Abstract
Background
Waning of COVID-19 vaccine efficacy/effectiveness (VE) has been observed across settings and epidemiological contexts. We conducted a systematic review of COVID-19 VE studies and performed a meta-regression analysis to improve understanding of determinants of waning.
Methods
Systematic review of PubMed, medRxiv and the WHO-International Vaccine Access Center database summarizing VE studies on 31 December 2022. Studies were those presenting primary adult VE data from hybrid immunity or third/fourth mRNA COVID-19 monovalent vaccine doses [due to limited data with other vaccines] against Omicron, compared with unvaccinated individuals or individuals eligible for corresponding booster doses but who did not receive them. We used meta-regression models, adjusting for confounders, with weeks since vaccination as a restricted cubic spline, to estimate VE over time since vaccination.
Results
We identified 55 eligible studies reporting 269 VE estimates. Most estimates (180/269; 67 %) described effectiveness of third dose vaccination; with 48 (18 %) and 41 (15 %) describing hybrid immunity and fourth dose effectiveness, respectively, mostly (200; 74 %) derived from test-negative design studies. Most estimates (176/269; 65 %) reported VE compared with unvaccinated comparison groups. Estimated VE against mild outcomes declined following third dose vaccination from 62 % (95 % CI: 58 % – 66 %) after 4 weeks to 48 % (41 % – 55 %) after 20 weeks. Fourth dose VE against mild COVID-19 declined from 48 % (41 % – 56 %) after 4 weeks to 47 % (19 % – 65 %) after 20 weeks. VE for severe outcomes was higher and declined in the three-dose group from 90 % (87 % – 92 %) after 4 weeks to 70 % (65 – 74 %) after 20 weeks.
Conclusions
Time-since vaccination is an important determinant of booster dose VE, a finding which may support seasonal COVID-19 booster doses. Integration of VE and immunological parameters – and longer-term data including from other vaccine types – are needed to better-understand determinants of clinical protection.
Keywords: COVID-19 vaccine booster shot, Vaccine effectiveness, Methods, Epidemiology
Text
Background
The first COVID-19 vaccine was approved for emergency use as a 2-dose schedule in December 2020 following randomized efficacy trials [1], [2]. Additional approvals quickly followed in the US and elsewhere, mostly for two-dose primary series 3 – 4 weeks apart; but also for a one-dose vaccine (Janssen’s JNJ-78436735) [3], [4]. Gradual reductions in protection, particularly against milder disease, were observed approximately 5–6 months after the end of the primary series [5], [6]. Third ‘booster’ doses of mRNA vaccines – intended to extend duration of immune protection through enhanced humoral responses, generate T-cell memory/expansion; broaden epitope recognition and/or support trained immunity [7], [8], [9], [10] – were subsequently shown to improve protection in vaccine effectiveness (VE) studies, first of the Pfizer vaccine in Israel [11], [12], and then with other vaccines elsewhere, irrespective of the primary schedule [13]. Following the emergence of the Omicron variant, vaccine recommendations were extended in late 2022 to include bivalent formulations targeting both ancestral and Omicron BA.4/BA.5 strains [14], before monovalent XBB.1.5. vaccines were recommended for the 2023/24 season [15].
Following the emergence of variants of concern (VoC), and after the distribution of > 11 billion doses of COVID-19 vaccines amidst a background of natural infection [16], current data indicate that neither vaccine- nor infection-derived immunity provides robust and durable protection from infection [17], [18]. Ongoing vaccination will therefore likely be needed to protect the most vulnerable population groups [19]. Optimal targeting of those doses in terms of the timing and frequency is necessary to define cost-effective vaccination strategies that minimize disease burden [4], [19], [20]. VE studies are needed to refine booster policies, and more than 100 COVID-19 VE studies have been published using different vaccine types, schedules, against different variants, clinical outcomes and study designs [21]. Interpretation of these studies is challenging because a high proportion of the population has been infected and most studies will compare with partially immune comparator populations where immunity might be influenced by vaccination history. Some studies measure the incremental benefit of the Nth dose by comparing with individuals who received N-1 doses but were eligible for the Nth dose, and therefore measure relatively smaller effect sizes which could be susceptible to bias due to variable infection histories between study groups [22], [23], [24].
VE estimates from individual studies may be influenced by terminology, interval post-primary series and varying exposure history in comparator groups and other confounding design features. Hybrid immunity likely offers improved protection over vaccination alone. We conducted a systematic review and meta-regression analysis to describe the literature and estimate the relationship between VE, hybrid immunity and time since vaccination, adjusted for important confounders, to inform COVID-19 booster vaccination policy.
Methods
Search strategy and selection criteria
We systematically searched PubMed, medRxiv and the WHO-International Vaccine Access Center database summarizing VE studies [25]. We considered peer-reviewed and preprint studies published until 31 December 2022 presenting primary data describing effectiveness of COVID-19 vaccine booster doses and/or hybrid immunity against mild and severe COVID-19 caused by the Omicron variant. The following keywords were used in the search; (“SARS-CoV-2” or “COVID-19” or “2019-nCov”) AND (“Vaccin*” or “Vaccination”) AND (“booster” or “booster shot” or “third dose” or “fourth dose” or “monovalent booster” or “first booster” or “second booster”) AND (“Efficacy” or “Effectiveness”). In addition, we screened the reference lists of included studies and identified review papers. The detailed search strategy is described in Supplementary Material.
Studies were included regardless of study setting (conducted in the community, primary care, or hospital), methodology and publication status. We excluded case reports, case series, modeling and review papers. In addition, letters to the editor, correspondence, reports and rapid communications were excluded if the methods are not adequately described. Because the rate of waning was a primary research question, studies were excluded if they did not report the period of time between vaccination and outcome. Studies reporting VE only in children, and in specific groups such as people living with diabetes or immunocompromised individuals, were also excluded due to different vaccination policies in these groups. We included peer-reviewed papers if both pre-print and peer-reviewed versions of the same study were available. This systematic review and meta-regression is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) [26].
Exposure and outcome definition
We defined boosters as third (first booster) and fourth (second booster) doses of mRNA vaccines (mRNA-1273 and BNT162b2) regardless of the vaccine used in the primary series (including mRNA and adenovirus vectored vaccines). Due to the timing of the search, only studies reporting data from monovalent booster vaccines were available. COVID-19 severity was considered either mild (asymptomatic or symptomatic disease) or severe (COVID-19-related hospitalisation, severe or fatal COVID-19), according to the definitions and language used in underlying papers. Multiple outcomes (e.g., hosptilisation and death reported separately) described in a single study were both eligible for inclusion, because we assumed they represented distinct patient populations. Due to evolving epidemiology and the near disappearance of Delta strains, the analysis was confined to infection with SARS-CoV-2 Omicron variants. Data were classified as “parenteral” if the original B.1.1.529 Omicron strain was circulating at the time of study, or “variants” if any subsequent strain (BA.1, BA.2, etc.) was already predominant, as described in the underlying literature.
Study selection, screening and data extraction
Records were imported into Covidence software (https://www.covidence.org), de-duplicated and sequentially screened for inclusion based on their titles, abstracts, and full text. Two reviewers (JN and YMM) did study screening and full-text review independently. Included studies were listed in Microsoft Excel and data extraction was conducted by one reviewer (YMM) and verified by a second reviewer (JN) using a pretested and standardized abstraction form. Discrepancies in study selection and data abstraction were resolved by discussion and consensus. For studies which reported > 1 VE estimate, multiple estimates were extracted if estimates were unique in terms of variables used in regression analyses, to prevent double counting. When > 1 estimate was eligible by duration of follow-up (time between vaccination and outcome event), the estimate with longest duration between vaccination and follow-up time was selected to provide variability and include data over periods over which appreciable waning was likely to have been observed.
The following data were extracted for each included VE estimate: time of study conduct and publication; country, study design, duration of follow-up, method of confounder control, participant age range in two categories (adults ≥ 60 years; and younger/mixed populations, reflecting the descriptions applied in most underlying studies), prior SARS-CoV-2 infection status of study population, type of exposure (vaccine booster dose or hybrid immunity, which was defined as any individual who had been identified as both infected and vaccinated, irrespective of sequence and number of doses), primary vaccine series schedule, booster dose type, prime-booster vaccine schedule (homologous, heterologous or unknown), period considered for immune delay post-vaccination, comparator group (unvaccinated, primary vaccine series vaccination or first booster vaccination), outcome type (asymptomatic, symptomatic, hospitalisation or severe disease), reported VE (calculated from reported data using 1-odds ratio or similar measure when these were reported) with adjustment for potential confounders and corresponding 95 % confidence intervals (CIs). For studies of hybrid immunity, we did not distinguish between studies where vaccine preceded infection, or the reverse.
Meta-analysis framework
The primary outcome of this study was to estimate effectiveness of monovalent mRNA booster doses and hybrid immunity against mild and severe COVID-19 over time since most recent vaccination. VE estimates were grouped based on a) disease severity (mild/severe), b) exposure type (3rd dose; 4th dose; hybrid immunity) and c) comparator group (by various categories of vaccination history and infection history). For each group, we estimated pooled VE as a function of time since most recent vaccine dose in a random-effects meta-regression model framework, adjusting for variables likely to impact VE measurement: prior infection status; age group; comparator group; prime booster type (homologous vs heterologous); VE study design used; period of immune delay considered between vaccination and protection. Maximum time in weeks between vaccination and outcome measurement was included as a restricted cubic spline with three internal knots to allow flexible estimation of VE as a function of time since vaccination.
Multivariable models were developed incorporating significant univariate predictors of VE, explicitly retaining a variable corresponding to maximum duration of follow-up, as the primary study objective. To avoid overfitting models with few estimates, an independent predictor with greatest confounding influence was included for every 6 estimates (and consequently we made no estimates for any groups with < 6 estimates because duration of follow-up; the primary study outcome; could not be reliably modelled) [27]. Redundant predictors (e.g., prior infection status when exploring only naïve comparators) were dropped from models. VE was estimated for each group at three different follow-up periods (4, 12 and 20 weeks since vaccination) which can be interpreted as “absolute” VE for studies comparing with naïve cohorts; and the “relative” VE of an additional dose, for studies comparing with vaccinated cohorts.
Statistical analyses were conducted using R software Version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria); using the metafor package for meta-analyses.
Role of the funding source
The funding bodies had no role in study design, the collection, analysis, and interpretation of data, the writing of the report or in the decision submit for publications.
Results
Characteristics of the literature
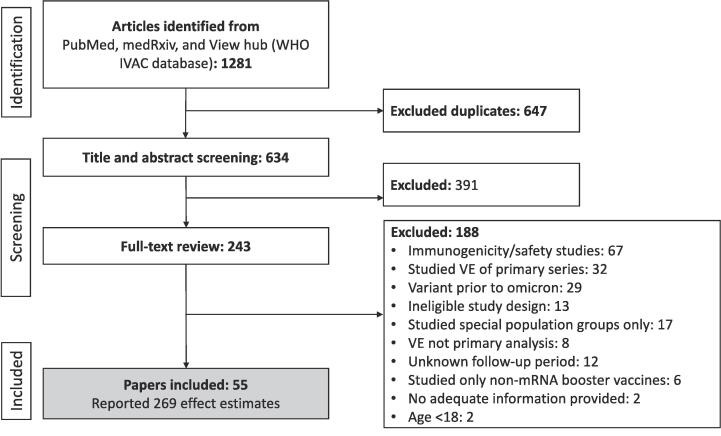
A total of 55 eligible COVID-19 booster studies were identified containing 269 unique, eligible VE estimates (Fig. 1), described in supplementary materials 2. Eighty five (32 %) originated from the Unites States, 39 (14 %) from Qatar, 31 (12 %) from Canada and 27 (10 %) from Brazil. Most estimates (180/269; 67 %) described effectiveness of third dose vaccination; with 48 (18 %) and 41 (15 %) describing hybrid immunity and fourth dose effectiveness, respectively (Table 1). The median duration of follow-up was 9 weeks and most estimates (200; 74 %) were derived from test-negative design studies. All estimates were derived from adjusted statistical analyses, using a broad range of potential confounders. Approximately half (114/269; 42 %) of estimates also used matching/stratification.
Fig. 1.
Study flow chart.
Table 1.
Characteristics of VE estimates in terms of number (%) of estimates identified, stratified by comparison groups (never-vaccinated vs previously vaccinated and eligible for this booster but has not received this booster).
| Characteristic | Naive, N = 1761 | Vaccinated, N = 931 | Overall, N = 2691 |
|---|---|---|---|
| Severity: | |||
| Mild | 106 (60 %) | 49 (53 %) | 155 (58 %) |
| Severe | 70 (40 %) | 44 (47 %) | 114 (42 %) |
| Immunity derived from: | |||
| 3-dose mRNA | 121 (69 %) | 59 (63 %) | 180 (67 %) |
| 4-dose mRNA | 9 (5.1 %) | 32 (34 %) | 41 (15 %) |
| 2-/3-dose hybrid | 46 (26 %) | 2 (2.2 %) | 48 (18 %) |
| Included omicron variant: | |||
| Omicron | 117 (66 %) | 77 (83 %) | 194 (72 %) |
| Omicron BA.1 | 29 (16 %) | 5 (5.4 %) | 34 (13 %) |
| Omicron BA.2 | 28 (16 %) | 3 (3.2 %) | 31 (12 %) |
| Omicron BA.2 and BA.5 | 0 (0 %) | 4 (4.3 %) | 4 (1.5 %) |
| Omicron BA.5 | 2 (1.1 %) | 4 (4.3 %) | 6 (2.2 %) |
| Age group: | |||
| Adults (mixed) | 145 (82 %) | 57 (61 %) | 202 (75 %) |
| 60 and older | 31 (18 %) | 36 (39 %) | 67 (25 %) |
| Maxiumum follow-up (weeks): | 9.0 (6.0, 17.0) | 8.0 (4.0, 16.0) | 9.0 (6.0, 16.0) |
| Study design: | |||
| Prospective cohort | 1 (0.6 %) | 3 (3.2 %) | 4 (1.5 %) |
| Retrospective cohort | 14 (8.0 %) | 51 (55 %) | 65 (24 %) |
| Test-negative case control | 161 (91 %) | 39 (42 %) | 200 (74 %) |
| 1n (%); Median (IQR) | |||
Treatment of SARS-CoV-2 infection history in comparator groups
Of identified estimates, the majority (176/269; 65 %) reported VE compared with never-vaccinated control groups. The remainder (93; 35 %) described booster VE compared with fully vaccinated individuals [including 4th dose studies using 3rd dose groups as comparators]. The impact of SARS-CoV-2 infection history on VE was addressed inconsistently in the included studies. Twenty-one out of 49 studies (42.9 % of the total) excluded previous COVID-19 cases from analysis; twelve (24.5 %) incorporated previous infection status into the analysis, for example as a model covariate; nine (18.4 %) applied a washout period (e.g., three months, corresponding to periods of elevated, post-infection immune system activation) prior to SARS-CoV-2 infection during which COVID-19 cases were excluded, and the remaining seven studies did not consider prior SARS-CoV-2 infection history of the study participants. Similarly, there was variation in the duration of immune delay assumed between vaccination and immune response, varying from zero to 14 days, with most studies (37; 67.3 %) choosing a period of six or seven days.
Meta-regression estimated vaccine effectiveness over time
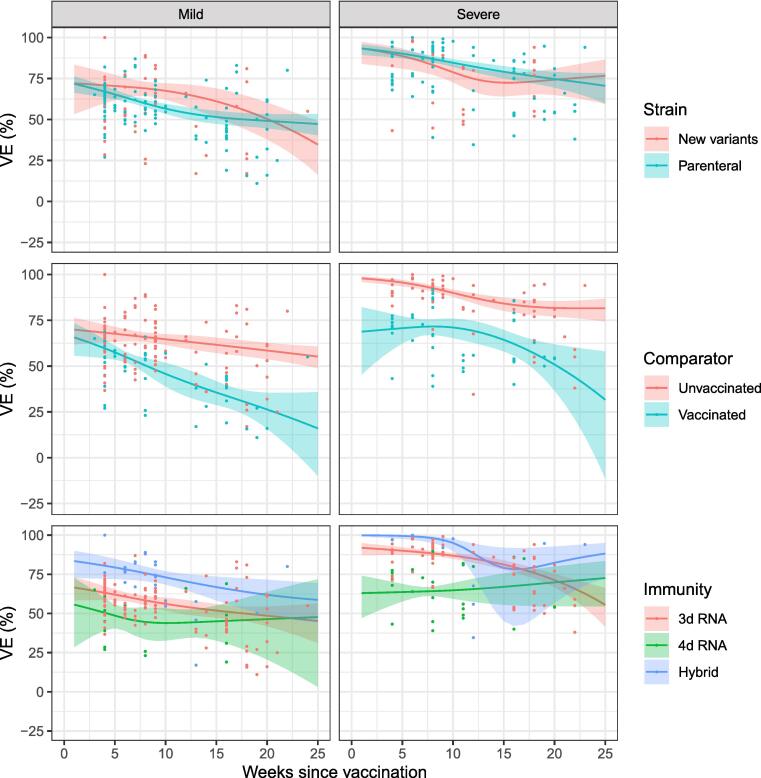
We developed a total of 14 meta-regression models to estimate VE according to infecting strain, comparator group or arising from different numbers of vaccine doses and natural infection (Fig. 2). Final estimates were adjusted for between one and six potential confounders (supplementary materials Fig. 1). Reported vaccine effectiveness was higher for severe outcomes, and studies estimating VE in comparison with a never-vaccinated group (referred to as ‘absolute’ VE’ in some studies) than from milder outcomes and studies calculating relative VE (using vaccinated or infected comparator populations; supplemental Table 1; Fig. 2). Estimated VE against mild outcomes declined following third dose mRNA vaccination from 62 % (95 % CI: 58 – 66 %) after 4 weeks to 48 % (41 – 55 %) after 20 weeks. Four dose mRNA VE [compared with three-dose vaccinated cohorts] against mild COVID-19 declined from 48 % (41 – 56 %) after 4 weeks to 47 % (19 – 65 %) after 20 weeks (Table 2). VE for severe outcomes was higher and declining in the three-dose group from 90 % (87 – 92 %) after 4 weeks to 70 % (65 – 74 %) after 20 weeks. VE was higher for hybrid immunity (declining from 100 %; 98 – 100 % after 4 weeks to 83 %; 59 – 93 % after 20 weeks) but lower for the four-dose relative VE group (64 %; 58 – 69 % after 4 weeks and 68 %, 45 – 82 % after 20 weeks; Table 2). Estimated VE was similar for parenteral and new Omicron strains (Fig. 2).
Fig. 2.
Confounder-adjusted estimated VE over time (≤25 weeks) for a) different infecting Omicron subvariants (top); b) studies comparing with vaccinated/never-vaccinated groups (middle); and c) individuals with different vaccination/infection profiles (bottom). Lines represent adjusted VE, shaded areas 95 % CIs; and dots original estimates.
Table 2.
VE estimates by time since vaccination. Data are stratified by severity (mild/severe), vaccine/immunity type and whether studies used vaccinated/never-vaccinated comparator groups.
|
Vaccine effectiveness, % (95 % CI) |
||||
|---|---|---|---|---|
| Immunity | n | 4 weeks | 12 weeks | 20 weeks |
| Outcome: mild | ||||
| 3d mRNA | 107 | 62 (58, 66) | 54 (49, 58) | 48 (41, 55) |
| Vax comparator | 35 | 61 (58, 63) | 45 (40, 50) | 26 (19, 32) |
| Naive comparator | 72 | 66 (58, 73) | 66 (56, 73) | 64 (52, 73) |
| Hybrid immunity | 32 | 79 (72, 85) | 70 (63, 75) | 61 (48, 71) |
| Vax comparator | 1 | |||
| Naive comparator | 31 | 79 (73, 84) | 71 (66, 76) | 65 (54, 73) |
| 4d mRNA | 16 | 49 (41, 56) | 44 (35, 53) | 47 (19, 65) |
| Vax comparator | 13 | 45 (39, 51) | 40 (29, 50) | 42 (16, 61) |
| Naive comparator | 3 | |||
| Outcome: severe | ||||
| 3d mRNA | 73 | 90 (87, 92) | 84 (81, 86) | 70 (65, 74) |
| Vax comparator | 24 | 75 (68, 81) | 69 (66, 72) | 61 (51, 69) |
| Naive comparator | 49 | 93 (90, 95) | 87 (85, 90) | 76 (71, 80) |
| Hybrid immunity | 16 | 100 (98, 100) | 85 (66, 94) | 83 (59, 93) |
| Vax comparator | 1 | |||
| Naive comparator | 15 | 100 (97, 100) | 88 (72, 95) | 87 (68, 95) |
| 4d mRNA | 25 | 64 (58, 69) | 65 (53, 74) | 68 (45, 82) |
| Vax comparator | 19 | 56 (50, 62) | 60 (45, 71) | 63 (28, 81) |
| Naive comparator | 6 | 68 (53, 79) | 81 (79, 83) | 89 (84, 92) |
Discussion
We conducted a systematic review of COVID-19 booster VE and hybrid immunity studies against the Omicron variant which had become the dominant circulating SARS-CoV-2 by early 2022 [28]. We conducted a meta-regression analysis to explore VE, adjusted for possibly influential covariates, against mild and severe COVID-19 disease as a function of time since receipt of last vaccine dose. Despite the short duration of booster-dose availability, a sizeable body of VE literature was identified, reflective of the recent prioritization of COVID-19 research and increasing availability of real-world data for epidemiological analysis. Over a period of approximately 6 months since booster vaccination, waning was observed almost immediately in populations who had received three vaccine doses or hybrid immunity, without noticeable inflection points within the first six months since vaccination. Three-dose VE against severe disease was estimated to decline on average from 90 % immediately after vaccination to 70 % after 20 weeks, and for mild infections, from 62 % to 48 %. This is broadly aligned with data from elsewhere showing 15 – 30 % reductions in mRNA VE against Omicron strains against mild, symptomatic disease within 10 weeks of vaccination [29]; and declining relative VE of booster vaccination against infection to < 20 %, three months after receipt of a booster dose [30].
Four dose vaccine protection appeared more durable with VE of approximately 50 % against mild COVID-19, and 65 % against severe outcomes, with no evidence of waning after 20 weeks, possibly a consequence of broad immunological responses in four-dose vaccine recipients who may also have been infected during the pandemic and thus benefited from broad and cross-neutralizing immunological profiles [31]. It is also likely that persistent fourth-dose VE was a consequence of study design features: these studies mostly compared outcome rates with individuals who had received three vaccine doses comparator groups. Because VE calculated from the incidence rate ratio between these two groups, declining three-dose VE would give the impression of stable/increasing four-dose VE, even under a scenario of stable absolute four-dose protection [32].
This principle also applies to third-dose booster VE studies: because booster vaccine doses aim to improve protection over and above what has already been provided by preceding vaccinations, the most relevant comparator group should be those who have received one fewer dose than that under study and would therefore report a ‘relative VE’ or ‘booster VE’, (sometimes referred to as an ‘incremental VE’. However, almost 2/3 of estimates we identified were derived from comparisons with reportedly uninfected and unvaccinated individuals, i.e. individuals without any pre-existing immunity in whom COVID-19 incidence rates would be expected to be higher than in fully vaccinated individuals or those with prior infections. Fourth-dose VE studies, which compared overwhelmingly with vaccinated comparator groups, reported lower VE than hybrid or three-dose vaccine studies which often used unvaccinated comparator groups. Nonetheless, given the high attack rate of Omicron and predisposition for breakthrough cases even in vaccinated individauls [33], [34], and because baseline serological testing to ascertain infection history is not conducted during effectiveness studies, it remains unlikely that unvaccinated comparator groups consist purely of uninfected individuals. If infection history is affected by vaccination history then the comparison may be affected by differential rates of infection-induced immunity in the two groups. This effect would give rise to lower-than-expected infection rates in comparator groups and thus potentially underestimate VE. Infection history is one of many important potential context-specific biases relating to changing treatment, diagnosis and healthcare utilization patterns for future VE estimation [35]. As booster doses continue to be administered seasonally and population immunity is shaped by future viral infections and immunological waning, approaches which consider time since the last dose, rather than the number of doses ever received, may be considered preferable methods for COVID-19 VE studies.
Additional methodological heterogeneity is contributed from the wide range of outcome definitions used in underlying VE studies, from asymptomatic infection through to fatal disease. As SARS-CoV-2 circulation becomes endemic, it is likely that reduction of the burden of severe COVID-19 will be a public health priority addressed by booster dose campaigns, whereas prevention of milder disease might take on lower importance, compared with other public health priorities. We identified higher VE for more severe endpoints including ‘hospitalization’. Studies using diagnostic codes to identify cases run the risk of identifying cases hospitalized “with” rather than “of” COVID-19 and more specific endpoints including admission diagnoses; or indicators of respiratory distress such as oxygen dependency, may be of benefit in identifying meaningful VE values, particularly when assessing vaccines against Omicron or future variants which evade humoral immunity [36], [37].
This review has several limitations. We observed considerable variability within reporting completeness and clarity, particularly in pre-print papers, some of which may never be published. Our approach was to collect only non-overlapping VE estimates and thus does not represent an exhaustive summary. We did not conduct a risk of bias assessment because the primary objective was to summarize the literature rather than assess study quality. Many studies had short follow-up periods, often collecting data for < 3 months after booster vaccination with sparser long-term data and are therefore biased towards time periods during which immunological response to vaccination would have been highest [38]. Most studies did not report vaccination histories of comparator groups, and residual immunity in these groups would be deterministic of reported VE. Due to the timing of the literature search, the review included only studies evaluated the real-world effectiveness of the monovalent mRNA vaccine booster dose.
We identified a broad body of COVID-19 VE literature, originating from 17 countries and using different study designs and analytical methods. Design aspects were heterogeneous and may have been influential in underlying VE estimates. Waning VE was observed in many vaccination categories, but greater consideration of comparison groups and longer follow-up periods will be needed to describe the magnitude and impact of waning of booster doses and the total benefit of an additional vaccine doses.
Data sharing
This study used no primary data and all data are available from the studies referenced in supplementary table 1.
Funding
This work was supported by a commissioned grant from the Health and Medical Research Fund of the Food and Health Bureau of the Hong Kong SAR Government, by the National Institute of General Medical Sciences (grant no. R01 GM139926), by the Theme-based Research Scheme (Project No. T11-705/21-N) from the Research Grants Council from the University Grants Committee of Hong Kong, and an RGC Senior Research Fellowship (grant number: HKU SRFS2021-7S03).
Declaration of interests
JN used to work for, and holds shares in, Sanofi. BJC: has received honoraria from AstraZeneca, Fosun Pharma, GlaxoSmithKline, Haleon, Moderna, Novavax, Roche and Sanofi. The other authors declare no potential conflicts of interest.
CRediT authorship contribution statement
Joshua Nealon: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing, Visualization. Yonatan M Mefsin: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Martina E. McMenamin: Conceptualization, Validation, Writing – review & editing. Kylie E.C. Ainslie: Methodology, Supervision, Writing – review & editing. Benjamin J. Cowling: Funding acquisition, Methodology, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Joshua Nealon reports a relationship with Sanofi that includes: equity or stocks. Benjamin Cowling has received honoraria from AstraZeneca, Fosun Pharma, GlaxoSmithKline, Haleon, Moderna, Novavax, Roche and Sanofi.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jvacx.2024.100451.
Contributor Information
Joshua Nealon, Email: joshnealon@gmail.com.
Benjamin J. Cowling, Email: bcowling@hku.hk.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The data are available in the paper
References
- 1.US Food and Drug Administration. FDA Takes Key Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for First COVID-19 Vaccine. 2020. https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19 (accessed 14 March 2022).
- 2.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Centers for Disease Control and Prevention. Different COVID-19 Vaccines. 2022. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines.html.
- 4.World Health Organization. COVID-19 advice for the public: Getting vaccinated. 2022. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice.
- 5.Thomas S.J., Moreira E.D., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine through 6 months. N Engl J Med. 2021;385(19):1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrews N., Tessier E., Stowe J., et al. Duration of protection against mild and severe disease by covid-19 vaccines. N Engl J Med. 2022;386(4):340–350. doi: 10.1056/NEJMoa2115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Covián C., Retamal-Díaz A., Bueno S.M., Kalergis A.M. Could BCG vaccination induce protective trained immunity for SARS-CoV-2? Front Immunol. 2020;11:970. doi: 10.3389/fimmu.2020.00970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeyanathan M., Afkhami S., Smaill F., Miller M.S., Lichty B.D., Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol. 2020;20(10):615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woodland D.L. Jump-starting the immune system: prime-boosting comes of age. Trends Immunol. 2004;25(2):98–104. doi: 10.1016/j.it.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Naranbhai V, Nathan A, Kaseke C, et al. T cell reactivity to the SARS-CoV-2 Omicron variant is preserved in most but not all prior infected and vaccinated individuals. medRxiv 2022: 2022.01.04.21268586.
- 11.Arbel R., Hammerman A., Sergienko R., et al. BNT162b2 vaccine booster and mortality due to covid-19. N Engl J Med. 2021;385(26):2413–2420. doi: 10.1056/NEJMoa2115624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bar-On Y.M., Goldberg Y., Mandel M., et al. Protection against covid-19 by BNT162b2 booster across age groups. N Engl J Med. 2021;385(26):2421–2430. doi: 10.1056/NEJMoa2115926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrews N., Stowe J., Kirsebom F., et al. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med. 2022 doi: 10.1038/s41591-022-01699-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moulia D.L., Wallace M., Roper L.E., et al. Interim recommendations for use of bivalent mRNA COVID-19 vaccines for persons aged ≥6 months - United States, April 2023. MMWR Morb Mortal Wkly Rep. 2023;72(24):657–662. doi: 10.15585/mmwr.mm7224a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US Food and Drug Administration. COVID-19 Vaccines for 2023-2024. 2023. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines-2023-2024 (accessed 15 October 2023).
- 16.Center JHCR. COVID-19 Dashboard. 2022. https://coronavirus.jhu.edu/map.html (accessed 5 May 2022.
- 17.Pilz S., Theiler-Schwetz V., Trummer C., Krause R., Ioannidis J.P.A. SARS-CoV-2 reinfections: overview of efficacy and duration of natural and hybrid immunity. Environ Res. 2022;209 doi: 10.1016/j.envres.2022.112911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeffery-Smith A., Rowland T.A.J., Patel M., et al. Reinfection with new variants of SARS-CoV-2 after natural infection: a prospective observational cohort in 13 care homes in England. Lancet Healthy Longev. 2021;2(12):e811–e819. doi: 10.1016/S2666-7568(21)00253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. SAGE updates COVID-19 vaccination guidance. 2023. https://www.who.int/news/item/28-03-2023-sage-updates-covid-19-vaccination-guidance (accessed 10 April 2023.
- 20.yona. asdf. asfd 1988.
- 21.Higdon MM, Wahl B, Jones CB, et al. A systematic review of COVID-19 vaccine efficacy and effectiveness against SARS-CoV-2 infection and disease. medRxiv 2022: 2021.09.17.21263549.
- 22.COVID-19 Cumulative Infection Collaborators. Estimating global, regional, and national daily and cumulative infections with SARS-CoV-2 through Nov 14, 2021: a statistical analysis. Lancet 2022. [DOI] [PMC free article] [PubMed]
- 23.McMenamin M.E., Bond H.S., Sullivan S.G., Cowling B.J. Estimation of relative vaccine effectiveness in influenza: a systematic review of methodology. Epidemiology. 2022;33(3) doi: 10.1097/EDE.0000000000001473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall V., Foulkes S., Insalata F., et al. Protection against SARS-CoV-2 after covid-19 vaccination and previous infection. N Engl J Med. 2022;386(13):1207–1220. doi: 10.1056/NEJMoa2118691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.International Vaccine Access Center. COVID-19 Data (Vaccine Studies). 2022. https://view-hub.org/covid-19/effectiveness-studies (accessed 31 March 2022.
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG, Group* P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151(4): 264-9. [DOI] [PubMed]
- 27.Fu R, Gartlehner G, Grant M, et al. AHRQ Methods for Effective Health Care Conducting Quantitative Synthesis When Comparing Medical Interventions: AHRQ and the Effective Health Care Program. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008. [PubMed]
- 28.Guo Y., Han J., Zhang Y., et al. SARS-CoV-2 omicron variant: epidemiological features, biological characteristics, and clinical significance. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.877101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrews N., Stowe J., Kirsebom F., et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022;386(16):1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patalon T., Saciuk Y., Peretz A., et al. Waning effectiveness of the third dose of the BNT162b2 mRNA COVID-19 vaccine. Nat Commun. 2022;13(1):3203. doi: 10.1038/s41467-022-30884-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bates T.A., McBride S.K., Leier H.C., et al. Vaccination before or after SARS-CoV-2 infection leads to robust humoral response and antibodies that effectively neutralize variants. Sci Immunol. 2022;7(68):eabn8014. doi: 10.1126/sciimmunol.abn8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis N.M., Chung J.R., Uyeki T.M., Grohskopf L., Ferdinands J.M., Patel M.M. Interpretation of relative efficacy and effectiveness for influenza vaccines. Clin Infect Dis. 2022;75(1):170–175. doi: 10.1093/cid/ciab1016. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. Statement – Update on COVID-19: Omicron wave threatening to overcome health workforce. 2022. https://www.euro.who.int/en/media-centre/sections/statements/2022/statement-update-on-covid-19-omicron-wave-threatening-to-overcome-health-workforce (accessed 5 May 2022 2022).
- 34.Chaguza C., Coppi A., Earnest R., et al. Rapid emergence of SARS-CoV-2 Omicron variant is associated with an infection advantage over Delta in vaccinated persons. Med (N Y) 2022 doi: 10.1016/j.medj.2022.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewnard J.A., Patel M.M., Jewell N.P., et al. Theoretical framework for retrospective studies of the effectiveness of SARS-CoV-2 vaccines. Epidemiology. 2021;32(4):508–517. doi: 10.1097/EDE.0000000000001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization. Evaluation of COVID-19 vaccine effectiveness, 2021.
- 37.Feikin D.R., Abu-Raddad L.J., Andrews N., et al. Assessing vaccine effectiveness against severe COVID-19 disease caused by omicron variant. Report from a meeting of the World Health Organization. Vaccine. 2022 doi: 10.1016/j.vaccine.2022.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lau C.S., Phua S.K., Liang Y.L., Oh M.L.H., Aw T.C. SARS-CoV-2 spike and neutralizing antibody kinetics 90 days after three doses of BNT162b2 mRNA COVID-19 vaccine in Singapore. Vaccines (Basel) 2022;10(2) doi: 10.3390/vaccines10020331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available in the paper