Abstract
Background:
Late initiation of HIV antiretroviral therapy in pregnancy is associated with failure to achieve viral suppression by delivery and increased infant transmission of HIV. We evaluated virological suppression by delivery with dolutegravir compared to efavirenz when initiated during the third trimester
Methods:
DolPHIN-2 (NCT03249181) randomized pregnant women in South Africa and Uganda initiating antiretroviral therapy in third trimester between January-August 2018 1:1 to open-label dolutegravir vs efavirenz-based therapy. Viral load was measured serially to delivery visit (0–14 days post-partum). We report the primary efficacy outcome (viral load<50 copies per mL at delivery visit), and the primary safety outcome (occurrence of drug-related adverse events in mothers and infants) to delivery visit. Longer-term follow-up of mothers and infants continues.
Findings:
Of 268 mothers randomized; all mothers and their infants were included in safety, and 250 mothers (125 dolutegravir, 125 efavirenz) and their infants in efficacy analyses by intention to treat analyses. The median duration of maternal therapy at delivery was 55 (IQR 33–77) days.
Frequency of viral load<50 copies/mL at delivery visit was higher with dolutegravir (89/120, 74·2%) vs efavirenz (50/117, 42·7%); risk ratio (RR) and 95% CI, 1·64 (1·31–2·06). Although well-tolerated, more mothers on dolutegravir reported serious adverse events (30/137 vs 14/131; P = 0·013), particularly surrounding pregnancy and puerperium. There were no differences in births <37 weeks and <34 weeks gestation (16·4% and 3·3% respectively across both groups). Three stillbirths in the dolutegravir arm, and one in the efavirenz arm were considered unrelated to drug. Three infant HIV infections were detected, all from the dolutegravir arm, and considered likely to be in-utero transmissions.
Interpretation:
Dolutegravir achieves more rapid virological suppression compared to efavirenz, is well-tolerated when initiated in late pregnancy and may be preferred in this group of high-risk individuals
Funding:
The DolPHIN-2 study was funded by Unitaid. Dolutegravir was donated by ViiV Healthcare.
Introduction
Delayed initiation of antiretroviral therapy until the third trimester of pregnancy is common in many settings where HIV is prevalent, and associated with increased mother-to-child transmission (MTCT) of HIV and infant mortality1–3. In the Uganda Demographic Health Survey, the first antenatal clinic appointment occurred at an average of 27·9 weeks1 and in South Africa, 11–19% of pregnant women presented at 28 weeks gestation or later 2,3.
Whilst the causes of infant HIV transmissions are multifactorial, one important factor may be the inability of current first line efavirenz-based therapy to suppress HIV viral load by labour and delivery, a time when transmission risk is highest. The integrase inhibitor dolutegravir reduces HIV viral load rapidly to <50 copies per mL after a median of 28 days in non-pregnant adults compared with 84 days for efavirenz4. Dolutegravir may consequently have special value for women presenting late in pregnancy, however safety and efficacy data are lacking.
In order to evaluate if the rapid virological decline, tolerability and high HIV resistance barrier of dolutegravir containing regimens4 conferred additional benefits to HIV-positive women initiating treatment in late pregnancy, we undertook a randomized trial comparing dolutegravir with efavirenz-based therapy in HIV-positive pregnant women initiating treatment in their third trimester of pregnancy in South Africa and Uganda.
Methods
Study Design:
DolPHIN-2 (NCT03249181) is a randomised open-label trial in pregnant mothers initiating antiretroviral therapy in the third trimester of pregnancy. Eligible participants were enrolled in South Africa and Uganda. In Cape Town, participants were enrolled at Gugulethu Community Health Centre, following recruitment from 8 primary antenatal facilities in the surrounding area. In Kampala, participants were enrolled at the Infectious Diseases Institute, following recruitment at Kawempe Hospital (a tertiary obstetric referral unit) or 8 primary antenatal facilities throughout Kampala and Wakiso District. All participants were randomised 1:1 to receive efavirenz versus dolutegravir-based regimens. The primary efficacy outcome was HIV viral load <50 copies per mL at delivery, and the primary safety outcome was frequency of drug-related adverse events. Secondary outcomes included viral load <1000 copies per mL at delivery, occurrence of MTCT, and safety and tolerability of dolutegravir in mothers and breastfed infants.
We report a pre-specified analysis of the primary efficacy outcome reached at the delivery visit (up to 14 days following birth), with accompanying safety data on mother-infant pairs to 6 weeks (+/− 2 weeks) post-partum.
Participants
We enrolled participants if they met all the following inclusion criteria: women aged ≥18 years with untreated, but confirmed HIV infection; positive pregnancy test; estimated gestation ≥28 weeks; willing to provide informed consent. We excluded individuals with any of the following: i) received antiretroviral therapy in the preceding year or ever received integrase inhibitors, ii) documented virological failure of a non-nucleoside containing antiretroviral regimen, iii) previous efavirenz toxicity or other clinical history that would preclude randomisation, iv) estimated glomerular filtration rate <50 mL/min, hemoglobin <8.0 g/dL, decompensated liver disease or alanine aminotransferase (ALT) >5 times the upper limit of normal (ULN) or ALT >3xULN and bilirubin >2xULN (with >35% direct bilirubin) v) severe pre-eclampsia vi) medical, psychiatric or obstetric condition that might affect participation in the study, or vii) receiving any drugs known to significantly interact with efavirenz or dolutegravir within the preceding 2 weeks. From 1st June 2018 onwards, the protocol was amended to exclude pre-treatment HIV viral load <50 copies per mL.
Ethics review committees in South Africa, Uganda and the UK approved the study. The Data and Safety Monitoring Board conducted a planned interim safety analysis after the first 125 mothers had delivered, and were notified of all infant HIV transmissions.
Randomisation and Masking
Since national policy required antiretroviral therapy to commence without delay in our study population, participants meeting eligibility criteria based on history and examination (prior to the availability of blood results) were enrolled with 1:1 block randomization (stratified by country, with concealment of allocation until assignment) to commence either dolutegravir or efavirenz on the same day. Any laboratory results which rendered the participant ineligible led to withdrawal at a confirmatory visit 7 days later.
Procedures:
Ultrasound examination was done at screening or within 2 weeks of enrolment across both sites. Gestational age at screening was a best estimate based on a combination of recall of last menstrual period, pubic symphysis-fundal height and fetal ultrasound (if undertaken at screening). The following tests were undertaken at screening: HIV-1 and −2 antibodies, CD4 count, full blood count, urea, creatinine and electrolytes, bilirubin, alanine aminotransferase and creatinine phosphokinase activity. HIV viral load and maternal safety bloods were also collected at 7 and 28 days following antiretroviral initiation, at 36 weeks gestation (if applicable), and at the delivery visit (0–14 days post-partum). Study site deliveries were attended by study staff and for offsite deliveries, the study team arranged review of mothers and infants within 14 days.
Antiretroviral therapy
Mothers randomized to dolutegravir received Tivicay® 50mg/day together with generic tenofovir disoproxil fumarate (300mg) co-formulated with emtricitabine 200mg (South Africa) or lamivudine 300mg (Uganda) once-daily. Mothers in the efavirenz arm received a generic single fixed combination pill of efavirenz 600mg with tenofovir plus either emtricitabine (South Africa) or lamivudine (Uganda). Each site had established protocols for supporting adherence, psychological counselling and health advice around pregnancy and breastfeeding. Use of traditional medications, supplements and other co-medications was checked at every study visit, and drug interactions managed as appropriate. In keeping with national guidelines in South Africa and Uganda, newborn infants were prescribed nevirapine for 6 weeks.
Outcomes
Safety evaluation and reporting in mothers and infants
We categorised adverse events (AE) and serious adverse events (SAE) according to MedDRA5 with severity classified according to the DAIDS Grading Scale (version 2·1, July 2017). A Safety Endpoint Review Committee (blinded to allocation arm) assessed all SAEs, stillbirths, infant deaths and infant transmissions for relationship to study medication (using the Liverpool Adverse Drug Reaction Causality Assessment Tool6) as well as likelihood of maternal Immune Reconstitution Inflammatory Syndrome (IRIS).
Assessment of maternal and birth outcomes
Case definitions for IRIS are heavily reliant on clinical judgement, given the relative lack of precision of symptoms and limited availability of diagnostic tests. For this study, we defined IRIS as any of the following developing within 12 weeks of treatment initiation, in the absence of an alternative diagnosis: i) fever and increased or new lymphadenopathy, or ii) pleural and pericardial effusions, ascites, abscess, cutaneous lesions and new or expanding central nervous lesions, iii) abnormal LFTs or hepatitis, or iv) atypical or exaggerated presentation of an opportunistic infection or tumour7–9. Liver function abnormalities, preterm delivery, pre-eclampsia and neurological toxicity were also identified a priori as adverse events of special interest.
Assessment of infant outcomes
We conducted a delivery visit as soon as possible, and within 14 days of birth. For live births, we recorded the following infant outcomes at the delivery visit: mode of delivery, duration of rupture of membranes, any complications, neonatal length, weight and head circumference, Apgar score and evidence of intrauterine growth retardation. At the delivery visit (0–14 days post-partum) we used COBAS TaqMan for HIV-1 DNA tests at the research sites.
We confirmed pre-term (<37 weeks gestation), and ‘premature’ (<34 weeks, classified as SAEs) births by Ballard Score10 if assessed within 7 days of delivery. Where any discrepancy arose, a pediatrician blinded to treatment arm assessed the infant and maternal dating evaluations within 48 hours of birth. We evaluated congenital anomalies using the WHO protocol11. For all infant deaths, we sought consent for verbal autopsy in an attempt to elucidate the cause of death.
We continue to follow-up mothers and infants to 72 weeks post-partum in order to assess the following secondary outcomes: maternal viral load response to 48 and 72 weeks (% under 50 and 1000 copies per mL), occurrence of infant HIV transmisisons at 48 and 72 weeks, dolutegravir exposure in maternal plasma, breast milk and infants and virological resistance.
Statistical analysis
Sample size calculations were based on clinical trials simulation (SAS v 9·3) using weighted probabilities (the weighted gestation-specific probabilities given different probabilities at different gestational ages) for achieving viral load <50 copies per mL for dolutegravir and efavirenz in treatment-naïve, non-pregnant adults4. Since the period of antiretroviral therapy prior to delivery could vary from a single dose to 12 weeks, we estimated statistical power from simulating five different distributions of gestational age at starting treatment in the third trimester12. Allowing for 20% dropout rate, recruitment of 250 evaluable HIV-positive women would retain ≥99% power to detect a superiority absolute difference of 28–38% between arms across all scenarios at the 5% level of significance.
Primary analyses were based on intention-to-treat. A generalised linear model (GLM) for the primary endpoint analysis included treatment as a study variable, baseline viral load (≥ or <100,000 copies per mL) and baseline CD4 (≥ or <200 cells per μL) as covariables, generating a risk ratio (RR; 95% CI) and risk difference (95% CI) between arms. Further covariate-adjusted GLM analysis of primary endpoint also incorporated age (< or ≥ median), country, and gestational age (< or ≥36 weeks) at baseline. Subgroup analysis was performed for each of the five pre-specified covariates. Sensitivity analysis of missing primary endpoint was also performed assuming (1) it did not achieve viral load <50 copies per mL; (2) it achieved viral load <50 copies per mL; and (3) it did not achieve suppression for dolutegravir group but achieved suppression for efavirenz group.
For time-to-event analyses, we used the actual visit dates to calculate time of viral load suppression. Kaplan-Meier curves for each treatment group were compared by the log rank test and hazard ratio (95% CI), calculated using the Cox regression model with viral load and CD4stratified covariables. Binary secondary outcomes were analyzed in a similar way as the primary endpoint analysis.
Role of the funding source
Neither ViiV Healthcare nor the study funder had any role in study design, data collection, data analysis, data interpretation, or writing of the report which remains the responsibility of the DolPHIN-2 trial management group, accountable to the Trial Steering Committee. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between 23rd January-15th August 2018, we screened and randomized 268 pregnant mothers (Figure 1), with mothers and infants comprising the safety evaluation population followed to delivery visit and beyond. Following withdrawals due to laboratory results falling outside eligibility criteria (including a pre-treatment viral load <50 copies per mL) an ‘Intent to Treat’ (ITT) population of 250 mothers (dolutegravir 125, efavirenz 125) was available for efficacy analyses with the last delivery visit on 21 December 2018.
FIGURE 1. CONSORT diagram for the DolPHIN-2 Study.
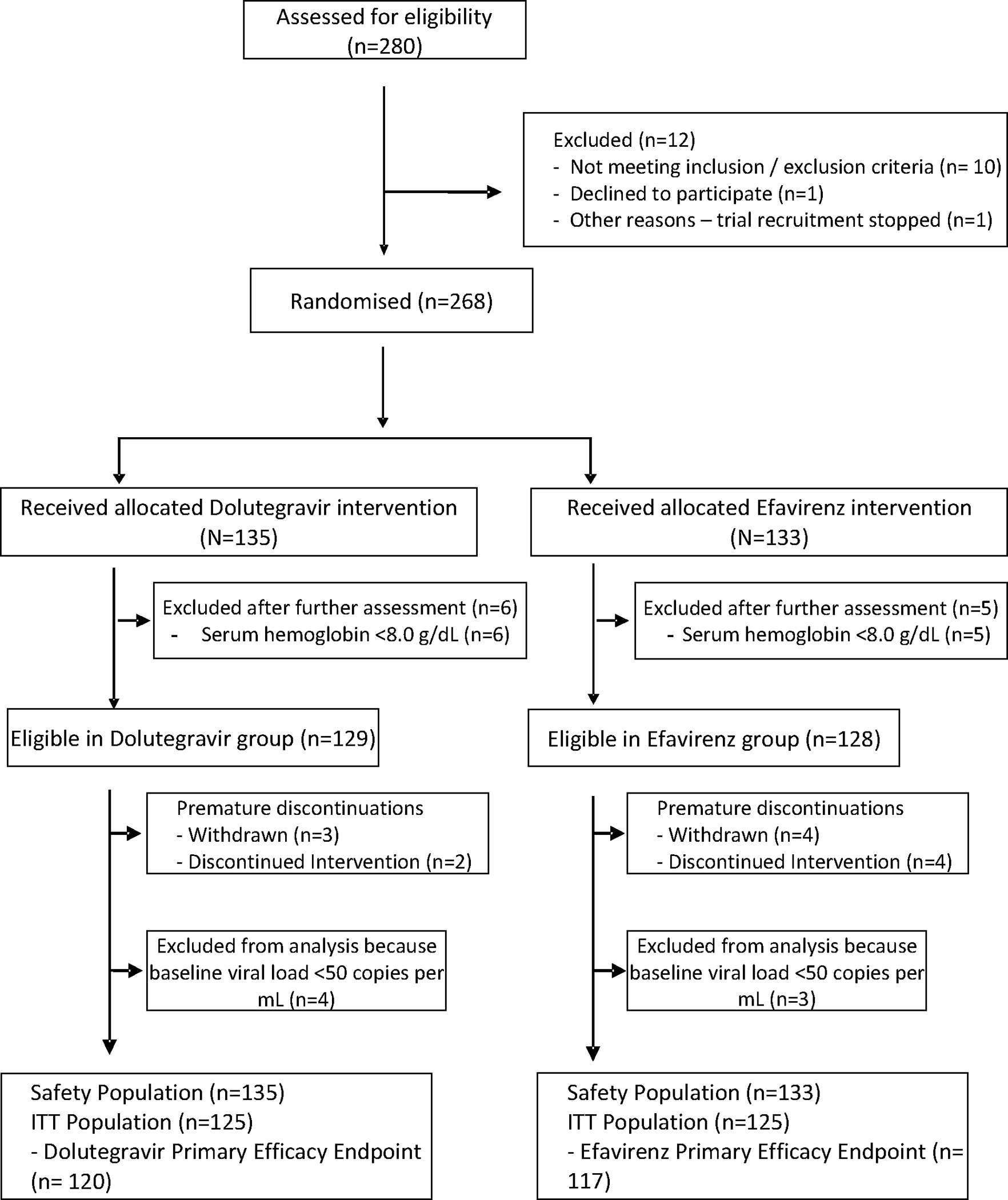
No significant differences were observed at baseline (Table 1) in median maternal age, gravidity, history of obstetric problems, median viral load or CD4 count. Use of herbal and traditional medicines was recorded in 34·8%, and vitamins or supplements in 37·6% of mothers. The median gestation at treatment initiation was 31 weeks with a median (IQR) time on treatment until delivery of 55 (33–77) days (52 (31–75) days for dolutegravir, and 59 (37–80) days for efavirenz).
TABLE 1.
Baseline Demographic Variables in the DolPHIN-2 Intent to Treat Population
| Variable | Statistic | Dolutegravir (N=125) | Efavirenz (N=125) | Total (N=250) |
|---|---|---|---|---|
|
| ||||
| Age (years) | Mean (SD) | 28·0 (5·3) | 27·4 (5·1) | 27·7 (5·2) |
| CD4 Count (cells per μL) | Median (IQR) | 464 (329– 664) | 414 (265 – 581) | 446 (296 – 633) |
| Log10 Viral Load | Median (IQR) | 4·4 (3·6 – 4·7) | 4.6 (3·9 – 4·8) | 4.4 (3·8 – 4·8) |
| Estimated gestation age (weeks) | Median (IQR) | 31 (29 –34) | 31 (29 –33) | 31 (29 –34) |
| Gravidity | Median (IQR) | 3 (2 – 4) | 3 (2 – 4) | 3 (2 – 4) |
| Previous live births | Median (IQR) | 2 (1 – 3) | 1 (1–2) | 2 (1–3) |
| Primigravida | Number (%) | 16 (12·8%) | 14 (11·2%) | 30 (12·0%) |
| Previous stillbirths | Yes (%) | 2 (1·6%) | 2 (1·6%) | 4 (1·6%) |
| Use of herbal/traditional medicine | Yes (%) | 42 (33·6%) | 45 (36·0%) | 87 (34·8%) |
| Use of supplements and vitamins | Yes (%) | 48 (38·4%) | 46 (36·8%) | 94 (37·6%) |
| Other co-medications | Yes (%) | 31 (24·8%) | 38 (30·4%) | 69 (27·6%) |
| Tobacco consumption in pregnancy | Yes (%) | 7 (5·6%) | 7 (5·7%) | 14 (5·6%) |
| Alcohol consumption in pregnancy | Yes (%) | 22 (17·6%) | 27 (21·6%) | 49 (19·6%) |
| History of psychiatric disorders | Yes (%) | 8 (6·5%) | 3 (2·4%) | 11 (4·4%) |
| Weight (kg) | Mean (SD) | 75·1 (16·1) | 71·8 (15·6) | 73·4 (16·0) |
| Height (cm) | Mean (SD) | 157·2 (7.8) | 158·0 (8·1) | 157·6 (7·9) |
| Site | South Africa | 57(45·60) | 57(45·60) | 114(45·60) |
| Uganda | 68(54·40) | 68(54·40) | 136(54·40) | |
Efficacy Outcomes
The primary endpoint of viral load <50 copies per mL at delivery by ITT was met in 74·2% (89/120) mothers receiving dolutegravir compared with 42·7% (50/117) in the efavirenz arm (risk ratio 1·64, 95%CI 1·31 – 2·06, P<0·0001; adjusted risk ratio 1·65, 95% CI 1·31 – 2·06, P <0·0001) – Table 2. The median time to achieve viral load<50 and <1000 copies per mL was 28 (95% CI: 28–34 ) and 7 (95% CI: 7–20) days for dolutegravir, and 82 (95% CI: 55–97) and 23(95% CI: 21–27) days for efavirenz based on Kaplan-Meier estimates (Figure 2). In a covariate-adjusted analysis, the risk ratio was unaltered by maternal age, country, baseline viral load, baseline CD4 count or gestation at enrolment, with consistent between-treatment differences across these covariates and no significant interactions observed. Sensitivity analysis (Table S1 p1) showed that results regarding the primary endpoint analyses are consistent and robust. The secondary endpoint of viral load<1000 copies per mL at delivery was also more likely achieved in women on dolutegravir vs efavirenz (93·3% vs 82·1%) with an adjusted risk difference of 10·36% (0·26 – 20·47; P = 0·044) and an adjusted risk ratio of 1·10 (95%CI 0·99–1·23; P = 0·089) (Table 2, Figure 2).
TABLE 2.
DolPHIN-2 Efficacy Results by General Linear Model
| no/No (%) of patients with events) | Estimate of relative risk (95% CI),P value | Estimate of risk difference (95% CI),P value | P-value for interaction test | |||
|---|---|---|---|---|---|---|
| Dolutegravir | Efavirenz | Dolutegravir vs Efavirenz | Dolutegravir vs Efavirenz | |||
|
| ||||||
| Outcome | ||||||
| Maternal HIV viral load <50 copies per mL at delivery(primary outcome) | Primary analysis * | 89/120 (74·2) | 50/117 (42·7) | 1.64 (1·31,2·06), <0·0001 | 29·78 (18·18,41·37), <0·0001 | |
| Covariate-adjusted analysis ** | 1.65 (1.31,2.06), <0·0001 | 30·48 (18·97,41·98), <0·0001 | ||||
| Maternal HIV viral load <1000 copies per mL at delivery | Primary analysis* | 112/120 (93·3) | 96/117(82·1) | 1·12 (1·00,1·25), 0·042 | 9·89 (0·94,18·84), 0·030 | |
| Covariate-adjusted analysis** | 1·10 (0·99,1·23), 0·089 | 10·36 (0·26,20·47), 0·044 | ||||
| By Subgroup | Subgroup analysis* | |||||
| Age | Age<28 | 39/54 (72·2) | 24/59 (40·7) | 1·71 (1·20,2·43), 0·003 | 31·60 (14·56,48·64), 0·0003 | 0·867 |
| Age≥28 | 50/66 (75·8) | 26/58 (44·8) | 1·61 (1·20,2·16), 0·001 | 26·66 (10·49,42·83), 0·001 | ||
| Country | South Africa | 43/56 (76·8) | 25/55 (45·5) | 1·64 (1·19,2·25), 0·002 | 30·01 (12·97,47·04), 0·0006 | 0·931 |
| Uganda | 46/64 (71·9) | 25/62 (40·3) | 1·55 (1·16,2·09), 0·004 | 28·45 (11·18,45·71), 0·001 | ||
| Viral load at baseline | Viral load<100,000 copies per mL | 81/103 (78·6) | 47/95 (49·5) | 1·59 (1·27,1·99), <0·0001 | 29·13 (16·34,41·91), <0·0001 | 0·274 |
| Viral load≥100,000 copies per mL | 8/17 (47·1) | 3/22 (13·6) | 3·41 (1·04,11·14), 0·042 | 32·93 (5·14,60·72), 0·020 | ||
| CD4 at baseline | CD4≥200 | 81/107 (75·7) | 46/99 (46·5) | 1·61 (1·27,2·02), <0·0001 | 28·95 (16·48,41·42), <0·0001 | 0·600 |
| CD4<200 | 8/13 (61·5) | 4/18 (22·2) | 2·30 (0·86,6·15), 0·097 | 35·39 (3·35,67·43), 0·030 | ||
| Gestational age at initiation of HIV therapy | Gestational age<36 | 72/97 (74·2) | 46/103 (44·7) | 1·64 (1·29,2·07), <0·0001 | 29·80 (17·34,42·25), <0·0001 | 0·522 |
| Gestational age≥36 | 17/23 (73·9) | 4/14 (28·6) | 1·35 (0·79,2·30), 0·277 | 27·90 (−9·55,65·35), 0·144 | ||
The generalized linear model has the treatment as a study variable, viral load (≥ or <100,000 copies) and CD4(≥ or <200 cells per μL) as covariables
The generalized linear model has the treatment as a study variable, viral load (≥ or <100,000 copies), CD4(≥ or <200 cells per μL), age (< or ≥ median in years), country (South African or Uganda), and gestational age at enrolment (< or ≥ 36 weeks) as covariables
FIGURE 2. Proportion with viral load <50 copies per mL and <1000 copies per mL following randomization.
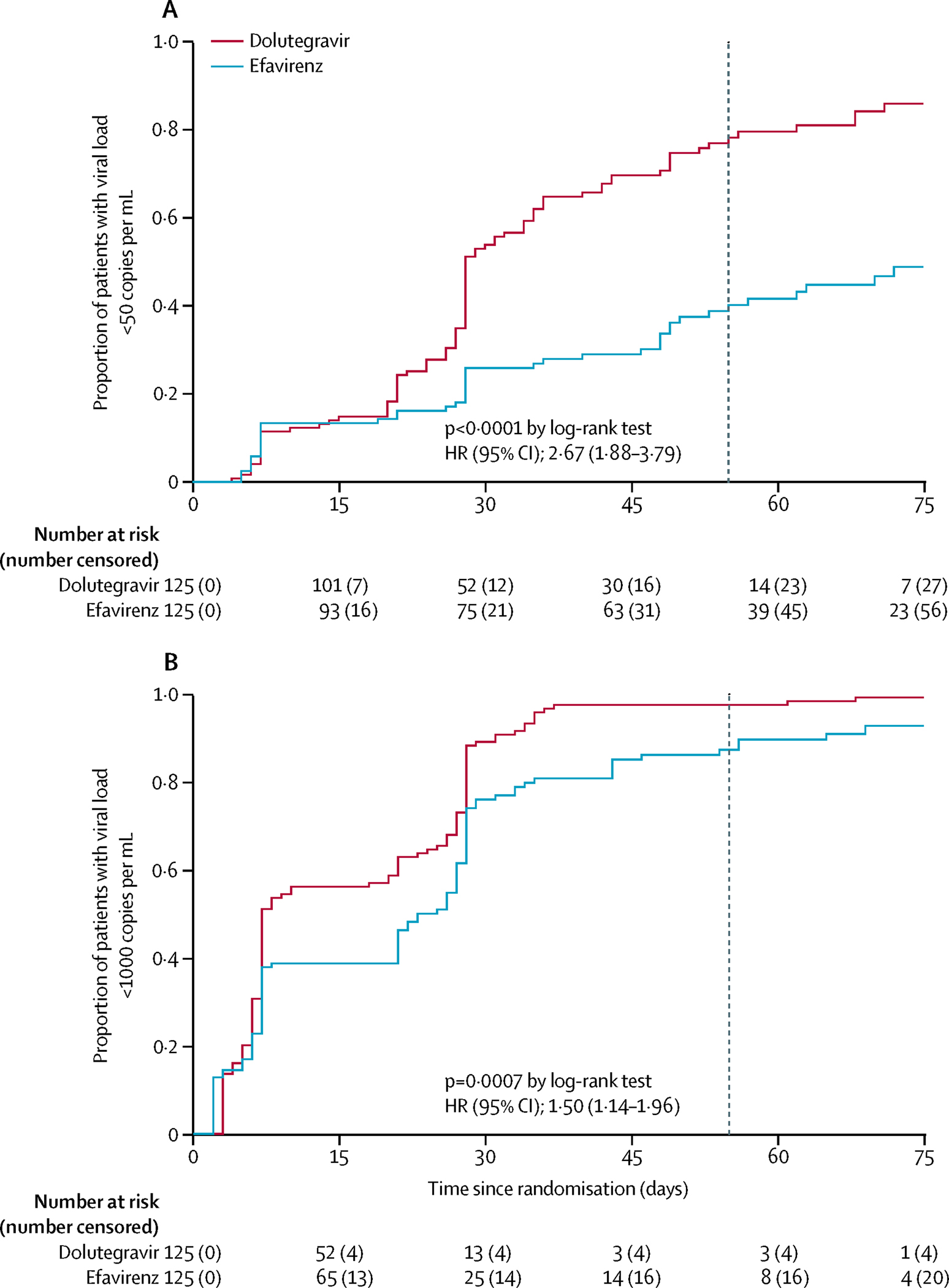
Kaplan-Meier plots of time from randomization to HIV viral load (a) <50 copies per mL and (b) <1000 copies per mL. Dashed vertical line in each plot represents the median time from randomization to delivery (55 days) in the total trial population.
Three mother-to-child transmissions of HIV were detected, all in the dolutegravir arm and each confirmed by two to four separate HIV DNA-positive tests. The first infant tested HIV-positive at five days of age, after 35 days of maternal dolutegravir from 32 weeks of gestation. The second infant tested HIV DNA-positive at three days of age, after 32 days of maternal dolutegravir from 32 weeks of gestation while the third tested positive at 11 days, after 24 days of maternal dolutegravir from 30 weeks of gestation. The maternal viral load at delivery visit was <50 copies per mL in the first two cases (with baseline viral loads of 48,969 and 32,844 copies per mL), and in the third case, was 31,354 copies per mL at baseline, and 200 copies per mL at delivery. We considered in-utero transmission likely given the early PCR-positivity of each infant, coupled with the low maternal viral loads at delivery s. All three mothers remained well, without signs of illness or IRIS.
Safety Outcomes
Maternal
Although antiretroviral therapy was well-tolerated across both arms, more mothers in the dolutegravir arm (30/137 vs 14/131; P = 0·013) reported any type of SAE. This was driven by a higher overall frequency of ‘Pregnancy, Puerperium and Perinatal’ events in mothers receiving dolutegravir (Table 3; supplementary table S2 p2–3), the majority of whom were judged to have prolonged pregnancy beyond term, with or without other conditions which led to Caesarean section being performed. Overall, there were 12 C-sections in the dolutegravir arm and 7 in the efavirenz arm (of which one was in the mother who had a stillbirth in the efavirenz arm). At the 28 day visit, we observed a modest increase in serum creatinine from baseline of 0.08mg/dL in the dolutegravir arm and of 0.02mg/dL for efavirenz; P<0·0001. Antepartum change in weight over the first 28 days of treatment did not differ between arms.
TABLE 3.
Serious adverse events, and pre-term deliveries in mothers and infants
| Dolutegravir | Efavirenz | Total | |
|---|---|---|---|
|
| |||
| Maternal SAEs | N = 137 (%) | N = 131 (%) | N = 268 (%) |
| Mothers with ≥1 SAE1 | 30 (21·9)2 | 14 (10·7)2 | 44 (16·4) |
| Mothers with ≥1 drug-related SAE | 1 (0·7) | 0 | 1 (0·4) |
| Mothers with ≥1 IRIS-related SAE | 1 (0·7) | 0 | 1 (0·4) |
| SYSTEM ORGAN CLASS | |||
| Blood and lymphatic system disorders | 2 (1·5) | 0 | 2 (0·7) |
| Gastrointestinal disorders | 1 (0·7) | 0 | 1 (0·4) |
| Infections and infestations | 5 (3·6) | 2 (1·5) | 7 (2·6) |
| Pregnancy, puerperium and perinatal conditions excl. stillbirths | 18 (13·1) | 10 (7·6) | 28 (10·4) |
| Renal and urinary disorders | 3 (2·2) | 0 | 3 (1·1) |
| Social circumstances | 1 (0·7) | 0 | 1 (0·4) |
| Vascular disorders | 0 | 1 (0·8) | 1 (0·4) |
|
| |||
| Deliveries with evaluable gestational age | N = 124 | N = 120 | N = 244 |
| Gestation3 at delivery (median; IQR) | 39 (37·2, 40·7) | 39 (37·3, 40·0) | 39 (37·3, 40·3) |
| Late pre-term (<37 weeks) | 21 (16·9) | 19 (15·8) | 40 (16·4) |
| Premature (< 34 weeks) | 3 (2·4) | 5 (4·2) | 8 (3·3) |
| Stillbirth | 3 (2·2) | 1 (0·8) | 4 (1·5) |
|
| |||
| Infant SAEs (242 live births) | N = 123 (%) | N = 119 (%) | N = 242 (%) |
| Birth weight (g; median; IQR) | 3180 (2800, 3440) (N = 109) | 3115(2860, 3420) (N = 118) | 3160 (2840, 3440) (N = 227) |
| Infants with ≥1 SAE | 61 (49·6) | 56(47·1) | 117 (48·3) |
| Infant deaths | 4 (3·3) | 2 (1·7) | 6 (2·5) |
| SYSTEM ORGAN CLASS | |||
| Congenital, familial and genetic disorders | 67 (54·5) | 71 (59·7) | 138 (57·0) |
| Ear and labyrinth disorders | 1 (0·8) | 0 | 1 (0·4) |
| Hepatobiliary disorders | 1 (0·8) | 0 | 1 (0·4) |
| Infections and infestations | 8 (6·5) | 6 (5·0) | 14 (5·8) |
| Injury, poisoning and procedural complications | 1 (0·8) | 0 | 1 (0·4) |
| Nervous system disorders | 1 (0·8) | 1 (0·8) | 2 (0·8) |
| Pregnancy, puerperium and perinatal conditions | 4 (3·3) | 7 (5·9) | 11 (4·5) |
| Respiratory, thoracic and mediastinal disorders | 8 (6·5) | 4 (3·4) | 12 (5·0) |
Including stillbirths.
P = 0·013, Chi-squared test
Gestation at delivery based on best estimate using recall of last menstrual period, fundal height and ultrasound dating, modified post-partum by the Ballard score.
Four stillbirths were reported (three in the dolutegravir arm; 2·2%). The first was a macerated stillbirth at 40w gestation, judged to be related to maternal syphilis. The second was at 36w gestation following uterine rupture and hemoperitoneum, with a history of maternal syphilis. The third fetus was a post-term pregnancy at 41w gestation, with evidence of fetal distress (efavirenz arm). The fourth was a macerated stillbirth at 41w gestation to a mother with a history of intermittent fevers (onset preceding antiretroviral therapy) and respiratory symptoms, presumptively treated for pulmonary tuberculosis; there was a history of traditional medicine use and treatment for malaria at 16 weeks gestation. All four cases were considered unlikely to be related to maternal antiretrovirals or IRIS.
Infant
A total of 242 evaluable live births (dolutegravir 123, efavirenz 119) were assessed, with a median gestation at delivery of 39 weeks for both arms, and no significant difference in pre-term and premature deliveries (Table 3), frequency of SAEs, or infant birthweights between arms. Six infant deaths (dolutegravir-4, efavirenz-2) were reported (Table S4 p6). Two deaths were related to extreme prematurity (a 1-day old twin born at 28 weeks gestation in the efavirenz arm, and a 19day old infant born at 32 weeks gestation in the dolutegravir arm, whose mother suffered an antepartum hemorrhage). A further four deaths were related to respiratory distress or asphyxia: three in the dolutegravir arm (aged 2, 47 and 88 days, full term), one in the efavirenz arm (aged 14 days, born at 35 weeks gestation).
Congenital disorders (Table S3 p4–5) also did not differ between arms, and comprised (dolutegravir vs efavirenz): umbilical hernias (37/124 (29·8%) vs 41/120 (34·2%)), birth marks (21/124 (16·9%) vs 21/120 (17·5%)), skin dimples (dolutegravir-3), acrochordon (dolutegravir-2, efavirenz-3), heterochromia iridis (dolutegravir-1), laryngomalacia (dolutegravir-1), strabismus (dolutegravir-1, efavirenz-1), talipes (dolutegravir-1, efavirenz-2), cleft palate (efavirenz-1), polydactyly (efavirenz2). No neural tube defects were reported.
Discussion
We found women on dolutegravir-based therapy were significantly more likely to achieve a VL of <50 copies per mL (or less likely to have a VL of ≥50 copies per mL) at delivery compared with those taking efavirenz regimens when initiated in the third trimester. These data address a critical knowledge gap around antepartum transmission of HIV in women initiating treatment late in pregnancy13–14 whereas other studies are assessing use of dolutegravir earlier in pregnancy. Undisclosed antiretroviral therapy was unlikely since we excluded mothers with VL <50 copies per mL at baseline. Smaller differences were observed in the proportion achieving HIV viral load <1000 copies per mL than that of achieving HIV viral load <50 copies per mL, an alternative threshold for transmission risk15. In calculating the time from randomisation to the occurrence of achieving viral suppression, we used the actual visit dates at the scheduled visits of 7 and 28 days. Such an algorithm may bias the true time of viral load suppression, however since it was equally applied in both arms, any bias should be similar. Given that peri-partum HIV transmission is strongly correlated with the prevailing maternal viral load, dolutegravir based regimens may reduce HIV transmission around birth and potentially during breast feeding versus efavirenz-based regimens in our population of pregnant mothers. The three HIV-infected infants detected were considered likely in-utero infections, although peripartum transmission could not be excluded as we did not test infants within two days of birth. Longer follow-up to detect transmissions during breastfeeding is ongoing.
We found both dolutegravir and efavirenz to be well-tolerated in mothers and infants. However, a higher proportion of mothers receiving dolutegravir developed SAEs. This was driven by a higher overall frequency of ‘Pregnancy, Puerperium and Perinatal’ events in mothers receiving dolutegravir (supplementary table S1), the majority of whom were judged to have prolonged pregnancy beyond term. The significance of this finding is unclear, since estimation of gestational age in late pregnancy is not always accurate. It is important to examine whether our findings are replicated in other studies examining dolutegravir in pregnancy (e.g. VESTED; NCT03048422 and the Antiviral Pregnancy Registry). The incidence of serious adverse events and treatment-related toxicities overall was comparable to previous large randomised trials in non-pregnant adults in sub-Saharan Africa7,18,19.
The infant deaths, stillbirths and infant transmissions observed attest to the poor outcomes previously reported in this group of mothers3. Detailed examination revealed that antiretroviral therapy or IRIS was considered unlikely to play a significant role in any of these cases. Of the four stillbirths, two were related to obstetric complications and two to severe maternal infection. The six infant deaths were related to conditions prevalent in Uganda and S Africa where neonatal mortality rates are four to nine times those of Western Europe20,21. We did not observe any increased risk of birth defects, although treatment was only initiated after organogenesis was completed.
Our sample size was not sufficiently large to study differences in infant transmissions, although we were powered to detect virological superiority by delivery (the best validated proxy for vertical HIV transmission). Safety follow-up of mothers and babies was limited given the relatively short time between third trimester initiation and our primary endpoint at delivery, however continued follow-up of post-partum and infant outcomes continues to 72 weeks. The higher frequency of SAEs observed with dolutegravir should be confirmed in other studies and observational datasets which are planned and in progress.
Policymakers weigh risk-benefit of new treatments before data in pregnancy are available22. A preliminary association with neural tube defects triggered precautionary measures globally, ranging widely in permissiveness for dolutegravir use in women23–25, increasing the burden of healthcare provision26, and delaying implementation of dolutegravir rollout. Population modelling suggests that net population health benefits accrue when transitioning to dolutegravir, including for women of child bearing potential27,28. This would hold true notwithstanding the differences in SAEs amongst mothers assigned to dolutegravir. The DolPHIN-2 results, taken together with the current evidence favoring dolutegravir use over efavirenz (especially in countries where HIV drug resistance is increasing29) strongly support global transition to dolutegravir use in first-line antiretroviral therapy.
Supplementary Material
Panel: Research in context.
Evidence before this study
Late initiation of antiretroviral therapy in the third trimester of pregnancy is associated with a seven-fold increased risk of infant HIV transmissions, and a doubling of infant mortality in the first year of life. The period of greatest risk for mother-to-child HIV transmission is during labour and delivery, and risks are highest for mothers who are not virologically suppressed at this time. Using a PubMed search (‘HIV’, ‘pregnancy’, ‘initiation’, ‘antiretroviral therapy’, ‘Africa’; 10th February 2020) we confirmed that across sub-Saharan Africa, late presentation for antiretroviral therapy during pregnancy is not uncommon (up to 1 in 5 pregnancies from South Africa). Conventional (efavirenz-based) antiretroviral regimens may simply have insufficient time to suppress maternal HIV viral load quickly enough in this scenario. Newer, dolutegravir-based regimens are associated with faster declines in HIV viral load in non-pregnant adults, reaching undetectable (<50 copies per mL) viral load after a median of 28 days in comparison with 84 days for efavirenz. However, the safety and efficacy of dolutegravir in pregnancy is unknown, and there is a general lack of randomised trial data informing selection of newer antiretroviral regimens for use in pregnant mothers. Moreover, recent data from a large birth registry in Botswana suggest peri-conception use of dolutegravir was associated with an excess risk of neural tube defects. This safety concern has led to precautionary alerts from WHO and regulatory agencies, and the restriction of use of dolutegravir in pregnancy or women of child-bearing potential.
Added value of this study
DolPHIN-2 randomised 268 pregnant mothers initiating antiretroviral therapy in third trimester to receive either efavirenz- or dolutegravir-based regimens. Dolutegravir was associated with superior rates of viral load reduction. Although both regimens were well-tolerated by mothers and infants, more mothers receiving dolutegravir developed serious adverse events (driven mainly by prolonged pregnancies). The significance of this finding is uncertain, and should be confirmed in other studies.
Implications of all the available evidence
Risk-benefit evaluation of drug use in pregnancy needs to be separately undertaken according to trimester of exposure, especially given risks may be higher in early pregnancy, whereas benefits may be greater for pregnant mothers starting treatment in late pregnancy. The large differences in rates of virological suppression between both regimens suggest that significant public health benefits could accrue if dolutegravir-based therapy is widely implemented. Our data support the recent revision to WHO guidelines recommending transition to dolutegravir in first line antiretroviral therapy for all adults, regardless of pregnancy or child-bearing potential.
ACKNOWLEDGEMENTS
We acknowledge the invaluable and generous contributions from all study participants, as well as from staff across health facilities who supported recruitment into our study. We thank Yusif Alhassan for advice. The DolPHIN-2 Study was funded by Unitaid. Dolutegravir was donated by ViiV Healthcare.
The DolPHIN-2 Study Team
Trial Steering Committee and Independent Data and Safety Monitoring Committee:
Marta Boffito, Polly Clayden, Tim Peto (chair IDSMB), Anton Pozniak (chair TSC), Graham Taylor
Infectious Diseases Institute, Kampala:
Tabitha Ayabo, Sabrina Bakeera Kitaka, Pauline Byakika-Kibwika, Daniel Kiiza, Isabella
Kyohairwe, Eva Laker, Andrew Luswata, Johnson Magoola, Hamza Mayanja, Flavia Vivian
Najjuma, Ritah Nakijoba, Diana Namuddu, Teopista Namuli, Peter Ntuyo, Annet Onzia, Emmanuel Sempijja, Jovia Tabwenda, Baluku William
University of Cape Town:
Nina Abrahams, Phakamani Magano, Sharon Bokako, Carmen Delport, Linda Hlwaya, Nai-Chung
Hu, Ushma Mehta, Dineo Molitsane, Megan Mrubata, Jasantha Odayar, Lee-Ann Stemmet, Sivuyile Tambula, Helene Theunissen, Mbuyiswa Tyam, Olga Venfolo
University of Liverpool:
Katie McAllister, Justin Chiong, Laura Else, Steve Potter
Royal Liverpool University Hospital NHS Trust:
Anne Neary
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DATA SHARING STATEMENT
We adhere to the principles of the UK Concordat on Open Research Data, which recognises that research data should wherever possible be made openly available for use by others in a manner consistent with relevant legal, ethical, disciplinary and regulatory frameworks and norms, and with due regard to the cost involved. Our data will be assigned a DOI through deposition in the University of Liverpool Research Data Catalogue (rdm@liverpool.ac.uk) and shared under a Data Transfer agreement (or equivalent- eg as part of a research collaboration agreement or confidentiality disclosure agreement), with all originating DolPHIN-2 data remaining the property of the University of Liverpool.
References
- 1.Statistics UBO. Uganda Demographic and Health Survey 2016. Rockville, Maryland, USA: 2018. [Google Scholar]
- 2.Kisuule I, Kaye DK, Najjuka F, et al. Timing and reasons for coming late for the first antenatal care visit by pregnant women at Mulago hospital, Kampala Uganda. BMC Pregnancy and Childbirth. 2013;13:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebonwu J, Mumbauer A, Uys M, Wainberg ML, Medina-Marino A. Determinants of late antenatal care presentation in rural and peri-urban communities in South Africa: A cross-sectional study. PloS One. 2018;13(3):e0191903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyers K, Qian H, Wu Y, et al. Early Initiation of ARV During Pregnancy to Move towards Virtual Elimination of Mother-to-Child-Transmission of HIV-1 in Yunnan, China. PloS One. 2015;10(9):e0138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. The New England Journal of Medicine. 2013;369(19):1807–18. [DOI] [PubMed] [Google Scholar]
- 6.International Council for Harmonisation. https://www.meddra.org/ version 22.0, accessed 18th June 2019
- 7.Gallagher RM, Kirkham JJ, Mason JR, et al. Development and inter-rater reliability of the Liverpool adverse drug reaction causality assessment tool. PloS One. 2011;6(12):e28096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hakim J, Musiime V, Szubert AJ, et al. Enhanced Prophylaxis plus Antiretroviral Therapy for Advanced HIV Infection in Africa. The New England Journal of Medicine. 2017;377(3):233–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.French MA, Price P, Stone SF. Immune restoration disease after antiretroviral therapy. AIDS. 2004;18(12):1615–27. [DOI] [PubMed] [Google Scholar]
- 10.Meintjes G, Lawn SD, Scano F, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. The Lancet Infectious Diseases. 2008;8(8):516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quinn JA, Munoz FM, Gonik B, et al. Preterm birth: Case definition & guidelines for data collection, analysis, and presentation of immunisation safety data. Vaccine. 2016;34(49):6047–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. https://www.who.int/tdr/news/2012/pregnancy_registry_protocol/en/, accessed 13th June 2019
- 13.Myer L, Phillips TK, Hsiao NY et al. Plasma viraemia in HIV-positive pregnant women entering antenatal care in South Africa . J Int AIDS Soc. 2015;18:20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mofenson LM. In-utero ART exposure and the need for pharmacovigilance. The Lancet Global Health 2018; 6:e716–e717 [DOI] [PubMed] [Google Scholar]
- 15.Black V, Schwartz SR. Issues about periconception use of dolutegravir are reminiscent of early concerns about efavirenz. The Lancet HIV 2018;5:e732–e736 [DOI] [PubMed] [Google Scholar]
- 16.Myer L, Phillips T, McIntyre J, et al. HIV viraemia and mother-to-child transmission risk after antiretroviral therapy initiation in pregnancy in Cape Town, South Africa. HIV Med 2017;18: 80–88. [DOI] [PubMed] [Google Scholar]
- 17.Waitt C, Orrell C, Walimbwa S, et al. The DolPHIN-1 study: A randomised trial of the sSafety and pharmacokinetics of dolutegravir in pregnant mothers with HIV infection and their neonates. PLoS Medicine 2019. Sep 20;16(9):e1002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulligan N, Best BM, Wang J, et al. Dolutegravir pharmacokinetics in pregnant and postpartum women living with HIV. AIDS. 2018. Mar 27;32(6):729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DART Trial Team. Fixed duration interruptions are inferior to continuous treatment in African adults starting therapy with CD4 cell counts <200 cells/microl. AIDS. 2008;22(2):237–47 [DOI] [PubMed] [Google Scholar]
- 20.Flynn PM, Taha TE, Cababasay M, et al. Prevention of HIV-1 Transmission Through Breastfeeding: Efficacy and Safety of Maternal Antiretroviral Therapy Versus Infant Nevirapine Prophylaxis for Duration of Breastfeeding in HIV-1-Infected Women With High CD4 Cell Count (IMPAACT PROMISE): A Randomized, Open-Label, Clinical Trial. Journal of Acquired Immune Deficiency Syndromes. 2018;77(4):383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tlou B, Sartorius B, Tanser F. Investigating risk factors for under-five mortality in an HIV hyper-endemic area of rural South Africa, from 2000–2014. PloS One. 2018;13(11):e0207294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unicef. Neonatal mortality 2018. Available from: https://data.unicef.org/topic/child-survival/neonatalmortality/., accessed 13th June 2019 [Google Scholar]
- 23.Colbers A, Mirochnick M, Schalkwijk S, Penazzato M, Townsend C, Burger D. Importance of prospective studies in pregnant and breastfeeding women living with HIV. Clinical Infectious Diseases 2019; Feb 13. pii: ciz121. doi: 10.1093/cid/ciz121. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zash R, Holmes L, Jacobson DL, et al. Neural-Tube Defects and Antiretroviral Treatment Regimens in Botswana. The New England Journal of Medicine. 2019; Jul 22. doi: 10.1056/NEJMoa1905230. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. https://www.who.int/medicines/publications/drugalerts/Statement_on_DTG_18May_2018final.pdf., accessed 13th June 2019
- 26.US Food and Drug Administration. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drugsafety-communication-fda-evaluate-potential-risk-neural-tube-birth-defects-hiv-medicine accessed 13th June 2019.
- 27.Arnold A, Laker Odongpiny EA, Owarwo N, et al. Ugandan clinic experience following potential teratogenicity alert for dolutegravir. CROI 2019; Seattle, Washington: 2019, March. [Google Scholar]
- 28.Phillips AN, Venter F, Havlir D, et al. Risks and benefits of dolutegravir-based antiretroviral drug regimens in sub-Saharan Africa: a modelling study. The Lancet HIV. 2019;6(2):e116–e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dugdale CM, Ciaranello AL, Bekker L, et al. Risks and Benefits of Dolutegravir- and Efavirenz-Based Strategies for South African Women With HIV of Child-Bearing Potential: A Modeling Study. Ann Intern Med. 2019. Apr 2. doi: 10.7326/M18-3358. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta R, Gregson J, Parkin N, et al. HIV-1 drug resistance before initiation or re-initiation of first-line antiretroviral therapy in low-income and middle-income countries: a systematic review and metaregression analysis. Lancet Infect Dis. 2018. Mar;18(3):346–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We adhere to the principles of the UK Concordat on Open Research Data, which recognises that research data should wherever possible be made openly available for use by others in a manner consistent with relevant legal, ethical, disciplinary and regulatory frameworks and norms, and with due regard to the cost involved. Our data will be assigned a DOI through deposition in the University of Liverpool Research Data Catalogue (rdm@liverpool.ac.uk) and shared under a Data Transfer agreement (or equivalent- eg as part of a research collaboration agreement or confidentiality disclosure agreement), with all originating DolPHIN-2 data remaining the property of the University of Liverpool.


