Abstract
Bannayan-Riley-Ruvalcaba syndrome (BRRS) is a congenital, autosomal-dominant disorder characterized by a triad of macrocephaly, lipomatosis, and pigmentation of the glans penis. The symptoms of this rare syndrome vary greatly and include multiple hamartomatous polyps, macrocephaly, increased birth weight, developmental delay, and intellectual disability. Vascular abnormalities, including arteriovenous malformations (AVMs), have rarely been reported as part of the vascular manifestations associated with BRRS. Congenital AVMs can rarely progress, resulting in limb- or life-threatening complications. We present the case of a young man with BRRS diagnosed in childhood and presenting with three AVMs involving the right upper extremity and chest. We also provide a brief literature summary of reported cases of BRRS with AVMs. Our paper highlights the importance of recognizing and understanding the vascular manifestations in patients with BRRS. Knowledge of the association between BRRS and AVMs is crucial for guiding patient diagnosis and management, optimizing treatment strategies, and improving overall patient outcomes.
Keywords: Arteriovenous malformation, Bannayan-Riley-Ruvalcaba syndrome, Vascular malformation
Case report
A 28-year-old White man presented with large bulging veins on his right arm, accompanied by throbbing, swelling, and discomfort that had been noted since childhood. Although the condition has worsened over time, it does not impact his daily activities or work. He was diagnosed with Bannayan-Riley-Ruvalcaba syndrome (BRRS) in childhood based on the presence of diffuse hamartomas, delayed speech, colonic polyps, macrocephaly, and a positive R130X mutation in the PTEN gene. Other than obesity, he had no other significant medical history. The patient has one healthy sister, no children, and no family history of BRRS. The patient provided written informed consent for the report of his case details and imaging studies.
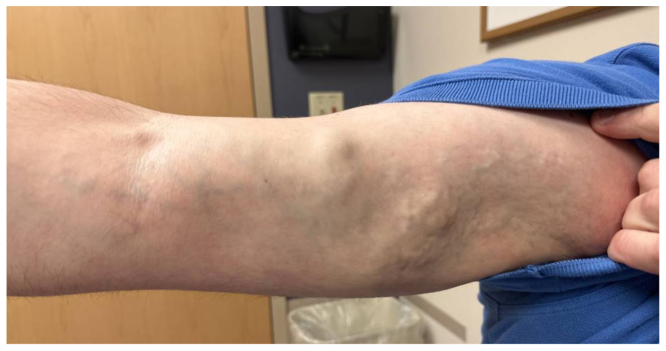
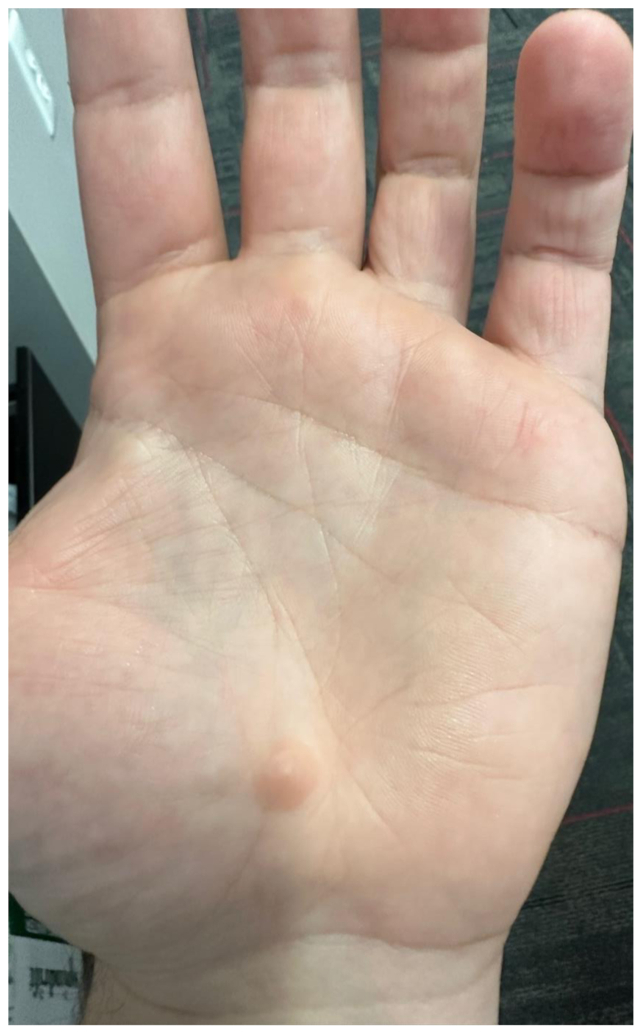
His vital signs included a blood pressure of 120/78 mm Hg, with a heart rate of 82 beats/min. The physical examination revealed large right arm varicosities, associated with bruising and swelling (Fig 1). Furthermore, the patient had scattered hamartomas, mostly involving the upper extremities (Fig 2). The blood workup revealed an unremarkable complete blood count and metabolic profile.
Fig 1.
Right upper extremity arteriovenous malformation (AVM).
Fig 2.
An example of hamartoma lesions on the patient's upper extremities, located on the left palm.
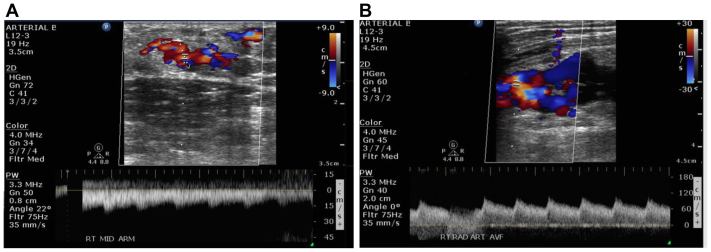
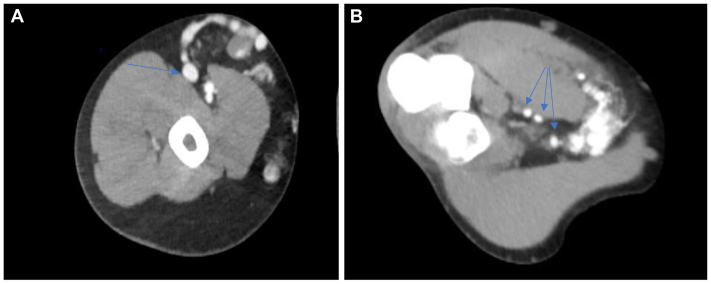
Right upper extremity arterial duplex ultrasound revealed evidence of an arteriovenous malformation (AVM) at the right arm measuring 2.2 × 1.0 cm with a connection between the brachial artery and vein through smaller branches. Another smaller AVM was found at the right proximal forearm, measuring 3.44 × 2.22 cm, with a connection between the proximal radial artery and vein (Fig 3). A computed tomography angiogram of the right upper extremity revealed early venous filling consistent with an AVM at the antecubital fossa level, which involves the distal brachial and proximal radial arteries. The right subclavian, axillary, brachial, radial, and ulnar arteries are otherwise patent (Fig 4). Computed tomography angiography of the chest, abdomen, and pelvis revealed a serpiginous vessel at the left breast and axillary region, measuring >2.5 cm.
Fig 3.
Transverse ultrasound image of the right arm (A) and forearm (B) showing arteriovenous malformations (AVMs) measuring 2.2 × 1.0 cm and 3.44 × 2.22 cm, respectively. Both sampled brachial (A) and radial (B) arteries had low resistive flow consistent with an AVM.
Fig 4.
Transverse computed tomography angiogram of the right arm (A) and forearm (B) showing arteriovenous malformations (AVMs) measuring 2.2 × 1.0 cm and 3.44 × 2.22 cm, respectively. Arrows indicate brachial (A) and radial (B) arteries (at different planes) feeding into the AVMs.
The patient was managed conservatively with intermittent right upper extremity compression and elevation. Furthermore, we plan annual surveillance office visits with repeat imaging studies to follow-up on the current AVM and rule out other new evolving vascular malformations.
Discussion
BRRS is a rare autosomal-dominant overgrowth condition that was first described by Riley and Smith1 in 1960. The prevalence of BRRS is currently unknown; however, multiple cases have been documented in the medical literature.2
Although specific criteria for a BRRS diagnosis have not been determined, the syndrome is usually diagnosed based on the clinical presentation.3 It is characterized by a triad of macrocephaly, genital lentiginosis, and lipomatosis.4 Other manifestations include multiple lipomas, hemangiomas, proximal myopathy, joint hyperextensibility, and scoliosis.5 Because hemangiomas are usually asymptomatic with no serious sequela, they do not require specific screening. They are usually discovered via clinical examinations or imaging studies. Infants with BRRS often have a higher weight at birth, >4000 g, although the adult height remains within the normal range.6,7 Patients usually have a head circumference greater than the 97th percentile.8 Approximately one half of the patients experience developmental delays, and mental retardation occurs in 20% to 50% of cases.6 Our patient meets the criteria for the diagnosis of BRRS based on the history of macrocephaly, speech delay, hamartomas, genetic test results, and concurrent AVMs.
As an autosomal dominant disorder, BRRS is a subset of the PTEN hamartoma tumor syndrome, marked by alterations in the phosphatase and tensin homolog (PTEN) gene.3 Although BRRS has multiple causes, ∼60% of cases arise from a mutation in the germline PTEN gene that encodes PTEN.9,10 Our patient falls into this category, as evidenced by the genetic test results, which unveiled an R130X mutation within the PTEN gene. In addition, 10% of BRRS patients without PTEN mutations harbor gene mutations and deletions in the PTEN promoter.11
The pathogenesis of the AVMs in BRRS remains unknown. Although many researchers have argued that AVMs are congenital and not neoplastic, this does not rule out the possibility of a noncongenital process that has undergone development over many years.12,13 The literature has added AVMs as a part of the spectrum of vascular pathology in BRRS.12,14
There is, however, ample evidence of the association between AVMs and mutations in PTEN. Déléris et al15 demonstrated that PTEN's involvement in the normal function of the PI3K–PKB/Akt–PTEN pathway is required for vascular development. PTEN is expressed in vascular smooth muscle cells and induces p53 activity to suppress tumor-induced angiogenesis.15,16 Therefore, mutations in the PTEN leading to the loss of its mediating effect on the pathway result in abnormal angiogenesis, which might, in turn, explain the incidence of AVMs.17
A small group of patients with BRRS exhibit vascular malformations, including arteriovenous shunts, anomalies, and fistulas. Vascular malformations are present in ≤10% of BRRS patients.18 These malformations can range from localized overgrowth of small vessels to the development of large aneurysms.14 As a form of vascular malformations, AVMs are an even rarer but recognized feature of BRRS. They are “high flow” lesions with the presence of arteries and shunting. Venous-only malformations are “low flow,” consisting of dilated veins without any arterial components.19 Managing AVMs poses significantly greater challenges compared with managing low-flow venous malformations or other vascular tumors.14 Surgical treatment of AVMs can be very challenging, because the lesions are generally less anatomically accessible, which can lead to significant bleeding complications. Furthermore, the recurrence rates are high.14,20 Therefore, endovascular treatment with embolic and sclerosing agents can be considered if intervention is indicated for AVM management.20
In 2001, Naidich et al12 first documented the association between AVM and BRRS, reporting the case of a 53-year-old man diagnosed with BRRS, who presented with truncal and upper extremity vascular malformations. Intracranial AVMs have also been associated with BRRS.17,21 AVMs can exhibit rapid angiogenesis that results in vascular remodeling, because they increase in size proportional to the growth of the patient.22 To the best of our knowledge, only 11 studies have reported 26 BRRS cases with AVMs (Table). Most reported cases of AVMs are located in the extremities. Surgical intervention was the most frequent approach for the cases with documented treatment, which might be due to a larger size (≤25 cm) and concurrent chronic symptoms such as pain and swelling.24 After intervention, most patients experienced an improvement in their symptoms.
Table.
Reported cases with Bannayan-Riley-Ruvalcaba syndrome (BRRS) and arteriovenous malformation (AVM)
| Pt. No. | Age, years/sex | Investigator | AVM Location | Management | Outcome |
|---|---|---|---|---|---|
| 1 | 9/Male | Srinivasa et al17 | Right posterior dural | Embolization of AVM on four separate occasions | Reduced periorbital venous congestion and resolved headaches |
| 2 | 53/Male | Naidich et al12 | Trunk and forearm | Excision of lesion | NR |
| 3 | 59/Male | Rujiwetpongstorn et al23 | Duodenum | Band ligation of duodenal AVM | NR |
| 4 | 4/Female | Kurek et al24 | Left thigh and calf | Excision | NR |
| 5 | 5/Female | Kurek et al24 | Right knee | Excision | NR |
| 6 | 5/Female | Kurek et al24 | Left thigh | NR | NR |
| 7 | 7/Male | Kurek et al24 | Left leg | Excision | NR |
| 8 | 8/Male | Kurek et al24 | Right pelvis, thigh, and calf | Debulking | NR |
| 9 | 8/Male | Kurek et al24 | Left foot | Excision | NR |
| 10 | 8/Male | Kurek et al24 | Left hand | NR | NR |
| 11 | 9/Male | Kurek et al24 | Entire right lower extremity and right pelvis | Amputation of right lower extremity | NR |
| 12 | 10/Female | Kurek et al24 | Right leg | Excision | NR |
| 13 | 11/Female | Kurek et al24 | Left thigh and calf | Excision | NR |
| 14 | 11/Female | Kurek et al24 | Left chest wall, left periclavicular region, and right forearm | Excision | NR |
| 15 | 16/Male | Kurek et al24 | Right thoracic paraspinal | Excision | NR |
| 16 | 17/Female | Kurek et al24 | Right knee | Excision | NR |
| 17 | 18/Female | Kurek et al24 | Right face and left knee | Excision | NR |
| 18 | 14/Male | Kurek et al24 | Right flank | Excision | NR |
| 19 | 19/Male | Kurek et al24 | Left face, neck, and left elbow | Excision | NR |
| 20 | 1/Female | Busa et al25 | Face | Conservative speech therapy | Received support for speech delay |
| 21 | NR | Palencia et al21 | Intracranial AVM | NR | NR |
| 22 | 24/Male | Anusic et al26 | Left forearm and right calf | Multiple transarterial embolization in right calf; contracture in calf treated with physiotherapy; excision of lesion in left forearm | Improved pain, worsened contracture, underwent genetic counseling |
| 23 | 14/Female | Moon et al27 | Dural arteriovenous fistula | Two stages of embolization | Improved symptoms |
| 24 | 8/Female | Soysal et al28 | Through the right buttock to the lower limbs | Amputation of right leg | NR |
| 25 | 11/Male | Erkek et al29 | Right lateral calf and ankle | Imaging and excisional biopsy | Discharged |
| 26 | 6/Male | Iacobas et al30 | Left forearm, hand, and left parietal lobe | Embolization and oral rapamycin | Left upper extremity regained full strength and mobility |
NR, not reported; Pt. No., patient number.
Recent literature has sought to expand our knowledge of the spectrum of vascular pathology in patients with BRRS by incorporating AVMs into the existing spectrum. Awareness of the vascular manifestations associated with BRRS has important implications for patient care. Healthcare providers caring for BRRS patients should consider and use appropriate imaging technologies to monitor their patients' vascular health. No guidelines have been established for the best approach to manage patients with AVMs. Based on the current evidence, treatment decisions should consider the severity of symptoms and their impact on patients' quality of life against the potential risks of interventional complications.31 Surgical and nonsurgical interventions have been associated with higher morbidity and should be reserved for severely symptomatic or disabled patients with vascular malformations.32 Because our patient remained asymptomatic, conservative treatment with annual follow-up visits and imaging surveillance was offered based on the current literature.33 If AVMs progress to become symptomatic and disabling, endovascular embolization or surgical resection could be considered. Until further research is reported, we suggest conservative therapy, including compression and elevation, for asymptomatic patients with AVMs, reserving endovascular or surgical intervention for patients with severe symptoms affecting their daily living activities.
Disclosures
None.
Footnotes
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Riley H.D., Smith W.R. Macrocephaly, pseudopapilledema and multiple hemangiomata : a previously undescribed heredofamilial syndrome. Pediatrics. 1960;26:293–300. [Google Scholar]
- 2.Lee S.H., Ryoo E., Tchah H. Bannayan-riley-ruvalcaba syndrome in a patient with a pten mutation identified by chromosomal microarray analysis: a case report. Pediatr Gastroenterol Hepatol Nutr. 2017;20:65–70. doi: 10.5223/pghn.2017.20.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yehia L., Eng C. In: Genereviews(®) Adam M.P., Feldman J., Mirzaa G.M., et al., editors. University of Washington, Seattle Copyright © 1993-2023, University of Washington, Seattle.; 1993. Pten hamartoma tumor syndrome. [PubMed] [Google Scholar]
- 4.Yehia L., Ni Y., Eng C. Germline ttn variants are enriched in pten-wildtype bannayan–riley–ruvalcaba syndrome. npj Genomic Medicine. 2017;2:37. doi: 10.1038/s41525-017-0039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gontijo G.M., Pinto C.A., Rogatto S.R., Cunha I.W., Aguiar S., Alves C.A. Bannayan-riley-ruvalcaba syndrome with deforming lipomatous hamartomas in infant--case report. An Bras Dermatol. 2013;88:982–985. doi: 10.1590/abd1806-4841.20132730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendriks Y.M.C., Verhallen J.T.C.M., van der Smagt J.J., et al. Bannayan–riley–ruvalcaba syndrome: further delineation of the phenotype and management of pten mutation-positive cases. Fam Cancer. 2003;2:79–85. doi: 10.1023/a:1025713815924. [DOI] [PubMed] [Google Scholar]
- 7.Hobert J.A., Eng C. Pten hamartoma tumor syndrome: an overview. Genet Med. 2009;11:687–694. doi: 10.1097/GIM.0b013e3181ac9aea. [DOI] [PubMed] [Google Scholar]
- 8.Miles J.H., Zonana J., Mcfarlane J., Aleck K.A., Bawle E. Macrocephaly with hamartomas: bannayan-zonana syndrome. Am J Med Genet. 1984;19:225–234. doi: 10.1002/ajmg.1320190204. [DOI] [PubMed] [Google Scholar]
- 9.Marsh D.J., Kum J.B., Lunetta K.L., et al. Pten mutation spectrum and genotype-phenotype correlations in bannayan-riley-ruvalcaba syndrome suggest a single entity with cowden syndrome. Hum Mol Genet. 1999;8:1461–1472. doi: 10.1093/hmg/8.8.1461. [DOI] [PubMed] [Google Scholar]
- 10.Pollock P.M. In: Encyclopedia of cancer. 2nd ed. Bertino J.R., editor. Academic Press; 2002. Melanoma: cellular and molecular abnormalities; pp. 141–152. [Google Scholar]
- 11.Zhou X.P., Waite K.A., Pilarski R., et al. Germline pten promoter mutations and deletions in cowden/bannayan-riley-ruvalcaba syndrome result in aberrant pten protein and dysregulation of the phosphoinositol-3-kinase/akt pathway. Am J Hum Genet. 2003;73:404–411. doi: 10.1086/377109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naidich J.J., Rofsky N.M., Rosen R., Karp N. Arteriovenous malformation in a patient with bannayan--zonana syndrome. Clin Imaging. 2001;25:130–132. doi: 10.1016/s0899-7071(01)00241-8. [DOI] [PubMed] [Google Scholar]
- 13.Turnbull M.M., Humeniuk V., Stein B., Suthers G.K. Arteriovenous malformations in cowden syndrome. J Med Genet. 2005;42:e50. doi: 10.1136/jmg.2004.030569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Litzendorf M., Hoang K., Vaccaro P. Recurrent and extensive vascular malformations in a patient with bannayan--riley--ruvalcaba syndrome. Ann Vasc Surg. 2011;25:1138.e15–1138.e19. doi: 10.1016/j.avsg.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 15.Déléris P., Bacqueville D., Gayral S., et al. Ship-2 and pten are expressed and active in vascular smooth muscle cell nuclei, but only ship-2 is associated with nuclear speckles. J Biol Chem. 2003;278:38884–38891. doi: 10.1074/jbc.M300816200. [DOI] [PubMed] [Google Scholar]
- 16.Su J.D., Mayo L.D., Donner D.B., Durden D.L. Pten and phosphatidylinositol 3'-kinase inhibitors up-regulate p53 and block tumor-induced angiogenesis: evidence for an effect on the tumor and endothelial compartment. Cancer Res. 2003;63:3585–3592. [PubMed] [Google Scholar]
- 17.Srinivasa R.N., Burrows P.E. Dural arteriovenous malformation in a child with bannayan-riley-ruvalcaba syndrome. AJNR Am J Neuroradiol. 2006;27:1927–1929. [PMC free article] [PubMed] [Google Scholar]
- 18.Garzon M.C., Huang J.T., Enjolras O., Frieden I.J. Vascular malformations. Part ii: associated syndromes. J Am Acad Dermatol. 2007;56:541–564. doi: 10.1016/j.jaad.2006.05.066. [DOI] [PubMed] [Google Scholar]
- 19.Zyck S., Davidson C.L., Sampath R. Disclosure: raghuram Sampath declares no relevant financial relationships with ineligible companies. StatPearls; 2023. Arteriovenous malformations. Statpearls. Treasure Island (FL) ineligible companies. Disclosure: caroline Davidson declares no relevant financial relationships with ineligible companies. [Google Scholar]
- 20.Kim R., Do Y.S., Park K.B. How to treat peripheral arteriovenous malformations. Korean J Radiol. 2021;22:568–576. doi: 10.3348/kjr.2020.0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palencia R., Ardura J. [bannayan syndrome with intracranial arteriovenous malformations] An Esp Pediatr. 1986;25:462–466. [PubMed] [Google Scholar]
- 22.Ernemann U., Kramer U., Miller S., et al. Current concepts in the classification, diagnosis and treatment of vascular anomalies. Eur J Radiol. 2010;75:2–11. doi: 10.1016/j.ejrad.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Rujiwetpongstorn R., Phowthongkum P., Panchaprateep R. Multiple lentigines in RASA1-associated capillary malformation-arteriovenous malformation syndrome. JAAD Case Rep. 2020;7:47–49. doi: 10.1016/j.jdcr.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurek K.C., Howard E., Tennant L.B., et al. Pten hamartoma of soft tissue: a distinctive lesion in pten syndromes. Am J Surg Pathol. 2012;36:671–687. doi: 10.1097/PAS.0b013e31824dd86c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Busa T., Milh M., Degardin N., et al. Clinical presentation of PTEN mutations in childhood in the absence of family history of Cowden syndrome. Eur J Paediatr Neurol. 2015;19:188–192. doi: 10.1016/j.ejpn.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 26.Anusic S., Clemens R.K., Meier T.O., Amann-Vesti B.R. Assessment of PTEN-associated vascular malformations in a patient with Bannayan-Riley-Ruvalcaba syndrome. BMJ Case Rep. 2016 doi: 10.1136/bcr-2016-215188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moon K., Ducruet A.F., Crowley R.W., Klas K., Bristol R., Albuquerque F.C. Complex dural arteriovenous fistula in Bannayan-Riley-Ruvalcaba syndrome. J Neurosurg PediatrJournal of neurosurgery. Pediatrics. 2013;12:87–92. doi: 10.3171/2013.3.PEDS12551. [DOI] [PubMed] [Google Scholar]
- 28.Soysal Y., Acun T., Lourenço C., Marques W., Jr., Yakıcıer M. Muscle hemangiomatosis presenting as a severe feature in a patient with the pten mutation: expanding the phenotype of vascular malformations in bannayan-riley-ruvalcaba syndrome. Balkan J Med Genet. 2012;15:45–50. doi: 10.2478/v10034-012-0007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erkek E., Hizel S., Sanlý C., et al. Clinical and histopathological findings in Bannayan-Riley-Ruvalcaba syndrome. J Am Acad Dermatol. 2005;53:639–643. doi: 10.1016/j.jaad.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 30.Iacobas I., Burrows P.E., Adams D.M., Sutton V.R., Hollier L.H., Chintagumpala M.M. Oral rapamycin in the treatment of patients with hamartoma syndromes and PTEN mutation. Pediatr Blood Cancer. 2011;57:321–323. doi: 10.1002/pbc.23098. [DOI] [PubMed] [Google Scholar]
- 31.Quintin S., Figg J.W., Mehkri Y., Hanna C.O., Woolridge M.G., Lucke-Wold B. Arteriovenous malformations: an update on models and therapeutic targets. J Neurosci Neurol Surg. 2023;13:250. [Google Scholar]
- 32.Lee B.B., Do Y.S., Yakes W., Kim D.I., Mattassi R., Hyon W.S. Management of arteriovenous malformations: a multidisciplinary approach. J Vasc Surg. 2004;39:590–600. doi: 10.1016/j.jvs.2003.10.048. [DOI] [PubMed] [Google Scholar]
- 33.Shen Y., Lixin S., Wang D., Fan X. Overview of peripheral arteriovenous malformations: from diagnosis to treatment methods. J Interv Med. 2023;6:170–175. doi: 10.1016/j.jimed.2023.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]