Highlights
-
•
The slightly acidic electrolyzed water (SAEW) used to treat Chinese shrimp has been optimized.
-
•
SAEW treatment significantly delayed the deterioration of Chinese quality.
-
•
SAEW treatment effectively extends the shelf life of Chinese shrimp.
Keywords: Slightly acidic electrolyzed water, Chinese shrimp, Sterilization process optimization, Response surface, Quality
Abstract
Chinese shrimps are popular among consumers for their delicious taste and high nutritional value, but they are highly susceptible to deterioration due to microbial contamination with degradation of texture, color and flavor. The aim of this study was to evaluate the effects of available chlorine concentration (ACC), processing time and material-liquid ratio on the bacterial inhibition rate of shrimp treated with slightly acidic electrolyzed water (SAEW). The effective parameters were optimized by response surface methodology to the optimal bactericidal conditions: ACC 88 mg/L, processing time 12 min, and material-liquid ratio 1:4. The actual bactericidal inhibition rate of shrimp under these conditions was 37.60 %. On this basis, the quality, color difference and textural changes of shrimp treated with SAEW, sodium hypochlorite and alkaline electrolytic water were compared and investigated during storage at 4 °C. The combined results showed that the SAEW treatment could extend the shelf-life by more than 2 d.
1. Introduction
Chinese shrimps are popular among consumers for their delicious flavor and high nutritional value. However, due to their high-moisture content and their own enzymatic and microbial action, Chinese shrimp are highly susceptible to spoilage with degradation of texture, color and flavor (Wu et al., 2021). Among them, microbial contamination may start at the beginning of harvesting or during contact with processing surfaces, and then under unfavorable storage conditions, causing the multiplication of spoilage microorganisms and accelerating their spoilage (Semeano et al., 2018). And the high spoilage rate of shrimp is the main reason limiting the transportation and storage of Chinese shrimp (Peng et al., 2022). Therefore, it is necessary to adopt appropriate preservation techniques to inhibit or decelerate the spoilage and deterioration of Chinese shrimp and prolong its storage period, which is of great significance to improve the level of consumption and increase the export of products to earn foreign exchange.
Slightly acidic electrolyzed water (SAEW) is increasingly valued for its broad-spectrum bactericidal effect and green safety, due to its chemical composition (HClO as the main chlorine compound) and near-neutral pH (Ding et al., 2015, Ding et al., 2015), there are few adverse effects on human health and the environment. Studies on the bactericidal effects of SAEW on a wide range of bacteria have been reported in the current literature (Al-Holy and Rasco, 2015, Li et al., 2017, Li et al., 2017, McCarthy and Burkhardt, 2012), and SAEW has been used for disinfection of food processing surfaces and other sites (Yan et al., 2023). The bactericidal mechanism of SAEW is mainly analyzed in terms of the dominant bactericidal factor and the target of action on microbial cells, and the characterization parameters of SAEW are available chlorine concentration (ACC), pH and oxidation reduction potential (ORP). Regarding the dominant bactericidal factor of SAEW, with the deepening of the research on bactericidal mechanism, the ACC alone is recognized as the dominant bactericidal factor of SAEW (Li et al., 2021, Liu et al., 2022, Liu et al., 2022). High concentrations of H+ cause hydrolysis of bacterial proteins and nucleic acids, and change the charge distribution of cell membranes, resulting in nutrient uptake; in addition, pH exceeding the range of enzyme activity can cause cellular metabolic disorders, which ultimately lead to bacterial death. High ORP affects electron flow in microbial cells, causing cell membrane rupture and promoting microbial death (Zhu et al., 2018). ACC disrupts microbial cell membranes and reacts with their DNA and mitochondria leading to death (Sun et al., 2022). Regarding the sterilization of seafood, relevant studies have been applied to squid (Xuan et al., 2017) and fish (Liu et al., 2023), whereas there are few reports on the application of SAEW to Chinese shrimp, and even more limited research on the process of SAEW in the sterilization of Chinese shrimp. Therefore, we took Chinese shrimp (Fenneropenaeus chinensis), an excellent shrimp species among Chinese shrimps, as the research object to investigate the effect of SAEW on its sterilization treatment, and compared the fence preservation technology by combining with three chemical bactericides on the basis of low-temperature storage.
A study evaluated the efficacy of slightly acidic electrolyzed water (SAEW) to reduce natural microbiota on celery and parsley at different effective chlorine concentrations (ACC), different treatment times and temperatures (Zhang et al., 2016). When SAEW was used as a bactericide for hatching eggs, its bactericidal efficiency increased with the increase of effective chlorine concentration (ACC), spray volume and sterilization time (Liu et al., 2022, Liu et al., 2022). The effect of temperature on the efficacy of SAEW was not considered because the preservation process of shrimp in this study was fixed at 4 °C. Therefore, this study investigated the optimal range of three single factors of SAEW, including ACC, processing time, and material-liquid ratio, to examine the inactivation of total colony counts in Chinese shrimp. Based on the results, a three-factor, five-level response surface method was designed to compare the effects of bacterial inhibition rate on Chinese shrimp and the interactions among the factors. The response surface model was used to predict the optimal sterilization process, which provides a process reference for SAEW to be used in the pre-treatment of shrimp for sterilization. The actual bacterial inhibition rate under the predicted process was determined by validation test, and the theoretical process of sterilization was further transformed into the actual process after the comparison of the error between the actual value and the predicted value. Subsequently, the quality changes of Chinese shrimp after treatment with sodium hypochlorite (NaClO), alkaline electrolyzed water (AlEW) and SAEW were compared with those of Chinese shrimp, using total number of colonies, pH, TVB-N, TBA, color difference and texture as freshness indexes, during the low temperature storage at 4 °C.
2. Materials and methods
2.1. Raw materials and pre-treatment
Chinese shrimp (Fenneropenaeus chinensis) was purchased from Yangling Trust-Mart Supermarket Chain Co. Individuals that were complete and of uniform size (length 15.0 cm ± 0.5 cm, width 2.0 cm ± 0.5 cm, weight 18.5 g ± 1.0 g) were selected, rinsed with tap water, drained of body surface water, and packaged in aseptic sealed bags for use.
2.2. SAEW preparation
Slightly acidic electrolyzed water (SAEW) is generated by electrolyzing 0.1 % NaCl and 0.01 % HCl solution at a rate of 50–80 L/h under 220 V, 18.3 A in a Harmony-II potentiostatic water generator (Harmony-II, Rui Andre Environmental Equipment Co., Ltd., Beijing, China), which consists of diaphragm-less electrolyzer. SAEW with corresponding available chlorine concentration (ACC) was prepared by electrolyzing for different times, and then the pH was adjusted to 5.0–6.5 by 0.1 mol/L HCl or NaOH solution and the oxidation reduction potential (ORP) was determined. The pH and ORP were determined using a pH/ORP meter (FE28, Mettler Toledo Instruments Co., Ltd, Switzerland), and the ACC was determined using a chlorine residual meter (PTH 027, Palintest Ltd, UK). The prepared SAEW must be stored in airtight containment away from light and standby before the test, and then its physicochemical indexes were measured again before use, and it was better to store it for less than 3 days. The physicochemical properties of SAEW are shown in Table S1.
2.3. Single factor experiment for SAEW treatment
2.3.1. Effect of ACC of SAEW treatment on sterilization effect
Chinese shrimps were immersed in SAEW with ACC of 20, 40, 60, 80, and 100 mg/L made from SAEW generator at a material-liquid ratio of 1:4 for 15 min, and the effect of ACC of SAEW on the bactericidal effect was investigated by determining the total number of colonies of the samples, as well as the control group (untreated) and the group with sterile water.
2.3.2. Effect of processing time of SAEW treatment on sterilization effect
Chinese shrimps were treated with SAEW made from a SAEW generator with an ACC of 60 mg/L at a material-liquid ratio of 1:4 for 5, 10, 15, 20, and 25 min, respectively, and the effect of the processing time of SAEW on the bactericidal effect was investigated by determining the total number of colonies of the samples, and the control (0 min) was determined at the same time.
2.3.3. Effect of material-liquid ratio of SAEW treatment on sterilization effect
Chinese shrimps were treated with SAEW made from the SAEW generator with an ACC of 60 mg/L at material-liquid ratios of 1:1, 1:2, 1:4, 1:6, and 1:8 (w/v) for 15 min, respectively, and the effects of material-liquid ratios of the SAEW-treated shrimp on the sterilizing effect were investigated by determining the total number of colonies of the samples, and the control (1:0) was determined at the same time.
2.4. Response surface experimental design
On the basis of the single-factor experiments, a three-factor, five-level response surface test was designed using the Central Composite Design (CCD) method of the Design Expert software, with ACC (A), processing time (B), and material-liquid ratio (C) as independent variables, and bacterial inhibition rate as the response value to study the interactions among the factors. The experimental factors and level coding values are detailed in Table S2.
Untreated shrimps were used as a control, and the shrimp were treated with the optimal conditions predicted by the response surface optimization test model, and the predicted conditions were validated by performing colony counts.
2.5. Determination of the total number of colonies
The total number of colonies was determined with reference to the method described in a previous study with slight modifications (Marasinghe et al., 2022). After SAEW treatment of shrimp, 10 g of shrimp meat was quickly taken with sterilized scissors in an ultra-clean bench and placed in a sterile homogenizing bag, 90 mL of sterile saline was added, and homogenized for 2 min using a tapping homogenizer (DH-11L, LAWSON Scientific Technology Co., Ltd, Ningbo, China) to make a 10-1 dilution suspension, and then a 10-fold series of diluted sample homogenates were prepared. Take 2–3 suitable gradients of suspension 0.1 mL coated on plate counting agar (PCA), each dilution to make 2 parallel, inverted in a 37 °C constant temperature incubator incubation 48 h, colony counting. The total number of colonies was finally expressed as log CFU/g.
2.6. Shrimp quality detection
2.6.1. Preparation of bactericides and raw material treatment
For the comparison test of chemical bactericides for fence preservation technology, alkaline electrolyzed water (AlEW) was prepared by electrolyzing 0.1 % NaCl solution through Harmony-II potentiostatic water generator, and then adjusting the pH to 10–11 with 0.1 mol/L HCl or NaOH solution and determining its oxidation reduction potential (ORP). The corresponding effective chlorine concentration (ACC) was determined by adjusting the electrolysis time using a residual chlorine meter, and the ACC of SAEW and AlEW was controlled at 88 mg/L. The sodium hypochlorite solution (NaClO) with an ACC of 88 mg/L was prepared by diluting the solution with a high concentration (analytically pure) of active chlorine content of 5.5 % and adjusting the pH to 10–11. The prepared biocides were stored in airtight containment at 4 °C protected from light for not more than 3 days prior to testing.
The shrimp were randomly divided into 4 groups, the treatment group was treated with the prepared SAEW, AlEW and NaClO respectively at the material-liquid ratio of 1:4 to soak the shrimp for 12 min, and no treatment was used as the control group. The water was removed and drained off, and the shrimp were stored in 4 °C refrigerator in sterile ziplock bags. Shrimp were randomly selected from each group every 2 d for the determination of the indexes. Three parallel samples were taken for all analyses.
2.6.2. Thiobarbituric acid (TBA) value and pH
Thiobarbituric acid (TBA) values were determined by referring to the method described in a previous study with slight modifications (Zhang et al., 2021). 5 g of crushed shrimp meat was weighed, added to 50 mL of TCA mixture, placed in a 50 °C shaker and shaken for 30 min, and filtered. 5 mL of filtrate and 5 mL of TBA solution were mixed and heated in a water bath at 90 °C for 30 min. Absorbance was measured by a microplate reader at 532 nm.
The pH was determined by referring to the method described in a previous study with slight modifications (Xu et al., 2019, Xu et al., 2019). The pH of the filtrate was determined by weighing 10 g of shrimp meat with sterile scissors, adding 90 mL of distilled water, homogenizing for 2 min, and filtering after 30 min of standing.
2.6.3. Total volatile basic nitrogen value (TVB-N)
The TVB-N content was determined by the method described in a previous study with slight modifications (Zhou et al., 2023, Zhou et al., 2023). Weighing 20 g of trimmed shrimp meat into a conical flask, it was macerated with 100 mL of ultrapure water for 30 min, and then filtered. 1 mL of boric acid absorbent and 1 drop of methyl red-bromocresol green ethanol mixed indicator were added to the inner chamber of the dish, and then 1 mL of shrimp extract and 1 mL of saturated potassium carbonate were added to the outer chamber of the dish, which was sealed and put into an incubator at 37 °C for 2 h. The color of the end point was titrated to purple-red with 0.01 mol/L hydrochloric acid solution. At the same time, ultrapure water was used to replace the filtrate as a reagent blank. TVB-N values were calculated according to the following formula:
Where: X is the content of volatile saline nitrogen, mg/100 g; V1 is the volume of hydrochloric acid consumed by the sample, mL; V2 is the volume of hydrochloric acid consumed by the reagent blank, mL; c is the concentration of hydrochloric acid solution, mol/L; 14 is the mass of nitrogen corresponding to 1 mL of hydrochloric acid solution, g/mol; m is the mass of the sample, g; V is the volume of filtrate, mL; V0 is the total volume, mL.
2.6.4. Color difference analysis
Referring to the method described by Sun et al. (Sun et al., 2019), a colorimeter (CS-820, Hangzhou Chroma Technology Co., Hangzhou, China) was used to determine the color difference values of shrimp. Prior to the determination, the instrument was calibrated using standard white and black boards. The color difference indexes included luminance (L*), chromaticity coordinates a* (+a*red, -a*green) and b* (+b*yellow, -b*blue), which were obtained by determining the second abdominal segment of the shrimp, and each sample was measured three times in parallel, and the color difference value ΔE* was expressed as the average of the three determinations. The formula for ΔE* was:
Where: L0, a0, b0 are the measured values of the color difference indicators L, a, b at day 0 of storage.
2.6.5. Textural analysis (physics)
The method described by Yan et al. (Yan et al., 2020) was referenced and slightly modified. The second and third abdominal segments of selected shrimps were used to determine their textural properties using the P/5 cylindrical probe of the texture analyser (TA.XT PLUS/50, STABLEMICVO, UK). The hardness, springiness, chewiness, resilience, gumminess, and cohesiveness of the specimens were determined by compression twice in TPA mode. The speed of the probe was 3 mm/s, 1 mm/s, and 5 mm/s before, during, and after the measurements, with 50 % deformation of the sample and a pressing interval of 5 s. Six points were measured.
2.7. Statistical analysis
Each experiment was repeated three times and the response surface killing effect was expressed as mean ± standard deviation. Design Expert 13 software was used for the design and analysis of response surface experiments, and Origin 2023 was used to make response surface plots and contour plots.
3. Results and discussion
3.1. Results of the single-factor test
A single-factor analysis of ACC was performed with a treatment time of 15.00 min and a material-liquid ratio of 1:4.00 for SAEW, and the results are shown in Fig. 1.A. Compared with the control group, the total number of colonies was significantly reduced in all concentration treatment groups, and the bactericidal effect was significantly enhanced with the increase of ACC (P < 0.05). However, the reduction of the total number of colonies became non-significant (P > 0.05) when the ACC of SAEW was greater than 80.00 mg/L. Tantratian et al. (Tantratian & Kaephen, 2020) found that the bactericidal effect of SAEW on oysters at ACC greater than 60.00 mg/L was also enhanced with the increase of ACC, which may be due to the differences of dominant bacterial taxa among the different types of seafood. This may be due to the different dominant bacterial groups in different species of seafood, thus there are some differences in the sensitivity to changes in ACC. According to the effect of ACC on the total number of colonies of Chinese shrimp, 80.00 mg/L was selected as the 0 level, and 60.00 mg/L, 68.11 mg/L, 80.00 mg/L, 91.89 mg/L, and 100.00 mg/L were selected as the five levels of SAEW ACC in the response surface optimization.
Fig. 1.
Effect of single factor test on the sterilization effect of SAEW and response surface with contour plots of the effects of three factors on shrimp bacterial inhibition rate. (A) Effect of ACC of SAEW on sterilizing effect; (B) Effect of processing time of SAEW on sterilizing effect; (C) Effect of material-liquid ratio of shrimp to SAEW on sterilizing effect;(D) Response surface of the effects of ACC and processing time on shrimp bacterial inhibition rate; (E) Response surface of the effects of ACC and material-liquid ratio on shrimp bacterial inhibition rate; (F) Response surface of the effects of processing time and material-liquid ratio on shrimp bacterial inhibition rate; (G) Contour plot of the effects of ACC and processing time on shrimp bacterial inhibition rate; (H) Contour plot of the interaction between ACC and material-liquid ratio on shrimp bacterial inhibition rate; (I) Contour plot of the effects of processing time and material-liquid ratio on shrimp bacterial inhibition rate.
The ACC and material-liquid ratio of SAEW were set at 60.00 mg/L and 1:4.00, and a single-factor analysis was performed for the processing time, and the results are shown in Fig. 1.B. Compared with the control group, the total number of colonies in the treatment groups at all times was significantly reduced (P < 0.05), and the bactericidal effect of SAEW was enhanced with the increase of processing time. However, there was no significant change (P > 0.05) in the bactericidal rate of shrimp when the treatment time exceeded 10.00 min. It may be attributed to the fact that with the prolongation of treatment time, the organic matter continued to interact with SAEW and continuously consumed the bactericidal active ingredients in SAEW (Jo et al., 2018). And with the growth of processing time and the consumption of ACC, the bactericidal effect of SAEW gradually became non-significant growth. Therefore, in this experiment, 10.00 min was taken as the 0 level of processing time, and 5.00 min, 7.03 min, 10.00 min, 12.97 min, and 15.00 min were selected as the 5 levels of SAEW processing time in further response surface optimization.
The ACC and processing time of SAEW were set at 60.00 mg/L and 15.00 min, and a single factor analysis of the material-liquid ratio was carried out, and the results are shown in Fig. 1.C. As the material-liquid ratio decreased, that is, the SAEW increased, the total bacterial counts of shrimp decreased significantly (P < 0.05). Therefore, the bactericidal effect of SAEW can be improved by appropriately decreasing the material-liquid ratio. It is worth noting that the bactericidal situation would stabilize with the decrease of material-liquid ratio when the material-liquid ratio was less than 1:4.00 (w/v), and the change of bactericidal rate became insignificant (P > 0.05). This may be due to the limited contact area between SAEW and shrimp, thus limiting the reduction of bacterial counts to some extent. Therefore, in this experiment, 1:4.00 (w/v) was used as the 0 level of the material-liquid ratio, and 1:2.00, 1:2.81, 1:4.00, 1:5.19, and 1:6.00 (w/v) were selected as the five levels of the material-liquid ratio of shrimp to SAEW in further response surface optimization.
3.2. Response surface optimization test result
The response surface optimization scheme and results are shown in Table 1. There were in total 20 experimental points, of which experimental groups 1–14 were analytical factorization tests, and experimental groups 15–20 were central tests. Taking the inhibition rate (%) of shrimp treated with SAEW as the response value, the response surface data in Table 1 were fitted with multiple regression using Design-Expert13 software, and the equations of quadratic multiple multinomial regression fitting were derived for ACC (A), processing time (B), material-liquid ratio (C) and shrimp inhibition rate (Y):
Table 1.
Response surface optimization scheme and results.
| Run number | A | B | C | Logarithmic bacterial number (lgCFU/g) | Sterilization logarithmic value (lgCFU/g) |
Inhibition rate (%) |
|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 2.91 ± 0.09 | 1.11 ± 0.09 | 27.51 ± 2.19 |
| 2 | 1 | 1 | −1 | 2.86 ± 0.07 | 1.16 ± 0.07 | 28.78 ± 1.82 |
| 3 | 1 | −1 | −1 | 2.74 ± 0.06 | 1.28 ± 0.06 | 31.79 ± 1.45 |
| 4 | 1 | −1 | 1 | 2.63 ± 0.06 | 1.39 ± 0.06 | 34.51 ± 1.56 |
| 5 | −1 | −1 | −1 | 2.90 ± 0.02 | 1.11 ± 0.02 | 27.71 ± 0.37 |
| 6 | −1 | −1 | 1 | 2.69 ± 0.09 | 1.33 ± 0.09 | 33.01 ± 2.19 |
| 7 | −1 | 1 | 1 | 2.87 ± 0.15 | 1.15 ± 0.15 | 28.58 ± 3.75 |
| 8 | −1 | 1 | −1 | 2.57 ± 0.04 | 1.45 ± 0.04 | 36.15 ± 0.99 |
| 9 | 1.68 | 0 | 0 | 2.90 ± 0.05 | 1.11 ± 0.05 | 27.71 ± 1.21 |
| 10 | −1.68 | 0 | 0 | 2.65 ± 0.03 | 1.37 ± 0.03 | 33.98 ± 0.68 |
| 11 | 0 | 1.68 | 0 | 2.79 ± 0.09 | 1.23 ± 0.09 | 30.60 ± 2.19 |
| 12 | 0 | −1.68 | 0 | 2.57 ± 0.03 | 1.45 ± 0.03 | 36.04 ± 0.83 |
| 13 | 0 | 0 | 1.68 | 2.94 ± 0.04 | 1.08 ± 0.04 | 26.84 ± 1.09 |
| 14 | 0 | 0 | −1.68 | 2.70 ± 0.08 | 1.32 ± 0.08 | 32.84 ± 1.93 |
| 15 | 0 | 0 | 0 | 2.56 ± 0.10 | 1.46 ± 0.10 | 36.36 ± 2.44 |
| 16 | 0 | 0 | 0 | 2.54 ± 0.02 | 1.47 ± 0.02 | 36.70 ± 0.52 |
| 17 | 0 | 0 | 0 | 2.50 ± 0.06 | 1.52 ± 0.06 | 37.72 ± 1.56 |
| 18 | 0 | 0 | 0 | 2.56 ± 0.08 | 1.45 ± 0.08 | 36.19 ± 1.93 |
| 19 | 0 | 0 | 0 | 2.54 ± 0.07 | 1.47 ± 0.07 | 36.70 ± 1.82 |
| 20 | 0 | 0 | 0 | 2.57 ± 0.06 | 1.45 ± 0.06 | 36.02 ± 1.36 |
A: Available chlorine concentration (ACC); B: Processing time; C: Material-liquid ratio.
Y = 36.62 + 2.01A + 1.70B + 0.9483C + 0.4644AB + 1.11AC-0.7503BC-2.04A2-1.17B2-2.40C2.
Table 2 shows the results of the significance analysis of the response surface model for the inhibition rate of shrimp by SAEW treatment. As shown in the table, the model was highly significant (P < 0.0001) and well fitted (p-value of the misfit error term was 0.0823 > 0.05). From the analysis of variance, we know that the effect of the three single factors A, B and C as well as the quadratic terms A2, B2 and C2 on the response values were all highly significant (P < 0.01). And from the significance of single factors on the inhibition rate, the magnitude of the F value of each factor indicates the degree of its influence on the experimental model (Xu et al., 2019, Xu et al., 2019), so the degree of influence of each factor in descending order of magnitude is A > B > C i.e., ACC > processing time > material-liquid ratio. In terms of the significance of the interaction terms, the difference in the effect of AC and BC terms on the response value was significant (P < 0.05), while the difference in AB term was not significant (P > 0.05). Taken together, the response surface model was a good fit to reality (R2 = 0.9670 > 0.9), had the ability to explain 93.73 % of the variability in the experimental data (AdjR2 = 0.9373), and had good accuracy and precision (CV = 2.88 %).
Table 2.
Significance analysis of the regression model.
| Variance source | Sum of squares | Degree of freedom | Mean square | F value | P value | Significance |
|---|---|---|---|---|---|---|
| Model | 261.52 | 9 | 29.06 | 32.58 | <0.0001 | Significant |
| A | 54.99 | 1 | 54.99 | 61.65 | <0.0001 | |
| B | 39.31 | 1 | 39.31 | 44.08 | <0.0001 | |
| C | 12.28 | 1 | 12.28 | 13.77 | 0.0040 | |
| AB | 1.73 | 1 | 1.73 | 1.93 | 0.1944 | |
| AC | 9.85 | 1 | 9.85 | 11.04 | 0.0077 | |
| BC | 4.50 | 1 | 4.50 | 5.05 | 0.0484 | |
| A2 | 60.06 | 1 | 60.06 | 67.34 | <0.0001 | |
| B2 | 19.62 | 1 | 19.62 | 22.00 | 0.0009 | |
| C2 | 82.91 | 1 | 82.91 | 92.96 | <0.0001 | |
| Residual | 8.92 | 10 | 0.89 | |||
| Lack of Fit | 7.08 | 5 | 1.42 | 3.86 | 0.0823 | Insignificant |
| Pure Error | 1.84 | 5 | 0.37 | |||
| Corr. Total | 270.44 | 19 | Adj R2 | 0.9373 | ||
| R2 | 0.9670 | CV | 2.88 |
A: Available chlorine concentration (ACC); B: Processing time; C: Material-liquid ratio.
Through the response surface data in Table 1, one of the dependent variables in ACC (A), processing time (B) and material-liquid ratio (C) were fixed at 0 level, respectively, and the equations were fitted through the regression of the sub-model composed of the other 2 factors, and the response surfaces and contour plots of the sub-models were constructed by using the Origin 2023 software as shown in Fig. 1. The response surface plots in Fig. 1.E and Fig. 1.F were obviously parabolic and the contour plots were elliptical, indicating that the interaction of ACC with material-liquid ratio (AC), processing time with material-liquid ratio (BC) had a significant effect on the inhibition rate, whereas, the surface of the response surface in Fig. 1.D was relatively flat and the ratio of the long and short axes of the ellipse in this plot was closer to 1, i.e., to a circle compared with the other two contour plots, indicating that the interaction of ACC with processing time (AB) did not have a significant effect on the inhibition rate, which is consistent with the results of ANOVA in Table 2. The response surface model was analyzed by Design-Expert 13 software, and the optimal treatment conditions were: ACC of 88.06 mg/L, processing time of 12.45 min, and material-liquid ratio of 1:4.18, which corresponded to the theoretical predictive value of 38.01 % of the inhibition rate of shrimp in SAEW. The optimal sterilization conditions were tested for validation. Considering the actual operation, the conditions were corrected to: ACC 88 mg/L, treatment time 12 min, material-liquid ratio 1:4. Validation experiments were carried out under these optimized conditions, and the total number of colonies in the control group was obtained to be (4.02 ± 0.06) log CFU/g, and the total number of colonies in the treatment group was obtained to be (2.51 ± 0.07) log CFU/g, and the bactericidal number was calculated to be (1.51 ± 0.07) log CFU/g, the inhibition rate was (37.60 ± 1.68)%, the relative error between the validation test and the theoretical prediction of the inhibition rate of 38.01 % was only 1.11 %, which indicated that the model was well fitted, and the optimized optimal bactericidal conditions for shrimp treated with SAEW were accurate and reliable, and could be used in the actual sterilization process of shrimp.
3.3. Effect of SAEW on shrimp quality
The microflora present on the surface and in the gastrointestinal tract of live shrimp decompose the shrimp body after their death, leading to gradual changes in the odor, pH, texture and appearance of Chinese shrimp (Ezati et al., 2021, Xu et al., 2018), which promotes proteolysis, enzymatic reactions (Chuesiang et al., 2020) and lipid oxidation (Majdinasab et al., 2020), thus causing deterioration. Due to the high correlation of TVB-N with the proliferation of protein-hydrolyzing bacteria and their own enzymatic degradation (Bekhit et al., 2021, Yu et al., 2018), the TBA value is an important quality parameter used to monitor the degree of lipid oxidation in meat (Dong et al., 2018). Therefore, total number of colonies, pH, TVB-N value and TBA value were used as indicators of freshness of Chinese shrimp.
Changes in the total number of colonies of Chinese shrimp treated with different bactericides during storage are shown in Fig. 2.A. During the 8-d storage period, the total number of colonies within the different treatment groups increased significantly (P < 0.05). According to the Chinese shrimp freshness grade: when the total colony count does not exceed 5.00 log CFU/g it can be assessed as first grade freshness; 5.00–5.70 log CFU/g is assessed as second grade freshness; and more than 6.00 log CFU/g is assessed as unacceptable (Li et al., 2017, Li et al., 2017). At 6 d of storage, the total number of colonies in the control group exceeded the applicable limit for shrimp colony counts, while the NaClO, AlEW and SAEW groups all met the secondary freshness, indicating that all three treatments inhibited the growth of microorganisms on shrimp to some extent. At 8 d of storage, the total colony counts of the SAEW group were within acceptable limits, while the shrimp in the NaClO and AlEW groups had lost their edible value, indicating that the SAEW treatment had the best inhibitory effect on the shrimp and prolonged the shelf-life of the shrimp by more than 2 d.
Fig. 2.
Changes in quality and color difference of shrimp during storage at 4 °C. (A) Total number of colonies; (B) pH; (C) TVB-N; (D) TBA; (E) L*Luminance value; (F) a*Red-Green value; (G) b*Yellow-Blue value; (H) ΔE*Total color difference value. Different superscripts (a, b, c, d) between different treatment groups at the same time indicate significant difference (P < 0.05). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Shrimp flesh pH above 7.8 was judged unacceptable (Balti et al., 2020). As seen in Fig. 2.B, the initial pH of Chinese shrimp was 6.69 ± 0.01 at fresh level. At 8 d of storage, the pH values of the control and AlEW groups had exceeded the acceptable limit, whereas the pH values of both the NaClO and SAEW groups did not exceed the acceptable limit, which more effectively inhibited the accumulation of alkaline compounds in shrimp during storage. However, only the pH of the SAEW group was significantly lower than that of the other groups (P < 0.05), suggesting that SAEW could effectively maintain the quality of Chinese shrimp and prolong the shelf life.
The shrimp is considered to be at the first grade freshness, the beginning of the deterioration stage, and completely deteriorated when the TVB-N values were < 25 mg /100 g, 25–30 mg /100 g, and > 30 mg /100 g, respectively (Huang et al., 2020). The results were shown in Fig. 2.C. The TVB-N content of each group gradually increased with the increase of storage time. At 6 d of storage, the corresponding values of NaClO, AlEW and SAEW treatment groups were significantly lower than those of the control group (P < 0.05). At 8 d of storage, the TVB-N values of the NaClO and AlEW groups were assessed as unacceptable, while the SAEW group remained within the acceptable range. Same as the results of other indicators, the SAEW group extended the shelf-life by at least 2 d compared to the control group.
The acceptable limit of TBA values for fish samples is 1–2 mg MDA/kg (Yu et al., 2017). As shown in Fig. 2.D, TBA values of shrimp from all groups increased but did not exceed the acceptable threshold at the end of storage. During the storage period, the NaClO and SAEW treated groups were always significantly lower than the control group (P < 0.05), suggesting that both 2 bactericides had some delaying effect on lipid oxidation. At 8 d of storage, the TBA values of the SAEW group were significantly lower than those of the other groups (P < 0.05), suggesting that SAEW could inhibit the rate of oxidative degradation of unsaturated fats in shrimp, and that it had a more pronounced effect on prolonging the shelf-life of Chinese shrimp.
3.4. Effect of SAEW on color difference of shrimp
Color is a key evaluation indicator of sea shrimp quality and is closely related to freshness (Wachirasiri et al., 2017). Appearance conditions such as color of sea shrimp are very important in consumers' perception of quality and are dominant factors in consumers' purchasing decisions as well as acceptable values (Zhang et al., 2015).
Decrease in L* values (brightness) are caused by degradation products of lipids and proteins in shrimp flesh and is a sign of browning (Sun et al., 2019). a* and b* values are elevated and can be attributed to the reaction of colored quinones, a product of oxidation of phenolics, and aldehydes, a product of autooxidation of fats, with amino acids of proteins, respectively, in shrimp body (Li et al., 2020, Verhaeghe et al., 2016). The overall trend of L* values in each group showed a decreasing trend with increasing storage time (Fig. 2.E), and the differences in L* values between NaClO and AlEW groups were always insignificant (P > 0.05), whereas the inhibitory effect of SAEW on the decrease of L* values was more significant (P < 0.05) in comparison to the other groups. a* values showed an increasing trend with the prolongation of storage period (Fig. 2.F), and the differences in the trend of the changes within the same group were significant (P < 0.05), and the delaying effect of SAEW on the increase of a* value was better than other treatments (P < 0.05). b* value showed an overall increase with the prolongation of the storage period (Fig. 2.G), and only the b* value of the SAEW group was consistently and significantly lower than that of the other groups during the storage period (P < 0.05), i.e., the SAEW was better than the other treatments. The a* and b* values in this study had the same increasing trend as reported by Sun et al. (Sun et al., 2018). ΔE* values indicate the magnitude of color difference values, which can effectively reflect the stability of shrimp color. The results showed (Fig. 2.H) that the increase in ΔE* value in the SAEW group was significantly lower than the other treatments (P < 0.05). Therefore, SAEW can delay the color change of shrimp body more effectively.
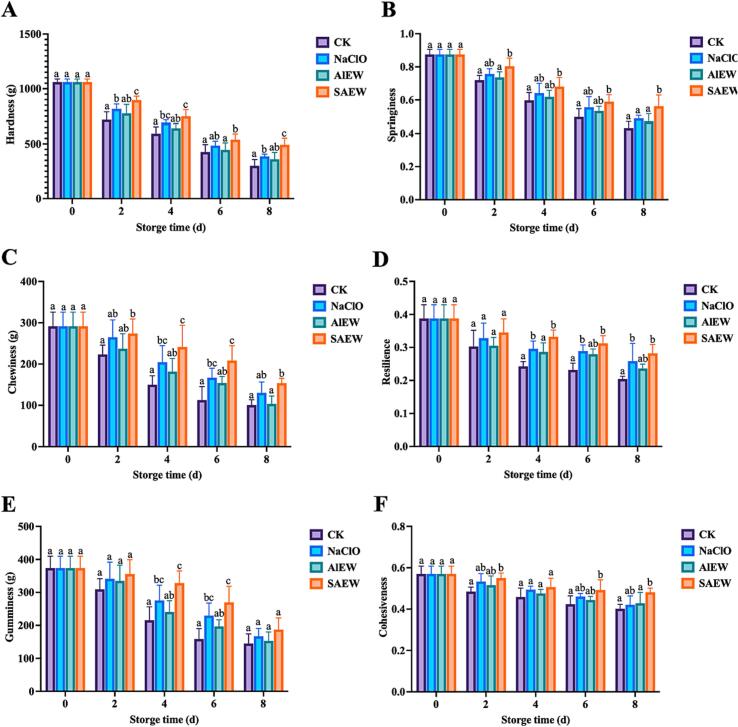
3.5. Effect of SAEW on the texture of shrimp
Food texture is a physical property that characterizes the organization and state of food. Chinese shrimps degrade myosin heavy chains and collagen by endogenous proteases after death, leading to the formation of paste-like texture and disintegration of microstructure (Xu et al., 2018). In this study, hardness, springiness, chewiness, resilience, gumminess, and cohesiveness were selected to determine the changes in muscle tissue of Chinese shrimp during storage after treatment with different bactericides, of which hardness and springiness were the two indicators that were most likely to change during storage. The six textural parameters of shrimp were examined simultaneously using the TPA model, and the results are shown in Fig. 3.
Fig. 3.
Changes in textural properties of shrimp during storage at 4 °C. (A) Hardness; (B) Springiness; (C) Chewiness; (D) Resilience; (E) Gumminess; and (F) Cohesiveness. Different superscripts (a, b, c, d) between different treatment groups at the same time indicate significant difference (P < 0.05).
The degree of initial structure of a food can be expressed by its hardness (Lan et al., 2022). As can be seen in Fig. 3.A, the hardness of each group decreased with increasing storage time, with the control group showing the greatest decrease in hardness. At 8 d, the hardness of the SAEW group was significantly higher than the other groups (P < 0.05), indicating the superiority of the SAEW treatment in maintaining the hardness of shrimp.
Springiness is defined as the ratio of the height of recovery of the sample after cessation of external forces to the height before deformation (Lan et al., 2022). The springiness of the groups continued to decrease with increasing storage time, which may be attributed to the disruption of the reticular structure composed of protein and hydrated layers in shrimp muscle during the continuous protein degradation process (Zhou et al., 2023, Zhou et al., 2023). The control group showed the most significant reduction in springiness, followed by the AlEW group, while the SAEW group samples showed the least reduction in springiness (Fig. 3.B). Moreover, at the 8 d of storage, the springiness of the SAEW group was significantly higher than that of the NaClO and AlEW treated groups (P < 0.05), indicating that SAEW was more effective in maintaining the springiness of the samples.
As shown in Fig. 3.C, the differences in chewiness during storage were not significant (P > 0.05) in the CK and AlEW groups; the chewiness in the SAEW group were significantly higher than those in the control group (P < 0.05), with the SAEW group being the most effective in maintaining the chewiness of the samples. In addition to this, the resilience (Fig. 3.D), gumminess (Fig. 3.E) and cohesiveness (Fig. 3.F) of the samples in each group showed an overall decreasing trend during storage, and the values within the same group were significantly different (P < 0.05) with the storage time; the data for the three parameters showed the fastest decrease in the control samples, and the slowest decrease in the samples of the SAEW group as compared to the other groups. Therefore, it was concluded that SAEW treatment improved the textural properties of Chinese shrimp during storage better than the other two bactericides.
3.6. Effect of SAEW on melanin deposition and shrimp coloration
Discoloration is considered to be one of the important characteristics in assessing deterioration of shrimp (Shiekh & Benjakul, 2020). Typically, fresh shrimp show a greenish color, then gradually lose their luster and become light red or even black during storage. Polyphenol oxidase (PPO) is responsible for the blackening. The enzyme reaction is catalyzed by PPO in or under the shrimp shell, and melanin accumulates mainly under the carapace of the cephalothorax (Nirmal & Benjakul, 2011).
Changes in melanin deposition in Chinese shrimp were monitored during 8 days of refrigeration, and black spot formation and shrimp body color changes are shown in Fig. 4. All groups showed no melanin deposition on day 0. The NaClO and AlEW groups showed more melanin deposition than the control group on day 2 and thereafter during storage. During refrigeration, the SAEW group showed the least melanosis of the four groups. Hemocyanin (Hc), a copper-binding protein in the hemolymph of mollusks and arthropods, has also been suggested to be PPO-active in the presence of activators. The activators and chemical agents (e.g., perchlorate, sodium dodecyl sulfate) can affect the tertiary and quaternary structure of Hc, enabling it to transform its form and acquire phenol oxidase activity (HcPO), which can lead to shrimp blackening (Gonçalves & De Oliveira, 2016). However, both the NaClO and AlEW groups were alkaline with a pH value of 10–11, so the occurrence of melanin in the two groups was more severe than that of the control group, which may be due to the alkaline environment affecting Hc. In addition to this, the orange-red coloration at the shrimp head appeared in the control group from day 2, and from day 4 in the NaClO and AlEW groups, and from day 6 in the SAEW. After the appearance of orange-red color at the head of shrimp in all groups, the color gradually spread to the abdomen of shrimp during the subsequent storage period. It may be due to the fact that endogenous proteases can spread from the head to the abdomen of shrimp when they are stored after death (Li et al., 2022). This color change trend is consistent with Fig. 2.F and G show. Thus, SAEW treatment retarded melanin production and color change during shrimp storage.
Fig. 4.
Images of shrimp under different treatments during storage at 4 °C.
4. Conclusion
In this study, we conducted a single-factor experiment with ACC, processing time and material-liquid ratio of SAEW-treated shrimp as the investigated variables, and the bacterial inhibition rate of shrimp as the response value and explored the effects of each factor of SAEW on the bacterial inhibition rate of shrimp based on the response surface methodology. This method can well predict the optimal sterilization process conditions of SAEW in shrimp treatment.
Considering the actual operation, the conditions were corrected to: ACC of 88 mg/L, processing time of 12 min, and material-liquid ratio of 1:4. The total number of colonies of Chinese shrimp at the zero point of storage was significantly reduced by the treatment of SAEW solution, and it effectively inhibited microbial growth and reproduction during the subsequent storage period, and delayed the increase of pH, TVB-N, and TBA values. SAEW reduced the deterioration of textural properties of shrimp meat during the storage period, and reduced the increase in pH, TVB-N, and TBA values of the shrimp meat. SAEW reduced the deterioration of textural properties of shrimp during storage and extended the shelf life of Chinese shrimp by more than 2 d. Therefore, the optimized optimal sterilization conditions for shrimp treated with SAEW are accurate and reliable and can be used in the actual sterilization process of shrimp. The data from this study can provide an effective reference for the seafood industry to utilize SAEW for shrimp preservation as well.
CRediT authorship contribution statement
Guanhong Chang: Conceptualization, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. Yang Liu: Conceptualization, Investigation, Methodology, Formal analysis. Zonghong Luo: Investigation, Methodology. Ke Ni: Investigation, Methodology. Pengfei Zhang: Methodology, Writing – review & editing. Ting Zhou: Resources. Li Bai: Project administration, Resources. Chunling Zhang: Supervision, Writing – review & editing. Xin Wang: Funding acquisition, Project administration, Resources, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was in supported by the Sichuan Natural Science Foundation (No. 2023NSFSC0178), National Natural Science Foundation of China (No. 31871894), and Project of science and technology of social development in Shaanxi Province (2021SF-470).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101180.
Contributor Information
Li Bai, Email: baili@cfsa.net.cn.
Chunling Zhang, Email: zbh545400370@163.com.
Xin Wang, Email: xinwang7516@nwsuaf.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Al-Holy M.A., Rasco B.A. The bactericidal activity of acidic electrolyzed oxidizing water against Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes on raw fish, chicken and beef surfaces. Food Control. 2015;54:317–321. doi: 10.1016/j.foodcont.2015.02.017. [DOI] [Google Scholar]
- Balti R., Ben Mansour M., Zayoud N., Le Balch R., Brodu N., Arhaliass A., Massé A. Active exopolysaccharides based edible coatings enriched with red seaweed (Gracilaria gracilis) extract to improve shrimp preservation during refrigerated storage. Food Bioscience. 2020;34 doi: 10.1016/j.fbio.2019.100522. [DOI] [Google Scholar]
- Bekhit A.-E.-D.-A., Holman B.W.B., Giteru S.G., Hopkins D.L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends in Food Science & Technology. 2021;109:280–302. doi: 10.1016/j.tifs.2021.01.006. [DOI] [Google Scholar]
- Chuesiang P., Sanguandeekul R., Siripatrawan U. Phase inversion temperature-fabricated cinnamon oil nanoemulsion as a natural preservative for prolonging shelf-life of chilled Asian seabass (Lates calcarifer) fillets. LWT. 2020;125 doi: 10.1016/j.lwt.2020.109122. [DOI] [Google Scholar]
- Ding T., Ge Z., Shi J., Xu Y.-T., Jones C.L., Liu D.-H. Impact of slightly acidic electrolyzed water (SAEW) and ultrasound on microbial loads and quality of fresh fruits. LWT - Food Science and Technology. 2015;60(2):1195–1199. doi: 10.1016/j.lwt.2014.09.012. [DOI] [Google Scholar]
- Ding T., Xuan X.-T., Liu D.-H., Ye X.-Q., Shi J., Warriner K., Xue S., Jones C.L. Electrolyzed Water Generated Using a Circulating Reactor. International Journal of Food Engineering. 2015;11(1):79–84. doi: 10.1515/ijfe-2014-0217. [DOI] [Google Scholar]
- Dong Z., Xu F., Ahmed I., Li Z., Lin H. Characterization and preservation performance of active polyethylene films containing rosemary and cinnamon essential oils for Pacific white shrimp packaging. Food Control. 2018;92:37–46. doi: 10.1016/j.foodcont.2018.04.052. [DOI] [Google Scholar]
- Ezati P., Bang Y.-J., Rhim J.-W. Preparation of a shikonin-based pH-sensitive color indicator for monitoring the freshness of fish and pork. Food Chemistry. 2021;337 doi: 10.1016/j.foodchem.2020.127995. [DOI] [PubMed] [Google Scholar]
- Gonçalves A.A., De Oliveira A.R.M. Melanosis in crustaceans: A review. LWT - Food Science and Technology. 2016;65:791–799. doi: 10.1016/j.lwt.2015.09.011. [DOI] [Google Scholar]
- Huang J., Chen M., Zhou Y., Li Y., Hu Y. Functional characteristics improvement by structural modification of hydroxypropyl methylcellulose modified polyvinyl alcohol films incorporating roselle anthocyanins for shrimp freshness monitoring. International Journal of Biological Macromolecules. 2020;162:1250–1261. doi: 10.1016/j.ijbiomac.2020.06.156. [DOI] [PubMed] [Google Scholar]
- Jo H.-Y., Tango C.N., Oh D.-H. Influence of different organic materials on chlorine concentration and sanitization of slightly acidic electrolyzed water. LWT. 2018;92:187–194. doi: 10.1016/j.lwt.2018.02.028. [DOI] [Google Scholar]
- Lan W., Zhao J., Liu L., Xie J. Relevance of cathepsins activity and texture in slightly acidic electrolyzed water-slurry iced mackerel (Pneumatophorus japonicus) Food Bioscience. 2022;49 doi: 10.1016/j.fbio.2022.101924. [DOI] [Google Scholar]
- Li D., Zhou D., Yin F., Dong X., Xie H., Liu Z., Li A., Li J., Rakariyatham K., Shahidi F. Impact of different drying processes on the lipid deterioration and color characteristics of Penaeus vannamei. Journal of the Science of Food and Agriculture. 2020;100(6):2544–2553. doi: 10.1002/jsfa.10280. [DOI] [PubMed] [Google Scholar]
- Li H., Liang D., Huang J., Cui C., Rao H., Zhao D., Hao J. The Bactericidal Efficacy and the Mechanism of Action of Slightly Acidic Electrolyzed Water on Listeria monocytogenes’ Survival. Foods. 2021;10(11):2671. doi: 10.3390/foods10112671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Ding T., Liao X., Chen S., Ye X., Liu D. Synergetic effects of ultrasound and slightly acidic electrolyzed water against Staphylococcus aureus evaluated by flow cytometry and electron microscopy. Ultrasonics Sonochemistry. 2017;38:711–719. doi: 10.1016/j.ultsonch.2016.08.029. [DOI] [PubMed] [Google Scholar]
- Li Y., Lei Y., Tan Y., Zhang J., Hong H., Luo Y. Efficacy of freeze-chilled storage combined with tea polyphenol for controlling melanosis, quality deterioration, and spoilage bacterial growth of Pacific white shrimp (Litopenaeus vannamei) Food Chemistry. 2022;370 doi: 10.1016/j.foodchem.2021.130924. [DOI] [PubMed] [Google Scholar]
- Li Y., Yang Z., Li J. Shelf-life extension of Pacific white shrimp using algae extracts during refrigerated storage: Shelf-life extension of Pacific white shrimp. Journal of the Science of Food and Agriculture. 2017;97(1):291–298. doi: 10.1002/jsfa.7730. [DOI] [PubMed] [Google Scholar]
- Liu C., Zheng W., Li Z., Zhou L., Sun Y., Han S. Slightly acidic electrolyzed water as an alternative disinfection technique for hatching eggs. Poultry Science. 2022;101(3) doi: 10.1016/j.psj.2021.101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Lan W., Wang Y., Xie J. Antibacterial activity and mechanism of slightly acidic electrolyzed water against Shewanella putrefaciens and Staphylococcus saprophytic. Biochemical and Biophysical Research Communications. 2022;592:44–50. doi: 10.1016/j.bbrc.2022.01.013. [DOI] [PubMed] [Google Scholar]
- Liu X., Sun X., Chen X., Zheng K., Li J., Li X. Effect of slightly acidic electrolyzed water(SAEW) combined with ultrasound sterilization on quality of Bigeye tuna (Thunnus obesus) during cryogenic storage. Journal of Food Composition and Analysis. 2023;115 doi: 10.1016/j.jfca.2022.104999. [DOI] [Google Scholar]
- Majdinasab M., Niakousari M., Shaghaghian S., Dehghani H. Antimicrobial and antioxidant coating based on basil seed gum incorporated with Shirazi thyme and summer savory essential oils emulsions for shelf-life extension of refrigerated chicken fillets. Food Hydrocolloids. 2020;108 doi: 10.1016/j.foodhyd.2020.106011. [DOI] [Google Scholar]
- Marasinghe B.N.A., Rathnayake R.M.N.P., Ranasinghe R.D.R., Madurakanthi A.A.G., Senevirathne W.S.M. Effect of gamma irradiation on microbial, physical and chemical parameters of postharvest Penaeus monodon F. Radiation Physics and Chemistry. 2022;192 doi: 10.1016/j.radphyschem.2021.109883. [DOI] [Google Scholar]
- McCarthy S., Burkhardt W. Efficacy of electrolyzed oxidizing water against Listeria monocytogenes and Morganella morganii on conveyor belt and raw fish surfaces. Food Control. 2012;24(1–2):214–219. doi: 10.1016/j.foodcont.2011.09.030. [DOI] [Google Scholar]
- Nirmal N.P., Benjakul S. Inhibition of melanosis formation in Pacific white shrimp by the extract of lead (Leucaena leucocephala) seed. Food Chemistry. 2011;128(2):427–432. doi: 10.1016/j.foodchem.2011.03.048. [DOI] [PubMed] [Google Scholar]
- Peng S., Wei H., Zhan S., Yang W., Lou Q., Deng S., Yu X., Huang T. Spoilage mechanism and preservation technologies on the quality of shrimp: An overview. Trends in Food Science & Technology. 2022;129:233–243. doi: 10.1016/j.tifs.2022.09.024. [DOI] [Google Scholar]
- Semeano A.T.S., Maffei D.F., Palma S., Li R.W.C., Franco B.D.G.M., Roque A.C.A., Gruber J. Tilapia fish microbial spoilage monitored by a single optical gas sensor. Food Control. 2018;89:72–76. doi: 10.1016/j.foodcont.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiekh K.A., Benjakul S. Effect of pulsed electric field treatments on melanosis and quality changes of Pacific white shrimp during refrigerated storage. Journal of Food Processing and Preservation. 2020;44(1) doi: 10.1111/jfpp.14292. [DOI] [Google Scholar]
- Sun J., Jiang X., Chen Y., Lin M., Tang J., Lin Q., Fang L., Li M., Hung Y.-C., Lin H. Recent trends and applications of electrolyzed oxidizing water in fresh foodstuff preservation and safety control. Food Chemistry. 2022;369 doi: 10.1016/j.foodchem.2021.130873. [DOI] [PubMed] [Google Scholar]
- Sun L., Sun J., Liu D., Fu M., Yang X., Guo Y. The preservative effects of chitosan film incorporated with thinned young apple polyphenols on the quality of grass carp (Ctenopharyngodon idellus) fillets during cold storage: Correlation between the preservative effects and the active properties of the film. Food Packaging and Shelf Life. 2018;17:1–10. doi: 10.1016/j.fpsl.2018.04.006. [DOI] [Google Scholar]
- Sun X., Guo X., Ji M., Wu J., Zhu W., Wang J., Cheng C., Chen L., Zhang Q. Preservative effects of fish gelatin coating enriched with CUR/βCD emulsion on grass carp (Ctenopharyngodon idellus) fillets during storage at 4 °C. Food Chemistry. 2019;272:643–652. doi: 10.1016/j.foodchem.2018.08.040. [DOI] [PubMed] [Google Scholar]
- Tantratian S., Kaephen K. Shelf-life of shucked oyster in epigallocatechin-3-gallate with slightly acidic electrolyzed water washing under refrigeration temperature. LWT. 2020;118 doi: 10.1016/j.lwt.2019.108733. [DOI] [Google Scholar]
- Verhaeghe T., Vlaemynck G., De Block J., Van Weyenberg S., Hendrickx M. Thermal inactivation kinetics of proteases and polyphenoloxidase in brown shrimp (Crangon crangon) Food Chemistry. 2016;197:641–647. doi: 10.1016/j.foodchem.2015.11.024. [DOI] [PubMed] [Google Scholar]
- Wachirasiri K., Wanlapa S., Uttapap D., Puttanlek C., Rungsardthong V. Changing in processing yield and physical properties of frozen white shrimp (Penaeus vannamei) treated with lysine and sodium bicarbonate. International Journal of Food Science & Technology. 2017;52(3):763–771. doi: 10.1111/ijfs.13333. [DOI] [Google Scholar]
- Wu S., Zhao M., Gao S., Xu Y., Zhao X., Liu M., Liu X. Change Regularity of Taste and the Performance of Endogenous Proteases in Shrimp (Penaens vannamei) Head during Autolysis. Foods. 2021;10(5):1020. doi: 10.3390/foods10051020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Sun L., Li C., Wang Y., Ye R. Inhibitory effect of glucose oxidase from Bacillus sp. CAMT22370 on the quality deterioration of Pacific white shrimp during cold storage. LWT. 2018;92:339–346. doi: 10.1016/j.lwt.2018.02.025. [DOI] [Google Scholar]
- Xu F., Wang B., Hong C., Telebielaigen S., Nsor-Atindana J., Duan Y., Zhong F. Optimization of spiral continuous flow-through pulse light sterilization for Escherichia coli in red grape juice by response surface methodology. Food Control. 2019;105:8–12. doi: 10.1016/j.foodcont.2019.04.023. [DOI] [Google Scholar]
- Xu N., Shi W., Wang X., Wang Z. Effect of ice water pretreatment on the quality of Pacific White Shrimps (Litopenaeus vannamei) Food Science & Nutrition. 2019;7(2):645–655. doi: 10.1002/fsn3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan X.-T., Fan Y.-F., Ling J.-G., Hu Y.-Q., Liu D.-H., Chen S.-G., Ye X.-Q., Ding T. Preservation of squid by slightly acidic electrolyzed water ice. Food Control. 2017;73:1483–1489. doi: 10.1016/j.foodcont.2016.11.013. [DOI] [Google Scholar]
- Yan P., Chen X., Chelliah R., Jo K.H., Shan L., Shin H., Kim S., Oh D.H. Biocontrol and anti-biofilm potential of aerosols sprayed slightly acidic electrolyzed water against Cronobacter sakazakii in infant food industry. LWT. 2023;178 doi: 10.1016/j.lwt.2023.114598. [DOI] [Google Scholar]
- Yan W., Zhang Y., Yang R., Zhao W. Combined effect of slightly acidic electrolyzed water and ascorbic acid to improve quality of whole chilled freshwater prawn (Macrobrachium rosenbergii) Food Control. 2020;108 doi: 10.1016/j.foodcont.2019.106820. [DOI] [Google Scholar]
- Yu D., Li P., Xu Y., Jiang Q., Xia W. Physicochemical, microbiological, and sensory attributes of chitosan-coated grass carp (Ctenopharyngodon idellus) fillets stored at 4°C. International Journal of Food Properties. 2017;20(2):390–401. doi: 10.1080/10942912.2016.1163267. [DOI] [Google Scholar]
- Yu L., Jiang Q., Yu D., Xu Y., Gao P., Xia W. Quality of giant freshwater prawn (Macrobrachium rosenbergii) during the storage at −18°C as affected by different methods of freezing. International Journal of Food Properties. 2018;21(1):2100–2109. doi: 10.1080/10942912.2018.1484760. [DOI] [Google Scholar]
- Zhang B., Ma L., Deng S., Xie C., Qiu X. Shelf-life of pacific white shrimp (Litopenaeus vannamei) as affected by weakly acidic electrolyzed water ice-glazing and modified atmosphere packaging. Food Control. 2015;51:114–121. doi: 10.1016/j.foodcont.2014.11.016. [DOI] [Google Scholar]
- Zhang C., Cao W., Hung Y.-C., Li B. Disinfection effect of slightly acidic electrolyzed water on celery and cilantro. Food Control. 2016;69:147–152. doi: 10.1016/j.foodcont.2016.04.039. [DOI] [Google Scholar]
- Zhang X., Lan W., Xie J. Combined citric acid and rosemary extract to maintain the quality of chilled Pacific white shrimp (Litopenaeus vannamei) Journal of Food Processing and Preservation. 2021;45(7) doi: 10.1111/jfpp.15614. [DOI] [Google Scholar]
- Zhou T., Ding Y.-X., Benjakul S., Shui S.-S., Zhang B. Characterization of endogenous enzymes in sword prawn (Parapenaeopsis hardwickii) and their effects on the quality of muscle proteins during frozen storage. LWT. 2023;177 doi: 10.1016/j.lwt.2023.114563. [DOI] [Google Scholar]
- Zhou Y., Jiao L., Wu J., Zhang Y., Zhu Q., Dong D. Non-destructive and in-situ detection of shrimp freshness using mid-infrared fiber-optic evanescent wave spectroscopy. Food Chemistry. 2023;422 doi: 10.1016/j.foodchem.2023.136189. [DOI] [PubMed] [Google Scholar]
- Zhu S., Wu H., Zhang C., Jie J., Liu Z., Zeng M., Wang C. Spoilage of refrigerated Litopenaeus vannamei: Eavesdropping on Acinetobacter acyl-homoserine lactones promotes the spoilage potential of Shewanella baltica. Journal of Food Science and Technology. 2018;55(5):1903–1912. doi: 10.1007/s13197-018-3108-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.