression (Fig. 1 and 2) with slower kinetics than those of proinflammatory cytokines. No measurable IFN-α production was seen in PBMC by LAB or GAS (data not shown). The kinetics of IL-12 and IFN-γ secretion induced by LAB and GAS was relatively slow and differed from that of IL-18. IL-18 protein production was already seen in the cell culture supernatant after 6 h of stimulation, whereas IL-12 and IFN-γ were detected only after 16 h of stimulation (Fig. 1). No IL-18 mRNA induction was detected in PBMC by any of the stimulants (data not shown). There were certain strain-specific differences in Th1 type cytokine production. L. rhamnosus E509 and S. pyogenes IH32030 stimulation elicited the highest secreted IL-12 p70 and IFN-γ protein levels (Fig. 1). Accordingly, in Northern blot analysis, these bacteria were the best inducers of IL-12 and IFN-γ mRNAs. All bacteria were able to induce IL-12 p35, IL-12 p40, and IFN-γ mRNAs except for L. bulgaricus E585, which was a poor inducer of IL-12 p35 and IFN-γ gene expression (Fig. 2).
FIG. 1.
ELISA measurements of cytokine production in PBMC induced by L. rhamnosus E509, L. rhamnosus E522, L. bulgaricus E585, and S. pyogenes IH32030. PBMC (6 × 106/ml) were stimulated with 6 × 106 live bacteria per ml for 6 h (hatched bars) or 16 h (black bars). Results are means of three independent experiments, each done with PBMC from three or four donors. Error bars represent standard deviations of the means.
FIG. 2.
Northern blot analysis of cytokine production in PBMC induced by LPS, L. rhamnosus E509, L. rhamnosus E522, L. bulgaricus E585, and S. pyogenes IH32030. PBMC (6 × 106/ml) from four donors were stimulated with 6 × 106 live bacteria per ml or with 100 ng of LPS per ml for 6 or 16 h.
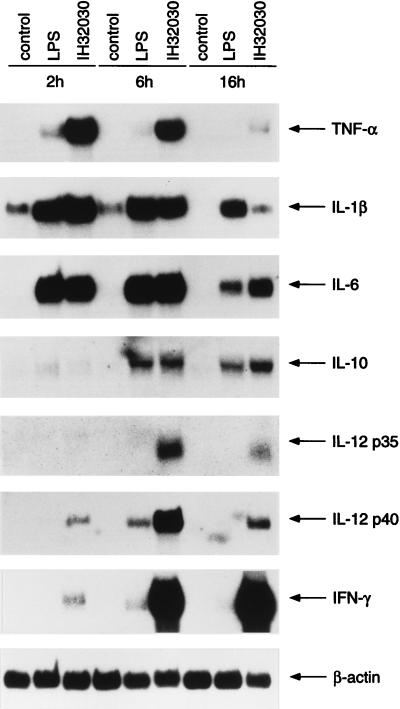
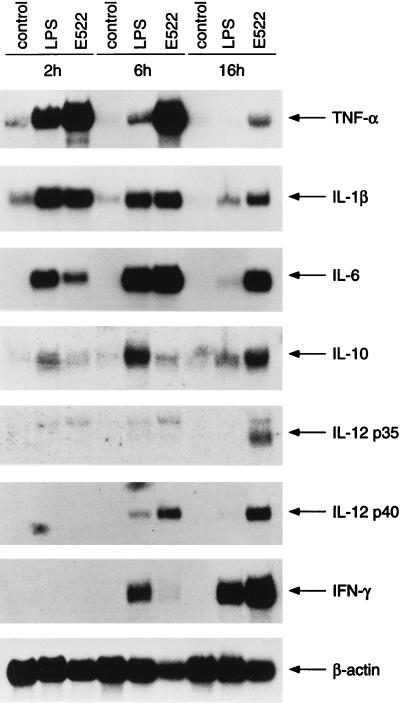
To analyze cytokine gene expression in more detail, PBMC were stimulated with LPS or with live L. rhamnosus E522 or S. pyogenes IH32030 for 2, 6, and 16 h. Cytokine mRNA expression in S. pyogenes IH32030-stimulated PBMC is shown in Fig. 3. Induction of TNF-α, IL-1β, and IL-6 mRNAs peaked at 2 h. IL-10 mRNA expression, on the contrary, peaked at 16 h. Weak induction of IL-12 p40 mRNA was seen after 2 h of stimulation. Both IL-12-specific mRNA chains p35 and p40 were strongly induced at 6 h by S. pyogenes IH32030 and appeared to decrease at 16 h. High IL-12 p40 and p35 mRNA levels at 6 h coincided with strong expression of IFN-γ. IFN-γ mRNA levels were high longer than IL-12 p35 and p40 levels. Cytokine mRNA expression levels after L. rhamnosus E522 stimulation are shown in Fig. 4. IL-1β mRNA levels were high at 2 h but started to decline thereafter. L. rhamnosus E522-induced TNF-α and IL-6 mRNA levels peaked at 6 h compared to the peak at 2 h by S. pyogenes IH32030. The expression of IL-10 and IL-12 p35 and p40 mRNAs were highest after 16 h of stimulation with L. rhamnosus E522. Some IL-12 p40 mRNA expression was seen already at 6 h, while IL-12 p35 mRNA expression was not seen then. IFN-γ induction by E522 L. rhamnosus was detectable at 6 h, and high levels were seen after 16 h of stimulation.
FIG. 3.
Kinetics of cytokine production in PBMC induced by S. pyogenes IH32030. PBMC (6 × 106/ml) from four donors were stimulated with 6 × 106 live bacteria per ml or with 100 ng of LPS per ml for 2, 6, or 16 h and then prepared for Northern blot analysis.
FIG. 4.
Kinetics of cytokine production in PBMC induced by L. rhamnosus E522. PBMC (6 × 106/ml) from four donors were stimulated with 6 × 106 live bacteria per ml or with 100 ng of LPS per ml for 2, 6, or 16 h and then prepared for Northern blot analysis.
Nonpathogenic LAB and pathogenic GAS did not appear to differ in their potency to induce TNF-α, IL-1β, and IL-6 secretion, and the kinetics of mRNA induction was fast, as shown by strong mRNA accumulation after only 2 h of stimulation. Similarities in the kinetics of TNF-α, IL-1β, and IL-6 induction and production by LPS, LAB, and GAS suggest that the most promising candidates for cells rapidly responding and being responsible for production of these cytokines are monocytes, which are known to respond rapidly after encountering pathogenic bacteria or bacterial components such as LPS and as a result produce proinflammatory cytokines (39).
LPS, LAB, or GAS did not induce any detectable IL-4 production in PBMC, while some IL-10 production was seen. Since the development of Th2 cells requires IL-4 (36), the lack of IL-4 production in LAB- and GAS-stimulated PBMC could suggest the lack of further Th2 type response. Our results on IL-4 and IL-10 production are in accordance with previous findings in which inactivated S. pyogenes preparations were found to induce IL-10 but not IL-4 (13). The kinetics of IL-10 induction and production was slower than those of proinflammatory cytokines, which is also consistent with previous observations (12).
Pathogenic streptococci and their cell walls or secreted components have been used to study Th1 type cytokine production in various cellular systems. Lipoteichoic acids of Streptococcus pneumoniae and S. pyogenes as well as heat-killed S. pneumoniae have been shown to induce IL-12 in THP-1 cells (8). Superantigens from GAS have been shown to induce IFN-γ in human PBMC (26, 27). In addition, nonpathogenic lactobacilli and streptococci have been shown to induce IFN-γ production in human lymphocytes and PBMC (1, 11). We show here that both live nonpathogenic LAB and pathogenic GAS induce IL-12 and IFN-γ production in human PBMC similarly. However, some kinetic differences in cytokine induction were seen. S. pyogenes was also a more potent inducer of IL-12 and IFN-γ secretion than LAB strains. Differences in the kinetics of IL-12 and IFN-γ induction could be due to differential use of leukocyte cell surface receptors by LPS, LAB, and GAS. While S. pyogenes and L. rhamnosus E522 induced IL-12 p35 and p40 and IFN-γ mRNA expression similarly, these bacteria did so at different time points. It is possible that S. pyogenes as a pathogen interacts more efficiently with PBMC than nonpathogenic LAB do. Cell size of bacterial strains used in our study varies, and the ratio of possible antigenic structures on bacterial cells could be different. These factors may affect interactions of LAB and GAS with PBMC and hence their cytokine production. Efficient interactions between GAS and PBMC could lead to a faster production of Th1 type cytokines by GAS than by LAB.
So far the only bacterium reported to induce IL-18 is Propionibacterium acnes (28). In the present work, we show that nonpathogenic lactobacilli and pathogenic S. pyogenes are equally potent in inducing IL-18 production in PBMC. IL-18 production in PBMC was induced by all Lactobacillus strains and S. pyogenes, and the kinetics of IL-18 production was fast, resembling those of proinflammatory cytokines. The similarity in the kinetics of IL-18 and proinflammatory cytokine production could reflect the recently suggested proinflammatory nature of IL-18 (29). While IL-18 secretion was induced by both LAB and GAS, the levels of IL-18 remained relatively low. It is possible that the amount of IL-18 produced by monocytes is small or that the vast majority of T (and B) cells present in PBMC use up at least some of the IL-18 produced during the experiment. Although IL-18 secretion was detected, no induction of IL-18 mRNA was seen. This could be explained by the findings that IL-18 production seems to be regulated primarily at a posttranscriptional level. Pro-IL-18 is processed to mature IL-18 by a proteolytic cleavage by caspase-1 enzyme, leading to secretion of mature IL-18 (3, 15, 17). IL-18 has been suggested to have an important role in mucosal immunity (20), and LAB most likely exert their immunological effects in vivo on intestinal mucosa. Our observation that lactobacilli induce IL-18 production in human PBMC could be of importance regarding their in vivo immunological effects in the gut.
Monocytes in PBMC are the mostly likely candidates producing IL-12 and IL-18 after LAB and GAS stimulation. Both IL-12 and IL-18 stimulate IFN-γ production in T cells, and they are known to act synergistically (10, 23, 28). All but one bacterial strain induced the production of IFN-γ in PBMC. The strain not able to induce IFN-γ also appeared to be the weakest inducer of IL-12. The differential induction of IL-12 could explain the observed differences in IFN-γ production, which correlates with IL-12 induction by LAB and GAS. Our data show that nonpathogenic LAB and pathogenic GAS are able to induce IL-12, IL-18, and IFN-γ, while production of IL-4 and IL-10 was limited. This suggests that gram-positive bacteria including nonpathogenic ones could elicit a Th1 type immune response.
Acknowledgments
This work was supported by the Jenny and Antti Wihuri Foundation, the Medical Research Council of the Finnish Academy, Nordic Industrial Fund project P93176, and the Sigrid Juselius Foundation.
REFERENCES
- 1.Aattouri N, Lemonnier D. Production of interferon induced by Streptococcus thermophilus: role of CD4+ and CD8+ lymphocytes. J Nutr Biochem. 1997;8:25–31. [Google Scholar]
- 2.Abbas A K, Murphy K M, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 3.Akita K, Ohtsuki T, Nukada Y, Tanimoto T, Namba M, Okura T, Takakura-Yamamoto R, Torigoe K, Gu Y, Su M S-S, Fuji M, Satoh-Itoh M, Yamamoto K, Kohno K, Ikeda M, Kurimoto M. Involvement of caspase-1 and caspase-3 in the production and processing of mature human interleukin 18 in monocytic THP.1 cells. J Biol Chem. 1997;272:26595–26603. doi: 10.1074/jbc.272.42.26595. [DOI] [PubMed] [Google Scholar]
- 4.Bhakdi S, Klonisch T, Nuber P, Fischer W. Stimulation of monokine production by lipoteichoic acids. Infect Immun. 1991;59:4614–4620. doi: 10.1128/iai.59.12.4614-4620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantell K, Hirvonen S, Kauppinen H-L, Kalkkinen N. Rapid production of interferon-γ in uninduced human leukocyte suspensions. J Interferon Res. 1991;11:231–236. doi: 10.1089/jir.1991.11.231. [DOI] [PubMed] [Google Scholar]
- 6.Cauwels A, Wan E, Leismann M, Tuomanen E. Coexistence of CD14-dependent and independent pathways for stimulation of human monocytes by gram-positive bacteria. Infect Immun. 1997;65:3255–3260. doi: 10.1128/iai.65.8.3255-3260.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chirgwin J M, Przybyla A E, MacDonald R J, Rutter W J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 8.Cleveland M G, Gorham J D, Murphy T L, Tuomanen E, Murphy K M. Lipoteichoic acid preparations of gram-positive bacteria induce interleukin-12 through a CD14-dependent pathway. Infect Immun. 1996;64:1906–1912. doi: 10.1128/iai.64.6.1906-1912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Andrea A, Aste-Amezaga M, Valiante N M, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon γ production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Andrea A, Rengaraju M, Valiante N M, Chehimi J, Kubin M, Aste M, Chan S H, Kobayashi M, Young D, Nickbarg E, Chizzonite R, Wolf S F, Trinchieri G. Production of natural killer cell stimulatory factor (interleukin-12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Simone C, Vesely R, Bianchi Salvadori B, Jirillo E. The role of probiotics in modulation of the immune system in man and in animals. Int J Immunother. 1993;9:23–28. [Google Scholar]
- 12.De Waal Malefyt R, Abrams J, Bennet B, Figdor C G, de Vries J E. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujimoto T, Duda R B, Szilvasi A, Chen X, Mai M, Donnel M A. Streptococcal preparation OK-432 is a potent inducer of IL-12 and T helper cell 1 dominant state. J Immunol. 1997;158:5619–5626. [PubMed] [Google Scholar]
- 14.Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989;66:363–378. [PubMed] [Google Scholar]
- 15.Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, Wong W, Kamen R, Tracey D, Allen H. Caspase-1 processes IFN-γ-inducing factor and regulates LPS-induced IFN-γ production. Nature. 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 16.Glisin V, Crkvenjakov R, Buys C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974;13:2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- 17.Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming M A, Hayashi N, Higashino K, Okamura H, Nakanishi K, Kurimoto M, Tanimoto T, Flavell R A, Sato V, Harding M W, Livingston D J, Su M S-S. Activation of interferon-γ inducing factor mediated by interleukin-1β converting enzyme. Science. 1997;275:206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 18.Heumann D, Barras C, Severin A, Glauser M P, Tomaz A. Gram-positive cell walls stimulate synthesis of tumor necrosis factor alpha and interleukin-6 by human monocytes. Infect Immun. 1994;62:2715–2721. doi: 10.1128/iai.62.7.2715-2721.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holm S E, Falsen E. An antigen-free medium for cultivation of β-hemolytic streptococci. Acta Pathol Microbiol Scand. 1967;69:264–276. [Google Scholar]
- 20.Kohno K, Kurimoto M. Interleukin 18, a cytokine which resembles IL-1 structurally and IL-12 functionally but exerts its effect independently of both. Clin Immunol Immunopathol. 1998;86:11–15. doi: 10.1006/clin.1997.4475. [DOI] [PubMed] [Google Scholar]
- 21.Kubin M, Chow J M, Trinchieri G. Differential regulation of interleukin-12 (IL-12), tumor necrosis factor α, and IL-1β production in human myeloid leukemia cell lines and peripheral blood mononuclear cells. Blood. 1994;83:1847–1855. [PubMed] [Google Scholar]
- 22.Lidbeck A, Nord C E. Lactobacilli and the normal human anaerobic microflora. Clin Infect Dis. 1993;16:S181–S187. doi: 10.1093/clinids/16.supplement_4.s181. [DOI] [PubMed] [Google Scholar]
- 23.Matsui K, Yoshimoto T, Tsutsui H, Hyodo Y, Hayashi N, Hiroishi K, Kawada N, Okamura H, Nakanishi K, Higashino K. Propionibacterium acnes treatment diminishes CD4+NK1.1+ T cells but induces type 1 T cells in the liver by induction of IL-12 and IL-18 production from Kupffer cells. J Immunol. 1997;159:97–106. [PubMed] [Google Scholar]
- 24.Miettinen M, Vuopio-Varkila J, Varkila K. Production of tumor necrosis factor alpha, interleukin-6, and interleukin-10 is induced by lactic acid bacteria. Infect Immun. 1996;64:5403–5405. doi: 10.1128/iai.64.12.5403-5405.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller-Alouf H, Alouf J E, Gerlach D, Ozegowski J-H, Fitting C, Cavaillon J-M. Comparative study of cytokine release by human peripheral blood mononuclear cells stimulated with Streptococcus pyogenes superantigenic erythrogenic toxins, heat-killed streptococci, and lipopolysaccharide. Infect Immun. 1994;62:4915–4921. doi: 10.1128/iai.62.11.4915-4921.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norrby-Tegelund A, Lustig R, Koth M. Differential induction of Th1 versus Th2 cytokines by group A streptococcal toxic shock syndrome isolates. Infect Immun. 1997;65:5209–5215. doi: 10.1128/iai.65.12.5209-5215.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norrby-Tegelund A, Norgren M, Holm S E, Andersson U, Andersson J. Similar cytokine induction profiles of a novel streptococcal exotoxin, MF, and pyrogenic exotoxins A and B. Infect Immun. 1994;62:3731–3738. doi: 10.1128/iai.62.9.3731-3738.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okamura H, Tsutsui H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, Akita K, Namba M, Tanabe F, Konishi K, Fukuda S, Kurimoto M. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 29.Puren A J, Fantuzzi G, Gu Y, Su S-S, Dinarello C A. Interleukin-18 (IFN-γ-inducing factor) induces IL-8 and IL-1β via TNFα production from non-CD14+ human blood mononuclear cells. J Clin Invest. 1998;101:711–721. doi: 10.1172/JCI1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riesenfeld-Orn I, Wolpe S, Garcia-Bustos J F, Hoffmann M K, Tuomanen E. Production of interleukin-1 but not tumor necrosis factor by human monocytes stimulated with pneumococcal cell surface components. Infect Immun. 1989;57:1890–1893. doi: 10.1128/iai.57.7.1890-1893.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romagnani S. Th1 and Th2 in human diseases. Clin Immunol Immunopathol. 1996;80:225–235. doi: 10.1006/clin.1996.0118. [DOI] [PubMed] [Google Scholar]
- 32.Romagnani S. Understanding the role of Th1/Th2 cells in infection. Trends Microbiol. 1996;4:470–473. doi: 10.1016/s0966-842x(97)82906-x. [DOI] [PubMed] [Google Scholar]
- 33.Ronni T, Sareneva T, Pirhonen J, Julkunen I. Activation of IFN-α, IFN-γ, MxA, and IFN regulatory factor 1 genes in influenza A virus-infected human peripheral blood mononuclear cells. J Immunol. 1995;154:2764–2774. [PubMed] [Google Scholar]
- 34.Sareneva T, Matikainen S, Kurimoto M, Julkunen I. Influenza A virus-induced IFN-α/β and IL-18 synergistically enhance IFN-γ gene expression in human T cells. J Immunol. 1998;160:6032–6038. [PubMed] [Google Scholar]
- 35.Sareneva T, Pirhonen J, Cantell K, Kalkkinen N, Julkunen I. Role of N-glycosylation in the synthesis, dimerization and secretion of human interferon-γ. Biochem J. 1994;303:831–840. doi: 10.1042/bj3030831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seder R A, Paul W E. Acquisition of lymphokine-producing phenotype by CD4+ cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 37.Stevens D L. Invasive group A streptococcus infections. Clin Infect Dis. 1992;14:2–13. doi: 10.1093/clinids/14.1.2. [DOI] [PubMed] [Google Scholar]
- 38.Taniguchi M, Nagaoka K, Kunikata T, Kayano T, Yamauchi H, Nakamura S, Ikeda M, Orita K, Kurimoto M. Characterization of anti-human interleukin-18 (IL-18)/interferon-γ-inducing factor (IGIF) monoclonal antibodies and their application in the measurement of human IL-18 by ELISA. J Immunol Methods. 1997;206:107–113. doi: 10.1016/s0022-1759(97)00094-x. [DOI] [PubMed] [Google Scholar]
- 39.Tracey K J, Cerami A. Tumor necrosis factor, other cytokines and disease. Annu Rev Cell Biol. 1993;9:317–343. doi: 10.1146/annurev.cb.09.110193.001533. [DOI] [PubMed] [Google Scholar]
- 40.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 41.Ushio S, Namba M, Okura T, Hattori K, Nukada Y, Akita K, Tanabe F, Konishi K, Micallef M, Fujii M, Torigoe K, Tanimoto T, Fukuda S, Ikeda M, Okamura H, Kurimoto M. Cloning of the cDNA for human IFN-γ-inducing factor, expression in Escherichia coli, and studies on the biologic activities of the protein. J Immunol. 1996;156:4274–4279. [PubMed] [Google Scholar]