Abstract
Background:
Overuse of preoperative cardiac testing contributes to high healthcare costs and delayed surgeries. A large body of research has evaluated factors associated with variation in preoperative cardiac testing. However, patient, provider, and system level factors associated with variation in testing have not been systematically studied.
Objectives:
To conduct a systematic review to better delineate the patient, provider, and system level factors associated with variation in preoperative cardiac testing.
Methods:
We included studies of an adult US population evaluating a patient, provider, or system level factor associated with variation in preoperative cardiac testing for non-cardiac surgery since 2012. Our search strategy used terms related to preoperative testing, diagnostic cardiac tests, and care variation, with Ovid MEDLINE and Embase from inception through January 2023. We extracted study characteristics and factors associated with variation and qualitatively analyzed them. We assessed risk of bias using the Newcastle-Ottawa Scale and Evidence Project Risk of Bias tool.
Results:
Twenty-eight articles met inclusion criteria. Older age and higher comorbidity were strongly associated with higher intensity testing. The evidence for provider and system level covariates was weaker. However, there was strong evidence that a focus on primary care and away from preoperative clinic and cardiac consultations was associated with less testing and that interventions to reduce low-value testing can be successful.
Conclusions:
There is significant inter-provider and inter-hospital variation in preoperative cardiac testing, the correlates of which are not well-defined. Further work should aim to better understand these factors.
Keywords: preoperative cardiac testing, low-value care, diagnostic overuse
Introduction
Variation in healthcare intensity has been studied at regional,1,2 health system,3,4 hospital,5 and provider levels.6-8 Much of this variation reflects low-value care which is defined as care for which “the harms or costs outweigh the benefits.”9 Recent estimates suggest that low-value care contributes between $76 and $101 billion to US healthcare costs annually.10 Preoperative testing, especially preoperative cardiac testing, is often low-value care when used on patients for whom it is unlikely to yield actionable information. In addition to increasing healthcare costs, low-value preoperative cardiac testing can delay surgeries leading to worse outcomes and increased length of stay.
As a result, six organizations have included preoperative cardiac testing among their list of low-value care to avoid as a part of the Choosing Wisely© campaign of the American Board of Internal Medicine, which began in 2012. The American College of Cardiology in 2012 recommended clinicians “avoid performing stress testing, coronary calcium scoring, or advanced cardiac imaging as part of preoperative cardiovascular risk assessment in patients scheduled for low-risk non-cardiac surgery.”11 Also in 2012, the American Society of Nuclear Cardiology recommended against preoperative cardiac imaging before low- or intermediate-risk non-cardiac surgery.12 In 2013, the American Society of Echocardiography recommended avoiding preoperative echocardiograms in patients without a history or symptoms of heart disease.13 That same year, the Society of General Internal Medicine recommended against routine preoperative testing (including electrocardiograms [EKGs]) before low-risk surgical procedures.14 The American Society of Anesthesiologists recommended against echocardiography and stress testing in “asymptomatic stable” patients undergoing low or moderate risk non-cardiac surgery.15 Additionally in 2013, the American Academy of Ophthalmology recommended against preoperative medical tests (including EKGs) without medical indications for eye surgery.16
These recommendations are consistent with the most recent perioperative testing guidelines from the American College of Cardiology and American Heart Association published in 2014.17 However, these guidelines do leave the question of ischemic evaluation up to the provider in patients at elevated risk of major adverse cardiac events and low or unknown metabolic equivalents. More recent preoperative guidelines published in 2022 by the European Society of Cardiology recommend against routine preoperative echocardiogram or stress testing but do allow for consideration of these tests in certain clinical scenarios.18
Many studies have quantified the use of low-value preoperative cardiac testing. However, the patient, provider, and system level drivers of this practice are not well-understood, which hampers development of effective interventions to mitigate it. Thus, we sought to systematically review the literature to determine which patient, provider, and system level characteristics are associated with higher levels of preoperative cardiac testing in the United States.
Methods
We registered the protocol in Prospero before beginning the review process (January 19, 2023; CRD42023390531).
Study Inclusion/Exclusion Criteria
We sought to include articles that assessed patient, provider, or system level factors associated with variation in preoperative cardiac testing. We excluded abstracts that did not primarily include a US population, primarily include adults, and study overuse of or variation in intensity of preoperative cardiac testing (rather than focusing on what preoperative cardiac testing should be done from a clinical standpoint) (Supplement Document 1). In the full text review, we also excluded: 1) articles that did not describe a patient, provider, or system level factor associated with variation in preoperative cardiac testing, 2) articles published before 2012 (the first year of the Choosing Wisely© campaign), 3) articles only examining preoperative cardiac testing before cardiac surgery, 4) articles presenting a survey that asked providers their preferences in hypothetical clinical scenarios, rather than what they actually do with respect to preoperative cardiac testing, and 5) abstract only references (Supplement Document 2).
Data Sources and Search Strategy
We developed a search strategy using keywords and medical subject headings relevant to preoperative testing, diagnostic cardiac tests, and variation in care (Supplement Document 3). We searched Ovid MEDLINE and Embase from inception through January 2023. These databases were accessed through our university library, and reference lists were downloaded and exported to Zotero version 6.0.26 (Bethlehem, Pennsylvania), which was used for de-duplication and reference management. The de-duplicated list was exported to Covidence (Melbourne, Australia), an online platform to facilitate collaboration. The team also hand-searched the reference lists of included articles for additional articles meeting inclusion criteria.
Review Process
Two reviewers independently screened titles/abstract for inclusion. Articles for which there was disagreement at the title/abstract screening stage were included in the full text review. Two independent reviewers then performed full text review. Disagreements between reviewers were resolved by discussion between the two reviewers.
Data Extraction Process
The research team developed a three-part extraction form: 1) study design (including details on type of intervention (if any), comparator, cardiac test, location, patient population, inclusion/exclusion criteria, data source, and type of surgery), 2) results (non-significant and significant patient, provider, and system level characteristics, as well as clinical/comorbidity variables), and 3) risk of bias. One reviewer extracted data, and a second reviewer checked for accuracy. We extracted the most fully adjusted results for each study.
Risk of Bias Assessment
Both reviewers conducted a risk of bias assessment. For observational studies, we used the Newcastle-Ottawa Scale. This was originally developed for case-control studies.19 However, more recently, it has been operationalized to be used with cross-sectional and cohort studies.20 For studies with an intervention, we used the Evidence Project Risk of Bias tool.21 Differences of opinion were resolved through discussion (Supplement Tables 1-4).
Data Synthesis and Analysis
We summarized the included studies by study characteristics: design, setting, inclusion criteria, type of surgery and preoperative testing, and risk of bias (Table 1). We grouped significant associations under subcategories of patient, provider, and system level factors, providing a qualitative description of the results for each relevant study within a subcategory and also a general interpretation of the findings of multiple studies within each subcategory (Table 2). Additionally, we summarized non-significant and significant findings by study at the patient (Supplement Table 5), provider (Supplement Table 6), and system level (Supplement Table 7). Given the heterogeneity of the studies, quantitative pooling of results was not feasible.
Table 1 –
Study Characteristics
| Study, year | Study design* (intervention if applicable) |
Data source | Setting | Inclusion (Exclusion) Criteria |
Type of surgery |
Type of preop cardiac testing |
Risk of Bias |
|---|---|---|---|---|---|---|---|
| Adair 201722 | Cross-sectional | Registry derived from EMR | AMC, 5/2009-11/2012 | >55 years old | Hip fracture surgery | Echo | Moderate |
| Aviki 202245 | Pre-post | EMR | AMC 7/2014-12/2015 (pre); 7/2016-12/2017 (post) | Adults | Ambulatory endometrial surgery | EKG | High |
| Chan 201832 | Cross-sectional | Collaborative registry | Southern California, 9/2012-5/2016 | No history of CAD | Various vascular surgeries | Stress test | Moderate |
| Charlesworth 201636 | Cross-sectional | Medicaid claims and All-Payer, All-Claims database | Oregon, 1/2013-12/2013 | Between 18 to 64 years of age enrolled in either Medicaid or commercial insurance (Excl – pregnant or dual-eligible patients) | Low or intermediate risk non-cardiothoracic surgery | Echo, stress test | Moderate |
| Clark 201937 | Cross-sectional | Interviews with thoracic surgeons | Online, 2018-2019 | Participation in the Society of Thoracic Surgeons | Lung resections | Echo, stress test | High |
| Colla 201823 | Retrospective cohort | 100 % Medicare claims and commercial claims from HCCI | National data from 2009 through 2011 | Enrollment in Medicare Part A and Part B (for Medicare beneficiaries) | Low-risk, noncardiac surgery | EKG, echo, stress tests, cardiac CT, MRI, and PET | Moderate |
| Ellenbogen 201938 | Cross-sectional | State Inpatient Databases from Maryland, New Jersey, and Washington | Acute care hospitals, 2011-2015 | Primary ICD procedure code for hip fracture repair (Excl – interhospital transfers) | Hip fracture repair | Echo, stress test, and cardiac catheterization | Moderate |
| Esper 202246 | Pre-post | Geriatric trauma database | Orthopedic specialty hospital 9/2015-6/2021 (Intervention-5/ 2019) | Adults | Hip fracture repair | Echo | Moderate |
| Ganguli 201924 | Retrospective cohort | FFS Medicare claims (20% sample) | Medicare FFS patients, (4/2013-9/2015) | Aged 66 or older and continuously enrolled in FFS Medicare during study period (Excl: diagnosis of cardiac disease in the last year) | Cataract surgery | EKG (associated with cascades of further testing) | Low |
| Gertz 201647 | Pre-post | EMR | AMC, 9/2010-2/2012 (washout period of 2/2011-9/2011) | Hospitalized adults receiving a stress test | Low-risk surgery | Stress test | Moderate |
| Hasan 202241 | Retrospective cohort | New York Statewide Planning and Research Cooperative System | 3/2016-6/2017 | Adults with a preoperative outpatient visit within two months of surgery | Total hip or knee replacement | EKG | Moderate |
| Kerr 201525 | Retrospective cohort | VA Corporate Data Warehouse, Medicare FFS claims (5% sample) | 2/2009-12/2009 | Ages 65 years or older | Cataract surgery, knee or shoulder arthroscopy | Stress test | Moderate |
| King 202331 | Cross-sectional | EMR | AMC, 1/2015-12/ 2019 | Adults (Excl – receiving cardiac testing for known or suspected angina or ischemic equivalent) | Laparoscopic bariatric surgery | EKG, echo, stress test, cardiac catheterization | Moderate |
| Langell 201648 | Pre-post | EMR | AMC, 2-3/2012 (pre), 2-3/2013 (post) | Outpatients | Elective non-cardiac surgery | EKG | High |
| Mafi 201944 | Non-randomized controlled trial | EMR | 2 safety net AMCs, 4/2015-4/2016; control was another AMC | Adults | Cataract surgery | EKG | Moderate |
| Marcantonio 201326 | Retrospective cohort | Internal billing database | AMC, 1/2004-2/2011 | Adults 65 and older | Hip fracture surgery | Echo | High |
| Muthappan 201434 | Retrospective cohort | Data collection forms and registry | 14 hospitals in Michigan | Adults (Excl – recent MI with intervention, cardiac arrest, or medications suggesting high cardiac complexity) | High risk, non-cardiac surgery | PCI | Low |
| Nelson 201949 | Pre-post | Perioperative data warehouse | AMC, 1/2010-10/2015 | Adults visiting the preoperative evaluation clinic | Any surgery | EKG | Low |
| Pappas 202135 | Cross-sectional | EMR | AMC, 2008-2018 | Adults receiving preoperative cardiac testing within 30 days of preoperative clinic visit | All except cardiac or ophthalmologic surgery | Stress test | Low |
| Peterson 201839 | Cross-sectional | EMR | AMC, 1/2012-12/2014 | Adults | All except cardiac or transplant | Stress test | Moderate |
| Pickering 202230 | Retrospective cohort | VA Corporate Data Warehouse | Veterans Hospital Administration, 10/2016-9/2018 | Continuously enrolled veterans | Low or intermediate risk non-cardiothoracic surgery | EKG (associated with cascades of cardiac and non-cardiac testing) | Low |
| Ponukumati 202233 | Retrospective cohort | Vascular Quality Initiative Registry | Vascular Quality Initiative Centers, 2015-2019 | Adults (Excl - urgent or emergent AAA repair, unknown indication for AAA repair) | AAA repair | Stress test | Moderate |
| Riggs 201842 | Retrospective cohort | MarketScan Commercial Claims Database | All states, 2010-2013 | Commercially insured patients, ages 18-64; at least one outpatient visit with a PCP one month to one year before surgery | Elective general, vascular, orthopedic, urologic, and gynecologic surgeries | EKG, echo, and stress test | Low |
| Rubin 202140 | Cross-sectional | MarketScan Research Databases, including Medicare supplemental coverage | All states, 2004-2017 | Adults | Elective total hip or total knee arthroplasty | Stress test | Low |
| Sheffield 201327 | Cross-sectional | Medicare claims (5% sample) | All states, 1996-2008 | Adults, 66 and over | Elective non-cardiac, non-vascular | Stress test | Low |
| Sigmund 201543 | Retrospective cohort | National ambulatory and national hospital ambulatory medical care surveys | All states, 1997-2010 | Adults with a preoperative visit or a general medical visit and an associated surgery | Any surgery | EKG, stress test | Low |
| Sinvani 202028 | Cross-sectional | EMR | 3 tertiary care and 4 community hospitals in a large integrated health system, 4/2014-12/2015 | Adults, 65 and older | Hip fracture repair | Echo, stress test | High |
| Valle 201829 | Retrospective cohort | VA Clinic Assessment Reporting and Tracking Program, Corporate Warehouse, and National Patient Care Database | 131 VA facilities, 2004-2011 | Adults who underwent surgery within 2 years of a PCI | Non-cardiac, non-emergent surgery | Stress test | Moderate |
In studies with multiple outcomes (that led to ambiguity with respect to retrospective cohort versus cross-sectional study), we focused on the primary outcome to determine the study type.
Abbreviations:
AAA – Abdominal Aortic Aneurysm
AMC – Academic Medical Center
CAD – Coronary Artery Disease
CT – Computed Tomography
EKG - Electrocardiogram
EMR – Electronic Medical Record
FFS – Fee-for-service
HCCI – Health Care Cost Institute
MI – Myocardial infarction
MRI – Magnetic Resonance Imaging
PCP – Primary Care Provider
PCI – Percutaneous Coronary Intervention
PET – Positron Emission Tomography
VA – Veterans Affairs
Table 2 –
Significant Factors Associated with Preoperative Cardiac Testing
| LEVEL | RESULTS | INTERPRETATION |
|---|---|---|
| Patient | ||
| Female gender | Fewer stress tests (Chan,32 Ellenbogen,38 Rubin40); fewer catheterizations (Ellenbogen38); more EKGs (Ganguli24), more stress tests (Sheffield27) | Generally, women received less testing |
| Older age | More EKGs and cascade events (Ganguli24); more echos (Adair,22 Ellenbogen,38 Marcantonio26); more stress tests (Charlesworth,36 Ellenbogen,38 Rubin,40 Valle29); more cardiac catheterizations (Ellenbogen38); more preoperative cardiac testing (King31) | Older age strongly associated with more testing. |
| White race | Fewer EKGs (Ganguli24), more EKGs (Pickering30) | Not a clear association between race or ethnicity and testing |
| Hispanic ethnicity | More EKGs (Ganguli24), fewer EKGs (Pickering30), more echos (Sinvani28) | |
| Area of residence | ||
| Urban area | More EKGs (Ganguli24) | Association between urban/large metro areas and more EKGs |
| Large metro (vs small metro) | More EKGs (Pickering30) | |
| Primary payer | ||
| Medicare (vs commercial) | More cardiac testing (Colla23) | Association of government-sponsored insurance with more testing and capitated insurance plans with less testing |
| Medicaid (vs commercial) | More echos and stress tests (Charlesworth36) | |
| Medicaid enrollment | More EKGs (Ganguli24) | |
| Commercial insurance (vs other insurance types) | Fewer stress tests and catheterizations (Ellenbogen38) | |
| Capitated (vs non- capitated) insurance plan | Fewer stress tests (Rubin40) | |
| Increasing comorbidity | ||
| Elixhauser Comorbidity Score | More EKGs (Ganguli24, Pickering30), more echos, stress tests, and catheterizations (Ellenbogen38), more cascade events (Ganguli24) | Strong association between higher levels of comorbidity and more testing |
| Charlson Comorbidity Index | More EKGs (Riggs42), more echos (Adair,22 Charlesworth,36 Riggs,42 Sinvani28), more stress tests (Charlesworth,36 Riggs,42 Sheffield27) | |
| ASA Physical Classification System | More echos (Marcantonio26), more preoperative cardiac testing (King31) | |
| MICA Score | More stress tests (Pappas35) | |
| RCRI | More cardiac testing (King31), more EKGs (Riggs42), more echos (Riggs42), more stress tests (Pappas,35 Riggs,42 Rubin,40 Valle29) | |
| More cascade events | More EKGs (Ganguli,24 Pickering30) | Strong evidence that low-value preoperative EKGs can lead to are associated with downstream testing |
| Provider | ||
| Preoperative clinic visit (vs primary care visit) before surgery | More EKGs and stress tests (Sigmund43) | Preoperative clinic visit (vs primary care visit) and preoperative cardiology visit associated with more testing |
| Preoperative cardiology referral | More preoperative cardiac tests (King31) | |
| Tendency of internal medicine physicians working at a preoperative evaluation clinic to order more tests | More stress tests (Pappas35) | Significant impact of preoperative clinic provider practice habits on volume of testing |
| Admitted to a medicine service (vs surgery service) | More echos (Sinvani28) | Admission to a medical (vs surgical) service associated with more echos |
| Transitioning from multiple specialties to just hospitalist deciding if echo is needed | Fewer echos (Esper46) | QI initiative streamlining decision on echo to only hospitalist associated with less testing |
| System | ||
| Increasing regional cardiologist density | More EKGs and more cascade events after EKG (Ganguli24), more stress tests (Sheffield27) | Regions with more cardiologists per capita, higher spending, or greater populations had more testing |
| Greater spending per Medicare beneficiary at HRR level | More stress tests (Sheffield27) | |
| Location in more populated MSA | More stress tests (Sheffield27) | |
| Higher VA Facility complexity level | More EKGs (Pickering30) | Higher health system patient complexity associated with more testing# |
| Higher overall RCRI scores for surgical patients at health system | More stress tests (Valle29) | |
| Location | Midwest and West (vs South and Northeast) associated with more EKGs (Pickering30); Midwest, Mountain West, and Northeast (vs Pacific Northwest) associated with more stress tests (Sheffield27); Washington state (vs Maryland and New Jersey) associated with fewer stress tests and catheterizations (Ellenbogen38) | Multiple studies showed regional variation in testing intensity, but with somewhat conflicting findings |
| Larger hospital or health system size | Fewer EKGs (Pickering30), more stress tests (Sheffield27), more catheterizations (Ellenbogen38), fewer echos (Ellenbogen38) | Does not seem to be a clear relationship between hospital/health system size and testing intensity |
| Community hospital (vs tertiary care center) | More echos (Sinvani28) | Community hospitals (vs tertiary care centers) associated with more echos |
| Implementation of preoperative testing guidelines and algorithms with or without educational curriculum | Fewer EKGs (Aviki,45 Langell,48 Mafi*,44 Nelson49) | Multiple successful QI intitatives to decrease EKG overutilization |
| Publication of 2002 ACC/AHA Perioperative Guidelines | Fewer EKGs (Sigmund43) | Publication of 2002 guidelines associated with fewer EKGs |
| Higher proportion of elective inpatient surgeries to outpatient surgeries | More stress tests (Valle29) | More elective inpatient surgeries relative to outpatient surgeries associated with mores stress tests |
Abbreviations:
ACC – American College of Cardiology
AHA – American Heart Association
ASA – American Society of Anesthesiologists
MICA – Myocardial Infarction or Cardiac Arrest
MSA – Metropolitan Statistical Area
QI – Quality Improvement
RCRI – Revised Cardiac Risk Index
Not all references are cited in this table because not all had significant findings for a patient, provider, or system level factor
Significance is defined as p<0.05
Mafi study had a large educational component
Both of these studies were done on VA populations
Results
Our search strategy yielded 681 titles, of which 129 underwent full text review and 28 met inclusion criteria for data extraction (Figure 1).
Figure 1 –
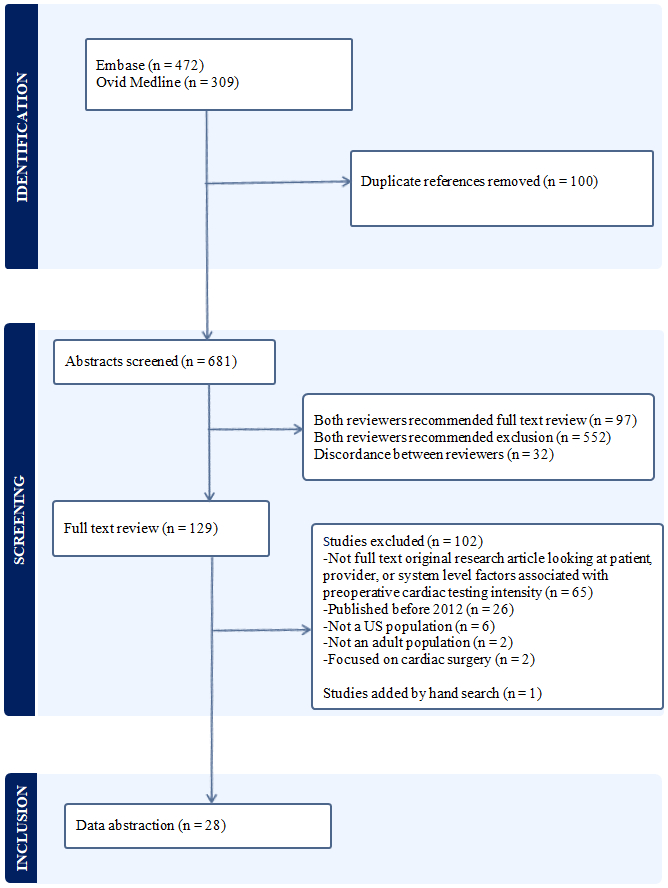
Evidence Search and Selection
Characteristics of Included Studies
Most of the studies were broadly focused on the adult population, but a minority were focused on the older adult population.22-28 Two studies were focused on Veterans.29,30 Only a minority of studies had specific cardiac exclusion criteria.24,29,31-35 Most of the included studies were observational using either cross-sectional22,27,28,31,32,35-40 or retrospective cohort designs (Table 1).23-26,29,30,33,34,41-43 Of those with an intervention,44-49 none was a randomized controlled trials although one was a non-randomized controlled trial (Supplement Table 8).44 Five other studies used pre-post designs45-49 of which all were prospective, interventional studies except one.49 The most frequently studied surgery was hip fracture repair.22,26,28,38,46 Fewer than half used a nationally representative patient sample.23-25,27,29,30,36,38,40,42,43 The preoperative study of interest varied: nine studies focused on stress tests,25,27,29,32,33,35,39,40,47 seven on EKGs24,30,41,44,45,48,49 (two of which24,30 also studied cascades of care after an EKG), three on echocardiograms,22,26,46 one on cardiac catheterization,34 and the remaining eight on a combination of tests.23,28,31,36-38,42,43
Risk of Bias
Fourteen of the 28 studies had a moderate risk of bias.22,23,25,29,31-33,36,38,39,41,44,46,47 Nine had a low risk of bias,24,27,30,34,35,40,42,43,49 and five had a high risk of bias (Table 1; Supplement Tables 1-4).26,28,37,45,48
Of the studies deemed to have a low-risk of bias, most24,27,30,42,43 were cohort studies that used a nationally representative data set with well-designed adjustment for patients’ clinical characteristics. Two were single-center studies – one pre-post49 and one cross-sectional35 – that were well-designed and benefited from granular data. Three used causal inference techniques – propensity score matching30,34 and difference-in-difference analysis.43
Patient Level Factors
Older patients22,24,26,29,31,38,40 were more likely to have preoperative cardiac testing, as were patients with greater comorbidity as measured by Elixhauser score,24,30,38 Charlson Comorbidity Index,22,27,28,36,42 American Society of Anesthesiologists Classification Score,26,31 Myocardial Infarction and Cardiac Arrest Score,35 and Revised Cardiac Risk Index (RCRI) Score29,31,35,40,42 (Table 2; Supplement Table 5). RCRI was the most commonly used perioperative risk stratification tool. The adjusted odds ratio of receiving a preoperative stress test with an RCRI of 1 (vs an RCRI of 0) ranged from 2.69 (95% CI, 2.46-2.93)42 to 4.35 (95% confidence interval (CI), 4.18-4.53).40 Individuals with Medicare or Medicaid, relative to commercial plans,23,24,36,38 were more likely to have preoperative cardiac testing; similarly, individuals in non-capitated health plans relative to capitated plans40 had more testing (adjusted odds ratio of stress testing for capitated plan 0.80 (95% CI, 0.73-0.87). Two studies found that low-value preoperative EKGs can lead to downstream testing.24,30
Provider Level Factors
Patients visiting a preoperative clinic, compared to a primary care clinic,43 or those having a preoperative referral to a cardiologist31 had more testing (Table 2; Supplemental Table 6). A single-center, quality improvement (QI) project demonstrated that when the hospitalist alone was tasked with determining the necessity of a preoperative echocardiogram, rather than the hospitalist, cardiologist, and anesthesiologist together, fewer echocardiograms were ordered.46 Another single-center study found that a preoperative clinic provider’s general tendency to order preoperative stress tests was strongly associated with the probability that a given patient would receive a stress test (a physician at the 95th percentile of stress test ordering intensity was three times more likely to order a stress test than a physician at the 5th percentile of ordering intensity).35
System Level Factors
A higher regional density of cardiologists,24,27 greater regional spending per Medicare beneficiary,27 and more populous metropolitan statistical areas27 were associated with more preoperative cardiac testing (Table 2; Supplemental Table 7). There was not a clear association between hospital size and intensity of preoperative cardiac testing.27,30,38 However, community hospital care relative to care at a tertiary hospital was associated with more preoperative echocardiograms.28 Implementation of preoperative testing guidelines at an institution were associated with fewer EKGs.44,45,48,49
Conclusions
Between 2012 and 2013, six organizations included avoidance of certain preoperative cardiac tests among their Choosing Wisely© recommendations. Since then, a large body of literature has critically evaluated preoperative cardiac testing, but to date, it has not been systematically reviewed. We conducted a systematic review to determine the patient, provider, and system level factors associated with higher levels of preoperative cardiac testing rates. Understanding drivers of high intensity preoperative cardiac evaluations will allow for development of successful QI initiatives and realignment of incentives to promote cost-effective care. About half of the included articles were explicitly evaluating overuse whereas the other half evaluated variation in diagnostic intensity, though this distinction was not one that we defined ex ante as an element for data extraction.
The included studies consistently reported that older age and increasing comorbidity are associated with more preoperative cardiac testing.22,24,26-31,35,36,38,40,42 However, significant inter-provider35 and inter-hospital29,32-34 variation in preoperative cardiac testing was observed, suggesting that variation in diagnostic intensity was not entirely driven by patient characteristics. We expected that use of low-value preoperative cardiac testing is a function of provider and system characteristics, specifically incentives and processes of care. Indeed, we found that a higher regional density of cardiologists,24,27 a preoperative cardiology referral,31 and a visit to a preoperative clinic rather than a primary care clinic43 were associated with more preoperative testing. These findings are consistent with previous work studying low-value care more generally, which showed that a higher ratio of specialists to primary care physicians was associated with more low-value care2 and that a higher regional concentration of cardiologists was associated with more low-value cardiac testing.50
The extent to which financial incentives contribute to low-value preoperative cardiac testing is unclear from our review. Patients with government sponsored insurance (Medicare and Medicaid) relative to commercial plans23,24,36,38 as the primary payer were more likely to receive preoperative testing. We do not know the mechanism for this relationship. While these studies did control for comorbidities, the finding may be due to residual confounding in that older, more medically complex patients (who are more likely to be on government sponsored insurance) typically receive more preoperative cardiac testing. Patients with non-capitated relative to capitated insurance plans received more testing.40 We suspect this has more to do with hospital or clinic payer mix rather than physicians basing management decisions on an individual patient’s insurance. Previous work has demonstrated a significant relationship between hospital payer mix and diagnostic intensity.51 A prior study also found that primary care physicians in capitated payment models ordered less low-value screening tests than those in non-capitated payment models.6
Our review included several studies testing interventions designed to reduce low-value preoperative cardiac testing,44-49 and all but one47 successful reduced testing. The one intervention that was not successful was aimed at reducing stress tests with imaging and not specifically preoperative stress tests, although it did provide results separately for preoperative stress testing. It is encouraging that five of the six interventional studies successfully reduced preoperative cardiac testing. These results speak to the extent to which social processes and dynamics might impact the intensity of preoperative cardiac testing. Of course, there could be an element of publication bias. Notably, four of the five successful interventions were focused on reducing preoperative EKGs.44,45,48,49 EKGs are the least expensive (and presumably least profitable, at least in a fee-for-service system) type of cardiac testing. Of note, one study which calculated the cost of the intervention at two safety net hospitals noted cost-savings due to the capitated nature of the safety net hospitals.44 However, in a simulation of the intervention at a fee-for-service hospital, they estimated significant financial losses.
Our review also found strong evidence suggesting that preoperative EKGs may lead to further downstream testing (“cascade” events).24,30 This is important because much of the research on this topic, and most of the QI research, focused on EKGs, which are a relatively low-cost diagnostic test without radiation or risk of direct clinical harm. This finding suggests that though the per-unit cost of EKGs is small, the implications of unnecessary EKG testing may still be significant. Moreover, previous work has shown that low and very low cost services contribute more to the total cost of low-value care than high and very high cost services, due to greater volume.52 Further supporting the concept of care cascades in preoperative cardiac testing, a study published after our literature search measured downstream effects of preoperative stress testing and used a causal inference strategy to show that they were associated with a higher incidence of coronary angiography, percutaneous coronary intervention, and delayed and cancelled non-cardiac surgery.53
This review has limitations, most notably that the heterogeneity of the research precluded quantitative pooling of the data. Additionally, we did not focus on one specific type of preoperative cardiac testing, and it is possible that different factors may perpetuate the overuse of different types of testing. Much of the research we reviewed focused on non-modifiable patient-level factors, and a relatively small portion focused on provider and system level factors that may be modifiable to reduce diagnostic overuse. An even smaller fraction dealt with easily modifiable care processes. Finally, much of the research that was focused on a single hospital or small number of hospitals was conducted at academic medical centers, so the results may not be applicable to community hospitals.
Future research should further evaluate provider and system (particularly hospital and health system) characteristics that are associated with, and potentially drivers of, low-value preoperative cardiac testing. At the provider level, degree and training (physician versus advanced practicing provider; years of experience) may have explanatory power. At the hospital level, provider compensation mechanisms and the relationship between surgeons and non-surgeons in managing surgical patients may yield insights. Additionally, understanding the impact of institutional culture on clinical care would be valuable. Culture likely informs differential emphasis placed on issues of patient safety and clinical outcomes, efficiency (like length of stay and time to surgery), avoidance of low-value care, and optimization of revenue streams.
The relationship between institutional culture and preoperative cardiac testing intensity could be elucidated though qualitative analyses involving structured interviews with surgeons, cardiologists, and generalists (primary care physicians and hospitalists) regarding their opinions on the value of preoperative cardiac testing. A recent positive deviance analysis utilizing interviews of health system leaders found that both health system culture and clinicians’ attitudes were important factors in allowing certain health systems to provide lower-than-average overuse while still delivering adequate quality of care.54 A mixed methods approaching quantitatively and qualitatively comparing preferences for and actual testing utilization among surgical subspecialties performing surgeries with a similar level of perioperative risk within a single hospital would be one way to help isolate various aspects of culture. Another way to better understand how institutional culture impacts low-value preoperative cardiac testing would be to evaluate the impact of hospital mergers and acquisitions. In these situations, processes of care and financial incentives are likely to change faster than clinicians’ attitudes and overall institutional culture and this would allow a causal inference approach to understanding the most important drivers.
At the regional level, the malpractice environment and payer and health system market power may have meaningful associations with preoperative cardiac testing. Identifying these drivers will help us understand the causes of low-value preoperative cardiac testing but also, more generally, low-value care. Special attention should be focused on identifying modifiable drivers of low-value care, for example, hospital processes of care.
In conclusion, increasing age and medical complexity are associated with higher intensity preoperative cardiac testing. There is also significant inter-provider and inter-hospital variation in preoperative cardiac testing, though the evidence supporting individual factors driving variation at these levels is weaker.
Supplementary Material
Acknowledgements:
We appreciate the technical input from Marcus Spann, informationist at Welch Medical Library.
Funding:
Dr. Ellenbogen is supported by the Agency for Healthcare Research and Quality 1K08HS028673-01A1. Dr. Segal is supported by National Institute of Aging K24AG049036.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to declare.
Role of the funder: The funding agents had no role in the study design, conduct, drafting, or decision to publish the manuscript.
References:
- 1.Fisher ES, Wennberg JE, Stukel TA, et al. Associations among hospital capacity, utilization, and mortality of US Medicare beneficiaries, controlling for sociodemographic factors. Health Serv Res. 2000;34(6):1351–1362. [PMC free article] [PubMed] [Google Scholar]
- 2.Oakes AH, Sen AP, Segal JB. Understanding Geographic Variation in Systemic Overuse Among the Privately Insured. Medical Care. 2020;58(3):257–264. doi: 10.1097/MLR.0000000000001271 [DOI] [PubMed] [Google Scholar]
- 3.Ganguli I, Morden NE, Yang CWW, Crawford M, Colla CH. Low-Value Care at the Actionable Level of Individual Health Systems. JAMA Intern Med. 2021;181(11):1490. doi: 10.1001/jamainternmed.2021.5531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Segal JB, Sen AP, Glanzberg-Krainin E, Hutfless S. Factors Associated With Overuse of Health Care Within US Health Systems: A Cross-sectional Analysis of Medicare Beneficiaries From 2016 to 2018. JAMA Health Forum. 2022;3(1):e214543. doi: 10.1001/jamahealthforum.2021.4543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellenbogen MI, Prichett L, Johnson PT, Brotman DJ. Development of a Simple Index to Measure Overuse of Diagnostic Testing at the Hospital Level Using Administrative Data. J Hosp Med. 2021;16(2):77–83. doi: 10.12788/jhm.3547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouck Z, Ferguson J, Ivers NM, et al. Physician Characteristics Associated With Ordering 4 Low-Value Screening Tests in Primary Care. JAMA Network Open. 2018;1(6):e183506. doi: 10.1001/jamanetworkopen.2018.3506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz AL, Jena AB, Zaslavsky AM, McWilliams JM. Analysis of Physician Variation in Provision of Low-Value Services. JAMA Internal Medicine. 2019;179(1):16. doi: 10.1001/jamainternmed.2018.5086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz AL, Zaslavsky AM, Landon BE, Chernew ME, McWilliams JM. Low-Value Service Use in Provider Organizations. Health Serv Res. 2018;53(1):87–119. doi: 10.1111/1475-6773.12597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oakes AH, Radomski TR. Reducing Low-Value Care and Improving Health Care Value. JAMA. 2021;325(17):1715. doi: 10.1001/jama.2021.3308 [DOI] [PubMed] [Google Scholar]
- 10.Shrank WH, Rogstad TL, Parekh N. Waste in the US Health Care System: Estimated Costs and Potential for Savings. JAMA. 2019;322(15):1501. doi: 10.1001/jama.2019.13978 [DOI] [PubMed] [Google Scholar]
- 11.THE AMERICAN COLLEGE OF CARDIOLOGY RELEASES LIST OF COMMONLY USED – BUT NOT ALWAYS NECESSARY – TESTS OR PROCEDURES. Published April 4, 2012. Accessed August 12, 2022. https://www.acc.org/About-ACC/Press-Releases/2012/04/04/13/34/ChoosingWisely [Google Scholar]
- 12.ASNC Joins Choosing Wisely Campaign. Accessed August 12, 2022. https://www.asnc.org/Files/Guidelines%20and%20Quality/Choosing%20Wisely%20Campaign(1).pdf
- 13.Choosing Wisely in Perioperative Echocardiography. Journal of the American Society of Echocardiography. 2015;28(1):A24–A25. doi: 10.1016/j.echo.2014.11.005 [DOI] [PubMed] [Google Scholar]
- 14.SGIM: Choosing Wisely. Accessed August 25, 2022. https://www.sgim.org/publications/choosing-wisely-recommendations# [Google Scholar]
- 15.American Society of Anesthesiologists releases list of commonly used tests and treatments to question-AS PART OF CHOOSING WISELY® CAMPAIGN. Published October 12, 2013. Accessed August 12, 2022. https://www.asahq.org/about-asa/newsroom/news-releases/2013/10/choosing-wisely [Google Scholar]
- 16.Choosing Wisely Part 1: Preoperative Testing. Accessed August 12, 2022. https://www.aao.org/eye-health/news/choosing-wisely-preoperative-testing
- 17.Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. JACC. 2014;64(22):e278 LP-e333. doi: 10.1016/j.jacc.2014.07.944 [DOI] [PubMed] [Google Scholar]
- 18.Halvorsen S, Mehilli J, Cassese S, et al. 2022 ESC Guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery. European Heart Journal. 2022;43(39):3826–3924. doi: 10.1093/eurheartj/ehac270 [DOI] [PubMed] [Google Scholar]
- 19.Wells G, Shea B, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Accessed January 28, 2023. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 20.Herzog R, Álvarez-Pasquin MJ, Díaz C, Del Barrio JL, Estrada JM, Gil Á. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? a systematic review. BMC Public Health. 2013;13(1):154. doi: 10.1186/1471-2458-13-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy CE, Fonner VA, Armstrong KA, et al. The Evidence Project risk of bias tool: assessing study rigor for both randomized and non-randomized intervention studies. Syst Rev. 2019;8(1):3. doi: 10.1186/s13643-018-0925-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adair C, Swart E, Seymour R, Patt J, Karunakar MA. Clinical practice guidelines decrease unnecessary echocardiograms before hip fracture surgery. J Bone Jt Surg Am Vol. 2017;99(8):676–680. doi: 10.2106/JBJS.16.01108 [DOI] [PubMed] [Google Scholar]
- 23.Colla CH, Morden NE, Sequist TD, Mainor AJ, Li Z, Rosenthal MB. Payer Type and Low-Value Care: Comparing Choosing Wisely Services across Commercial and Medicare Populations. Health Services Research. 2018;53(2):730–746. doi: 10.1111/1475-6773.12665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganguli I, Lupo C, Mainor AJ, et al. Prevalence and Cost of Care Cascades After Low-Value Preoperative Electrocardiogram for Cataract Surgery in Fee-for-Service Medicare Beneficiaries. JAMA Internal Medicine. 2019;179(9):1211. doi: 10.1001/jamainternmed.2019.1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerr EA, Chen J, Sussman JB, Klamerus ML, Nallamothu BK. Stress testing before low-risk surgery - so many recommendations, so little overuse. JAMA Internal Medicine. 2015;175(4):645–647. doi: 10.1001/jamainternmed.2014.7877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marcantonio A, Steen B, Kain M, Bramlett KJ, Tilzey JF, Iorio R. The Clinical and Economic Impact of Preoperative Transthoracic Echocardiography in Elderly Patients with Hip Fractures. Bull Hosp Jt Dis (2013). 2015;73(4):239–242. [PubMed] [Google Scholar]
- 27.Sheffield KM, McAdams PS, Benarroch-Gampel J, et al. Overuse of preoperative cardiac stress testing in medicare patients undergoing elective noncardiac surgery. Annals of Surgery. 2013;257(1):73–80. doi: 10.1097/SLA.0b013e31826bc2f4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinvani L, Mendelson DA, Sharma A, et al. Preoperative Noninvasive Cardiac Testing in Older Adults with Hip Fracture: A Multi-Site Study. J Am Geriatr Soc. 2020;68(8):1690–1697. doi: 10.1111/jgs.16555 [DOI] [PubMed] [Google Scholar]
- 29.Valle JA, Graham L, Thiruvoipati T, et al. Facility-level association of preoperative stress testing and postoperative adverse cardiac events. Heart. 2018;((Valle J.A., javier.valle@Ucdenver.edu; Grunwald G.; Armstrong E.J.) Department of Cardiology, Veterans Affairs Eastern Colorado Health Care System, Denver, CO, United States). doi: 10.1136/heartjnl-2018-313047 [DOI] [PubMed] [Google Scholar]
- 30.Pickering AN, Zhao X, Sileanu FE, et al. Prevalence and Cost of Care Cascades Following Low-Value Preoperative Electrocardiogram and Chest Radiograph Within the Veterans Health Administration. J Gen Intern Med. 2022;((Pickering A.N., pickeringan@upmc.edu; Zhao X.; Sileanu F.E.; Lovelace E.Z.; Oakes A.H.; Hale J.A.; Schleiden L.J.; Gellad W.F.; Fine M.J.; Thorpe C.T.; Radomski T.R.) Center for Health Equity Research and Promotion (CHERP), VA Pittsburgh Healthcare System, Pittsburgh, PA, United States). doi: 10.1007/s11606-022-07561-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King S, Calisi O, Caldwell C, et al. Frequency and Predictors of Preoperative Cardiac Testing Overuse in Low-Risk Patients Before Laparoscopic Bariatric Surgery. Am J Cardiol. 2023;186((King S.; Calisi O.; Caldwell C.; Berger D.; Rich A.M.; Dan Y.; Qureshi U.; Ramedani S.) Department of Medicine, Penn State College of Medicine, Hershey, Pennsylvania, United States):181–185. doi: 10.1016/j.amjcard.2022.09.024 [DOI] [PubMed] [Google Scholar]
- 32.Chan K, Abou-Zamzam AM, Woo K. Preoperative Cardiac Stress Testing in the Southern California Vascular Outcomes Improvement Collaborative. Ann Vasc Surg. 2018;49((Chan K.) University of California at Los Angeles, Los Angeles, CA, United States):234–240. doi: 10.1016/j.avsg.2017.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ponukumati AS, Columbo JA, Suckow BD, et al. The financial implications of cardiac stress testing prior to abdominal aortic aneurysm repair. Vasc Med. 2022;27(5):469–475. doi: 10.1177/1358863X221112180 [DOI] [PubMed] [Google Scholar]
- 34.Muthappan P, Smith D, Aronow HD, et al. The epidemiology and outcomes of percutaneous coronary intervention before high-risk noncardiac surgery in contemporary practice: insights from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2) Registry. J Am Heart Assoc. 2014;3(3):e000388. doi: 10.1161/JAHA.113.000388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pappas MA, Sessler DI, Auerbach AD, et al. Variation in preoperative stress testing by patient, physician and surgical type: A cohort study. BMJ Open. 2021;11(9). doi: 10.1136/bmjopen-2020-048052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charlesworth CJ, Meath THA, Schwartz AL, McConnell KJ. Comparison of Low-Value Care in Medicaid vs Commercially Insured Populations. JAMA Internal Medicine. 2016;176(7):998. doi: 10.1001/jamainternmed.2016.2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clark JM, Marrufo AS, Kozower BD, et al. Cardiopulmonary Testing Before Lung Resection: What Are Thoracic Surgeons Doing? Ann Thorac Surg. 2019;108(4):1006–1012. doi: 10.1016/j.athoracsur.2019.04.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ellenbogen MI, Brotman DJ, Prichett L, Li X, Feldman LS. Contemporary Rates of Preoperative Cardiac Testing Prior to Inpatient Hip Fracture Surgery. Journal of Hospital Medicine. 2019;14(4):224–228. [DOI] [PubMed] [Google Scholar]
- 39.Peterson B, Ghahramani M, Emerich M, Foy AJ. Frequency of Appropriate and Low-Risk Noncardiac Preoperative Stress Testing Across Medical Specialties. Am J Cardiol. 2018;122(5):744–748. doi: 10.1016/j.amjcard.2018.05.007 [DOI] [PubMed] [Google Scholar]
- 40.Rubin DS, Hughey R, Gerlach RM, Ham SA, Ward RP, Nagele P. Frequency and Outcomes of Preoperative Stress Testing in Total Hip and Knee Arthroplasty from 2004 to 2017. JAMA Cardiol. 2021;6(1):13–20. doi: 10.1001/jamacardio.2020.4311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hasan MM, Kang R, Lee J, et al. Is there variation in utilization of preoperative tests among patients undergoing total hip and knee replacement in the US, and does it affect outcomes? A population-based analysis. BMC Musculoskelet Disord. 2022;23(1). doi: 10.1186/s12891-022-05945-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riggs KR, Bass EB, Segal JB. Role of Patient- and Surgery-Specific Risk in Receipt of Outpatient Preoperative Testing. Perioperative Care and Operating Room Management. Published online March 2018:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sigmund AE, Stevens ER, Blitz JD, Ladapo JA. Use of preoperative testing and physicians’ response to professional society guidance. JAMA Intern Med. 2015;175(8):1352–1359. doi: 10.1001/jamainternmed.2015.2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mafi JN, Godoy-Travieso P, Wei E, et al. Evaluation of an Intervention to Reduce Low-Value Preoperative Care for Patients Undergoing Cataract Surgery at a Safety-Net Health System. JAMA Intern Med. 2019;179(5):648–657. doi: 10.1001/jamainternmed.2018.8358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aviki EM, Gordhandas SB, Velzen J, et al. Implementation of Evidence-Based Presurgical Testing Guidelines in Patients Undergoing Ambulatory Surgery for Endometrial Cancer. JCO Oncol Pract. 2022;18(2):E219–E224. doi: 10.1200/OP.21.00247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Esper G, Anil U, Konda S, Furgiuele D, Zaretsky J, Egol K. Standardized Preoperative Pathways Determining Preoperative Echocardiogram Usage Continue to Improve Hip Fracture Quality. Geriatr Orthop Surg Rehabit. 2022;13((Esper G.; Anil U.; Konda S.; Egol K., kenneth.egol@nyulangone.org) Department of Orthopedic Surgery, NYU Langone Health, Grossman School of Medicine, New York, NY, United States). doi: 10.1177/21514593221094730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gertz ZM, O’Donnell W, Raina A, Balderston JR, Litwack AJ, Goldberg LR. Implementation of a Computerized Order Entry Tool to Reduce the Inappropriate and Unnecessary Use of Cardiac Stress Tests With Imaging in Hospitalized Patients. Am J Cardiol. 2016;118(8):1123–1127. doi: 10.1016/j.amjcard.2016.07.021 [DOI] [PubMed] [Google Scholar]
- 48.Langell JT, Bledsoe A, Vijaykumar S, Anderson T, Zawalski I, Zimmerman J. Implementation of national practice guidelines to reduce waste and optimize patient value. J Surg Res. 2016;203(2):287–292. doi: 10.1016/j.jss.2016.03.033 [DOI] [PubMed] [Google Scholar]
- 49.Nelson SE, Li G, Shi H, Terekhov M, Ehrenfeld JM, Wanderer JP. The impact of reduction of testing at a Preoperative Evaluation Clinic for elective cases: Value added without adverse outcomes. J Clin Anesth. 2019;55:92–99. doi: 10.1016/j.jclinane.2018.12.027 [DOI] [PubMed] [Google Scholar]
- 50.Colla CH, Sequist TD, Rosenthal MB, Schpero WL, Gottlieb DJ, Morden NE. Use of non-indicated cardiac testing in low-risk patients: Choosing Wisely. BMJ Quality & Safety. 2015;24(2):149–153. doi: 10.1136/bmjqs-2014-003087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ellenbogen MI, Prichett L, Brotman DJ. Characterizing the Relationship Between Payer Mix and Diagnostic Intensity at the Hospital Level. J GEN INTERN MED. 2022;37(15):3783–3788. doi: 10.1007/s11606-022-07453-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mafi JN, Russell K, Bortz BA, Dachary M, Hazel WA, Fendrick AM. Low-Cost, High-Volume Health Services Contribute The Most To Unnecessary Health Spending. Health Affairs. 2017;36(10):1701–1704. doi: 10.1377/hlthaff.2017.0385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pappas MA, Auerbach AD, Kattan MW, Blackstone EH, Rothberg MB, Sessler DI. Consequences of preoperative cardiac stress testing—A cohort study. Journal of Clinical Anesthesia. 2023;90:111158. doi: 10.1016/j.jclinane.2023.111158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ellenbogen MI, Wiegand AA, Austin JM, Schoenborn NL, Kodavarti N, Segal JB. Reducing Overuse by Healthcare Systems: A Positive Deviance Analysis. J GEN INTERN MED. Published online February 13, 2023. doi: 10.1007/s11606-023-08060-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


