Abstract
Background:
Mechanical circulatory support (MCS) with the Impella device (Abiomed, Danvers, MA) has been associated with higher in-hospital mortality than intra-aortic balloon pump (IABP) in the Premier Healthcare Database and National Cardiovascular Data Registry.
Methods:
The objective of this retrospective cohort study was to describe trends and outcomes of Impella usage in acute myocardial infarction complicated by cardiogenic shock (AMICS) treated with MCS (Impella or IABP) using real-world observational data from the National Inpatient Sample (NIS) including hospitalizations for AMICS managed with MCS between January 2012 to December 2017. The primary outcomes included in-hospital mortality, transfusion, acute kidney injury, stroke, total costs, and length of stay. Propensity score matching was performed with hierarchical models using risk factor and Elixhauser comorbidity variables.
Results and Conclusion:
We identified 54,480 hospitalizations for AMICS managed with MCS including 5750 (10.5%) utilizing Impella. Throughout the study period, Impella usage increased yearly to 19.9% of AMICS cases in 2017. After propensity score matching, Impella was associated with higher in-hospital mortality (odds ratio [OR] 1.74, 95% confidence interval [CI] 1.41–2.13) and transfusions (OR 1.97, 95% CI 1.40–2.78) than IABP, without association with acute kidney injury or stroke. Impella use was associated with higher hospital costs (mean difference $22,416.80 [95% CI $17,029–27,804]). Impella usage for AMICS increased significantly from 2012 to 2017 and was associated with increased in-hospital mortality and costs. Randomized controlled trials are urgently needed to assess the safety and efficacy of Impella.
Keywords: acute myocardial infarction/STEMI, cardiogenic shock, ECMO/IABP/tandem/impella, mechanical circulatory support
1 |. INTRODUCTION
Acute myocardial infarction complicated by cardiogenic shock (AMICS) is associated with 30-day mortality around 50%.1 Intravascular microaxial left ventricular assist devices can provide up to 5 liters per minute of flow, greater hemodynamic support than intra-aortic balloon pumps (IABPs).2 Numerous studies confirm the hemodynamic effects of the Impella device (Abiomed, Danvers, MA) in the setting of cardiogenic shock and high-risk percutaneous coronary intervention (PCI).3–5 Whether these hemodynamic effects translate to sustained clinical survival benefit remains less well characterized.6–9
Current cardiogenic shock guidelines condone the use of mechanical circulatory support (MCS) devices such as Impella or IABP based on observational data suggesting improvement in mortality.10 However, the landmark IABP-SHOCK II trial (Intra-aortic Balloon Pump in Cardiogenic Shock II) showed no mortality benefit for IABP use in AMICS.2,11 Recently, Amin et al. published a real-world observational study comparing Impella and IABP in patients undergoing PCI: they found an association between Impella utilization and higher rates of death and stroke.6 Furthermore, in patients undergoing PCI for AMICS, Dhruva et al. showed increased in-hospital mortality and bleeding associated with Impella utilization.12 Therefore, we sought to compare outcomes in a large, real-world, nationally representative dataset between Impella and IABP among patients presenting with AMICS.
2 |. METHODS
2.1 |. Data
The National Inpatient Sample (NIS) from the Agency for Healthcare Research and Quality (AHRQ) includes discharge-level data from approximately eight million annual hospitalizations from 28 U.S. states. Adult patients (age ≥ 18) hospitalized for AMICS from January 2012 through December 2017 were selected utilizing International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification (ICD-9-CM and ICD-10-CM) codes among the first three admission diagnoses (Supplement Figure 1). From this population, we identified patients who underwent Impella or IABP placement during the hospital course. To be included in the study patients in both arms could have cardiogenic shock, cardiac arrest, or both. Propensity matching included the presence of cardiac arrest. Patients who received both devices were excluded for purposes of direct comparison. This study was exempt from the Yale University institutional review board because NIS is publicly available, deidentified data.
2.2 |. Variables
Patient demographics and hospital characteristics including age, sex, race, payor, hospital location and size, and length of stay were obtained from NIS. Clinical characteristics (hypertension, diabetes, hyperlipidemia, chronic kidney disease, ST-elevation myocardial infarction) and hospital course (ventricular tachycardia, ventricular fibrillation, respiratory failure, acute kidney injury, sepsis) were determined using ICD-9-CM and ICD-10-CM codes (Supplement Table 1). Elixhauser mortality risk score was derived using 29 comorbidity classification variables, each defined by a list of ICD-9/10-CM codes.13 Procedures (PCI, cardiopulmonary resuscitation, transfusion of blood products) and MCS devices (Impella and IABP) were identified using the ICD-9 and ICD-10 procedure coding system (ICD-9/10-PCS). Primary outcomes were in-hospital mortality, transfusions, acute kidney injury, stroke, and length of stay. Transfusions, acute kidney injury, and stroke were derived using ICD-9/10-CM and ICD-9/10-PCS codes (Supplement Table 1).
2.3 |. Statistical analysis
National admission trends were approximated using weight trends recommended by the AHRQ for analysis of survey data.14 We compared survey-specific statements (e.g., SURVEYFREQ, SURVEYMEANS) for descriptive statistics, and discharge weights were used to obtain national estimates.8 Temporal trends for proportion of Impella use (annual number of Impella hospitalizations divided by total hospitalizations included) were evaluated for the study time period. The Cochran-Armitage trend test was used to determine the annual trend in the proportion of Impella use. Next, we compared baseline patient characteristics and hospital course using the Rao-Scott χ2-test for categorical variables and survey-specific t-tests for continuous variables. Total hospital charges and cost-to-charge ratio files were used to determine average costs. Missing cost data and extreme outliers (<1% or >99%) were removed.
Lastly, we used propensity score matching to account for differences between patients who received Impella versus IABP. Hospitalizations with Impella use were matched by logit of propensity score using a greedy neighbor approach with a caliper distance of 0.25 times the SD of the logit of the propensity score using a proc PSMATCH statement. Propensity score matching included patient demographics (age and sex), and other risk variables (hypertension, hyperlipidemia, diabetes, coronary artery disease, chronic kidney disease, congestive heart failure, ST-elevation myocardial infarction, cardiac arrest, cardiopulmonary resuscitation, and PCI). Elixhauser comorbidity classification software was used to derive variables used in the propensity score matching (chronic pulmonary disease, peripheral vascular disease, fluid/electrolyte disorders, obesity, coagulation deficiency, and blood loss). After matching, we used survey logistic regression to compare primary outcomes. All analyses were performed using SAS version 9.4 (Cary, NC) with a 2-tailed p value of 0.05 for statistical significance. Regional heatmaps were generated with Python 3.6 (Wilmington, DE) using the Plotly package.
3 |. RESULTS
Between January 2012 and December 2017, the NIS recorded 54,480 hospitalizations for AMICS managed with either Impella (10.6%) or IABP (89.4%). There were no significant differences in age, gender, or race between the Impella and IABP groups. Patients receiving Impella were less likely to have hypertension, hyperlipidemia, coronary artery disease, chronic obstructive pulmonary disease, congestive heart failure, and atrial fibrillation and more likely to have peripheral artery disease. Small and medium sized hospitals were less likely to use Impella during the study period than larger hospitals. Regarding in-hospital events, patients receiving Impella had more acute kidney injury, cardiac arrest, arrhythmias, respiratory failure, sepsis, and stroke; were more likely to undergo PCI, transfusions, and CPR; and had a longer length of stay. Details of patient demographics, comorbidities, and hospital courses appear in Supplement Tables 2 and 3.
MCS device utilization increased in all regions of the United States except the Northeast. The greatest increase occurred in the Southern region (Figure 1(A)). Overall, the proportion of Impella use increased approximately 5-fold from 4.1% in 2012 to 19.9% in 2017 (p for trend <0.01) (Figure 1(B)), while the proportion of IABP use declined from 92% to 76%. Based upon annual Elixhauser mortality risk scores (Supplement Figure 2), Impella use over the study period accelerated most in patients who were younger with fewer comorbidities.
FIGURE 1.
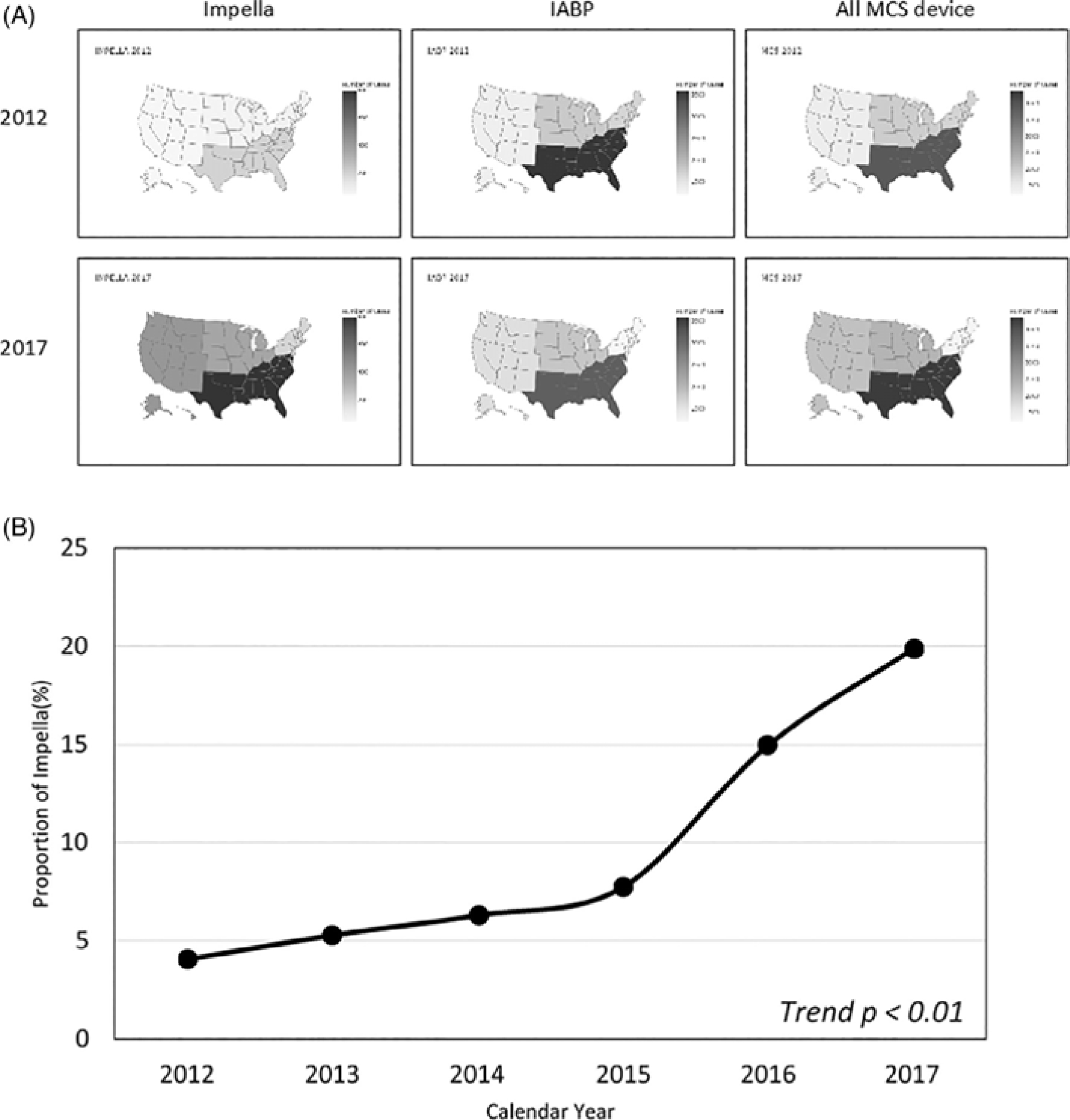
Heatmap of Impella, IABP, and total MCS device used in the United States by region in 2012 and 2017 (A) and proportion of Impella use annually (B). IABP, intra-aortic balloon pumps; MCS, mechanical circulatory support
After propensity score matching, there were 5750 matched pairs with mean difference of <0.05 for all matched covariates (Table 1). In the study population, 345 (8.9%) hospitalizations in the Impella arm had cardiac arrest, while 1170 (6.5%) in the IABP arm had cardiac arrest; these diagnoses were not mutually exclusive. Following propensity matching, cardiac arrest was present in 22%–23% of patients in each arm (OR 0.96, 95%CI 0.79–1.17). Overall, matched pairs showed no statistically significant differences in demographics, comorbidities, or in-hospital events except for respiratory failure (52.6% for Impella versus 46.1% for IABP, p < 0.01). After propensity score matching, Impella use was still associated with increased in-hospital mortality (OR 1.73 [95%CI 1.41–2.13]) and transfusions (OR 1.97 [95%CI, 1.40–2.78]) (Table 2). There were no significant differences in acute kidney injury (OR 1.12 [95%CI, 0.95–1.32]) or stroke (OR 1.10 [95%CI, 0.67–1.80]) between the groups. Impella use was associated with increased hospital costs (mean difference $22,416.80 [95%CI $17,029–$27,805]) compared to IABP. Mean length of stay was numerically longer for Impella without reaching statistical significance (mean difference 0.86 days, 95%CI [−0.31 to 2.02]).
TABLE 1.
Propensity score matched patient characteristics (N = 5750 | 5750)
| Impella (%) | IABP (%) | Standardized mean difference (matched variables) | OR 95%CIa, p-value± | |
|---|---|---|---|---|
| Age (years, SD) | 64.6 (13) | 65.4 (13) | 0.03 | 0.15 |
| Female | 31 | 31 | 0.00 | 1.00 (0.83–1.19) |
| White | 70.5 | 71.0 | NA | 0.97(0.74–1.29) |
| Hypertension | 44.4 | 45.6 | 0.02 | 0.96 (0.81–1.13) |
| Hyperlipidemia | 46.5 | 46.1 | 0.01 | 1.02 (0.86–1.20) |
| Diabetes | 39.0 | 38.0 | 0.02 | 1.04(0.88–1.23) |
| CKD | 22.8 | 24.4 | 0.04 | 0.92 (0.76–1.11) |
| CAD | 84.5 | 86.4 | 0.05 | 0.86 (0.68–1.09) |
| CHRNLUNG | 17.3 | 17.8 | 0.01 | 0.96 (0.78–1.20) |
| CHF | 45.1 | 45.1 | 0.00 | 1.00 (0.85–1.18) |
| PVD | 16.0 | 17.6 | 0.04 | 0.89 (0.72–1.11) |
| Obesity | 13.6 | 13.7 | 0.00 | 0.99 (0.78–1.26) |
| Fluid disorders | 50.1 | 49.5 | 0.01 | 1.02 (0.87–1.21) |
| Coagulopathy | 21.7 | 20.1 | 0.04 | 1.01 (0.90–1.34) |
| Blood loss | 1.4 | 1.8 | 0.04 | 0.76 (0.39–1.46) |
| STEMI | 63.5 | 63.0 | 0.01 | 1.02 (0.86–1.21) |
| AKI | 44.1 | 41.3 | NA | 1.12 (0.95–1.32) |
| Cardiac arrest | 22.1 | 22.8 | 0.02 | 0.96 (0.79–1.17) |
| PCI | 73.8 | 74.9 | 0.02 | 0.95 (0.79–1.14) |
| VT | 21.9 | 19.0 | NA | 1.20 (0.98–1.47) |
| CPR | 10.7 | 10.9 | 0.01 | 0.98 (0.75–1.28) |
| Transfusions | 8.7 | 4.6 | NA | 1.97 (1.40–2.78) |
| Respiratory failure | 52.6 | 46.1 | NA | 1.30(1.10–1.53) |
| Sepsis | 7.1 | 7.0 | NA | 1.01 (0.74–1.39) |
| Stroke | 3.0 | 2.7 | NA | 1.10 (0.67–1.80) |
| Length of stayb (days, SD) | 11.4 (12) | 10.5 (10) | NA | 0.15 |
| Death | 24.6 | 15.9 | NA | 1.73 (1.41–2.13) |
| Hospital costs (USD, SD) | 73,172.80 (68,730) | 50,756.00 (61,805) | NA | <0.01 |
Abbreviations: AKI, acute kidney injury; CAD, coronary artery disease; CHRNLUNG, chronic lung disease (Elixhauser variable); CHF, congestive heart failure; CI, confidence interval; CKD, chronic kidney disease; CPR, cardiopulmonary resuscitation; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease (Elixhauser variable); STEMI, ST-elevation myocardial infarction; USD, US dollars; VT, ventricular tachycardia.
Based on univariate analysis derived from 2 × 2 contingency tables
2 tailed student’s t-test; NA, not matched by propensity score matching.
Length of stay evaluated only in patients discharged from hospital stay.
TABLE 2.
Primary outcomes in propensity score matched cohort and unmatched study population
| Outcome(s) | Matched cohort OR (95%CI)a | Unmatched cohort OR (95%CI)* |
|---|---|---|
| Death | 1.74(1.41–2.13) | 2.07 (1.79–2.44) |
| Transfusions | 1.97 (1.40–2.78) | 1.94 (1.55–2.44) |
| Acute kidney injury | 1.12 (0.95–1.32) | 1.36 (1.20–1.54) |
| Stroke | 1.10 (0.67–1.80) | 1.28 (0.89–1.85) |
| Continuous outcome (mean difference [95%CI]) | ||
| Length of stay (days) | 0.9 (−0.31–2.02) | 1.23 (0.41–2.05) |
| Total hospital cost | $22,416.80 (17,029–27,804) | $25,746.00 (21,632–29,859) |
Abbreviations: CI, confidence interval; OR, odds ratio.
Based on univariate analysis.
4 |. DISCUSSION
In this large U.S. national database, there was a 5-fold increase in Impella implantation in patients presenting with AMICS from 2012 to 2017. However, Impella was associated with greater in-hospital mortality, blood transfusion, and hospital costs than IABP. The association with increased mortality remained in a propensity score matched analysis. The study aimed to compare outcomes of IABP and Impella as the primary strategy in a billing dataset, as such patients who received both IABP and Impella were excluded from our study. The methods and results model the prior studies performed in observational datasets, which also excluded patients who received both devices.6,15 Unfortunately, given the limitations of the data available, inclusion of these patients would result in undue speculation rather than further insight. For example, the use of both devices could indicate that IABP failed, IABP caused harm, initial Impella placement attempt was unsuccessful, or the patient was too sick and Impella was used as salvage and did not further add to the risk profile. Indeed, it would be difficult to speculate as to the reasons for using both devices and, resultantly, difficult to explain the effect on mortality. Additionally, patients receiving Impella were more likely to receive care at tertiary centers. However, in a prior study, Amin et al. reported that there was wide variation in Impella use across hospitals even after propensity matching (>5 fold variation).6 Therefore, we did not adjust for hospital size in our propensity matching, focusing on matching at the patient level rather than at the hospital level.
Cardiogenic shock complicates between 5% and 10% of acute myocardial infarction with incidence increasing.16,17 Despite marked improvements in cardiac care over the past 30 years, there have been unchanging mortality rates in AMICS18–20 and few evidence-based therapies available for improved survival beyond early revascularization.19,21–23 Thus, there is clearly a need for MCS devices that can improve outcomes in this population. In line with evidence that IABP provides no survival benefit in AMICS over medical therapy alone,2 we found IABP use declining over the study period. Impella provides a promising—but under-investigated—alternative.
Despite its theoretical hemodynamic superiority, Impella has been consistently associated with similar or higher mortality than IABP in this and prior smaller studies.6,8 Previously published data are limited to single arm registry studies, small trials, and retrospective comparisons. An initial 2013 multicenter registry feasibility study of Impella in very high-risk patients showed 30-day mortality around 64%.24 A small (n = 48) exploratory randomized, open-label prospective trial (IMPRESS) showed similar survival rates between Impella (50%) and IABP (46%) at 30 days but higher bleeding rate in the Impella group among a population of patients who were critically ill with a majority having cardiac arrest.20 Pooled analyses of smaller trials showed no difference in 30-day mortality between percutaneous ventricular assist devices including Impella compared to IABP.20,25 Subsequent retrospective data comparing matched patients from the IABP-SHOCK trial showed equivalent 30-day mortality rates with Impella (48.5%) and IABP (46.3%).26 Prospective, single-arm registry data does suggest improved survival with earlier compared to later device implantation,4,5 however the overall survival rate to hospital discharge in one registry was 44%4 and 53% survival to device explant in the other,5 similar to mortality rates without Impella from other trials between 40% and 50%.2,19,27 The present study, the largest of in the field by far, builds upon the landscape of prior observational studies suggesting no improvement in AMICS mortality using Impella versus IABP.28 In fact, compared to IABP, which provides no survival benefit over medical therapy,2 Impella exhibited a signal for harm in this study and others.6,15 Building on previous studies, this study adds to existing literature adding propensity matching to help to control for unmeasured confounders.
The findings of this study indicate a pattern of increased Impella usage over the study period, and, unsurprisingly, increased costs attendant to the device usage. Although our data showed a trend toward increased mortality with Impella versus IABP, this may well have been predominantly a function of case selection wherein sicker patients received Impella support. The intrinsic methodological limitations of the present study preclude firm conclusion whether this mortality observation reflects patient selection or actual contributions of device complications. These findings, however, underscore the need for rigorous, large-scale prospective clinical trials in AMICS patients to evaluate the safety and efficacy of Impella implantation.
5 |. LIMITATIONS
This study had several limitations. First, using retrospective observational data may result in selection bias and missing unmeasured confounding factors. Propensity matching can be used to control for some selection bias, but it cannot substitute for a prospective study, where equipoise can be attempted by design. Second, reliance on administrative codes for identification of cardiogenic shock may lead to unrecognized miscoding of diagnostic and procedural information. For example, is not possible to determine whether shock was present on admission or developed during the hospital stay. Additionally, although this study utilized propensity matching, there is a possibility of unmeasured confounders that resulted in Impella patients being sicker than non-Impella patients. In summary, these observational findings underscore the need for high-quality, prospective randomized studies for patients suffering AMICS managed with MCS.
6 |. CONCLUSION
This retrospective cohort study describes trends and outcomes of Impella usage in 54,480 hospitalizations for AMICS treated with MCS (Impella or IABP) using real-world observational data. Impella usage increased yearly to 19.9% of AMICS cases in 2017. After propensity score matching, Impella was associated with higher in-hospital mortality and transfusions than IABP, without association with acute kidney injury or stroke, as well as with higher hospital costs. Randomized controlled trials are urgently needed to assess the safety and efficacy of Impella.
Supplementary Material
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Babaev A, Frederick PD, Pasta DJ, et al. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. Jama. 2005;294(4):448–454. [DOI] [PubMed] [Google Scholar]
- 2.Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367(14):1287–1296. [DOI] [PubMed] [Google Scholar]
- 3.Basir MB, Kapur NK, Patel K, et al. Improved outcomes associated with the use of shock protocols: updates from the national cardiogenic shock initiative. Catheter Cardiovasc Interv. 2019;93(7):1173–1183. [DOI] [PubMed] [Google Scholar]
- 4.Basir MB, Schreiber TL, Grines CL, et al. Effect of early initiation of mechanical circulatory support on survival in cardiogenic shock. Am J Cardiol. 2017;119(6):845–851. [DOI] [PubMed] [Google Scholar]
- 5.O’Neill WW, Grines C, Schreiber T, et al. Analysis of outcomes for 15,259 US patients with acute myocardial infarction cardiogenic shock (AMICS) supported with the Impella device. Am Heart J. 2018;202:33–38. [DOI] [PubMed] [Google Scholar]
- 6.Amin AP, Spertus JA, Curtis JP, et al. The evolving landscape of Impella use in the United States among patients undergoing percutaneous coronary intervention with mechanical circulatory support. Circulation. 2020;141(4):273–284. [DOI] [PubMed] [Google Scholar]
- 7.Cheng JM, den Uil CA, Hoeks SE, et al. Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: a meta-analysis of controlled trials. Eur Heart J. 2009;30(17):2102–2108. [DOI] [PubMed] [Google Scholar]
- 8.Khera R, Cram P, Lu X, et al. Trends in the use of percutaneous ventricular assist devices: analysis of national inpatient sample data, 2007 through 2012. JAMA Intern Med. 2015;175(6):941–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thiele H, Sick P, Boudriot E, et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2005;26(13):1276–1283. [DOI] [PubMed] [Google Scholar]
- 10.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124(23):2574–2609. [DOI] [PubMed] [Google Scholar]
- 11.Thiele H, Zeymer U, Thelemann N, et al. Intraaortic balloon pump in cardiogenic shock complicating acute myocardial infarction: long-term 6-year outcome of the randomized IABP-SHOCK II trial. Circulation. 2018;139(3):395–403. [DOI] [PubMed] [Google Scholar]
- 12.Dhruva SS, Ross JS, Mortazavi BJ, et al. Association of use of an intravascular microaxial left ventricular assist device vs intra-aortic balloon pump with in-hospital mortality and major bleeding among patients with acute myocardial infarction complicated by cardiogenic shock. Jama. 2020;323(8):734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. [DOI] [PubMed] [Google Scholar]
- 14.Houchens R, Elixhauser A. 2005. Final Report on Calculating Nationwide Inpatient Sample (NIS) Variances, 2001. HCUP Method Series Report 2003–02. In: Quality AfHRa, editor. Rockville, MD. [Google Scholar]
- 15.Dhruva SS, Mortazavi BJ, Desai NR. Intravascular microaxial left ventricular assist device vs intra-aortic balloon pump for cardiogenic shock-reply. Jama. 2020;324(3):303–304. [DOI] [PubMed] [Google Scholar]
- 16.Kolte D, Khera S, Aronow WS, et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J Am Heart Assoc. 2014;3(1):e000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helgestad OKL, Josiassen J, Hassager C, et al. Temporal trends in incidence and patient characteristics in cardiogenic shock following acute myocardial infarction from 2010 to 2017: a Danish cohort study. Eur J Heart Fail. 2019;21(11):1370–1378. [DOI] [PubMed] [Google Scholar]
- 18.Lee L, Bates ER, Pitt B, Walton JA, Laufer N, O’Neill WW. Percutaneous transluminal coronary angioplasty improves survival in acute myocardial infarction complicated by cardiogenic shock. Circulation. 1988;78(6):1345–1351. [DOI] [PubMed] [Google Scholar]
- 19.Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic SHOCK. SHOCK Investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med. 1999;341(9):625–634. [DOI] [PubMed] [Google Scholar]
- 20.Ouweneel DM, Eriksen E, Seyfarth M, Henriques JP. Percutaneous mechanical circulatory support versus intra-aortic balloon pump for treating cardiogenic shock: meta-analysis. J Am Coll Cardiol. 2017;69(3):358–360. [DOI] [PubMed] [Google Scholar]
- 21.van Diepen S, Katz JN, Albert NM, et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136(16):e232–e268. [DOI] [PubMed] [Google Scholar]
- 22.Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization and long-term survival in cardiogenic shock complicating acute myocardial infarction. Jama. 2006;295(21):2511–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldberg RJ, Samad NA, Yarzebski J, Gurwitz J, Bigelow C, Gore JM. Temporal trends in cardiogenic shock complicating acute myocardial infarction. N Engl J Med. 1999;340(15):1162–1168. [DOI] [PubMed] [Google Scholar]
- 24.Lauten A, Engstrom AE, Jung C, et al. Percutaneous left-ventricular support with the Impella-2.5-assist device in acute cardiogenic shock: results of the Impella-EUROSHOCK-registry. Circ Heart Fail. 2013;6(1):23–30. [DOI] [PubMed] [Google Scholar]
- 25.Thiele H, Jobs A, Ouweneel DM, et al. Percutaneous short-term active mechanical support devices in cardiogenic shock: a systematic review and collaborative meta-analysis of randomized trials. Eur Heart J. 2017;38(47):3523–3531. [DOI] [PubMed] [Google Scholar]
- 26.Schrage B, Ibrahim K, Loehn T, et al. Impella support for acute myocardial infarction complicated by cardiogenic shock. Circulation. 2019;139(10):1249–1258. [DOI] [PubMed] [Google Scholar]
- 27.Hunziker L, Radovanovic D, Jeger R, et al. Twenty-year trends in the incidence and outcome of cardiogenic shock in AMIS plus registry. Circ Cardiovasc Interv. 2019;12(4):e007293. [DOI] [PubMed] [Google Scholar]
- 28.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58(24):e44–e122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


