Abstract

The reductive Heck hydroarylation of unactivated alkenes has emerged as an essential reaction for regioselective hydroarylation. Herein, we report a palladium-catalyzed reductive Heck hydroarylation of unactivated alkenes under mild conditions with enhanced functional group tolerance using hydrosilane as the reducing reagent. Under the optimal conditions, the alkylarene yields increased, resulting in minimal undesired products. Mechanistic studies using deuterated reagents indicated the involvement of two competing catalytic cycles.
The Mizoroki–Heck coupling1−4 of aryl halides and alkenes is an effective protocol for C(sp2)–C(sp2) bond formation, which enables the synthesis of functionalized alkenes5−7 used for producing pharmaceuticals, agrochemicals, and natural products. Reductive Heck hydroarylation of alkenes,8−10 a variant of Mizoroki–Heck coupling, is an attractive approach for the single-step construction of alkylarenes via C(sp2)–C(sp3) bond formation using hydride reagents. Aiming for a protecting group-free total synthesis,11 the reductive Heck hydroarylation has recently been applied to construct complex carbon skeletons of natural products.
The first palladium-catalyzed reductive Heck hydroarylation was reported by Cacchi12 in 1983 using α,β-unsaturated ketones and aryl iodides. Since then, inter- and intramolecular reductive Heck hydroarylation reactions have been developed using various substrates that have been classified into four types: activated (conjugated),12−14 unactivated (nonconjugated),15−20 strained21 (lack β-hydrogens), and tethered alkenes22,23 (aryl halide groups in the same molecules). However, unactivated alkenes have not been widely used in this reaction, except for intramolecular coupling, because substrates with no electronic differences between two olefinic sites cannot undergo regioselective hydroarylation. Therefore, inter- and intramolecular reductive Heck hydroarylations have been conducted using electronically biased alkenes, such as activated alkenes.
In 2018, Wu and Loh16 reported the first regioselective reductive Heck hydroarylation of unactivated alkenes with a directing group using proton sponge as the hydride source (Figure 1a). The directing group, Dauglis’s 8-aminoquinoline24 (AQ), controlled the regioselectivity of the migratory insertion to achieve the regioselective hydroarylation of unactivated alkenes.25−28 Subsequently, Loh and Wu18,19 and Gong and Song20 independently developed the reductive Heck hydroarylations of unactivated alkenes with the same directing group using alcohols as the hydride sources (Figures 1b and c). These pioneering studies revealed that AQ is an effective directing group for regioselectivity and that α-hydrogen-bearing alkylamines or alcohols can be used as hydride sources. Fatty acid alkylarene derivatives are important skeletons frequently found in different pharmaceuticals (Figure 1e).29−31 However, despite affording valuable alkylarenes of fatty acids, certain drawbacks are associated with these reactions: (I) high temperatures (>100 °C) are required; (II) polar functional groups, such as amino groups, are unsuitable because they undergo side reactions such as N-alkylation32,33 with aldehydes or imine generation when alcohols or amines are used as hydride sources; and (III) easily reducible substituents, such as bromo and benzyl-protecting hydroxy groups, are not tolerated by palladium–hydride species. Overcoming these drawbacks will lead to significant progress in the reductive Heck hydroarylation of unactivated alkenes. Therefore, using a new reducing reagent, we investigated the palladium-catalyzed reductive Heck hydroarylation of unactivated alkenes at room temperature under mild conditions using hydrosilane as the hydride source (Figure 1d).
Figure 1.
Intermolecular reductive Heck hydroarylation of unactivated alkenes and pharmaceuticals. (a) Amine and (b and c) alcohol as the hydride sources. (d) This study: hydrosilane as the hydride source. (e) FDA-approved pharmaceuticals.
For the initial screening, different reported hydride sources8−10,15−20 were investigated using the 4-pentenoic acid derivative 1a with an AQ directing group and p-iodoanisole 2a (3.0 equiv) in the presence of Pd(OAc)2 (10 mol %) and 6,6′-dimethyl-2,2′-bipyridyl (10 mol %) (Table 1a, entries 1–4). These hydride sources afforded either no reaction or a low desired product (3aa) yield. Therefore, we focused on triethylsilane, which is a reducing reagent used in the dehalogenation34,35 of aryl halides and the palladium-catalyzed cycloreductions of haloene-ynes,36 particularly the room-temperature dehalogenation of aryl halides using triethylsilane. Based on previous studies, we used triethylsilane as the hydride source; however, no reaction occurred (Table 1a, entry 5). Subsequently, we examined the effects of adding cesium fluoride. Fluoride anions coordinate with organosilane reagents to form highly reactive pentacoordinated silicates,37,38 which can undergo transmetalation39,40 with the PdII ligand. Consequently, 3aa was obtained in 16% yield in our developed reaction (Table 1a, entry 6). Combining hydrosilane and cesium fluoride was effective at room temperature. Thus, we screened various hydrosilanes (Table 1a, Entries 6–9, See Supporting Information (SI), p. S5), among which polymethylhydrosiloxane (PMHS) gave the highest yield of the desired product. Next, we examined the reaction with and without bipyridyl ligands and found that the reaction without bipyridyl ligands afforded the highest yield among these trials (Table 1a, entries 9–12, see SI, p S6). To further improve the yield, we analyzed the crude mixture after the reaction reached completion (Table 1a, entry 12). The crude mixture contained the starting material 1a (38%), desired compound 3aa (21%), undesired olefin migration product 1b (19%), and undesired reduced product 1c (16%). Based on this result, we hypothesized different pathways for the formation of the desired and undesired products (Table 1b) depending on the coupling partner that underwent oxidative addition to the palladium catalyst. In the desired pathway, the reaction proceeds via the Ar–PdII–I species generated via oxidative addition of Ar–I to Pd0, whereas in the undesired pathway side reactions such as olefin migration (chain walking reaction41,42) and C=C double bond reduction43 proceed via the Si–PdII–H44 species generated from the Pd0 catalyst and Si–H. We hypothesized that decreasing the yield of the undesired products, such as 1b and 1c, would improve the yield of 3aa. To ensure that the reaction proceeded via the desired pathway, we attempted to create a condition under which Ar–PdII–I was produced in larger excess than Si–PdII–H. As the formation of the Si–PdII–H species could be avoided with a limited amount of hydrosilane, we proceeded with slow addition of Si–H over 6 h using a syringe pump (see SI, p S7); the yield of 3aa increased (82%), whereas those of the byproducts decreased (0% 1b and 11% 1c) (Table 1c, entries 12 and 13). Screening of the reaction solvents indicated that the highest yield of 3aa (87%) was obtained using ethyl acetate (Table 1a, entries 13–15).
Table 1.
(a) Reaction optimization. (b) Overview: desired and undesired paths from 1a. (c) Effect of slow addition (entries 12 and 13).
NMR yield was determined using dibenzylether as an internal standard.
Slow addition of Si–H over 6 h.
Under the optimized reaction conditions, the substrate scope of aliphatic alkenes for the room-temperature reductive Heck hydroarylation (Figure 2a) was examined. As expected, using butenoic and pentenoic acid derivatives gave the desired γ- and δ-arylated products, respectively, in high yields (3aa, 3da, 3ea, and 3ha). Although alkenes bearing branched alkyl chains were expected to afford high yields owing to the Thorpe–Ingold effect,45,46 the yields were lower than those achieved using the derivatives with nonsubstituted alkyl chains (3fa, 3ga, and 3ha). By contrast, internal alkenes gave higher yields than terminal alkenes, although the migratory insertion of internal alkenes was less likely than that of terminal alkenes (3da and 3ea). Further, we evaluated aryl iodides containing electron-withdrawing groups (EWGs), electron-donating groups (EDGs), and polar functional groups (Figure 2b). The coupling reagent had the following reactivity characteristics: (I) The ortho-, meta-, and para-substituents did not significantly affect the reactivity; however, the ortho-substituted reagents had relatively low reactivity (3aa, 3ab, and 3ac). (II) The reaction with the coupling reagents and EWGs proceeded slower than that with the EDGs (selected examples: 57% 3an and 73% 3aq). (III) Functional groups that could be reduced by hydrosilanes, such as aldehydes,47,48 methyl ketones,47,48 and nitriles,49,50 were also applicable. Additionally, easily reducible substituents, such as bromo, benzyl-protecting hydroxy, and nitro groups, were applicable under these reductive conditions (3af, 3am, and 3av). (IV) Polar functional groups, such as amino or hydroxy groups, were applicable (3ad and 3aj). (V) The yields were comparable to or higher than those previously reported for the reductive Heck hydroarylation,8−10,15−20 which required high reaction temperatures.
Figure 2.
(a) Scope of aliphatic alkenes. (b) Scope of aryl iodides. aReaction conditions: 1 (0.25 mmol), p-iodoanisole (0.75 mmol), Pd(OAc)2 (0.025 mmol), CsF (0.5 mmol), PMHS (0.5 mmol, slow addition over 6 h), and AcOEt (1.1 mL). Reaction time of 24 h. b3.1 equiv of PMHS (0.75 mmol, slow addition over 9 h) was used. c4.1 equiv of PMHS (1.0 mmol, slow addition over 12 h) was used. Reaction time of 48 h. d4.1 equiv of PMHS (1.0 mmol, slow addition over 12 h) and 2.4 mL of AcOEt were used. Reaction time of 48 h. eYield determined after purification.
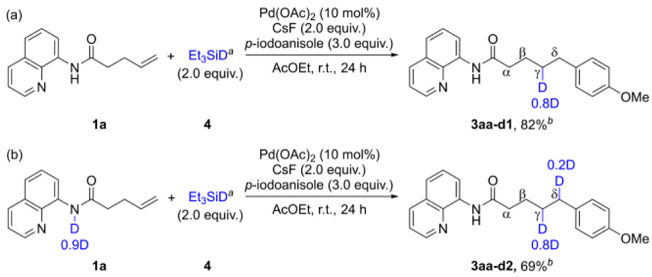
To gain mechanistic insights into the reductive Heck hydroarylation using hydrosilanes, we examined the incorporation of deuterium using deuterated triethylsilane (Et3SiD). The results indicated that 3aa-d1 was deuterated (0.8 D) only at the γ-position of the alkyl chain (Figure 3a), suggesting that the alkyl–palladium species generated by migratory insertion underwent transmetalation by triethylsilane, thereby affording the deuterated products, which is consistent with the reported mechanism.8−10 The partial incorporation of deuterium (0.8 D) at the γ-position suggests competition between protodepalladation51,52 by an amide proton and transmetalation by Si–D. Therefore, we examined reductive Heck hydroarylation using a deuterated amide (1a, 0.9D) (Figure 3b). Incorporation of deuterium at the γ-(0.8 D) and δ-positions (0.2 D) was observed, and the occurrence of protodepalladation by amide deuterons was supported.
Figure 3.
Mechanistic experiments. (a) Deuteration experiment using Si–D. (b) Using deuterated amide 1a (0.9D). aSi–D added over 6 h using a syringe pump. bYield determined after column chromatography.
The results reported herein and previously8−10 suggest two plausible catalytic cycles for the reductive Heck hydroarylation: main and side cycles (Figure 4). In the main cycle, PdIII is first reduced to Pd0II by hydrosilane,43 followed by the oxidative addition of Ar–I to Pd0. The resulting Ar–PdII–I species III is coordinated by the AQ directing group of the substrate and undergoes regioselective migratory insertion53 to form the key intermediate (V) of the five- or six-membered palladacycle. After transmetalation40 with the pentacoordinated silicate generated from Si–H and CsF, the alkyl–PdII–H intermediate (VI) is obtained. Finally, reductive elimination completes the catalytic cycle, thus producing the γ- or δ-arylated desired product (X). However, the main cycle does not fully rationalize the results of incorporating deuterium, in which the δ- (0.2 D) and γ-positions (0.8 D) are deuterated (Figure 3b). This recovered deuterated product (3aa-d2) suggested the formation of a benzyl palladium intermediate as the product precursor. Thus, we propose a side cycle where, following the regioselective β-H elimination of palladacycle Vn=1 (see SI, p S9), the Pd–H species undergoes rotation and migratory insertion, thereby affording the benzyl palladium intermediate (IX). This intermediate is protonated by an amide proton, thus producing X. Finally, PdIII, released by the protodepalladation of product precursor IX, is reduced again by Si–H to regenerate Pd0II, which becomes available for the next catalytic cycle.
Figure 4.
Proposed catalytic/main and side cycles (n = 0 or 1).
In summary, we developed the room-temperature reductive Heck hydroarylation of unactivated alkenes. The key to this process was the slow addition of PMHS as the hydride source. These findings indicate that the reaction proceeds at room temperature with high functional group tolerance, thereby affording the products in moderate-to-high yields. This reaction system does not require specific ligands, and each reagent is relatively inexpensive. Therefore, this reaction is useful for laboratory-scale and industrial synthesis. Further studies to elucidate this reaction mechanism are ongoing in our laboratory.
Acknowledgments
This work was supported in parts by JSPS KAKENHI Grant 21K15228 (Takahiro SHIRAI), as well as by the Natural Science Center for Basic Research and Development (NBARD-00066).
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.3c02488.
Investigation of optimal conditions, control experiments, preparation of substrates, typical procedure and synthesis for alkylarenes, characterization data, and NMR spectra (PDF)
Author Contributions
T.S. and Y.M. performed the experiments and analyzed the data. T.S., Y.M., R.N., and T.K. conceived and designed the experiments and prepared the manuscript.
The authors declare no competing financial interest.
This paper was published ASAP on February 1, 2024, with errors in Table 1. The corrected version was reposted on February 6, 2024.
Supplementary Material
References
- Fujiwara Y.; Moritani I.; Danno S.; Asano R.; Teranishi S. Aromatic Substitution of Olefins. Vi. Arylation of Olefins with Palladium(II) Acetate. J. Am. Chem. Soc. 1969, 91, 7166–7169. 10.1021/ja01053a047. [DOI] [PubMed] [Google Scholar]
- Mizoroki T.; Mori K.; Ozaki A. Arylation of Olefin with Aryl Iodide Catalyzed by Palladium. Bull. Chem. Soc. 1971, 44, 581. 10.1246/bcsj.44.581. [DOI] [Google Scholar]
- Heck R. F.; Nolley J. P. Jr. Palladium-Catalyzed Vinylic Hydrogen Substitution Reactions with Aryl, Benzyl, and Styryl Halides. J. Org. Chem. 1972, 37, 2320–2322. 10.1021/jo00979a024. [DOI] [Google Scholar]
- Zeng Z.; Chen Y.; Zhu X.; Yu L. Polyaniline-supported nano metal-catalyzed coupling reaction: Opportunities and challenges. Chin. Chem. Lett. 2023, 34, 107728 10.1016/j.cclet.2022.08.008. [DOI] [Google Scholar]
- Torborg C.; Beller M. Recent Applications of Palladium-Catalyzed Coupling Reactions in the Pharmaceutical, Agrochemical, and Fine Chemical Industries. Adv. Synth. Catal. 2009, 351, 3027–3043. 10.1002/adsc.200900587. [DOI] [Google Scholar]
- Zhuang C.; Zhang W.; Sheng C.; Zhang W.; Xing C.; Miao Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. 10.1021/acs.chemrev.7b00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devendar P.; Qu R. Y.; Kang W. M.; He B.; Yang G. F. Palladium-Catalyzed Cross-Coupling Reactions: A Powerful Tool for the Synthesis of Agrochemicals. J. Agric. Food Chem. 2018, 66, 8914–8934. 10.1021/acs.jafc.8b03792. [DOI] [PubMed] [Google Scholar]
- Ghosh T. Reductive Heck Reaction: An Emerging Alternative in Natural Product Synthesis. ChemistrySelect. 2019, 4, 4747–4755. 10.1002/slct.201804029. [DOI] [Google Scholar]
- Oxtoby L. J.; Gurak J. A. Jr.; Wisniewski S. R.; Eastgate M. D.; Engle K. M. Palladium-Catalyzed Reductive Heck Coupling of Alkenes. Trends Chem. 2019, 1, 572–587. 10.1016/j.trechm.2019.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J.-Q.; Liang R.-X.; Jia Y.-X. Recent Advances of Catalytic Enantioselective Heck Reactions and Reductive-Heck Reactions. Chin. J. Chem. 2021, 39, 710–728. 10.1002/cjoc.202000464. [DOI] [Google Scholar]
- Baran P. S.; Maimone T. J.; Richter J. M. Total Synthesis of Marine Natural Products without Using Protecting Groups. Nature 2007, 446, 404–408. 10.1038/nature05569. [DOI] [PubMed] [Google Scholar]
- Cacchi S.; Arcadi A. Palladium-Catalyzed Conjugate Addition Type Reaction of Aryl Iodides with α,β-Unsaturated Ketones. J. Org. Chem. 1983, 48, 4236–4240. 10.1021/jo00171a016. [DOI] [Google Scholar]
- Cacchi S.; La Torre F.; Palmieri G. The Palladium-Catalyzed Conjugate Addition Type Reaction of Aryl Iodides with α,β-Unsaturated Aldehydes. J. Organomet. Chem. 1984, 268, c48–c51. 10.1016/0022-328X(84)80252-1. [DOI] [Google Scholar]
- Torii S.; Tanaka H.; Morisaki K. Pd(0)-Catalyzed Electro-reductive Hydrocoupling of Aryl Halides with Olefins and Acetylenes. Chem. Lett. 1985, 14, 1353–1354. 10.1246/cl.1985.1353. [DOI] [Google Scholar]
- Hu X.; Hu B.; Shen Z.; Jin L.; Qian J.; Sun N. Pd-Catalyzed Reductive Heck Reaction of Olefins with Aryl Bromides for Csp2 – Csp3 Bond Formation. Chem. Commun. 2018, 54, 5752–5755. 10.1039/C8CC02571A. [DOI] [PubMed] [Google Scholar]
- Wang C.; Xiao G.; Guo T.; Ding Y.; Wu X.; Loh T. P. Palladium-Catalyzed Regiocontrollable Reductive Heck Reaction of Unactivated Aliphatic Alkenes. J. Am. Chem. Soc. 2018, 140, 9332–9336. 10.1021/jacs.8b03619. [DOI] [PubMed] [Google Scholar]
- Gurak J. A.; Engle K. M. Practical Intermolecular Hydroarylation of Terminal Alkenes via Reductive Heck Coupling. ACS Catal. 2018, 8, 8987–8992. 10.1021/acscatal.8b02717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng K.; Xiao G.; Guo T.; Ding Y.; Wang C.; Loh T. P.; Wu X. Intermolecular Reductive Heck Reaction of Unactivated Aliphatic Alkenes with Organohalides. Org. Lett. 2020, 22, 694–699. 10.1021/acs.orglett.9b04474. [DOI] [PubMed] [Google Scholar]
- Guo T.; Ding Y.; Zhou L.; Xu H.; Loh T.-P.; Wu X. Palladium-Catalyzed Anti-Michael Reductive Heck Reaction of α,β-Unsaturated Esters. ACS Catal. 2020, 10, 7262–7268. 10.1021/acscatal.0c02414. [DOI] [Google Scholar]
- Li Y.; Gong J.-F.; Song M.-P. Palladium-Catalyzed δ-Selective Reductive Heck Reaction of Alkenyl Carbonyl Compounds with Aryl Iodides and Bromides. Org. Chem. Front. 2020, 7, 2216–2223. 10.1039/D0QO00428F. [DOI] [Google Scholar]
- Arcadi A.; Marinelli F.; Bernocchi E.; Cacchi S.; Ortar G. Palladium-Catalyzed Preparation of Exo-Aryl Derivatives of the Norbornane Skeleton. J. Organomet. Chem. 1989, 368, 249–256. 10.1016/0022-328X(89)85320-3. [DOI] [Google Scholar]
- Larock R. C.; Babu S. Synthesis of Nitrogen Heterocycles via Palladium-Catalyzed Intramolecular Cyclization. Tetrahedron Lett. 1987, 28, 5291–5294. 10.1016/S0040-4039(00)96710-8. [DOI] [Google Scholar]
- Diaz P.; Gendre F.; Stella L.; Charpentier B. New Synthetic Retinoids Obtained by Palladium-Catalyzed Tandem Cyclisation-Hydride Capture Process. Tetrahedron 1998, 54, 4579–4590. 10.1016/S0040-4020(98)00169-0. [DOI] [Google Scholar]
- Zaitsev V. G.; Shabashov D.; Daugulis O. Highly Regioselective Arylation of sp3 C-H Bonds Catalyzed by Palladium Acetate. J. Am. Chem. Soc. 2005, 127, 13154–13155. 10.1021/ja054549f. [DOI] [PubMed] [Google Scholar]
- Gurak J. A.; Yang K. S.; Liu Z.; Engle K. M. Directed, Regiocontrolled Hydroamination of Unactivated Alkenes via Protodepalladation. J. Am. Chem. Soc. 2016, 138, 5805–5808. 10.1021/jacs.6b02718. [DOI] [PubMed] [Google Scholar]
- Yang K. S.; Gurak J. A. Jr.; Liu Z.; Engle K. M. Catalytic, Regioselective Hydrocarbofunctionalization of Unactivated Alkenes with Diverse C-H Nucleophiles. J. Am. Chem. Soc. 2016, 138, 14705–14712. 10.1021/jacs.6b08850. [DOI] [PubMed] [Google Scholar]
- Wang H.; Bai Z.; Jiao T.; Deng Z.; Tong H.; He G.; Peng Q.; Chen G. Palladium-Catalyzed Amide-Directed Enantioselective Hydrocarbofunctionalization of Unactivated Alkenes Using a Chiral Monodentate Oxazoline Ligand. J. Am. Chem. Soc. 2018, 140, 3542–3546. 10.1021/jacs.8b00641. [DOI] [PubMed] [Google Scholar]
- Matsuura R.; Jankins T. C.; Hill D. E.; Yang K. S.; Gallego G. M.; Yang S.; He M.; Wang F.; Marsters R. P.; McAlpine I.; Engle K. M. Palladium(II)-Catalyzed γ-Selective Hydroarylation of Alkenyl Carbonyl Compounds with Arylboronic Acids. Chem. Sci. 2018, 9, 8363–8368. 10.1039/C8SC03081B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The 2021 WHO Essential Medicines List can be found at:; WHO Model List of Essential Medicines - 22nd list, 2021; WHO/MHP/HPS/EML/2021.02; World Health Organization: Geneve, Switzerland, 2021.
- Seferovic J. P.; Claggett B.; Seidelmann S. B.; Seely E. W.; Packer M.; Zile M. R.; Rouleau J. L.; Swedberg K.; Lefkowitz M.; Shi V. C.; Desai A. S.; McMurray J. J. V.; Solomon S. D. Effect of Sacubitril/Valsartan versus Enalapril on Glycaemic Control in Patients with Heart Failure and Diabetes: A Post-Hoc Analysis from the PARADIGM-HF trial. Lancet Diabet. Endocrinol. 2017, 5, 333–340. 10.1016/S2213-8587(17)30087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnik N. D.; Oza Y. K.; Sane S. P.; Kaushik R.; Bhatt A. D.; Chawla K. P.; Vaidya A. B.; Yajnik V. H.; Khokhani R. C. An Open Clinical Trial of Benazepril--A New ACE Inhibitor in Mild-Moderate Hypertension. J. Assoc. Physicians India 1998, 46, 283–285. [PubMed] [Google Scholar]
- Hamid M. H. S. A.; Slatford P. A.; Williams J. M. J. Borrowing Hydrogen in the Activation of Alcohols. Adv. Synth. Catal. 2007, 349, 1555–1575. 10.1002/adsc.200600638. [DOI] [Google Scholar]
- Xu C. P.; Xiao Z. H.; Zhuo B. Q.; Wang Y. H.; Huang P. Q. Efficient and Chemoselective Alkylation of Amines/Amino Acids Using Alcohols as Alkylating Reagents under Mild Conditions. Chem. Commun. 2010, 46, 7834–7836. 10.1039/c0cc01487g. [DOI] [PubMed] [Google Scholar]
- Boukherroub R.; Chatgilialoglu C.; Manuel G. PdCl2-Catalyzed Reduction of Organic Halides by Triethylsilane. Organometallics 1996, 15, 1508–1510. 10.1021/om950514k. [DOI] [Google Scholar]
- Iranpoor N.; Firouzabadi H.; Azadi R. Diphenylphosphinite Ionic Liquid (Il-OPPh2): A Solvent and Ligand for Palladium-Catalyzed Silylation and Dehalogenation Reaction of Aryl Halides with Triethylsilane. J. Organomet. Chem. 2010, 695, 887–890. 10.1016/j.jorganchem.2010.01.001. [DOI] [Google Scholar]
- Oh C. H.; Park S. J. Palladium-Catalyzed Cycloreductions of Haloene-Ynes in the Presence of Triethylsilane. Tetrahedron Lett. 2003, 44, 3785–3787. 10.1016/S0040-4039(03)00713-5. [DOI] [Google Scholar]
- Rendler S.; Oestreich M. Hypervalent Silicon as a Reactive Site in Selective Bond-Forming Processes. Synthesis 2005, 2005, 1727–1747. 10.1055/s-2005-869949. [DOI] [Google Scholar]
- Orito Y.; Nakajima M. Lewis Base Catalyzed Asymmetric Reactions Involving Hypervalent Silicate Intermediates. Synthesis 2006, 2006, 1391–1401. 10.1055/s-2006-926405. [DOI] [Google Scholar]
- Hatanaka Y.; Hiyama T. Alkenylfluorosilanes as Widely Applicable Substrates for the Palladium-Catalyzed Coupling of Alkenylsilane/Fluoride Reagents with Alkenyl Iodides. J. Org. Chem. 1989, 54, 268–270. 10.1021/jo00263a003. [DOI] [Google Scholar]
- Sugiyama A.; Ohnishi Y. Y.; Nakaoka M.; Nakao Y.; Sato H.; Sakaki S.; Nakao Y.; Hiyama T. Why Does Fluoride Anion Accelerate Transmetalation between Vinylsilane and Palladium(II)-Vinyl Complex? Theoretical Study. J. Am. Chem. Soc. 2008, 130, 12975–12985. 10.1021/ja801362e. [DOI] [PubMed] [Google Scholar]
- Werner E. W.; Mei T. S.; Burckle A. J.; Sigman M. S. Enantioselective Heck Arylations of Acyclic Alkenyl Alcohols Using a Redox-Relay Strategy. Science 2012, 338, 1455–1458. 10.1126/science.1229208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L.; Hilton M. J.; Zhang X.; Norrby P. O.; Wu Y. D.; Sigman M. S.; Wiest O. Mechanism, Reactivity, and Selectivity in Palladium-Catalyzed Redox-Relay Heck Arylations of Alkenyl Alcohols. J. Am. Chem. Soc. 2014, 136, 1960–1967. 10.1021/ja4109616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza-Aghayan M.; Boukherroub R.; Bolourtchian M. A Mild and Efficient Palladium-Triethylsilane System for Reduction of Olefins and Carbon-Carbon Double Bond Isomerization. Appl. Organometal. Chem. 2006, 20, 214–219. 10.1002/aoc.1036. [DOI] [Google Scholar]
- Hurst M. R.; Zakharov L. N.; Cook A. K. The Mechanism of Oxidative Addition of Pd(0) to Si-H Bonds: Electronic Effects, Reaction Mechanism, and Hydrosilylation. Chem. Sci. 2021, 12, 13045–13060. 10.1039/D1SC04419B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh K. K.; van Gemmeren M. Pd-Catalyzed β-C(sp3)-H Arylation of Propionic Acid and Related Aliphatic Acids. Chem. Eur. J. 2017, 23, 17697–17700. 10.1002/chem.201705449. [DOI] [PubMed] [Google Scholar]
- Zhu B.; Li Z.; Chen F.; Xiong W.; Tan X.; Lei M.; Wu W.; Jiang H. Palladium-Catalyzed Oxidative Heck Reaction of Non-Activated Alkenes Directed by Fluorinated Alcohol. Chem. Commun. 2022, 58, 12688–12691. 10.1039/D2CC04921J. [DOI] [PubMed] [Google Scholar]
- Boyer J.; Corriu R. J. P.; Perz R.; Reye C. Reduction Selective de Composes Carbonyles par Catalyse Heterogene a la Surface Des Sels. Tetrahedron 1981, 37, 2165–2171. 10.1016/S0040-4020(01)97975-X. [DOI] [Google Scholar]
- Corriu R. J. P.; Perz R.; Reye C. Activation of Silicon-Hydrogen, Silicon-Oxygen, Silicon-Nitrogen Bonds in Heterogeneous Phase. Tetrahedron 1983, 39, 999–1009. 10.1016/S0040-4020(01)88599-9. [DOI] [Google Scholar]
- Bornschein C.; Werkmeister S.; Junge K.; Beller M. TBAF-Catalyzed Hydrosilylation for the Reduction of Aromatic Nitriles. New J. Chem. 2013, 37, 2061–2065. 10.1039/c3nj00171g. [DOI] [Google Scholar]
- Clarke J. A.; van der Est A.; Nikonov G. I. Base-Catalyzed Hydrosilylation of Nitriles to Amines and Esters to Alcohols. Eur. J. Org. Chem. 2021, 2021, 4434–4439. 10.1002/ejoc.202100834. [DOI] [Google Scholar]
- Jia C.; Lu W.; Oyamada J.; Kitamura T.; Matsuda K.; Irie M.; Fujiwara Y. Novel Pd(II)- and Pt(II)-Catalyzed Regio- and Stereoselective Trans-Hydroarylation of Alkynes by Simple Arenes. J. Am. Chem. Soc. 2000, 122, 7252–7263. 10.1021/ja0005845. [DOI] [Google Scholar]
- Panda N.; Mothkuri R. Stereoselective Synthesis of Enamides by Pd-Catalyzed Hydroamidation of Electron Deficient Terminal Alkynes. J. Org. Chem. 2012, 77, 9407–9412. 10.1021/jo301772f. [DOI] [PubMed] [Google Scholar]
- Bahamonde A. Mechanistically Guided Survey of Enantioselective Palladium-Catalyzed Alkene Functionalization. Trends Chem. 2021, 3, 863–876. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.