Cutaneous reflexes to stimulation of non-nociceptive Aβ afferents convey cutaneous sensory information about the foot, involve an extensive spinal interneuronal network, and are thought to help shape lower limb movement behaviors (Zehr and Stein, 1999). In people with chronic incomplete spinal cord injury (SCI), cutaneous reflexes are present in the soleus but their amplitudes and modulation are reduced (Phipps and Thompson, 2023), suggesting impaired somatosensory processing in this population. Although it may be impaired, presence of a reflex response presents an opportunity of the pathway reflected in that reflex to be modified through a neurobehavioral training approach, such as operant conditioning; operant conditioning of a spinal reflex can guide beneficial plasticity in the targeted pathway (Thompson and Wolpaw, 2015). To date, almost all the reflex operant conditioning studies have targeted simpler spinal reflexes (i.e., spinal stretch reflex and its partial electrical analog H-reflex (Mrachacz-Kersting et al., 2019, Thompson and Wolpaw, 2015)), and therefore, it is unknown if it can target a polysynaptic reflex such as a cutaneous reflex. If we can induce targeted beneficial plasticity in a cutaneous reflex to non-noxious stimulation of Aβ afferents in the plantarflexor soleus, which is essential during standing and walking, we might be able to improve sensorimotor function in which the soleus participates. Here, as the first step in testing the hypothesis that operantly conditioning a cutaneous reflex to non-noxious stimuli is possible and that it can alter impaired somatosensory processing due to chronic SCI, we examined operant conditioning of the soleus cutaneous reflex in a person with chronic incomplete SCI.
An adult male (61 years old) with chronic (9 years post-injury) C3-C7 incomplete SCI (classified as American Spinal Injury Association Impairment Scale D) from a traumatic event was studied with the cutaneous reflex operant conditioning protocol. At the time of study enrollment, the participant presented with spasticity, had been on chronic stable dose (60 mg daily) of baclofen, and could ambulate independently with a two-wheeled walker. The participant gave written informed consent to participation in the procedures that were approved by the Institutional Review Board of the Medical University of South Carolina.
The protocol consisted of 6 baseline and 30 up-conditioning sessions over 12 weeks at a rate of 3 sessions/week. Electromyography (EMG) was recorded from the soleus and tibialis anterior (TA) of the stimulated leg. The same investigator (AP) administered each session’s procedures throughout the study. In each session, to elicit cutaneous reflexes in the soleus, the sural nerve (SRn) that innervates the lateral aspect of the foot was stimulated with a train of five 1.0-ms pluses at 200 Hz. SRn stimulation was delivered at ~1.5 × radiating threshold (RT) to target non-nociceptive afferents. Soleus cutaneous reflexes were measured over a medium latency response (MLR) period, centered around the greatest excitation; the MLR period shifted from 103.5 – 122 ms post-stimulus onset during baseline sessions to 96 – 114.5 ms post-stimulus onset during conditioning sessions. To calculate the reflex amplitude, the EMG signal was full-wave rectified first, and then the background EMG averaged over 50 ms period prestimulus was subtracted from the EMG amplitude over the MLR window for each trial. In all sessions, 225 reflex trials were administered in 3 blocks of 75 trials each, while the participant stood and maintained his natural standing level of soleus EMG activity. During baseline sessions, reflexes were simply measured (i.e., no feedback was given to the participant on reflex size). During conditioning trials of conditioning sessions, the participant was encouraged to increase the MLR size and was given immediate feedback as to whether the reflex was larger than a criterion (Figure 1A). Perceptual threshold (PerT: barely perceptible), RT (minimum stimulus intensity to cover the maximum skin area), and pain threshold (PainT: intensity at which the stimulation became painful) were determined at the beginning of each session. 10-meter and 6-minute walk tests were administered before baseline and after the 30th conditioning sessions. MLR amplitude, PerT, RT, and PainT (expressed as % mean baseline value) and background EMG were compared between the 6 baseline and final 6 conditioning sessions using a two-sided paired t test. All statistical analyses were completed using SPSS Version 28 and α was set at 0.05.
Figure 1.
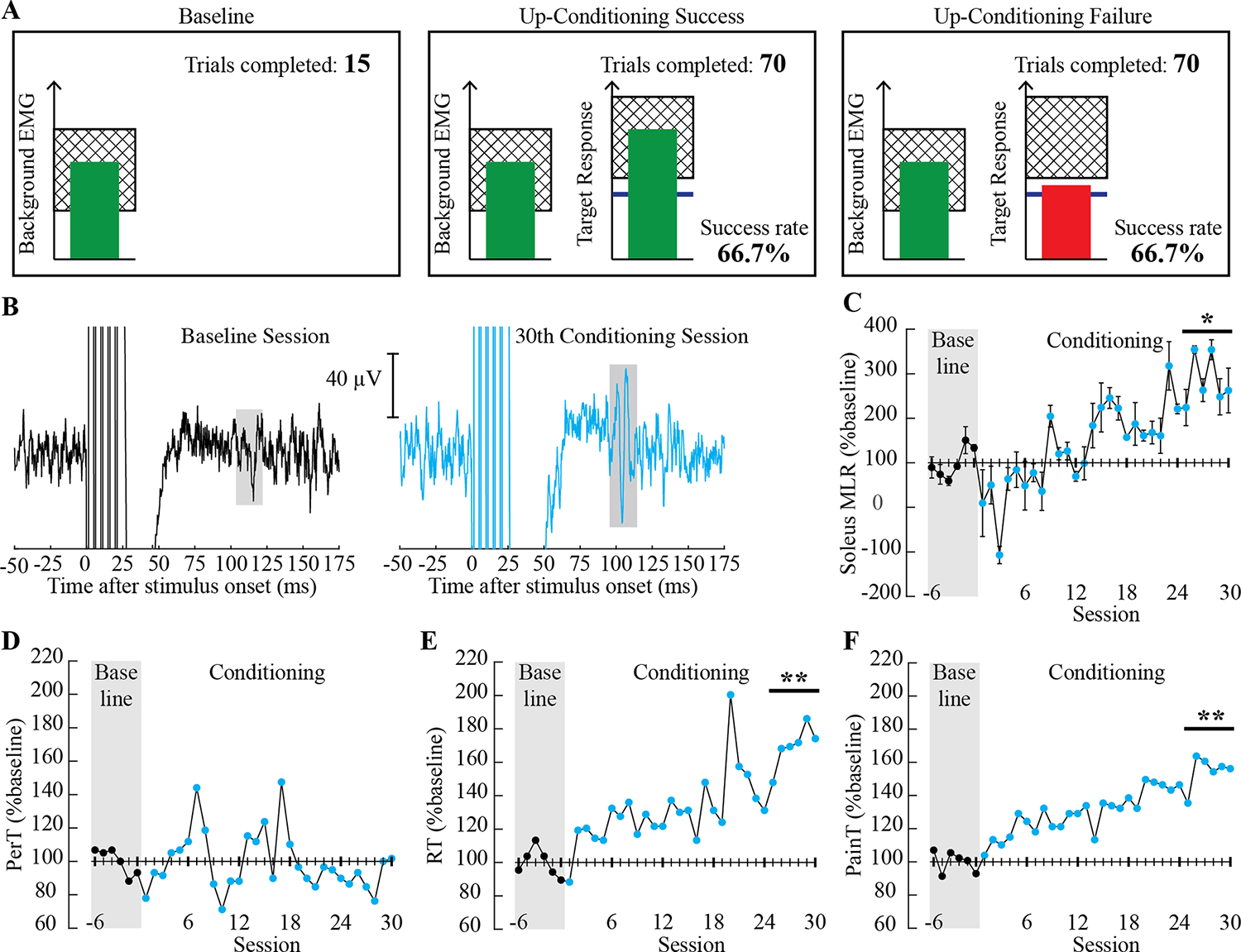
Visual feedback during baseline and operant conditioning trials and outcome measurements. A: Visual feedback that was presented to the participant on a computer monitor during sessions. In all trials, the number of trials completed within its block is displayed, and the ongoing soleus EMG activity level is shown in the green vertical bar (updated every 200 ms). When the soleus EMG has remained in a pre-set range (indicated as a hatched zone, which matches the individual’s natural standing level) for at least 2 s, the sural nerve is stimulated, and a cutaneous reflex is elicited. During control trials (left), the size of the targeted reflex (i.e., medium latency response: MLR) is not shown. During conditioning trials (middle and right), the shading in the target response panel indicates the rewarded range for the reflex size with up-conditioning. The blue horizontal line indicates the average reflex size for the 6 baseline sessions. When the elicited reflex size (shown as the height of the vertical bar) reaches into the shaded area, the bar becomes green and the trial is a success (middle); if the reflex bar falls under the shaded area, the bar becomes red and the trial is a failure (right). The running success rate (%) for the current block of 75 trials is also shown. B: Representative examples of soleus EMG after a train of five pulses at 200 Hz from a baseline session (black, left) and the 30th conditioning session (cyan, right). Six trials were averaged for each sweep. The reflex window is highlighted in grey. C-F: MLR reflex size (session mean±SD C), perceptual threshold [(PerT) D], radiating threshold [(RT) E], and pain threshold [(PainT) F] over the course of 6 baseline (filled black) and 30 conditioning sessions (filled cyan) are shown in % mean baseline value. Asterisks over the final 6 conditioning sessions indicate significant differences from the 6 baseline sessions (by paired t test, * for P ≤ 0.05 and ** for P < 0.001).
Background EMG did not differ between the baseline and the final 6 conditioning sessions for the soleus (21±1 [mean±SD] μV vs. 22±3 μV, P = 0.49) nor TA (12±1 μV vs. 13±2 μV, P = 0.37). Figure 1B shows examples of soleus EMG sweeps from a baseline (black) and the 30th conditioning session (cyan). MLR over the final 6 conditioning sessions (5.9 μV) was significantly larger than that of the baseline (2.1 μV); the final MLR was 184±56% larger than the baseline MLR (P = 0.002). The change over the course of 30 conditioning sessions was not significant for PerT (−10±10%, P = 0.20). RT and PainT increased gradually, with the final values over the last 6 conditioning sessions being 170±12% for RT (P < 0.001) and 155±10% for PainT (P < 0.001) (Figure 1C–F). (Note that along with the RT increase, the stimulus current for MLR elicitation increased from 62±4 mA [baseline] to 97±6 mA [for the final 6 conditioning sessions]; this might have contributed to observed increases in MLR size. Currently whether and how cutaneous reflex size may increase with increasing stimulus intensity are not established.) From baseline to after the 30th conditioning session, 10-meter walk speed and 6-minute walk distance increased from 0.09 to 0.22 m/s and 28 to 39 meters, respectively.
Findings from this study lead to three important implications. First, although in a single case, the increase in soleus MLR amplitude appeared to be systematic and consistent across 30 conditioning sessions, suggesting that operant conditioning of a cutaneous reflex is possible in persons with chronic incomplete SCI. Second, gait function improved in the participant, supporting the possibility that cutaneous reflex conditioning could become a viable tool for enhancing gait rehabilitation after SCI. Third, the pain threshold to cutaneous nerve stimulation (stimulus intensity that is perceived as painful) increased systematically over the course of 30 conditioning sessions, indicating systematic tuning down of pain processing in this individual. These findings suggest that operant up-conditioning of the MLR to non-noxious cutaneous stimulation may produce beneficial changes in multiple spinal pathways, leading to improvements in sensory and motor functions in lower limb of people with SCI. Together with the theorized interaction between the non-nociceptive and nociceptive pathways (Melzack and Wall, 1965), the present findings support potential clinical values of cutaneous reflex operant conditioning, which is a non-invasive, non-pharmacological neurobehavioral training, in individuals with neuropathic pain due to SCI. If proven effective in suppressing heightened spinal pain processing after SCI, cutaneous reflex conditioning could serve as an alternative to invasive and pharmacological pain treatments after SCI (Siddall, 2009).
Funding:
This work was supported in part by the Assistant Secretary of Defense for Health Affairs endorsed by the Department of Defense through the Spinal Cord Injury Research Program (W81XWH-22-1-1099), South Carolina Spinal Cord Injury Research Fund (SCIRF#2019 PD-01, 2021 PD-01), National Institutes of Health (NINDS R01 NS114279 to AKT, NIBIB P41EB018783 to Wolpaw, NICHD P2C HD086844 to Kautz), and the Doscher Neurorehabilitation Research Program. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by these funding agencies.
Footnotes
Conflict of Interest Statement: The authors declare no competing interests, financial or otherwise.
References
- Melzack R, Wall PD. Pain Mechanisms: A New Theory: A gate control system modulates sensory input from the skin before it evokes pain perception and response. Science 1965;150(3699):971–9. [DOI] [PubMed] [Google Scholar]
- Mrachacz-Kersting N, Kersting UG, de Brito Silva P, Makihara Y, Arendt-Nielsen L, Sinkjaer T, et al. Acquisition of a simple motor skill: task-dependent adaptation and long-term changes in the human soleus stretch reflex. J Neurophysiol 2019;122(1):435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps AM, Thompson AK. Altered cutaneous reflexes to non-noxious stimuli in the triceps surae of people with chronic incomplete spinal cord injury. J Neurophysiol 2023;129(3):513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddall PJ. Management of neuropathic pain following spinal cord injury: now and in the future. Spinal Cord 2009;47(5):352–9. [DOI] [PubMed] [Google Scholar]
- Thompson AK, Wolpaw JR. Targeted neuroplasticity for rehabilitation. Prog Brain Res 2015;218:157–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr EP, Stein RB. What functions do reflexes serve during human locomotion? Prog Neurobiol 1999;58(2):185–205. [DOI] [PubMed] [Google Scholar]


