Abstract
Background.
This scoping review and analysis were designed to assess the amount of time spent delivering photobiomodulation (PBM) light therapy after dental extraction to improve postoperative pain and wound healing.
Types of Studies Reviewed.
The scoping review was performed according to the Cochrane Collaboration and Preferred Reporting Items for Systematic Reviews and Meta-Analyses criteria. Publications were specific for human randomized controlled clinical trials, PBM after dental extraction therapy, and related clinical outcomes. Online databases searched included PubMed, Embase, Scopus, and Web of Science. Analyses were conducted to analyze the prescribed intervals of time (seconds) per application of PBM.
Results.
Of the 632 studies initially identified, 22 studies fulfilled the inclusion criteria. Postoperative pain and PBM were reported in 20 articles for 24 treatment groups, with treatment times ranging from 17 through 900 seconds and wavelengths from 550 through 1,064 nm. Clinical wound healing outcomes were reported in 6 articles for 7 groups with treatment times ranging from 30 through 120 seconds and wavelengths from 660 through 808 nm. PBM therapy was not associated with adverse events.
Conclusions and Practical Implications.
There is future potential to integrate PBM after dental extraction therapy to improve postoperative pain and clinical wound healing. The amount of time spent delivering PBM will vary by wavelength and the type of device. Further investigation is needed to translate PBM therapy into human clinical care.
Keywords: Laser; laser therapy; low-level light therapy; tooth extraction; pain management, facial pain; oral surgery procedures; dentistry; photobiomodulation
Photobiomodulation (PBM) therapy is a nonthermal, nonionizing treatment of red and near-infrared light.1,2 PBM applications typically involve laser wavelengths from 600 through 1,000 nm. Incorporating PBM as an adjunctive therapy can promote wound healing, reduce inflammation, and provide analgesia. Dentistry research groups have documented PBM applications to improve dental postextraction pain and wound healing.3–6 The amount of time spent delivering PBM should be adjusted, as protocols vary by wavelength and between devices.7 PBM can be applied safely by optimizing laser settings (irradiation parameters) that are calibrated for the optical properties of alveolar bone and soft tissues.8
PBM’s photochemical effects transmit light through various tissue types and tissue optical properties.8–11 The PBM mechanisms are categorized as intracellular, cell membrane receptors, or extracellular components. Intracellular mechanisms involve the absorption of PBM wavelengths via cytochrome c oxidase, which is contained in the respiratory chain located within the mitochondria.12 It is hypothesized that PBM light energy is absorbed by cytochrome c oxidase,13 leading to enhancement of enzyme activity,14 electron transport,15 increasing mitochondrial respiration, and adenosine triphosphate production.12 By altering the cellular redox state, PBM can activate numerous intracellular signaling pathways, including transcription factors concerned with cell proliferation, survival, tissue repair, and healing.12,15 The cell membrane receptor mechanism involves PBM modulation of photosensitive ion transporters and cell receptors on the cell membrane, such as Opsins 2–4, TRPV1, AHR, and P2X7.16,17
The extracellular mechanisms can lead to expected therapeutic results1,2,18 that directly photoactivate latent transforming growth factor β1 (TGF-β1) via a redox-mediated physiochemical process.19–24 TGF- β1 is a pluripotent family of cytokines,22,25 significantly involved as multifunctional growth factors for reepithelialization, inflammation, angiogenesis, and granulation tissue formation during wound healing.20,22,25 Specific PBM wavelengths predictably upregulate signaling of TGF- β1,19–21,23,24 thus affecting keratinocyte function and migration, which is essential to wound reepithelialization.25 There is evidence to support that TGF- β1 will appear immediately after PBM treatment, which indicates activity from degranulating platelets in the serum of freshly wounded tissues.19,23
PBM applications in dentistry are guided by randomized controlled trials, systematic reviews, and recommendations from international associations.26–30 These bodies of evidence can help the clinician determine the amount of PBM necessary to safely achieve a desired effect. The purpose of our scoping review was to examine emerging evidence for PBM, tooth extraction, pain discomfort, and wound healing. Scoping reviews can identify, assess, and map the available evidence, inform of different types of practice in a specific field, and report on ways that research has been conducted.31–34 The aim of our scoping review was to summarize time (seconds) and irradiation settings prescribed for PBM after dental extraction therapy to improve postoperative pain and wound healing. These findings will inform clinicians about available evidence and drive future research without guiding clinical decision making.
METHODS
We conducted a scoping review and irradiation parameter analyses according to the Preferred Reporting Items for Systematic Review and Meta-Analyses guidelines.35 The Population, Intervention, Comparison, Outcomes question formulated was as follows: In patients receiving dental extraction therapy (P), does the amount of time prescribed for PBM therapy (I), compared with placebo PBM therapy(C), differ by wavelength for postoperative pain and wound healing (O)? We conducted a detailed review of the literature from January 1, 1970, through June 1, 2022, in the following databases: National Library of Medicine PubMed (MEDLINE), Elsevier Embase, Scopus, and Web of Science. The comprehensive search strategy is available via PROSPERO (registration CRD42022341395). We used the Cochrane Risk of Bias tool for randomized controlled trials (Cochrane RoB 2.0) to assess bias.
The inclusion criteria were
human prospective randomized controlled trials comparing PBM therapy and a placebo as an adjunct treatment after dental extraction surgery
studies that reported PBM protocols for clinical outcomes of pain and clinical wound healing
test groups using a single PBM system at the same laser wavelength throughout the entire treatment
studies reporting the following irradiation parameters: wavelength (nm), treatment time (seconds), amount of contact points per visit, and the total amount of visits
studies reporting adverse events, safety, and efficacy
statistical analysis
The exclusion criteria were
nonhuman studies
cohort, case-control, case series, expert opinion, and review studies
inadequate site standardization
use of multiple laser systems at different wavelengths or the same PBM system at varying wavelengths
no placebo or control group
non-English language
Analysis and data synthesis
We created an electronic data conversion form (Microsoft Excel) to include author, year, wavelength (nm), laser power (W), beam area spot size (cm2), treatment time per point (seconds), irradiance or power (W/cm2), energy dose (J), and fluence (J/cm2). Notations were made if data were misreported or corrected and if values were not reported and were added via synthesis (Table 1). We calculated fluence (J/cm2) as (power × time)/area spot size, energy (J) as power × time, power density (W/cm2) as power/area spot size, and spot size (cm2) as π(radius 1 × radius 2), noting that many laser diode beams are elliptical and not round. As applicable, we generated and plotted the mean, median, mode, and upper and lower quartile ranges. We applied wavelength (nm)-specific analyses of irradiation parameters, when possible, to avoid bias caused by the difference in effect.
Table 1.
Summary of characteristics in studies reporting on pain
| AUTHOR | YEAR | WAVELENGTH, NM | LASER TYPE | LASER POWER, W | BEAM AREA AND SPOT SIZE, cm2 | TREATMENT TIME PER POINT, S | ENERGY DOSE PER POINT, J | FLUENCE, J/cm2 | CLINICAL SITE | CONTACT POINTS, NO. | APPOINTMENT INTERVALS, NO. | APPOINTMENT DAYS | SURGICAL SITE | PAIN SCALE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fernando and Colleagues 36 | 1993 | 830 | –* | 0.03 | – | 120 | 4 | – | Intraoral, contact | 1 | 1 | 1 | Mandibular third molar | Giles |
| Mozzati and Colleagues 37 | 2011 | 904 | Gallium arsenide | 0.2† | 1 | 900† | 180 | 180 | Intraoral, contact | 1 | 1 | 4 | Maxillary and mandibular molar | VAS‡ |
| Lopez and Colleagues 1 | 2012 | 810 | Gallium aluminum arsenide | 0.4 | 3.2§ | 32 | 12.8 | 4 | Intraoral, contact | 1 | 1 | 1 | Mandibular third molar | VAS |
| Mozzati and Colleagues 38 | 2012 | 904 | Gallium arsenide | 0.2† | 1 | 900† | 180 | 18‡‡ | Intraoral, contact | 1 | 1 | 3 | Maxillary and mandibular molar | VAS |
| Paschoal and Santos-Pinto 39 | 2012 | 830 | GaAlAs¶ | 0.1 | 0.01† | 17 | 1.7 | 170† | Intraoral, noncontact | 3 | 3 | 1–3 | Maxillary and mandibular premolar | VAS |
| Ferrante and Colleagues 40 | 2013 | 980 | – | 0.3 | – | 60 | 18‡‡ | – | Intraoral, noncontact; extraoral, contact | 3 | 2 | 1, 2 | Mandibular third molar | VAS |
| Alan and Colleagues 41 | 2016 | 810 | GaAlAs | 0.3 | – | 40 | 12 | – | Extraoral, contact | 1 | 2 | 1, 2 | Mandibular third molar | VAS |
| Pedreira and Colleagues 42 | 2016 | 808 | Aluminum gallium arsenide | – | – | 30§ | – | 2† | Extraoral, contact; intraoral, contact | 12 | 4 | 1, 3, 5, 7 | Mandibular third molar | Pain analog |
| Sierra and Colleagues 43 | 2016 | 660 | – | 0.1† | 0.028§ | 30 | 3 | 106 | Intraoral, contact | 4 | 1 | 1 | Mandibular third molar | VAS |
| Sierra and Colleagues 43 | 2016 | 660 | – | 0.1† | 0.028§ | 30 | 3 | 106 | Extraoral, contact | 4 | 1 | 1 | Mandibular third molar | VAS |
| Sierra and Colleagues 43 | 2016 | 808 | – | 0.1† | 0.028§ | 30 | 3 | 106 | Intraoral, contact | 4 | 1 | 1 | Mandibular third molar | VAS |
| Sierra and Colleagues 43 | 2016 | 808 | – | 0.1† | 0.028§ | 30 | 3 | 106 | Extraoral, contact | 4 | 1 | 1 | Mandibular third molar | VAS |
| Farhadi and Colleagues 44 | 2017 | 550 | – | 0.1† | 0.5 | 25 | 2.5 | 5 | Intraoral, contact | 1 | 1 | 1 | Mandibular third molar | VAS |
| Feslihan and Eroglu 45 | 2019 | 810 | GaAlAs | 0.3 | 3 | 60 | 18§ | 6 | Extraoral, contact | 1 | 2 | 1, 2 | Mandibular third molar | VAS |
| Ahrari and Colleagues 46 | 2020 | 660 | Aluminum gallium indium phosphide | 0.02† | – | 30 | 6 | – | Extraoral, noncontact | 3 | 2 | 1, 3 | Mandibular molar | VAS |
| Ahrari and Colleagues 46 | 2020 | 810 | GaAlAs | 0.02† | 0.28 | 30 | 6 | 2.14† | Extraoral, noncontact | 3 | 2 | 1, 3 | Mandibular molar | VAS |
| Salem 4 | 2020 | 660 | – | 0.025† | – | 60 | – | – | Intraoral, noncontact | 1 | 1 | 1 | Mandibular third molar | Universal Pain Assessment Tool |
| Santos and Colleagues 47 | 2020 | 780 | – | 0.07† | 0.04 | 30 | 2.1§ | 52.5 | Intraoral, contact | 5 | 1 | 1 | Mandibular third molar | Modified VAS |
| Da Silva and Colleagues 48 | 2021 | 808 | – | 0.04 | 0.28 | 70 | 2.8 | 100 | Intraoral, contact | 5 | 4 | 0, 7, 14, 21 | Maxilla and mandibula | VAS |
| Isolan and Colleagues 6 | 2021 | 808 | GaAlAs | 0.05† | 0.4 | 73† | 11 | 9.125† | Intraoral, contact | 6 | 1 | 1 | Maxillary and mandibular third molar | VAS |
| Momeni and Colleagues 5 | 2021 | 940 | – | 0.5 | 1.5§ | 30 | 15 | 10 | Intraoral, noncontact | 3 | 1 | 2 | Mandibular third molar | VAS |
| Scarano and Colleagues 49 | 2021 | 1064 | Neodymium-doped yttrium aluminum garnet. | 1 | – | 17.1† | – | – | Intraoral, noncontact | 7 | 5 | 1, 2, 4, 9, 14 | Mandibular third molar | VAS |
| Hadad and Colleagues 50 | 2022 | 810 | GaAlAs | 0.1 | 0.0283§ | 60 | 6 | 212 | Intraoral, contact | 4 | 1 | 1 | Mandibular third molar | VAS |
| Le and Colleagues 51 | 2022 | 810 | GaAlAs | 0.43§ | 3.2§ | 30 | 12.8 | 4 | Intraoral, noncontact | 1 | 2 | 1, 2 | Mandibular third molar | Likert |
A value that cannot be derived from other reported values.
Indicates value that was initially misreported and corrected by the authors’ calculations.
VAS: Visual analog scale.
Indicates value was not reported in the literature and was calculated by the authors with values given in the article.
GaAlAs: Gallium aluminum arsenide.
RESULTS
The literature search is detailed in Figure 1. The search strategy resulted in a total of 632 articles. We identified 67 articles after reviewing titles and abstracts for full-text assessment. We excluded 45 articles for not fulfilling the inclusion and exclusion criteria (Figure 1). We included 22 articles1,3–6,36–52 for the review and analysis (Tables 1 and 2). Postoperative pain and PBM were reported in 20 articles with 24 treatment groups (Table 1). Red visible wavelength devices (550–660 nm) were used with 4 articles and 5 treatment groups. PBM treatment times ranged from 30 through 60 seconds per application point, depending on the protocol (Figure 2A). Near-infrared devices (780–1,064 nm) were reported in 18 articles and 19 treatment groups, with treatment times ranging from 17 through 900 seconds per application point (Figure 2B). Some articles had multiple treatment arms testing both red visible and near-infrared wavelengths.
Figure 1.
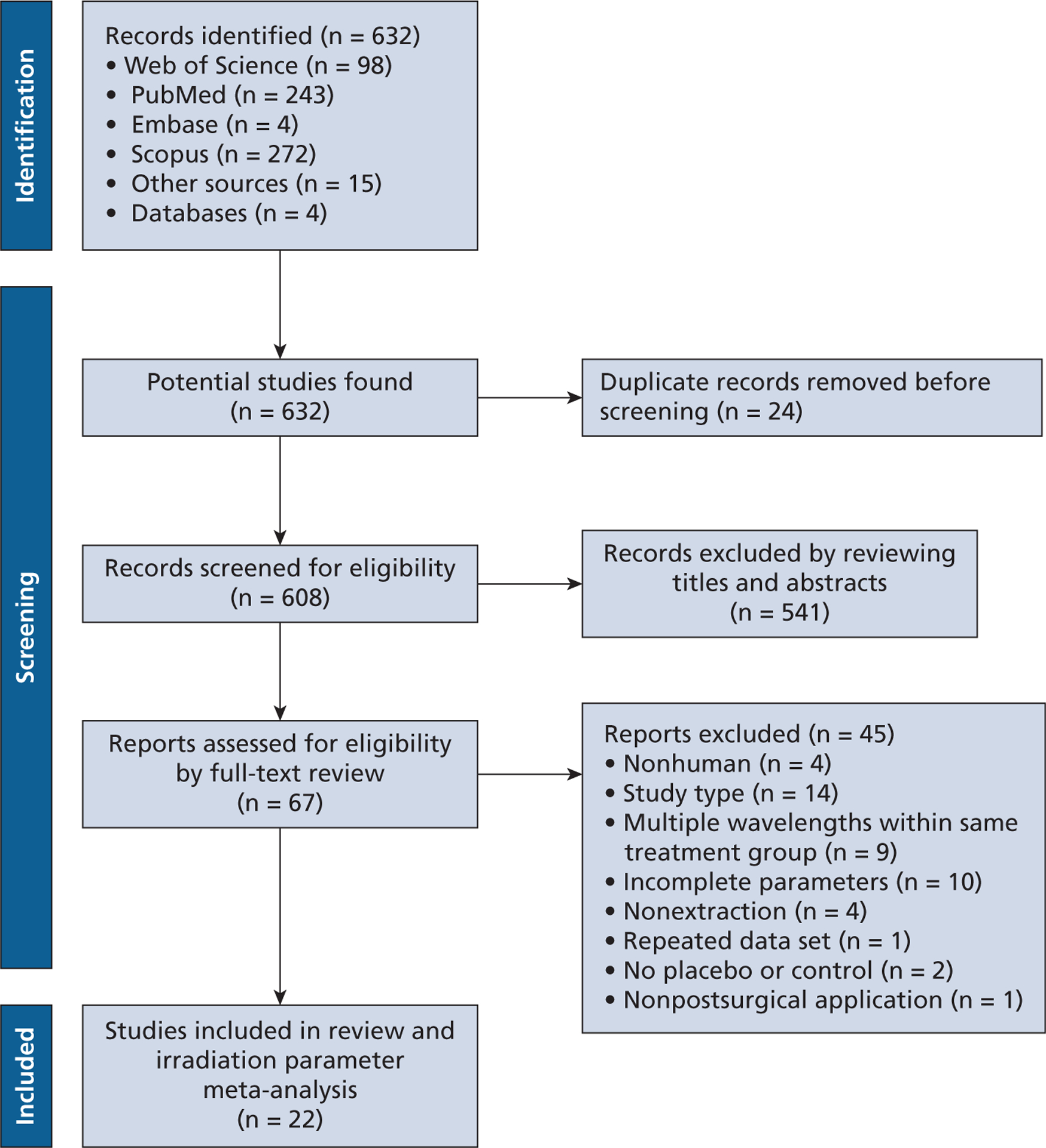
Preferred Reporting Items for Systematic Reviews and Meta-analyses flowchart of study search and selection process.35
Table 2.
Summary of characteristics in studies reporting on wound healing
| AUTHOR | YEAR | WAVELENGTH, NM | LASER TYPE | LASER POWER, W | BEAM AREA AND SPOT SIZE, cm2 | TREATMENT TIME PER POINT, S | ENERGY DOSE PER POINT, J | FLUENCE, J/cm2 | CLINICAL SITE | CONTACT POINTS, NO. | APPOINTMENT INTERVALS, NO. | APPOINTMENT DAYS | WOUND HEALING | SURGICAL SITE | DAYS, WOUND HEALING SCALE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fernando and Colleagues 36 | 1993 | 830 | –* | 0.03 | – | 120 | 4 | – | Intraoral, contact | 1 | 1 | 1 | Soft tissue | Mandibular third molar | 0, 7; healing scale |
| Romao and Colleagues 3 | 2015 | 808 | GaAlAs† | 0.1‡ | 0.04 | 30 | 3 | 75 | Intraoral, contact | 5 | 5 | 1, 3, 5, 7, 14 | Bone tissue | Mandibular molar | 40; micro–computed tomography, histology |
| Ahrari and Colleagues 46 | 2020 | 810 | Aluminum gallium indium phosphide | 0.02‡ | 0.28 | 30 | 6 | 2.14‡ | Extraoral, noncontact | 3 | 2 | 1, 3 | Soft tissue | Mandibular molar | 3, 7; degree of healing |
| Ahrari and Colleagues 35 | 2020 | 660 | GaAlAs | 0.02‡ | – | 30 | 6 | – | Extraoral, noncontact | 3 | 2 | 1, 3 | Soft tissue | Mandibular molar | Photos on 3, 7; degree of healing |
| Rosero and Colleagues 52 | 2020 | 808 | GaAlAs | 0.1 | 0.028 | 25 | 2.5 | 89 | Intraoral, contact | 5 | 5 | 1, 3, 5, 7, 14 | Bone tissue | Mandibular molar | 45; micro–computed tomography, histology |
| Salem 4 | 2020 | 660 | – | 0.025‡ | – | 60 | – | – | Intraoral, noncontact | 1 | 1 | 1 | Soft tissue | Mandibular third molar | 1, 2, 7; Laundry and Turnbull |
| Da Silva and Colleagues 48 | 2021 | 808 | – | 0.04 | 0.28 | 70 | 2.8 | 100 | Intraoral, contact | 5 | 4 | 0, 7, 14, 21 | Soft tissue | Maxilla and mandible | 7, 14, 18, 21; alveolar mucosa healing |
A value that cannot be derived from other reported values.
GaAlAs: Gallium aluminum arsenide.
Indicates value that was initially misreported and corrected by the authors’ calculations.
Figure 2.
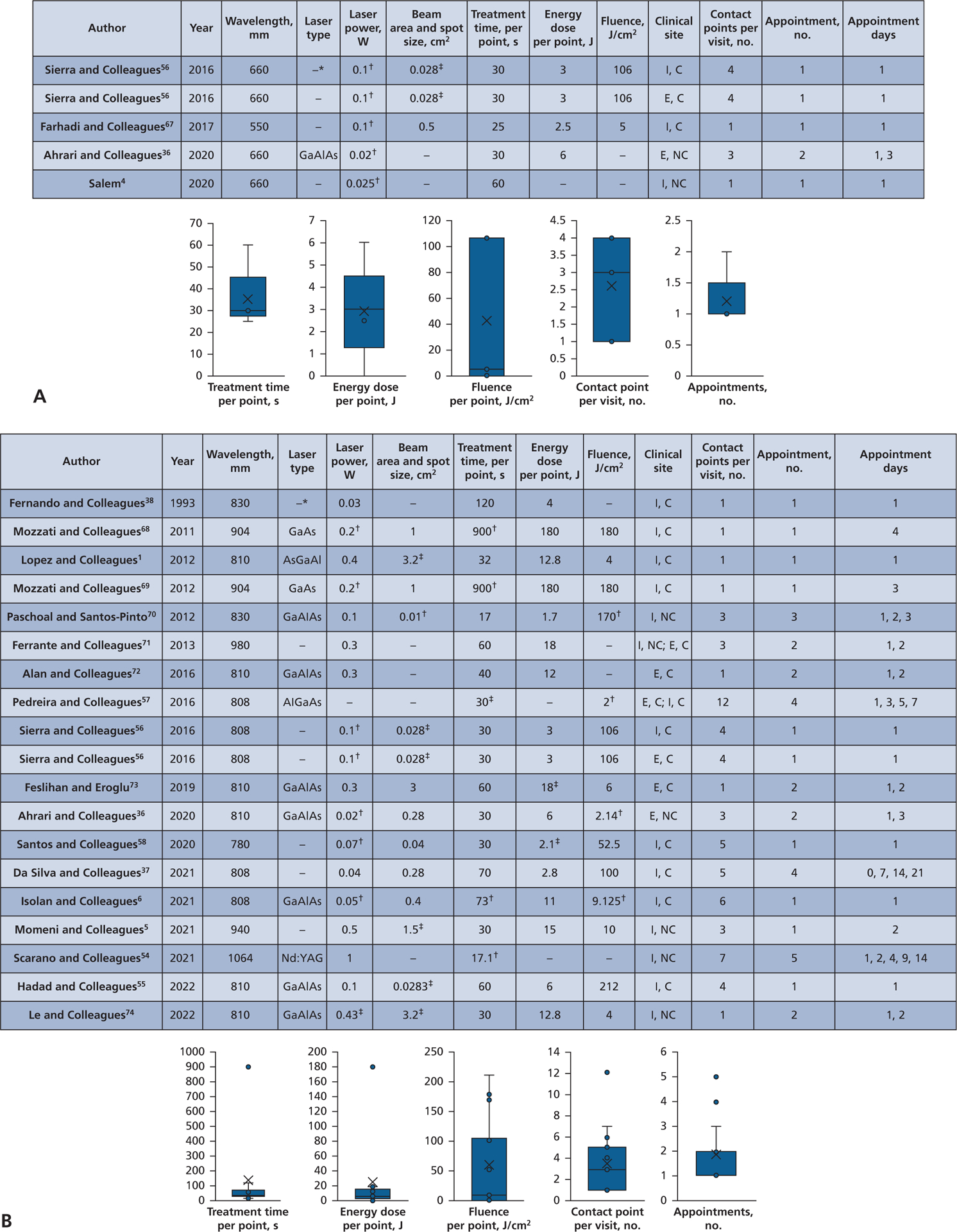
Pain, analyses for time and irradiation parameters: 24 treatment groups reported from 20 studies. A. Red visible light wavelengths, 550 through 660 nm. B. Near-infrared wavelengths, 780 through 1,064 nm. AlGaAs: Aluminum gallium arsenide. AsGaAl: Arsenide gallium aluminium. C: Contact. E: Extraoral. GaAlAs: Gallium aluminum arsenide. GaAs: Gallium arsenide. I: Intraoral. NC: Noncontact. Nd:YAG: Neodymium-doped yttrium aluminum garnet. * –: A value that cannot be derived from other reported values. † Indicates value that was initially misreported and corrected by the authors’ calculations. ‡ Indicates value was not reported in the literature and was calculated by the authors with values given in the article.
Clinical wound healing outcomes were reported in 6 articles and 7 treatment groups (Table 2). Of the 6 wound healing articles, 4 reported for PBM and soft-tissue wound healing,4,36,46,48 and 2 reported for PBM and bone-tissue wound healing3,52 (Table 2). Soft-tissue wound healing was reported for both red visible and near-infrared devices. Red visible wavelength devices (660 nm) were used with 2 articles and 2 treatment groups, with PBM delivery times ranging from 30 through 60 seconds (Figure 3A). Near-infrared wavelength devices (808–830 nm) were reported in 3 articles and 3 treatment groups with PBM delivery times ranging from 70 through 120 seconds per application point (Figure 3B). Bone-tissue wound healing was reported for near-infrared devices (808 nm) in 2 articles and 2 treatment groups, with PBM delivery times ranging from 25 through 30 seconds per application (Figure 3C).
Figure 3.
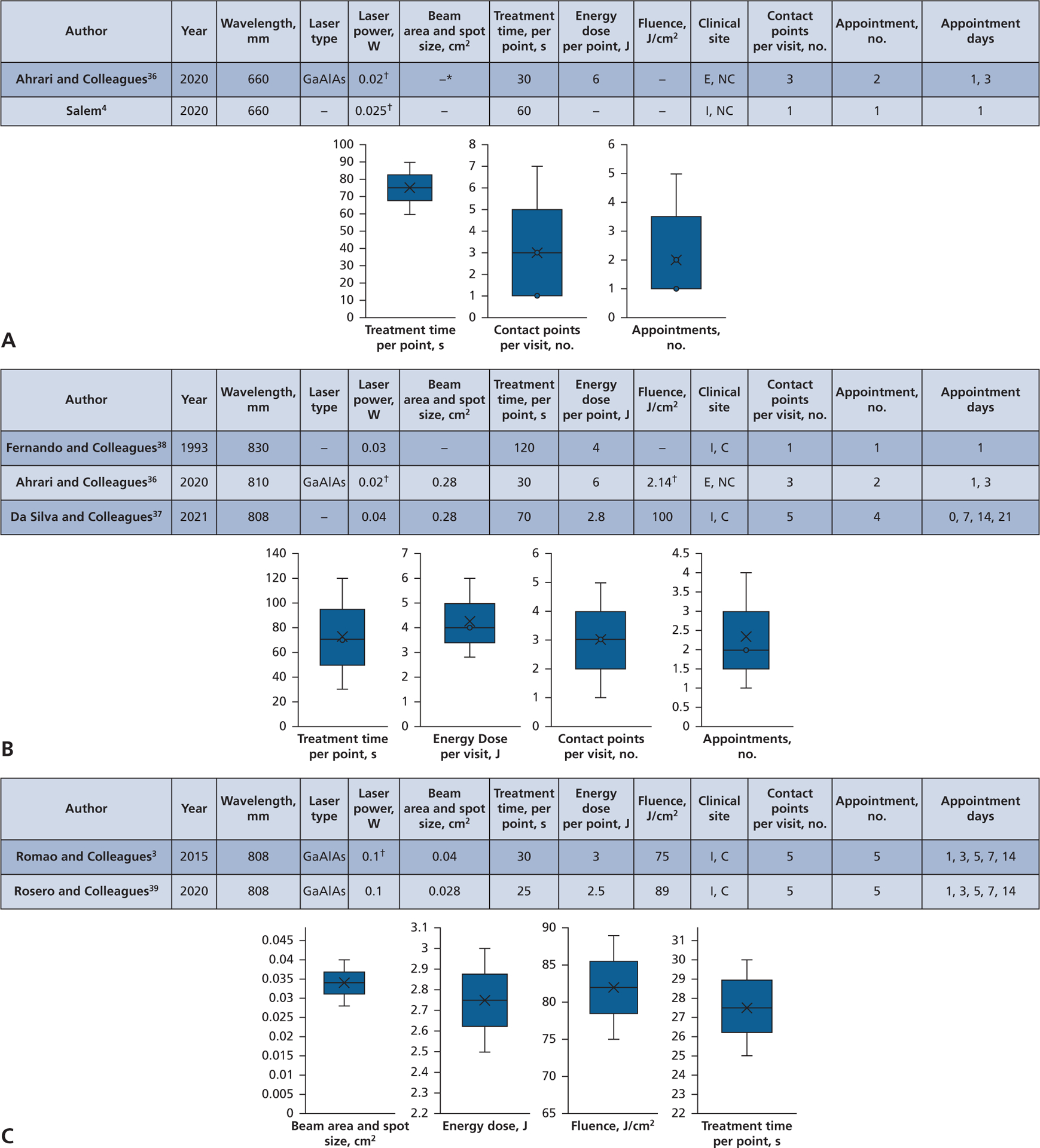
Wound healing, analyses for time and irradiation parameters. A. Red visible light wavelengths, soft tissue, 660 nm. B. Near-infrared wavelengths, soft tissue, 808 through 830 nm. C. Near-infrared wavelengths, bone tissue, 808 nm. C: Contact. E: Extraoral. GaAlAs: Gallium aluminum arsenide. I: Intraoral. NC: Noncontact. * –: A value that cannot be derived from other reported values. † Indicates value that was initially misreported and corrected by the authors’ calculations.
Risk of bias assessment
We summarized the risk of bias in each study according to the Revised Cochrane Risk of Bias 2.0 classification from 2019 (Table 3).53 We considered 16 studies as having a low risk of bias, 4 as having a moderate risk of bias, and 2 as having a high risk of bias (Table 3).
Table 3.
Risk of bias assessment of the included studies.
| AUTHOR | YEAR | RANDOMIZATION PROCESS | DEVIATIONS FROM THE INTENDED INTERVENTION | MISSING OUTCOME DATA | MEASUREMENT OF THE OUTCOME | SELECTION OF THE REPORTED RESULTS | OVERALL RISE OF BIAS |
|---|---|---|---|---|---|---|---|
| Fernando and Colleagues 36 | 1993 | Low | Low | Low | Low | Low | Low |
| Mozzati and Colleagues 37 | 2011 | Low | Low | Low | Low | Low | Low |
| Lopez and Colleagues 1 | 2012 | Low | Low | Low | Low | Low | Low |
| Mozzati and Colleagues 38 | 2012 | Low | Low | Low | Low | Low | Low |
| Paschoal and Santos-Pinto 39 | 2012 | Low | Low | Some concern | Low | Low | Some concern |
| Ferrante and Colleagues 40 | 2013 | Low | Low | Low | Low | Low | Low |
| Romao and Colleagues 3 | 2015 | Low | Low | Low | Low | Low | Low |
| Alan and Colleagues 41 | 2016 | Low | Low | Low | Low | High | High |
| Pedreira and Colleagues 42 | 2016 | Low | Low | Low | Low | Low | Low |
| Sierra and Colleagues 43 | 2016 | Low | Low | Low | Low | Low | Low |
| Farhadi and Colleagues 44 | 2017 | Low | Low | Low | Low | Low | Low |
| Feslihan and Eroglu 45 | 2019 | Low | Low | Low | Low | Low | Low |
| Ahrari and Colleagues 46 | 2020 | Some concern | Low | Low | Low | High | High |
| Rosero and Colleagues 52 | 2020 | Low | Low | Low | Low | Low | Low |
| Salem 4 | 2020 | Low | Low | Low | Low | Low | Low |
| Santos and Colleagues 47 | 2020 | Low | Low | Low | Low | Low | Low |
| Da Silva and Colleagues 48 | 2021 | Low | Low | Low | Low | Low | Low |
| Isolan and Colleagues 6 | 2021 | Some concern | Low | Low | Low | Low | Some concern |
| Momeni and Colleagues 5 | 2021 | Low | Low | Low | Low | Low | Low |
| Scarano and Colleagues 49 | 2021 | Low | Low | Low | Low | Low | Low |
| Hadad and Colleagues 50 | 2022 | Some concern | Low | Some concern | Low | Low | Some concern |
| Le and Colleagues 51 | 2022 | Low | Low | Some concern | Low | Low | Some concern |
Time: irradiation parameter analysis
A total of 22 studies reported laser irradiation parameter settings with respect to time for each indication of PBM. Some groups reported on more than 1 indication and had multiple treatment groups. Twenty studies reported results for pain (Table 1), and 6 studies reported results for wound healing (Table 2). Of the 6 studies that reported on wound healing, 4 defined parameters for soft-tissue healing and 2 for bone-tissue healing (Table 2). Not all studies fully reported the entire panel of irradiation parameters: laser type, laser power (W), beam area and spot size (cm2), treatment time per point (seconds), energy dose (J), fluence (J/cm2), contact points (per visit), and several appointment intervals.
The pain reduction analysis (Table 1) for treatment time included 20 studies, with reporting on red visible wavelength devices in 4 articles and 5 treatment groups (Figure 2A) and on near-infrared wavelengths in 18 articles and 19 treatment groups (Figure 2B). The reports on red visible devices included the following irradiation parameters: treatment times, 25 through 60 seconds; wavelength, 550 through 660 nm; laser power, 0.02 through 0.1 W; beam area and spot size, 0.028 through 0.05 cm2 (3 groups); energy dose, 2.5 through 6 J (4 groups); fluence, 5 through 106 J/cm2 (3 groups); contact points, 1 through 4; and appointment intervals, 1 and 2 (Figure 2A). The reports on near-infrared devices included the following irradiation parameters: treatment time, 17.1 through 900 seconds; wavelengths, 780 through 1,064 nm; laser power, 0.04 through 1 W; beam area and spot size, 0.01 through 3.2 cm2 (14 groups); energy dose, 2.1 through 180 J (17 groups); fluence, 2 through 212 J/cm2 (15 groups); contact points, 1 through 12; and appointment intervals, 1 through 5 (Figure 2B).
The wound healing analysis (Table 2) included 6 studies, with reports on soft-tissue healing for red visible wavelengths in 2 studies and 2 groups (Figure 3A) and on near-infrared devices in 3 studies and 3 groups (Figure 3B). Bone-tissue healing was reported using near-infrared devices in 2 studies and 2 groups (Figure 3C). Reports on soft-tissue wound healing with red visible devices included the following irradiation parameters: treatment times, 30 through 60 seconds; wavelength, 660 nm; laser power, 0.02 through 0.025 W; beam area and spot size, (none); energy dose, 6 J (1 group); fluence, (none); contact points, 1 through 3; and appointment intervals, 1 through 3 (Figure 3A). Reports on near-infrared devices included the following irradiation parameters for 3 articles and 3 groups: treatment time, 70 through 120 seconds; wavelengths, 808 through 830 nm; laser power, 0.02 through 0.04 W; beam area and spot size, 0.28 cm2 (2 groups); energy dose, 2.8 through 6 J; fluence, 2.14 through 100 J/cm2 (2 groups); contact points, 1 through 5; and appointment intervals, 1 through 4 (Figure 3B). Reports on bone-tissue healing with near-infrared devices included the following irradiation parameters in 2 articles and 2 groups: treatment time, 25 through 30 seconds; wavelength, 808 nm; laser power, 0.1 W; beam area and spot size, 0.028 through 0.04 cm2; energy dose, 2.5 through 3 J; fluence, 75 through 89 J/cm2; contact points, 5; and appointment intervals, 5 (Figure 3C).
DISCUSSION
In our scoping review of available evidence, we evaluated the use of PBM in postsurgical dental extraction therapy and determined that time spent delivering PBM can range from 17.1 through 900 seconds per treatment application. The amount of time prescribed for each PBM protocol differed according to the wavelengths reported. Delivering PBM light after extraction can influence pain reduction and clinical wound healing. The specific amount of time spent delivering PBM to improve pain and wound healing differs per device and wavelength (Tables 1 and 2, Figures 2 and 3). Achieving these outcomes is possible and requires further investigation for red and near-infrared wavelengths. Future research is needed to better understand the wide heterogeneity reported in the available evidence of this review, specifically for PBM time delivery and irradiation parameter settings.
The standard for postoperative dental extraction therapy requires solutions that promote healing, prevent complications, and provide comfort. Dentistry is transitioning away from dispensing narcotics due to increased rates of opioid misuse.54–56 Practitioners have adopted alternatives by prescribing analgesics, corticosteroids, and antibiotics. Although these medications are effective nonnarcotic treatment options, they may not be viable for all patient populations. This can be due to patient preference, noncompliance, or history of medical compromise. The results of our scoping review provide a summary of emerging evidence that can be investigated further to support the use of PBM in dental extraction therapy. There is potential to improve the standard of postoperative care for dental extractions by expanding future PBM research through recognized dose-escalation trial formats.57–59
We selected dental extraction therapy for our analysis because it is a procedure that is well documented in the literature.60–63 It is well understood that complications from dental extractions can present as excessive inflammation, postoperative surgical pain, swelling, and trismus.64 A dental extraction procedure will initiate biological events that immediately alter soft- and hard-tissue structures.65 Extraction therapies will release inflammatory mediators, provoke tissue damage, and activate nociceptors, which can be altered by PBM.3,6,42,43,49,50,52,66
Translating the findings reported in this review may pose challenges for future investigators. We determined that the amount of time spent delivering PBM ranged from 17.1 through 900 seconds per application for wavelengths ranging from 550 through 1,064 nm (Tables 1 and 2, Figures 2 and 3). In the ideal circumstance, the amount of time spent delivering PBM will predictably stimulate a biological effect at the targeted site. To further explore the potential for achieving a PBM biological effect on tissue, future research should investigate the cumulative amount of time spent delivering PBM with specific irradiation parameters (Figures 2 and 3). This will allow a greater understanding of the behavioral characteristics specific to each wavelength and each device.
Additional investigations are necessary to determine the appointment intervals required for PBM light delivery after a dental extraction procedure. The results of our review reported PBM protocols requiring from 1 through 5 postoperative appointments (Tables 1 and 2, Figures 2 and 3). Establishing a PBM protocol with multiple PBM treatment visits may not be feasible in the nonresearch setting because of scheduling limitations and patient availability. Despite this wide range of PBM appointment intervals (Figures 2 and 3), several studies reported promising pain reduction results with a single PBM application on the day of surgery.4,6,47,50 The amount of time spent delivering PBM for these studies with a single intervention varied by device from 30 through 73 seconds per application. The total cumulative treatment time for a single appointment in this group ranged from 60 through 438 seconds. In addition, 1 study that applied PBM immediately after extraction showed improvements in soft-tissue wound healing and a total treatment time of 60 seconds in 1 application.4
The results from our analysis reported on protocols describing the placement of the PBM probe at different anatomic locations. Clinical sites varied from external points of contact, internal points of contact, being in direct contact, or having a fixed distance from the anatomy (Tables 1 and 2, Figures 2 and 3). An optimal amount of PBM light can be delivered at the intended target site and requires the dose distribution at the target tissue to ensure adequate coverage of the intended pathology. Tissue optical properties can vary spatially due to the presence of optical nonuniformities. These properties can vary due to hemodynamic and metabolic processes that occur between tissue types with different biological structures and as a function of wavelength.67,68 It is possible to model total tissue dose distribution over the entire tissue volume by using the diffusion approximation equation for light in tissue.69,70 PBM devices are manufactured with distinct specifications for the shape of the lens and the beam area and spot size of the handheld probe. It is important to recognize these details as they will help clinicians better deliver PBM light, fluence, and energy.
Prescribing a PBM delivery protocol can follow a predictable and safe evidence-based approach. An animal study has helped determine therapeutic dose limits, biological safety, and molecular pathways involved with phototoxicity.71 Human tissue optical properties vary from person to person and are characterized by coefficients of absorption, scattering, and depth of penetration and attenuation.9,72 PBM can be prescribed via the following parameters: wavelength (nm), laser power (W), treatment time (seconds), beam area and spot size (cm2), and fluence (J/cm2).2 Optimizing these settings to specific tissue optical properties will deliver a light dose received at the intended target site (fluence, J/cm2).18,66,73,74 Accurately quantifying irradiation parameters will guide the prescription formula for an effective PBM treatment.
Ng and colleagues’8 2018 article focused on the amount of energy loss when laser energy penetrates through human alveolar bone. They investigated a total of 27 extraction sockets and determined that each millimeter of bone thickness amounted to a 6.81% reduction in laser energy. Understanding how to prescribe for depth of penetration (attenuation) can guide dosing protocols toward biomolecular targets like the hemoglobin coefficient of absorption.11 Specifically, when oxygen is coupled to hemoglobin, the 500 through 600 nm red visible wavelength laser penetration peaks at a shallow depth of 0.6 mm, maintaining a steady decline through 2.0 mm. Asimov and colleagues11 also noted that when oxygen is decoupled from hemoglobin, the laser at the 800 nm near-infrared range penetrates at 2.0 mm depth and remains steady through 3.0 mm.
Our scoping review has limitations in the variation in the amount of time spent delivering PBM and the wide range of wavelengths (Tables 1 and 2, Figures 2 and 3). Given the unique characteristics of each type of device and wavelength, the total number of validated PBM protocols for pain reduction and wound healing that are available in the published databases is limited. Substantial efforts are needed to standardize PBM therapy by reporting all irradiation parameters (Tables 1 and 2, Figures 2 and 3). This will allow future research groups to validate any outcomes with further investigation.
Another limitation of this review was the inconsistent standardization of dental extraction sites and the array of pain and wound healing indexes used for the PBM trials reported. The pain outcomes reported included 14 studies reporting on mandibular third molars, 1 study on mandibular molars, 4 studies on both maxillary and mandibular third molars, and 1 study on maxillary and mandibular premolars. The 4 soft-tissue wound healing studies included mandibular molars, maxillary and mandibular teeth, and mandibular third molars. Both bone tissue studies reported on mandibular molars.
Pain reduction and wound healing indexes were not standardized in type and appointment intervals. Although 15 pain studies used a visual analog pain scale, measurement intervals were not consistent among all the groups. The remaining 5 pain studies used a visual numerical scale, Giles scale, Likert scale, pain analog scale, and the Universal Pain Assessment Tool scale. The soft-tissue healing indexes were not standardized, with each study using a different type of measurement with varying assessment intervals. The bone-tissue wound healing analyses were consistent with micro–computed tomography and histology analysis at the time of biopsy on days 40 and 45.
A challenge realized from this analysis was that several PBM settings should have been reported by the authors. We recognize that multiple PBM settings (power, beam area and spot size, energy, fluence) were unique to our analysis, as they are necessary to calculate the amount of light delivered. However, we also acknowledge that these criteria may be considered trade secrets, as the manufacturer does not always release device specifications. Knowledge of these settings is necessary for scientific validation in a clinical setting. A complete understanding of these criteria will guide clinical calculations for PBM light delivery that are needed to accurately reproduce a protocol. Authors were not contacted for these specifications to highlight the lack of reporting PBM settings (Tables 1 and 2). A complete analysis of all settings was only possible with bone-tissue wound healing (Figure 3C).
CONCLUSION
To our knowledge, this is the first PBM scoping review to present a summary analysis of available evidence for time (seconds) and irradiation parameters prescribed for PBM after dental extraction therapy to improve postoperative pain and wound healing. The results of this study determined that the amount of time prescribed for PBM differs by wavelength for postoperative pain and wound healing after tooth extraction. Further studies are needed before PBM can be introduced to clinical practice after a routine dental extraction procedure.
Acknowledgments
Research reported in this publication was supported by award T90DE030854 from the National Institutes of Health National Institute of Dental and Craniofacial Research. Grants T90DE030854 and TL1TR001880 from the National Institutes of Health were awarded to Dr. Sourvanos. Grants R01 EB028778, R01 EB032821, and PO1 CA08797 from the National Institutes of Health were awarded to Dr. Zhu. The Thouron Award (London, UK) supports Dr. Lander.
ABBREVIATION KEY
- AlGaAs
Aluminum gallium arsenide
- AsGaAl
Arsenide gallium aluminium
- C
Contact
- E
Extraoral
- GaAlAs
Gallium aluminum arsenide
- GaAs
Gallium arsenide
- I
Intraoral
- NC
Noncontact
- Nd:YAG
Neodymium-doped yttrium aluminum garnet
- PBM
Photobiomodulation
- TGF- β1
Transforming growth factor β1
- VAS
Visual analog scale
Biographies
Dr. Sourvanos is a National Institute of Dental and Craniofacial Research T90 postdoctoral fellow, Center for Innovation and Precision Dentistry, School of Dental Medicine, School of Engineering, University of Pennsylvania, Philadelphia, PA; a periodontics surgical resident, Department of Periodontics, School of Dental Medicine, University of Pennsylvania, Philadelphia, PA; and an advanced graduate student, Department of Periodontics, School of Dental Medicine, University of Pennsylvania, Philadelphia, PA.
Dr. Lander is a Thouron Scholar, advanced graduate student, and periodontics surgical resident, Department of Periodontics, School of Dental Medicine, University of Pennsylvania, Philadelphia, PA.
Dr. Sarmiento is a clinical assistant professor, Department of Periodontics, School of Dental Medicine, University of Pennsylvania, Philadelphia, PA, and in private practice, New York, NY.
Mr. Carroll is an associate, THOR Photomedicine, Chesham, Buckinghamshire, UK.
Mr. Hall is a medical physics associate, Department of Radiation Oncology, Perelman Center for Advanced Medicine, University of Pennsylvania, Philadelphia, PA.
Dr. Zhu is a professor, Department of Radiation Oncology, Perelman Center for Advanced Medicine, Hospital of the University of Pennsylvania, University of Pennsylvania, Philadelphia, PA.
Dr. Fiorellini is a professor, Department of Periodontics, School of Dental Medicine, University of Pennsylvania, Philadelphia, PA.
Footnotes
Disclosures. None of the authors reported any disclosures.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Dennis Sourvanos, University of Pennsylvania, Philadelphia, PA.
Bradley Lander, Department of Periodontics, School of Dental Medicine, University of Pennsylvania, Philadelphia, PA..
Hector Sarmiento, Department of Periodontics, School of Dental Medicine, University of Pennsylvania, Philadelphia, PA, and in private practice, New York, NY..
James Carroll, THOR Photomedicine, Chesham, Buckinghamshire, UK..
Ryan D. Hall, Department of Radiation Oncology, Perelman Center for Advanced Medicine, University of Pennsylvania, Philadelphia, PA..
Timothy C. Zhu, Department of Radiation Oncology, Perelman Center for Advanced Medicine, Hospital of the University of Pennsylvania, University of Pennsylvania, Philadelphia, PA..
Joseph P. Fiorellini, Department of Periodontics, School of Dental Medicine, University of Pennsylvania, Philadelphia, PA..
References
- 1.López-Ramírez M, Vílchez-Pérez MÁ, Gargallo-Albiol J, Arnabat-Domínguez J, Gay-Escoda C. Efficacy of low-level laser therapy in the management of pain, facial swelling, and postoperative trismus after a lower third molar extraction: a preliminary study. Lasers Med Sci. 2012;27(3):559–566. [DOI] [PubMed] [Google Scholar]
- 2.Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng. 2012;40(2):516–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romão MM, Marques MM, Cortes AR, Horliana AC, Moreira MS, Lascala CA. Micro-computed tomography and histomorphometric analysis of human alveolar bone repair induced by laser phototherapy: a pilot study. Int J Oral Maxillofac Surg. 2015;44(12):1521–1528. [DOI] [PubMed] [Google Scholar]
- 4.Salem S. Consequences of 660 nm diode laser following postsurgical exodontia in patients under contraceptive pills: a randomized double-blinded clinical trial. J Contemp Dent Pract. 2020;21(1):2–10. [PubMed] [Google Scholar]
- 5.Momeni E, Barati H, Arbabi MR, Jalali B, Moosavi MS. Low-level laser therapy using laser diode 940 nm in the mandibular impacted third molar surgery: double-blind randomized clinical trial. BMC Oral Health. 2021;21(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isolan C, Kinalski MD, Leão OA, Post LK, Isolan TM, Dos Santos MB. Photobiomodulation therapy reduces postoperative pain after third molar extractions: a randomized clinical trial. Med Oral Patol Oral Cir Bucal. 2021;26(3):e341–e348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young NC, Maximiano V, Arany PR. Thermodynamic basis for comparative photobiomodulation dosing with multiple wavelengths to direct odontoblast differentiation. J Biophotonics. 2022;15(6):e202100398. [DOI] [PubMed] [Google Scholar]
- 8.Ng DY, Chan AK, Dalci O, Petocz P, Papadopoulou AK, Darendeliler MA. A pilot study of laser energy transmission through bone and gingiva. JADA. 2018;149(8):704–711. [DOI] [PubMed] [Google Scholar]
- 9.Sandell JL, Zhu TC. A review of in-vivo optical properties of human tissues and its impact on PDT. J Biophotonics. 2011;4(11–12):773–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacques SL. Optical properties of biological tissues: a review. Phys Med Biol. 2013;58(11):R37–61. [DOI] [PubMed] [Google Scholar]
- 11.Asimov MM, Asimov RM, Rubinov AN. Efficiency of laser action on hemoglobin and oxyhemoglobin in skin blood vessels. Proc SPIE. 1998;3254:407–412. 10.1117/12.308190 [DOI] [Google Scholar]
- 12.Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg. 2005;23(4):355–361. [DOI] [PubMed] [Google Scholar]
- 13.Zecha JA, Raber-Durlacher JE, Nair RG, et al. Low level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer, part 1: mechanisms of action, dosimetric, and safety considerations. Support Care Cancer. 2016;24(6): 2781–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong-Riley MT, Liang HL, Eells JT, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280(6):4761–4771. [DOI] [PubMed] [Google Scholar]
- 15.Peplow PV, Chung TY, Baxter GD. Laser photo-stimulation (660 nm) of wound healing in diabetic mice is not brought about by ameliorating diabetes. Lasers Surg Med. 2012;44(1):26–29. [DOI] [PubMed] [Google Scholar]
- 16.Suh S, Choi EH, Atanaskova Mesinkovska N. The expression of opsins in the human skin and its implications for photobiomodulation: a systematic review. Photodermatol Photoimmunol Photomed. 2020;36(5):329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zupin L, Ottaviani G, Rupel K, et al. Analgesic effect of photobiomodulation therapy: an in vitro and in vivo study. J Biophotonics. 2019;12(10):e201900043. [DOI] [PubMed] [Google Scholar]
- 18.Zhu TC, Finlay JC, Dimofte A, Hahn SM. Light dosimetry at tissue surfaces for oblique incident circular fields. Proc SPIE Int Soc Opt Eng. 2004;5315:113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arany PR, Chen AC-H, Hunt TH, Mooney DJ, Hamblin M. Role of ROS-mediated TGF beta activation in laser photobiomodulation. Proc SPIE. 2009:7165. [Google Scholar]
- 20.Tang E, Khan I, Andreana S, Arany PR. Laser-activated transforming growth factor-β1 induces human β-defensin 2: implications for laser therapies for periodontitis and peri-implantitis. J Periodontal Res. 2017;52(3):360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosseinpour S, Fekrazad R, Arany PR, Ye Q. Molecular impacts of photobiomodulation on bone regeneration: a systematic review. Prog Biophys Mol Biol. 2019; 149:147–159. [DOI] [PubMed] [Google Scholar]
- 22.Finnson KW, Arany PR, Philip A. Transforming growth factor beta signaling in cutaneous wound healing: lessons learned from animal studies. Adv Wound Care (New Rochelle). 2013;2(5):225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arany PR, Nayak RS, Hallikerimath S, Limaye AM, Kale AD, Kondaiah P. Activation of latent TGF-beta1 by low-power laser in vitro correlates with increased TGF-beta1 levels in laser-enhanced oral wound healing. Wound Repair Regen. 2007;15(6):866–874. [DOI] [PubMed] [Google Scholar]
- 24.Arany PR, Cho A, Hunt TD, et al. Photoactivation of endogenous latent transforming growth factor-β1 directs dental stem cell differentiation for regeneration. Sci Transl Med. 2014;6(238):238ra269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramirez H, Patel SB, Pastar I. The role of TGFβ signaling in wound epithelialization. Adv Wound Care (New Rochelle). 2014;3(7):482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robijns J, Nair RG, Lodewijckx J, et al. Photobiomodulation therapy in management of cancer therapy-induced side effects: WALT position paper 2022. Front Oncol. 2022;12:927685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elad S, Cheng KKF, Lalla RV, et al. ; for the Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO). MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2020;126(19):4423–4431.32786044 [Google Scholar]
- 28.Santinoni CD, Oliveira HF, Batista VE, Lemos CA, Verri FR. Influence of low-level laser therapy on the healing of human bone maxillofacial defects: a systematic review. J Photochem Photobiol B. 2017;169:83–89. [DOI] [PubMed] [Google Scholar]
- 29.Rosso MPO, Buchaim DV, Pomini KT, et al. Photobiomodulation therapy (PBMT) applied in bone reconstructive surgery using bovine bone grafts: a systematic review. Materials (Basel). 2019;12(24):4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amaroli A, Colombo E, Zekiy A, Aicardi S, Benedicenti S, De Angelis N. Interaction between laser light and osteoblasts: photobiomodulation as a trend in the management of socket bone preservation—a review. Biology (Basel). 2020;9(11):409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson S, Allen P, Peckham S, Goodwin N. Asking the right questions: scoping studies in the commissioning of research on the organisation and delivery of health services. Health Res Policy Sys. 2008;6(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armstrong R, Hall BJ, Doyle J, Waters E. “Scoping the scope” of a cochrane review. J Public Health (Oxf). 2011;33(1):147–150. [DOI] [PubMed] [Google Scholar]
- 33.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Social Res Methodol. 2005; 8(1):19–32. [Google Scholar]
- 34.Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernando S, Hill CM, Walker R. A randomised double blind comparative study of low level laser therapy following surgical extraction of lower third molar teeth. Br J Oral Maxillofac Surg. 1993;31(3):170–172. [DOI] [PubMed] [Google Scholar]
- 37.Mozzati M, Martinasso G, Cocero N, et al. Influence of superpulsed laser therapy on healing processes following tooth extraction. Photomed Laser Surg. 2011;29(8):565–571. [DOI] [PubMed] [Google Scholar]
- 38.Mozzati M, Martinasso G, Cocero N, et al. Super-pulsed laser therapy on healing process after tooth extraction in patients waiting for liver transplantation. Lasers Med Sci. 2012;27(2):353–359. [DOI] [PubMed] [Google Scholar]
- 39.Paschoal MA, Santos-Pinto L. Therapeutic effects of low-level laser therapy after premolar extraction in adolescents: a randomized double-blind clinical trial. Photomed Laser Surg. 2012;30(9):559–564. [DOI] [PubMed] [Google Scholar]
- 40.Ferrante M, Petrini M, Trentini P, Perfetti G, Spoto G. Effect of low-level laser therapy after extraction of impacted lower third molars. Lasers Med Sci. 2013; 28(3):845–849. [DOI] [PubMed] [Google Scholar]
- 41.Alan H, Yolcu Ü, Koparal M, Özgür C, Öztürk SA, Malkoç S. Evaluation of the effects of the low-level laser therapy on swelling, pain, and trismus after removal of impacted lower third molar. Head Face Med. 2016;12(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedreira AA, Wanderley FG, Sa MF, et al. Thermographic and clinical evaluation of 808-nm laser photobiomodulation effects after third molar extraction. Minerva Stomatol. 2016;65(4):213–222. [PubMed] [Google Scholar]
- 43.Sierra SO, Deana AM, Bussadori SK, et al. Choosing between intraoral or extraoral, red or infrared laser irradiation after impacted third molar extraction. Lasers Surg Med. 2016;48(5):511–518. [DOI] [PubMed] [Google Scholar]
- 44.Farhadi F, Eslami H, Majidi A, Fakhrzadeh V, Ghanizadeh M, KhademNeghad S. Evaluation of adjunctive effect of low-level laser therapy on pain, swelling and trismus after surgical removal of impacted lower third molar: a double blind randomized clinical trial. Laser Ther. 2017; 26(3):181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feslihan E, Eroğlu CN. Can photobiomodulation therapy be an alternative to methylprednisolone in reducing pain, swelling, and trismus after removal of impacted third molars? Photobiomodul Photomed Laser Surg. 2019;37(11):700–705. [DOI] [PubMed] [Google Scholar]
- 46.Ahrari F, Eshghpour M, Zare R, Ebrahimi S, Fallahrastegar A, Khaki H. Effectiveness of low-level laser irradiation in reducing pain and accelerating socket healing after undisturbed tooth extraction. J Lasers Med Sci. 2020;11(3):274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santos PL, Marotto AP, Zatta da Silva T, et al. Is low-level laser therapy effective for pain control after the surgical removal of unerupted third molars? A randomized trial. J Oral Maxillofac Surg. 2020;78(2):184–189. [DOI] [PubMed] [Google Scholar]
- 48.da Silva TMV, Melo TS, de Alencar RC, et al. Photobiomodulation for mucosal repair in patients submitted to dental extraction after head and neck radiation therapy: a double-blind randomized pilot study. Support Care Cancer. 2021;29(3):1347–1354. [DOI] [PubMed] [Google Scholar]
- 49.Scarano A, Lorusso F, Postiglione F, Mastrangelo F, Petrini M. Photobiomodulation enhances the healing of postextraction alveolar sockets: a randomized clinical trial with histomorphometric analysis and immunohistochemistry. J Oral Maxillofac Surg. 2021;79(1):57.e51–57.e12. [DOI] [PubMed] [Google Scholar]
- 50.Hadad H, Santos AFP, de Jesus LK, et al. Photobiomodulation therapy improves postoperative pain and edema in third molar surgeries: a randomized, comparative, double-blind, and prospective clinical trial. J Oral Maxillofac Surg. 2022;80(1):37.e31–37.e12. [DOI] [PubMed] [Google Scholar]
- 51.Le HT, Huynh NC, Nguyen-Ho QA, Nguyen TT, Le SH, Nguyen LT. Effect of Photobiomodulation therapy on reducing acute pain and inflammation following surgical removal of impacted mandibular third molars: a randomized, split-mouth clinical trial. Photobiomodul Photomed Laser Surg. 2022;40(4):245–251. [DOI] [PubMed] [Google Scholar]
- 52.Rosero KAV, Sampaio RMF, Deboni MCZ, et al. Photobiomodulation as an adjunctive therapy for alveolar socket preservation: a preliminary study in humans. Lasers Med Sci. 2020;35(8):1711–1720. [DOI] [PubMed] [Google Scholar]
- 53.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 54.Thornhill MH, Suda KJ, Durkin MJ, Lockhart PB. Is it time US dentistry ended its opioid dependence? JADA. 2019;150(10):883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Denisco RC, Kenna GA, O’Neil MG, et al. Prevention of prescription opioid abuse: the role of the dentist. JADA. 2011;142(7):800–810. [DOI] [PubMed] [Google Scholar]
- 56.Wehler CJ, Panchal NH, Cotchery DL 3rd, et al. Alternatives to opioids for acute pain management after dental procedures: a Department of Veterans Affairs consensus paper. JADA. 2021;152(8):641–652. [DOI] [PubMed] [Google Scholar]
- 57.Hansen AR, Graham DM, Pond GR, Siu LL. Phase 1 trial design: is 3 + 3 the best? Cancer Control. 2014;21(3): 200–208. [DOI] [PubMed] [Google Scholar]
- 58.Le Tourneau C, Lee JJ, Siu LL. Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst. 2009;101(10):708–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Araujo DV, Oliva M, Li K, Fazelzad R, Liu ZA, Siu LL. Contemporary dose-escalation methods for early phase studies in the immunotherapeutics era. Eur J Cancer. 2021;158:85–98. [DOI] [PubMed] [Google Scholar]
- 60.Cardaropoli G, Araújo M, Lindhe J. Dynamics of bone tissue formation in tooth extraction sites: an experimental study in dogs. J Clin Periodontol. 2003;30(9):809–818. [DOI] [PubMed] [Google Scholar]
- 61.Araújo MG, Lindhe J. Ridge alterations following tooth extraction with and without flap elevation: an experimental study in the dog. Clin Oral Implants Res. 2009;20(6):545–549. [DOI] [PubMed] [Google Scholar]
- 62.Araújo MG, Lindhe J. Dimensional ridge alterations following tooth extraction: an experimental study in the dog. J Clin Periodontol. 2005;32(2):212–218. [DOI] [PubMed] [Google Scholar]
- 63.Araújo MG, Silva CO, Misawa M, Sukekava F. Alveolar socket healing: what can we learn? Periodontol 2000. 2015;68(1):122–134. [DOI] [PubMed] [Google Scholar]
- 64.Kim K, Brar P, Jakubowski J, Kaltman S, Lopez E. The use of corticosteroids and nonsteroidal antiin-flammatory medication for the management of pain and inflammation after third molar surgery: a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(5):630–640. [DOI] [PubMed] [Google Scholar]
- 65.Araújo MG, Sukekava F, Wennström JL, Lindhe J. Ridge alterations following implant placement in fresh extraction sockets: an experimental study in the dog. J Clin Periodontol. 2005;32(6):645–652. [DOI] [PubMed] [Google Scholar]
- 66.Ebrahimi T, Moslemi N, Rokn A, Heidari M, Nokhbatolfoghahaie H, Fekrazad R. The influence of low-intensity laser therapy on bone healing. J Dent (Tehran). 2012;9(4):238–248. [PMC free article] [PubMed] [Google Scholar]
- 67.Dimofte A, Finlay JC, Sharikova AV, et al. Determination of tissue optical properties in PDT treated head & neck patients. Proc SPIE Int Soc Opt Eng. 2014:8926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bashkatov A, Berezin K, Dvoretskiy K, et al. Measurement of tissue optical properties in the context of tissue optical clearing. J Biomed Optics. 2018;23(9):091416. [DOI] [PubMed] [Google Scholar]
- 69.Jacques SL. Light distributions from point, line and plane sources for photochemical reactions and fluorescence in turbid biological tissues. Photochem Photobiol. 1998;67(1):23–32. [PubMed] [Google Scholar]
- 70.Star WM. Light dosimetry in vivo. Phys Med Biol. 1997;42(5):763–787. [DOI] [PubMed] [Google Scholar]
- 71.Khan I, Tang E, Arany P. Molecular pathway of near-infrared laser phototoxicity involves ATF-4 orchestrated ER stress. Sci Rep. 2015;5:10581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang HW, Zhu TC, Putt ME, et al. Broadband reflectance measurements of light penetration, blood oxygenation, hemoglobin concentration, and drug concentration in human intraperitoneal tissues before and after photodynamic therapy. J Biomed Opt. 2005;10(1):14004. [DOI] [PubMed] [Google Scholar]
- 73.Dimofte A, Finlay JC, Zhu TC. A method for determination of the absorption and scattering properties interstitially in turbid media. Phys Med Biol. 2005;50(10):2291–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dimofte A, Finlay JC, Liang X, Zhu TC. Determination of optical properties in heterogeneous turbid media using a cylindrical diffusing fiber. Phys Med Biol. 2012; 57(19):6025–6046. [DOI] [PMC free article] [PubMed] [Google Scholar]


