Abstract
Background
Myopia is the most prevalent form of refractive error that has a major negative impact on visual function and causes blurring of vision. We aimed to determine if Repeated Low-Level Red Light (RLRL) treatment is beneficial in treating childhood myopia in terms of axial length (AL), spherical equivalent refraction (SER), and sub foveal choroidal thickness (SFCT).
Methods
This systematic review was performed on RLRL for treatment of myopia in children compared to single vision spectacles (SVS). We employed the search strategy with key terms myopia and low-level light therapy then we searched PubMed, Scopus, Cochrane, and Web of Science databases. The mean differences (MD) were used to evaluate the treatment effects. Heterogeneity was quantified using I2 statistics and explored by sensitivity analysis.
Results
Five randomized controlled trials (RCTs) were included in our meta-analysis with a total of 833 patients, 407 in treatment group and 426 in control group. At a 3 month follow up period, pooled studies show a statistical difference in AL between RLRL and SVS group (MD = -0.16; 95% CI [-0.19, -0.12], SER (MD = 0.33; 95% CI [0.27, 0.38]), and SFCT (MD = 43.65; 95% CI [23.72, 45.58]). At a 6 month follow up period, pooled studies show a statistical difference in AL between RLRL and SVS group (MD = -0.21; 95% CI [-0.28, -0.15]), SER (MD = 0.46; 95% CI [0.26, 0.65]), and SFCT (MD = 25.07; 95% CI [18.18, 31.95]). At a 12 month follow up period, pooled studies show a statistical difference in AL between RLRL and SVS group (MD = -0.31; 95% CI [-0.42, -0.19]) and SER (MD = 0.63; 95% CI [0.52, 0.73]).
Conclusion
This is the first systematic review and meta-analysis investigating only RCTs evidence supporting the efficacy of 650 nm RLRL for myopia control in the short term of 3, 6, and 12 months follow up. The present review revealed the clinical significance of RLRL as a new alternative treatment for myopia control with good user acceptability and no documented functional or structural damage. However, the effect of long-term RLRL treatment and the rebound effect after cessation require further investigations.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12886-024-03337-5.
Keywords: Childhood myopia, Repeated Low-Level Red Light (RLRL), Spherical equivalent refraction (SER), Axial length (AL), Sub foveal choroidal thickness (SFCT)
Introduction
Myopia is the most prevalent form of refractive error [1] which has a major negative impact on visual function and causes blurring of vision. It is estimated that the number of myopic patients will be 4.7 billion in the world by 2050 [2, 3]. Myopia prevalence in children aged 6 to 8 years increased 1.4–3 times during COVID-19's social isolation [4]. High myopia is linked to a significant risk of disorders that permanently impair vision, such as myopic maculopathy, glaucoma, staphyloma, and retinal detachment [5–7]. As a result myopia is a significant public health issue, and there is an urgent need for a strategy to stop its progression.
Myopia is most frequently characterized by an increase in the axial length of the eyeball [8] and can be brought on by both environmental [9] and hereditary causes [10, 11]. The threshold for myopia progression is a spherical equivalent refraction (SER) of -1.00 diopters (D) or less is [12]. The primary characteristic of high myopia-related irreversible vision loss is choroidal thinning [13]. Research on both animals and human indicates that the choroid is crucial for controlling the development of the eye's refractive system [14, 15]. As potential indicators of treatment response, axial length (AL), spherical equivalent refraction (SER), and macular choroidal thickness (mCT) have all been identified [16].
Several techniques have already been proven to be efficient preventative elements for the growth of myopia in children. Increased exposure to bright light outdoors [3, 17] is used with myopia. Increased time outdoors has been shown to prevent or delay myopia onset in several studies. Furthermore, increased outdoor time has been shown to have a protective effect on the cumulative incidence rate of myopia in children enrolled in randomized clinical trials in China and Taiwan. On the other hand, increased near-work time and reduced outdoor activities have been suggested to be at the origin of the increased myopia prevalence in older children [18]. A previous review mentioned that several studies conducted in various countries concluded that greater average daily light exposure is associated with a reduced axial elongation during childhood. For example, a study cluster-randomized intervention-controlled trial showed that exposure to outdoor light leads to less myopic shifts, reduced axial elongation and a 54% lower risk of myopia progression [19]. Additionally, single vision spectacle lenses (SVS), refractive procedures, and implantable collamer lens (ICL) are all frequently used to treat myopia. Additional methods such as orthokeratology, various contact lens options, peripheral defocus-modifying spectacle lenses, pharmacy therapy in the form of atropine eye drops, and orthokeratology were also used [20–23]. However, all of these methods have disadvantages being potentially difficult and harmful in administration. For example, the risk of adverse events associated with orthokeratology, such as infective keratitis, is well recognized [24]. Photophobia, among other side effects, is possible with atropine eye solutions [25]. Thus, there is a pressing need for more effective and practical treatments for halting myopia progression.
While increasing exposure to outdoor light levels can successfully be implemented through national outdoor programs, implementation remains suboptimal in some circumstances. On the other hand, the optimization of architectural lighting or development of light-therapy devices requires a holistic understanding of the benefits and side effects of light characteristics on ocular growth and neurophysiology [19]. The characteristics of wavelength, intensity, and other variables all have an impact on how light affects the refractive power of lenses [26]. According to research, red light has been shown to be more efficient than other wavelengths in slowing the progression of myopia in rhesus monkeys [27]. Studies have shown that repeated low-intensity red light (RLRL) can slow the progression of myopia in children [12, 25, 28] Laser therapy induces various molecular, cellular, and tissue effects [29]. Differentiating from high-power laser therapy, RLRL utilizes low doses of red and near-infrared light, with an output ranged from 1 to 500 mW, and a wavelength ranging from 600 to 1100 nm. As a result, it is described as a sort of phototherapy that emits low energy to cause tissue response [30].
Clinical studies that compared RLRL to single-vision glasses over a six-month period discovered that it significantly delayed the development of myopia and axial elongation (SVS) [28]. Several randomized clinical trials including children showed that RLRL therapy was more effective than SVS in reducing myopia, and no structural or functional impairment was detected [12]. To accurately assess the effects, it was necessary to do a meta-analysis collectively with RCT which is much better than using non-RCT. Potential biases are likely to be greater for non-randomized studies compared with randomized trials when evaluating the effects of interventions. Additionally, randomization provides a rigorous tool to examine cause-effect relationships between an intervention and outcome. Therefore, we conducted this systematic review to determine the potential benefits of RLRL treatment for childhood myopia. Our research objectives include understanding how RLRL influences the development of myopia, spherical equivalent refraction (SER), axial length (AL), and sub foveal choroidal thickness (SFCT).
Materials and methods
This review followed the guidance of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement [31]. The filled checklist is attached in the supplementary data (Additional file 1). This systematic review was registered in PROSPERO (registration ID: CRD42023410702).
Search strategy
A systematic review of the published literature was performed on Repeated Low-Level Red Light (RLRL) for treatment of myopia in Children. The Population-Intervention-Comparison-Outcome (PICO) question was formulated as follows: Do myopic children (P) treated with Repeated Low-level red-light therapy in addition to single vision spectacles (I) compared with Singles vision spectacles (C) have a slowed down progression of myopia with no damage or serious adverse effects? (O) We employed the search strategy attached to the supplementary data (Additional file 2): we searched PubMed, Scopus, Cochrane, and Web of Science databases. The bibliographies of all relevant articles were checked for additional articles that were not identified in our search.
Eligibility criteria and study selection
Inclusion criteria
The relevant articles that met the following criteria were selected for inclusion:
(1) randomized control trials (RCTs) (2) patients with myopia younger than 16 years old, with cycloplegic spherical equivalent refraction (SER) of at least—0.50 diopter (D), (3) with the use of RLRL in addition to single vision spectacles compared to single vision spectacles only, (4) studies reported at least one of the outcomes of interest: Axial length (mm), Spherical equivalent refraction (SER) and Subfoveal choroid thickness (SFChT) with minimum 3 months follow up.
Exclusion criteria
Exclusion criteria included: Studies not meeting inclusion criteria, animal studies, In vitro studies, case reports, case series, non-English articles.
The duplicate records were automatically removed via the EndNote X9 computer program by (A.S) and (M.Y). Then, the remaining citations were imported to the Rayyan web application [32], which is a free web and mobile app, that helps expedite the initial screening of abstracts and titles using a process of semi-automation while incorporating a high level of usability. Thus, we used it for screening eligible studies for our systematic reviews. (R.F), (H.F) and (M.R) independently screened the citations for eligibility based on our inclusion and exclusion criteria, and they were blinded to decisions until the end of the screening process. Any conflicts that arose about the inclusion of the studies were first solved by discussion between them. (A.S) and (M.Y) were considered referees if there were unresolved discussions.
Data extraction
For the included studies, a group of the authors extracted the data and exported the collected data into excel sheet for the following characteristics: First: Summary sheet (H.F), (R.F) and (M.R) extracted the following characteristics: Study design, location and number of center(s), sample size, follow up duration, study arms (treatment and control), patients main inclusion criteria, description of RLRL used and study conclusion. (A.S) then reviewed the final data extracted.
Second: Baseline sheet (M.R) and (A.M) extracted the following baseline characteristics of the participants: Number of patients in each arm, age, Sex (M/F), Uncorrected visual acuity, Axial length (mm), Spherical equivalent refraction and Subfoveal choroidal thickness. (A.S) then reviewed the final data extracted.
Third: Outcome sheet (A.S) and (M.Y) extracted the following outcome characteristics of the participants: Axial length (mm), Spherical equivalent refraction and Subfoveal choroid thickness.
Risk of bias assessments of eligible studies
Using the National Institutes of Health (NIH) quality assessment tools for controlled intervention study [33], (M.R) and (A.M) independently assessed the risk of bias in the included studies, with all discrepancies being resolved in a discussion in the presence of (A.S) and (M.Y). The NIH tool consisted of 14 dichotomous (yes/no) questions. If the answer to any question was "No", then there is a potential of bias in a study.
Data analysis
Baseline characteristics were summarized for each study and reported as mean ± standard-deviation (SD) for continuous variables, and number (%) for categorical variables. Some of the data of baseline or outcomes were presented in a study [34] as median and interquartile range (IQR). Thus, we converted these data to be presented as mean ± SD using an equation on excel sheet [35].
Standard deviations of mean changes from baseline are specified as frequently missing outcome data [36] and problems in conducting a meta-analysis without missing SDs are explained by prior systematic reviews [37, 38]. A formula was established as follows to calculate missing SDs: [39, 40]
SD final corresponds to the SD of the post-test, SD baseline denotes the SD of the pre-test, and SD change denotes the SD of the mean changes from baseline. The r denotes the correlations between the baseline and final measurements; this correlation value is typically not presented in studies. For instance, among the included studies for this systematic review, we determined a study's coefficient (r) from its data file, and then we used the aforementioned equation to determine the missed SD change [41]. We used an equation to transform the confidence intervals (CI) from two additional included studies [12, 42] into standard deviation [43].
The I2 statistic, which shows the percentage of the variability in effect estimates caused by heterogeneity rather than chance [44], was used to determine the level of heterogeneity and to quantify the heterogeneity of a meta-analysis. Low (25%), moderate (50%) and high (75%) I2 results were assigned [45]. A meta-analysis was carried out for a continuous data, inverse variance, fixed-effect model [44] and a 95% confidence interval (95% CI) when statistical heterogeneity was absent (p > 0.05 in the Chi2 statistics). However, a meta-analysis was carried out using a more cautious random effect model for continuous data, inverse variance, and a 95% CI when statistical heterogeneity was detected [46]. Sensitivity analyses were performed by sequentially removing individual studies to determine whether each resulted in a substantial change in the magnitude or direction of the pooled estimates and heterogeneity. When the excluded study substantially changed I2 value, it is reported in the results. Meta-analyses of this review were conducted via the Cochrane Collaboration Review Manager (RevMan) software, v.5.4.1 for Windows.
Results
Literature search results
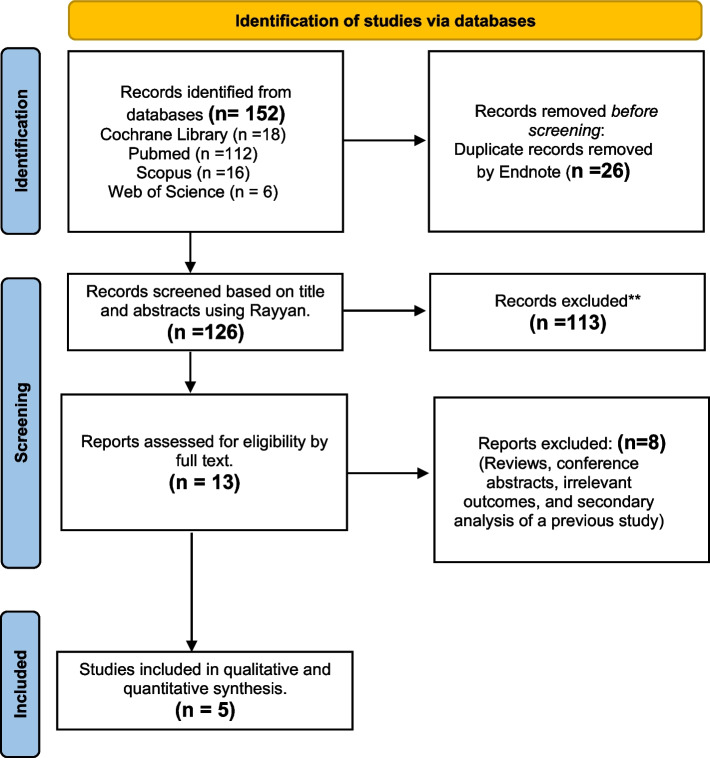
Following a specific search strategy for each database. The search yielded 152 studies, after removing duplication 126 studies remain. Full-text screening results in five studies [12, 28, 34, 41, 42] available for data extraction and analysis after exclusion of irrelevant studies. PRISMA flow diagrams show the search results (Fig. 1).
Fig. 1.
PRISMA flowchart of paper selection. PRISMA Flowchart outlining the search strategy and details on the studies finally included in the meta-analysis
Characteristics of the included studies
Five studies were included in our mete-analysis with a total of 833 patients, 407 in treatment group and 426 in control group. These studies were published in the period from 2021 to 2023 with only two multi-center studies.
All the included studies are randomized controlled trials (RCTs). (Additional file 3) and Table 1 present the summary and the baseline characteristic of the included studies respectively. The mean age of the patients is about 10 years ranged from (7–13) years with no statistically significant difference in baseline characteristics for included children. All children in the treatment group were subjected to repeated low repeated low-level red-light (RLRL) in addition to single-vision spectacle (SVS) compared with SVS only in control group. Patients in control group didn't undergo any intervention except in Dong 2023 where the patients subjected to a sham device.
Table 1.
Baseline characteristics of the patients
| Study ID | Arm | Patients | AGE, Mean ± SD | SEX (Male/Female) | Axial length (mm), Mean ± SD | Spherical equivalent refraction(D), Mean ± SD | Subfoveal choroid thickness (μm), Mean ± SD |
|---|---|---|---|---|---|---|---|
| Xiong 2021 [27] | Treatment | 74 | 10.22 ± 2.38 | 40/ 34 | 25.07 ± 1.15 | − 3.39 ± 2.17 | 288.61 ± 59.59 |
| Control | 74 | 10.33 ± 2.03 | 40 /34 | 25.07 ± 0.87 | − 3.32 ± 1.36 | 286.81 ± 63.67 | |
| Jiang 2022 [12] | Treatment | 119 | 10.46 ± 3.75 | 57/62 | 24.54 ± 0.67 | - 2.49 ± 0.92 | NA |
| Control | 145 | 10.53 ± 3.66 | 73/72 | 24.62 ± 0.86 | - 2.67 ± 1.06 | NA | |
| Tian 2022 [33] | Treatment | 112 | 9.66 ± (1.65) | 55/57 | 24.31 ± 0.92 | -2.17 ± 1.50 | 295 ± 82.98 |
| Control | 112 | 9.47 ± (1.59) | 57/55 | 24.20 ± 0.85 | -2 ± 1.13 | 297.33 ± 81.11 | |
| Chen 2022 [40] | Treatment | 46 | 9.00 ± (1.90) | 27/19 | 24.62 ± 0.97 | - 2.54 ± 1.04 | 259.00 ± 51.46 |
| Control | 40 | 8.98 ± (1.92) | 25/15 | 24.57 ± 0.76 | - 2.29 ± 0.77 | 273.08 ± 54.37 | |
| Dong 2023 [41] | Treatment | 56 | 10.3 ± (2.07) | 26/30 | 24.7 ± 1.04 | - 3.13 ± 1.91 | NA |
| Control | 55 | 9.86 ± (1.41) | 30/26 | 24.6 ± 0.96 | - 2.82 ± 1.86 | NA |
Quality assessments
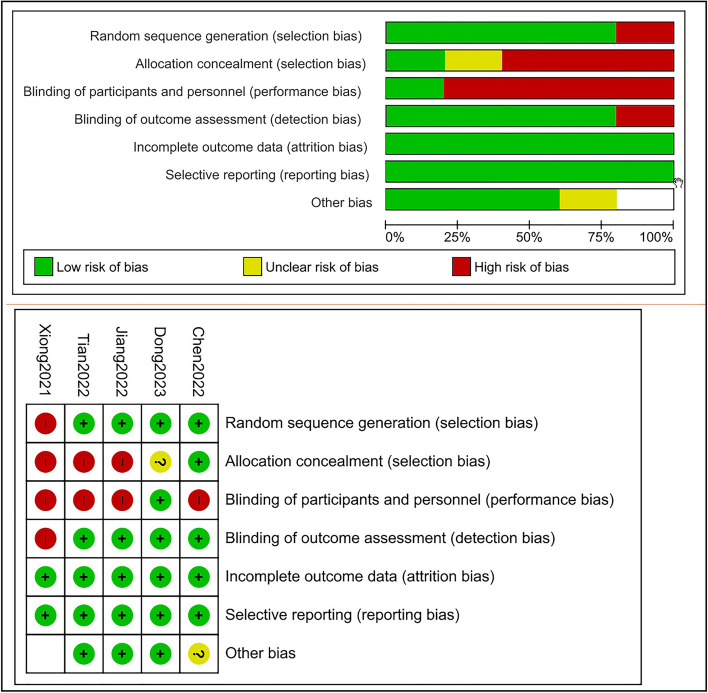
According to NIH quality assessment tool, four studies have low risk of bias with a good quality and a score more than 10 except Xiong 2021 which is a low-quality study with high risk of bias. This study didn't provide sufficient details about its methodology. Most of the studies were single blinded, the patients and ophthalmologists knew the treatment group from the controlled group, and this may be due to the nature of the laser intervention; however, outcome assessors including technicians, optometrists, and statisticians were masked to the treatment allocation. On the other hand, one included study was double blinded as it used the Sham device (Dong 2023). Furthermore, Xiong 2021 which didn't report the masking. Figure 2 shows risk of bias summary and the details of the studies quality are attached in the supplementary files. (Additional file 4).
Fig. 2.
Summary of risk of bias assessment. Shows the risk of bias assessment for each trial using the Cochrane risk of bias tool. Green-colored symbol corresponds to low risk of bias, yellow corresponds to unclear risk of bias, and red corresponds to high risk of bias
Statistical analysis
We used mean change and standard deviation (SD) to compare results between treatment and control group. Most studies recorded data as a mean change and SD.
Outcomes
We analyzed the difference in the three main parameters: Axial length. Spherical equivalent refraction and macular choroidal thickness during different follow up periods.
Axial length
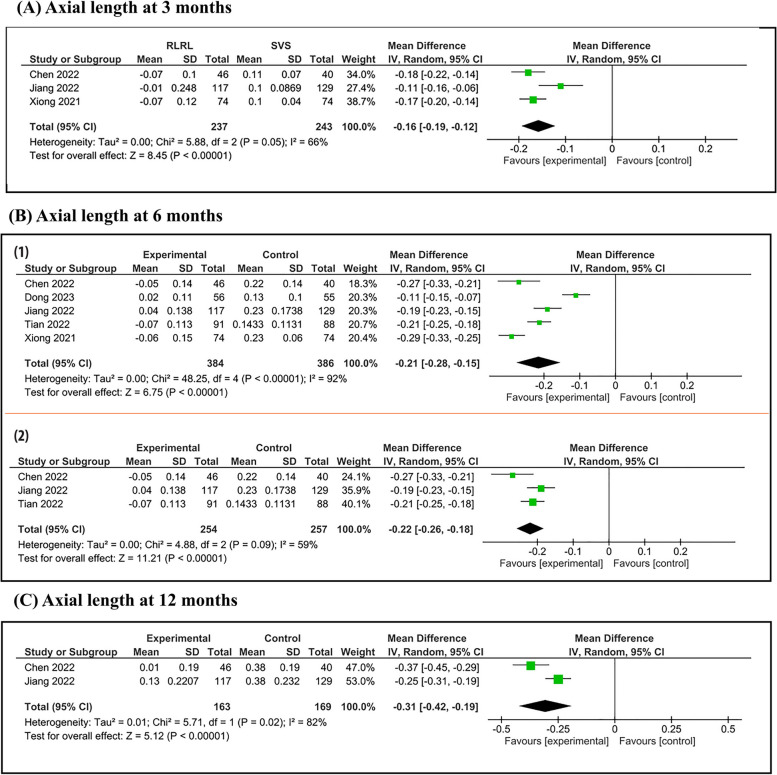
Axial length analysis was conducted at 3, 6 and 12 months. Figure 3 shows that:
Fig. 3.
Forest plot of change in AL. Forest plot of change in AL with RLRL and single vision lens groups: (A) 3 months, (B) 6 months (1) before and (2) after sensitivity analysis, and (C) 12 months follow up interval
At a 3 month follow up period, pooled studies show a statistical difference in axial length between RLRL and SVS group (MD = -0.16; 95% CI [-0.19, -0.12]). Pooled results were heterogenous (I2 = 66%, P = 0.05).
At a 6 month follow up period, there were 5 studies that reported the change in axial length from the baseline. Pooled studies show a statistical difference in axial length between RLRL and SVS group (MD = -0.21; 95% CI [-0.28, -0.15]). Pooled results were heterogeneous (I2 = 92%, P < 0.00001).
At a 12 month follow up period, pooled studies show a statistical difference in axial length between RLRL and SVS group (MD = -0.31; 95% CI [-0.42, -0.19]). Pooled results were heterogeneous (I2 = 82%, P = 0.02).
Spherical equivalent refraction
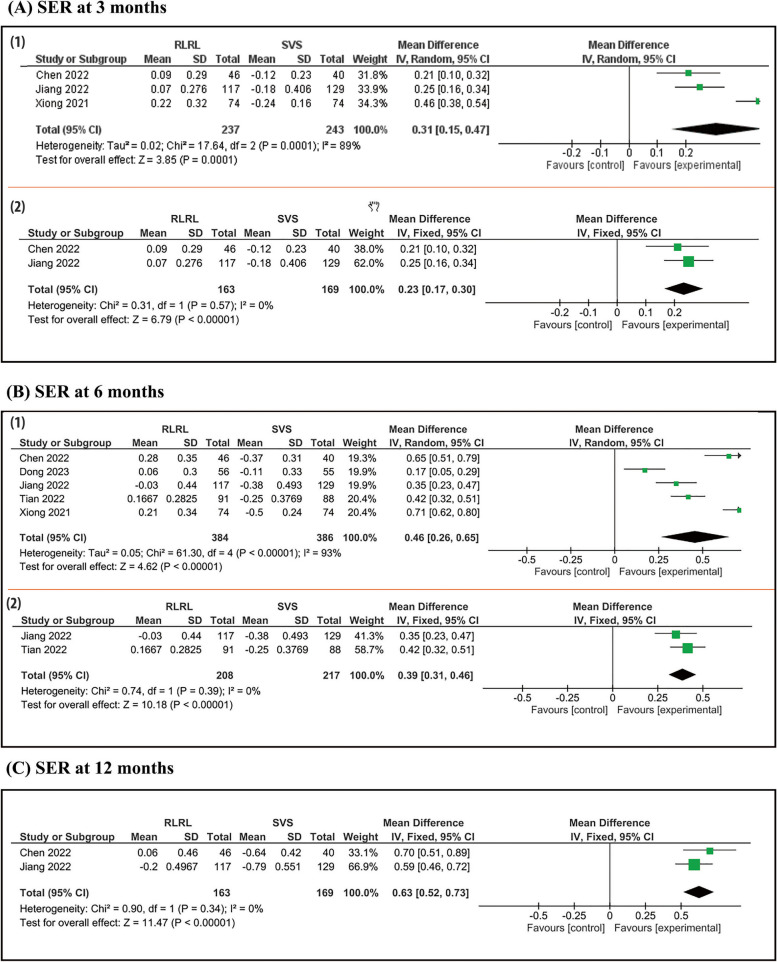
SER analysis was conducted at 3 and 6 and 12 months. Figure 4 shows that:
At a 3 month follow up period, we analyzed 3 studies, pooled studies show a statistical difference in SER between RLRL and SVS group (MD = 0.33; 95% CI [0.27, 0.38]). Pooled results were heterogeneous (I2 = 89%, P = 0.0001).
At a 6 month follow up period, we analyzed 5 studies, pooled studies show a statistical difference in SER between RLRL and SVS group (MD = 0.46; 95% CI [0.26, 0.65]). Pooled results were heterogeneous (I2 = 93%, P < 0.00001).
At a 12 month follow up period, pooled studies show a statistical difference in SER between RLRL and SVS group (MD = 0.63; 95% CI [0.52, 0.73]). Pooled results were homogeneous (I2 = 0%, P = 0.34).
Fig. 4.
Forest plot of change in SER. Forest plot of change in SER with RLRL and single vision lens groups: (A) 3 months (1) before and (2) after sensitivity analysis (B) 6 months (1) before and (2) after sensitivity analysis, and (C) 12 months follow up interval
Macular choroidal thickness
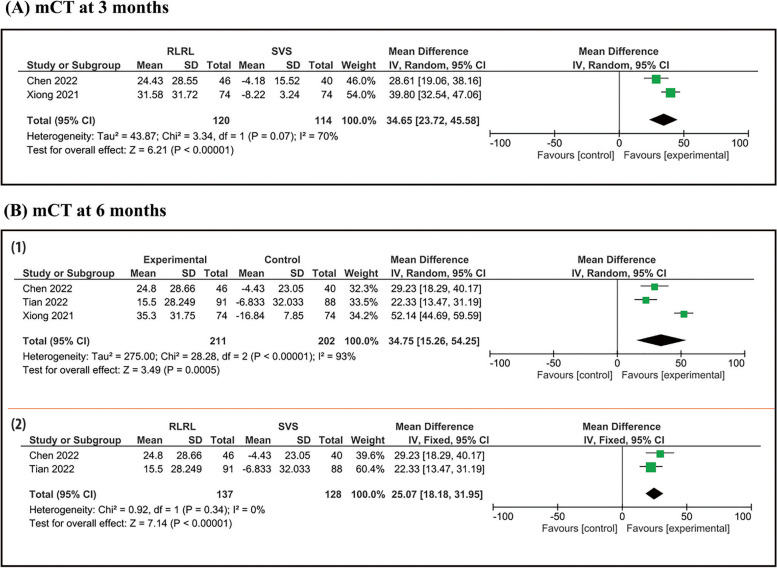
mCT analysis was conducted at 3 and 6 months. Figure 5 shows that:
At a 3-month, pooled studies show a statistically difference in macular choroidal thickness between RLRL and SVS group (MD = 34.65; 95% CI [23.72, 45.58]). Pooled results were heterogenous (I2 = 70%, P = 0.07).
At a 6-month, pooled studies show a statistically difference in macular choroidal thickness between RLRL and SVS group (MD = 34.75; 95% CI [15.26, 54.25]). Pooled results were heterogenous (I2 = 93%, P < 0.00001).
Fig. 5.
Forest plot of change in SFCT. Forest plot of change in SFCT with RLRL and single vision lens groups: (A) 3 months and (B) 6 months (1) before and (2) after sensitivity analysis
Sensitivity analysis and heterogeneity
Meta-analysis showed heterogeneity in some studies, to overcome this heterogeneity we used random effect model instead of fixed effect model. There was no sufficient data to do subgroup analysis so we couldn't overcome the heterogeneity. Finally, we used leave one out method in trying to overcome the heterogeneity, however, it still presents in some outcomes.
Axial length
At a 6 month follow up period, a significant heterogeneity was noted when pooling all the studies, so we made a sensitivity analysis by removing two studies Dong 2023 and Xiong 2021 and there was still a statistically significant difference and the heterogenicity decreased (I2 = 59%, P = 0.09). (Fig. 3-b-2).
At a 12 month follow up period, pooled results were heterogeneous (I2 = 82%, P = 0.02) and we couldn't solve this heterogeneity by sensitivity or subgroup analysis due to limited number of studies.
Spherical equivalent refraction
At a 3 month follow up period, pooled results were heterogeneous (I2 = 89%, P = 0.0001). After sensitivity analysis, we removed Xiong 2021 and there was still a statistically significant difference with no heterogenicity (I2 = 0%, P = 0.57). (Fig. 4-a-2).
At a 6 month follow up period, pooled results were heterogeneous (I2 = 93%, P < 0.00001). After sensitivity analysis, we removed Dong 2023 and Xiong 2021 and there was no heterogenicity (I2 = 0%, P = 0.39). (Fig. 4-b-2).
Macular choroidal thickness
At a 6 month follow up period, pooled results were heterogeneous (I2 = 93%, P < 0.00001). After sensitivity analysis, we Xiong 2021 and there was no heterogenicity (I2 = 0%, P = 0.34). (Fig. 5-b-2).
At a 3-month, pooled results were heterogenous (I2 = 70%, P = 0.07) and we couldn't solve this heterogeneity by sensitivity or subgroup analysis due to limited number of studies.
Discussion
This is a systematic review specifically conducted to investigate whether repeated low-level red light (RLRL) therapy can control the progression of myopia in children. The efficacy of the RLRL can introduce an alternative which seems at least competitive with other treatment methods. Our results showed that RLRL can better control axial length (AL) elongation, spherical equivalence refraction (SER), and subfoveal choroidal thickness (SFCT) better than single vision spectacles (SVS) within 3, 6, 12 months when compared to wearing single-vision glasses. All the outcomes were reported at 6 months follow up; however, AL was reported in 3 months and SER was reported in 12 months additionally. AL was defined as the distance from the corneal vertex to the retinal pigment epithelium, representing myopia. AL was improved compared to baseline values at 3 month follow up and 6 month follow up periods. SER was improved after using RLRL compared to baseline at 3 months follow up, 6 months follow up, and 12 months follow up. SFCT was increased compared to baseline at 6 months follow up.
The heterogeneity was significantly high when pooling all studies. To investigate the heterogeneity, we couldn’t do subgroup analysis due to limited data. However, we excluded from the quantitative analysis the studies that were responsible for heterogeneity by conducting sensitivity analysis. Sensitivity analysis showed changes in the heterogeneity, however, there was still a statistically significant difference. The studies removed by leave one out may have some limitations due to the design, sample size, or the procedures. For example, we removed the data of Xiong 2021 due to significant heterogeneity detected in all outcomes except AL of 3 months. This study was of low quality according to the quality assessments tool which may have resulted from the methods of randomization and allocation. Additionally, the laser power was very different in this paper compared to other papers (Additional file 3). Another example, we removed the data of Dong 2023 also due to significant heterogeneity detected in all outcomes without exception. The heterogeneity of this study could be related to using sham device that used 10% of the original device’s power compared with the SVS control group which improved the cases to an extent. Sensitivity analysis showed solving of some heterogeneity however, the significant differences were still found between the two groups.
Our results are consistent with a recently published systematic review and meta-analysis (Tang et al. 2023) [47] discussing the RLRL therapy effectiveness. Tang et al. 2023 concluded that the weighted mean difference (WMD) for myopia progression between RLRL and the control group was 0.68 D per 6 months for SER change, -0.35 mm per 6 months for AL elongation and 36.04 μm per 6 months for SFChT change. However, this recently published study included non-randomized controlled trials as cohort and post-hoc analysis studies that may affected the heterogeneity and validity of the results.
No severe adverse events, such as scotoma that occurred during the trial linked to the intervention or sudden vision loss by 2 lines happening over the course of a few seconds or minutes to a few days, were reported in any of the included studies. This is consistent with Tang et al. 2023 which reported that none of the studies in their meta-analysis reported vison-threatening events and no structural damage was observed in the photosensory layers. However, a recent case report of 12-year-old female mentioned the possibility of retinal damage after RLRL exposure [48]. Based on the findings of the short-term follow-up, RLRL intervention has a promising control impact on myopia. Contrarily, the risk of adverse events associated with orthokeratology, such as infective keratitis, is well recognized [24]. Photophobia, among other side effects, is possible with atropine eye solutions [49–51].
Currently, much empirical evidence suggests that oxidative stress and inflammation may be responsible for the altered regulatory pathways in myopia, and that oxidative damage of hypoxic myopia can change how nitric oxide and dopamine are neuromodulated during eye development [52, 53]. It may be feasible to protect patients from the effects of oxidative stress and reduce inflammation associated with myopia by investigating the potential mechanisms underlying the inhibitory effects of RLRL treatment [54]. In both animal research [55] and clinical studies [28, 56], RLRL has the greatest effects on the nitric oxide system and reduces the intensity of oxidative stress. By inhibiting inflammatory cytokines like interleukin-1 (IL-1) and tumor necrosis factor-α, RLRL may lower their amounts [57]. Additionally, severe myopia may substantially raise IL-1 and IL-6 levels, which may be related to the myopic control mechanism [58, 59]. According to a generally accepted theory, bright light causes the retina to produce and release more dopamine [60]. In the development of refractive eyes, dopamine functions as a termination signal [61].
The choroid has a variety of functions, including nourishing the retina [62] and can affect the refractive state [63, 64]. Furthermore, the choroid has a crucial role in relaying signals derived from the retina to the sclera, further altering the synthesis of scleral extracellular matrix and changing the ocular size, resulting in refractive changes that have a vital function in the etiology of myopia [65, 66]. Additionally, the release of nitric oxide from the retina or choroid by the dopamine in the retina may cause choroidal thickening and inhibit ocular growth [67, 68]. After 650 nm RLRL treatment, previous research found a significant increase in choroidal thickness. Similarly, Gawne et al. [69] noted a refractive shift brought on by a decrease in vitreous chamber depth and a rise in choroid thickness. Two of the included studies [12, 41] hypothesized that RLRL may control myopia by increasing choroidal blood flow and reducing oxidative stress and inflammation [70, 71], and thus ameliorating scleral hypoxia.
Other presently available non-invasive interventions, such as more outdoor exercise, orthokeratology, and atropine eye drops, may not have the same myopia-controlling effects as 650 nm RLRL. Over the past few years, outdoor time has drawn a lot of focus. According to He et al. [72], spending more time outside did reduce the progression of myopia compared to the control group. Despite an extra 40 min of outdoor activity being added to each school day, children still on average underwent a -1.42 D myopic shift and a 0.95 mm elongation of AL during the 3-year follow-up. Outdoor exercise, in contrast, appeared to be less effective than 650 nm RLRL, let alone the difficulty of implementing an extra 40 min of outdoor exercise given the demands of students' academic work.
In terms of clinical implication from this review, repeated low-level red-light intervention was needed twice daily, 3 min per session, five days per week which is the same procedure for treating amblyopia. The investigators of one of the included studies (Jiang 2022) gave parents the device to allow this daily treatment schedule so they could carry out this treatment at home. Users must log in to the system using a specified username and password given to start treatment because the device is connected to the internet. The research coordinator will be able to watch, record, and keep track of treatment adherence when using devices. Jiang et al.'s study [12] showed that greater treatment compliance greatly increased treatment efficacy. This significant dose response effect may further support the effectiveness of RLRL in controlling myopia, but it also emphasizes the importance of putting up a suitable incentive system to motivate kids to use the device and to maximize treatment efficacy. This potent dose–response relationship also suggests that treatment efficacy may be enhanced by increasing the treatment duration from 3 min to an extended treatment time per session.
However, there were some limitations in our review. First, the number of studies included in this meta-analysis was limited. Because of the limited number of included studies and hence limited statistical power, publication bias test was not performed in our review according to Begg’s and Egger’s recommendation [73]. Second, considering the significant methodological heterogeneity among included studies, we couldn't conduct subgroup analysis due to limited data. Third, the follow up periods were short to evaluate the potential dose–response relationship and the optimal power intensity of the RLRL treatment for myopia control. Fourth, all the published studies were limited to centers in China. Thus, we recommend further studies with long-term follow-up involving various centers in different countries to confirm our findings. Furthermore, we do not know whether there is a rebound effect like atropine eye drops once treatment is stopped, which is a knowledge gap for future study.
Conclusion
In summary, this is the first systematic review and meta-analysis investigating only RCTs evidence supporting the efficacy of 650 nm RLRL for myopia control in the short term of 3, 6, and 12 months follow up. The present review revealed the clinical significance of RLRL for myopia control in terms of AL, SER, and SFCT. It has slowed down and reversed the myopia progression in a large proportion of children. RLRL therapy is an effective new alternative treatment for myopia control with good user acceptability and no documented functional or structural damage. However, the effect of long-term RLRL treatment and the rebound effect after cessation require further investigation.
Supplementary Information
Additional file 1. PRISMA 2020 Checklist.
Additional file 3. Table showing summary sheet of characteristic of the included studies.
Additional file 4. Table showing risk of bias summary and the details of the studies quality.
Acknowledgements
Not applicable.
Abbreviations
- RLRL
Repeated Low-Level Red Light
- SVS
Singe vision spectacles
- AL
Axial length
- SER
Spherical equivalent refraction
- SFCT
Sub foveal choroidal thickness
- MD
Mean difference
- RCTs
Randomized controlled trials
- D
Diopters
- mCT
Macular choroidal thickness
- ICL
Implantable collamer lens
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PICO
The Population-Intervention-Comparison-Outcome
- Sex (M/F)
Male/Female
- NIH
National Institutes of Health
- SD
Standard-deviation
- IQR
Interquartile range
- r
Denotes the correlations between the baseline and final measurements
- CI
Confidence intervals
- IL-1
Interleukin-1
- NMA
Network meta-analysis
Authors’ contributions
M.Y and A.S formed the search strategy after checking validity and were third reviewers for all steps. R.F, H.F and M.R independently screened the citations for eligibility. R.F, H.F and M.R formed the summary sheet. R.F and H.F wrote the introduction section and A.T revised and corrected it. A.M formed the baseline sheet and quality assessment along with M.R and they also wrote materials and methods section. A.S wrote and conducted the results section. M.Y conducted the discussion and A.T revised and corrected it.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dolgin E. The myopia boom. Nature. 2015;519(7543):276. doi: 10.1038/519276a. [DOI] [PubMed] [Google Scholar]
- 2.Yu Y, Liu J. The effect of 0.01% atropine and orthokeratology on ocular axial elongation for myopia children: a meta-analysis (a PRISMA-compliant article). Medicine. 2022;101(18):e29191. 10.1097/MD.0000000000029191. [DOI] [PMC free article] [PubMed]
- 3.Bourne RR, Stevens GA, White RA, Smith JL, Flaxman SR, Price H, Jonas JB, Keeffe J, Leasher J, Naidoo K, Pesudovs K. Causes of vision loss worldwide, 1990–2010: a systematic analysis. Lancet Glob Health. 2013;1(6):e339–e349. doi: 10.1016/S2214-109X(13)70113-X. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Li Y, Musch DC, Wei N, Qi X, Ding G, Li X, Li J, Song L, Zhang Y, Ning Y. Progression of myopia in school-aged children after COVID-19 home confinement. JAMA Ophthalmol. 2021;139(3):293–300. doi: 10.1001/jamaophthalmol.2020.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holden BA, Wilson DA, Jong M, Sankaridurg P, Fricke TR, Smith EL, III, Resnikoff S. Myopia: a growing global problem with sight-threatening complications. Commun Eye Health. 2015;28(90):35. [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell P, Hourihan F, Sandbach J, Wang JJ. The relationship between glaucoma and myopia: the Blue Mountains eye study. Ophthalmology. 1999;106(10):2010–2015. doi: 10.1016/S0161-6420(99)90416-5. [DOI] [PubMed] [Google Scholar]
- 7.Ohno-Matsui K. What is the fundamental nature of pathologic myopia? Retina. 2017;37(6):1043–1048. doi: 10.1097/IAE.0000000000001348. [DOI] [PubMed] [Google Scholar]
- 8.Chamberlain P, Lazon de la Jara P, Arumugam B, Bullimore MA. Axial length targets for myopia control. Ophthal Physiol Optics. 2021;41(3):523–31. doi: 10.1111/opo.12812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Q. Genetics of refraction and myopia. Prog Mol Biol Transl Sci. 2015;134:269–279. doi: 10.1016/bs.pmbts.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Czepita DA, Zejmo M. Environmental factors and myopia. Ann Acad Med Stetin. 2011;57(3):88–92. [PubMed] [Google Scholar]
- 11.Pärssinen O, Kauppinen M, Viljanen A. The progression of myopia from its onset at age 8–12 to adulthood and the influence of heredity and external factors on myopic progression. A 23-year follow-up study. Acta Ophthalmol. 2014;92(8):730–9. doi: 10.1111/aos.12387. [DOI] [PubMed] [Google Scholar]
- 12.Jiang Y, Zhu Z, Tan X, Kong X, Zhong H, Zhang J, Xiong R, Yuan Y, Zeng J, Morgan IG, He M. Effect of repeated low-level red-light therapy for myopia control in children: a multicenter randomized controlled trial. Ophthalmology. 2022;129(5):509–519. doi: 10.1016/j.ophtha.2021.11.023. [DOI] [PubMed] [Google Scholar]
- 13.Ohno-Matsui K. Pathologic myopia. Asia-Pac J Ophthalmol. 2016;5(6):415–423. doi: 10.1097/APO.0000000000000230. [DOI] [PubMed] [Google Scholar]
- 14.Biswas S, Muralidharan AR, Betzler BK, Busoy JM, Barathi VA, Tan RK, Low WY, Milea D, Kathrani BK, Brennan NA, Najjar RP. A duration-dependent interaction between high-intensity light and unrestricted vision in the drive for myopia control. Invest Ophthalmol Visual Sci. 2023;64(3):31. doi: 10.1167/iovs.64.3.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z, Wang W, Liu R, Wang D, Zhang J, Xiao O, Guo X, Jong M, Sankaridurg P, Ohno-Matsui K, He M. Choroidal thickness predicts progression of myopic maculopathy in high myopes: a 2-year longitudinal study. Br J Ophthalmol. 2021;105(12):1744–1750. doi: 10.1136/bjophthalmol-2020-316866. [DOI] [PubMed] [Google Scholar]
- 16.Li Z, Hu Y, Cui D, Long W, He M, Yang X. Change in subfoveal choroidal thickness secondary to orthokeratology and its cessation: a predictor for the change in axial length. Acta Ophthalmol. 2019;97(3):e454–e459. doi: 10.1111/aos.13866. [DOI] [PubMed] [Google Scholar]
- 17.Naidoo KS, Fricke TR, Frick KD, Jong M, Naduvilath TJ, Resnikoff S, Sankaridurg P. Potential lost productivity resulting from the global burden of myopia: systematic review, meta-analysis, and modeling. Ophthalmology. 2019;126(3):338–346. doi: 10.1016/j.ophtha.2018.10.029. [DOI] [PubMed] [Google Scholar]
- 18.Karthikeyan SK, Ashwini DL, Priyanka M, Nayak A, Biswas S. Physical activity, time spent outdoors, and near work in relation to myopia prevalence, incidence, and progression: an overview of systematic reviews and meta-analyses. Indian J Ophthalmol. 2022;70(3):728. doi: 10.4103/ijo.IJO_1564_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muralidharan AR, Lança C, Biswas S, Barathi VA, Shermaine LWY, Seang-Mei S, Milea D, Najjar RP. Light and myopia: from epidemiological studies to neurobiological mechanisms. Therapeut Adv Ophthalmol. 2021;13:25158414211059246. doi: 10.1177/25158414211059246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho P, Tan Q. Myopia and orthokeratology for myopia control. Clin Exp Optom. 2019;102(4):364–377. doi: 10.1111/cxo.12839. [DOI] [PubMed] [Google Scholar]
- 21.Logan N. Myopia control and contact lenses. Cont Lens Anterior Eye. 2020;43(1):3. doi: 10.1016/j.clae.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Sankaridurg P, Donovan L, Varnas S, Ho A, Chen X, Martinez A, Fisher S, Lin Z, Smith EL, III, Ge J, Holden B. Spectacle lenses designed to reduce progression of myopia: 12-month results. Optomet Vision Sci. 2010;87(9):631. doi: 10.1097/OPX.0b013e3181ea19c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yam JC, Jiang Y, Lee J, Li S, Zhang Y, Sun W, Yuan N, Wang YM, Yip BH, Kam KW, Chan HN. The association of choroidal thickening by atropine with treatment effects for myopia: two-year clinical trial of the low-concentration atropine for myopia progression (LAMP) study. Am J Ophthalmol. 2022;237:130–138. doi: 10.1016/j.ajo.2021.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Leo SW. Current approaches to myopia control. Curr Opin Ophthalmol. 2017;28(3):267–275. doi: 10.1097/ICU.0000000000000367. [DOI] [PubMed] [Google Scholar]
- 25.Zhou L, Xing C, Qiang W, Hua C, Tong L. Low-intensity, long-wavelength red light slows the progression of myopia in children: an Eastern China-based cohort. Ophthalmic Physiol Opt. 2022;42(2):335–344. doi: 10.1111/opo.12939. [DOI] [PubMed] [Google Scholar]
- 26.Rucker F. Monochromatic and white light and the regulation of eye growth. Exp Eye Res. 2019;184:172–182. doi: 10.1016/j.exer.2019.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hung LF, Arumugam B, She Z, Ostrin L, Smith EL., III Narrow-band, long-wavelength lighting promotes hyperopia and retards vision-induced myopia in infant rhesus monkeys. Exp Eye Res. 2018;176:147–160. doi: 10.1016/j.exer.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiong F, Mao T, Liao H, Hu X, Shang L, Yu L, Lin N, Huang L, Yi Y, Zhou R, Zhou X. Orthokeratology and low-intensity laser therapy for slowing the progression of myopia in children. BioMed Res Int. 2021;2021:8915867. 10.1155/2021/8915867. [DOI] [PMC free article] [PubMed]
- 29.Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng. 2012;40:516–533. doi: 10.1007/s10439-011-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.AlGhamdi KM, Kumar A, Moussa NA. Low-level laser therapy: a useful technique for enhancing the proliferation of various cultured cells. Lasers Med Sci. 2012;27:237–249. doi: 10.1007/s10103-011-0885-2. [DOI] [PubMed] [Google Scholar]
- 31.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 32.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:1. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Heart, Lung, and Blood Institute. Quality assessment tools for controlled intervention and cohort observation studies. Recuperado de: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. 2017.
- 34.Tian L, Cao K, Ma DL, Zhao SQ, Lu LX, Li A, Chen CX, Ma CR, Ma ZF, Jie Y. Investigation of the efficacy and safety of 650 nm low-level red light for myopia control in children: a randomized controlled trial. Ophthalmol Therapy. 2022;11(6):2259–2270. doi: 10.1007/s40123-022-00585-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:1–3. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions. Wiley; 2019. [DOI] [PMC free article] [PubMed]
- 37.Shida N, Yagiz G, Yamada T. The effects of exergames on muscle architecture: a systematic review and meta-analysis. Appl Sci. 2021;11(21):10325. doi: 10.3390/app112110325. [DOI] [Google Scholar]
- 38.Yagiz G, Akaras E, Kubis HP, Owen JA. Heterogeneous effects of eccentric training and nordic hamstring exercise on the biceps femoris fascicle length based on ultrasound assessment and extrapolation methods: a systematic review of randomised controlled trials with meta-analyses. PLoS ONE. 2021;16(11):e0259821. doi: 10.1371/journal.pone.0259821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Effect sizes based on means. Introduction to meta-analysis. 2009:21–32.
- 40.Higgins JP. Cochrane handbook for systematic reviews of interventions. Version 5.1. 0 [updated March 2011]. The Cochrane Collaboration. www.cochrane-handbook.org. 2011.
- 41.Chen H, Wang W, Liao Y, Zhou W, Li Q, Wang J, Tang J, Pei Y, Wang X. Low-intensity red-light therapy in slowing myopic progression and the rebound effect after its cessation in Chinese children: a randomized controlled trial. Graefes Arch Clin Exp Ophthalmol. 2023;261(2):575–584. doi: 10.1007/s00417-022-05794-4. [DOI] [PubMed] [Google Scholar]
- 42.Dong J, Zhu Z, Xu H, He M. Myopia control effect of repeated low-level red-light therapy in Chinese children: a randomized, double-blind, controlled clinical trial. Ophthalmology. 2023;130(2):198–204. doi: 10.1016/j.ophtha.2022.08.024. [DOI] [PubMed] [Google Scholar]
- 43.Cochrane Collaboration. 7.7. 3.2 Obtaining standard deviations from standard errors and confidence intervals for group means. Cochrane handbook for systematic reviews of interventions. 2011.
- 44.Deeks JJ, Higgins JPT, Altman DG. Analysing data and undertaking meta-analyses. In: Cochrane Handbook for Systematic Reviews of Interventions, Version 5.0.2; The Cochrane Collaboration: London, 2011.
- 45.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 47.Tang J, Liao Y, Yan N, Dereje SB, Wang J, Luo Y, Wang Y, Zhou W, Wang X, Wang W. Efficacy of repeated low-level red-light therapy for slowing the progression of childhood myopia: a systematic review and meta-analysis. Am J Ophthalmol. 2023. [DOI] [PubMed]
- 48.Liu H, Yang Y, Guo J, Peng J, Zhao P. Retinal damage after repeated low-level red-light laser exposure. JAMA Ophthalmol. 2023;141(7):693–695. doi: 10.1001/jamaophthalmol.2023.1548. [DOI] [PubMed] [Google Scholar]
- 49.Chia A, Chua WH, Wen L, Fong A, Goon YY, Tan D. Atropine for the treatment of childhood myopia: changes after stopping atropine 0.01%, 0.1% and 0.5% Am J Ophthalmol. 2014;157(2):451–7. doi: 10.1016/j.ajo.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 50.Wei S, Li SM, An W, Du J, Liang X, Sun Y, Zhang D, Tian J, Wang N. Safety and efficacy of low-dose atropine eyedrops for the treatment of myopia progression in Chinese children: a randomized clinical trial. JAMA Ophthalmol. 2020;138(11):1178–1184. doi: 10.1001/jamaophthalmol.2020.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chia A, Chua WH, Cheung YB, Wong WL, Lingham A, Fong A, Tan D. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2) Ophthalmology. 2012;119(2):347–54. doi: 10.1016/j.ophtha.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 52.Francisco BM, Salvador M, Amparo N. Oxidative stress in myopia. Oxid Med Cell Longevity. 2015;2015:750637. 10.1155/2015/750637. [DOI] [PMC free article] [PubMed]
- 53.Dai L, Yang W, Qin X, Li Y, Cao H, Zhou C, Wang Y. Serum metabolomics profiling and potential biomarkers of myopia using LC-QTOF/MS. Exp Eye Res. 2019;186:107737. doi: 10.1016/j.exer.2019.107737. [DOI] [PubMed] [Google Scholar]
- 54.Jówko E, Płaszewski M, Cieśliński M, Sacewicz T, Cieśliński I, Jarocka M. The effect of low level laser irradiation on oxidative stress, muscle damage and function following neuromuscular electrical stimulation. A double blind, randomised, crossover trial. BMC Sports Sci Med Rehabil. 2019;11:1–4. doi: 10.1186/s13102-019-0147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ojaghi R, Sohanaki H, Ghasemi T, Keshavarz F, Yousefifard M, Sadeghipour H. Role of low-intensity laser therapy on naloxone-precipitated morphine withdrawal signs in mice: is nitric oxide a possible candidate mediator? Lasers Med Sci. 2014;29:1655–1659. doi: 10.1007/s10103-014-1530-7. [DOI] [PubMed] [Google Scholar]
- 56.Rubis LM. Chiropractic management of bell palsy with low level laser and manipulation: a case report. J Chiropr Med. 2013;12(4):288–291. doi: 10.1016/j.jcm.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamaura M, Yao M, Yaroslavsky I, Cohen R, Smotrich M, Kochevar IE. Low level light effects on inflammatory cytokine production by rheumatoid arthritis synoviocytes. Lasers Surg Med. 2009;41(4):282–290. doi: 10.1002/lsm.20766. [DOI] [PubMed] [Google Scholar]
- 58.Chiou GC, Xuan BO, Liu Q, Yamasaki T, Okawara T. Inhibition of interleukin-1-induced uveitis and corneal fibroblast proliferation by interleukin-1 blockers. J Ocul Pharmacol Therapeut. 2000;16(5):407–18. doi: 10.1089/jop.2000.16.407. [DOI] [PubMed] [Google Scholar]
- 59.Yuan J, Wu S, Wang Y, Pan S, Wang P, Cheng L. Inflammatory cytokines in highly myopic eyes. Sci Rep. 2019;9(1):3517. doi: 10.1038/s41598-019-39652-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cohen Y, Peleg E, Belkin M, Polat U, Solomon AS. Ambient illuminance, retinal dopamine release and refractive development in chicks. Exp Eye Res. 2012;103:33–40. doi: 10.1016/j.exer.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 61.Feldkaemper M, Schaeffel F. An updated view on the role of dopamine in myopia. Exp Eye Res. 2013;114:106–119. doi: 10.1016/j.exer.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 62.Cicinelli MV, Rabiolo A, Marchese A, de Vitis L, Carnevali A, Querques L, Bandello F, Querques G. Choroid morphometric analysis in non-neovascular age-related macular degeneration by means of optical coherence tomography angiography. Br J Ophthalmol. 2017;101(9):1193–1200. doi: 10.1136/bjophthalmol-2016-309481. [DOI] [PubMed] [Google Scholar]
- 63.Queirós A, Amorim-de-Sousa A, Lopes-Ferreira D, Villa-Collar C, Gutiérrez ÁR, González-Méijome JM. Relative peripheral refraction across 4 meridians after orthokeratology and LASIK surgery. Eye Vision. 2018;5(1):1–8. doi: 10.1186/s40662-018-0106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29(2):144–168. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wallman J, Wildsoet C, Xu A, Gottlieb MD, Nickla DL, Marran L, Krebs W, Christensen AM. Moving the retina: choroidal modulation of refractive state. Vision Res. 1995;35(1):37–50. doi: 10.1016/0042-6989(94)E0049-Q. [DOI] [PubMed] [Google Scholar]
- 66.Summers JA. The choroid as a sclera growth regulator. Exp Eye Res. 2013;114:120–127. doi: 10.1016/j.exer.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nickla DL, Damyanova P, Lytle G. Inhibiting the neuronal isoform of nitric oxide synthase has similar effects on the compensatory choroidal and axial responses to myopic defocus in chicks as does the non-specific inhibitor L-NAME. Exp Eye Res. 2009;88(6):1092–1099. doi: 10.1016/j.exer.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sekaran S, Cunningham J, Neal MJ, Hartell NA, Djamgoz MB. Nitric oxide release is induced by dopamine during illumination of the carp retina: serial neurochemical control of light adaptation. Eur J Neurosci. 2005;21(8):2199–2208. doi: 10.1111/j.1460-9568.2005.04051.x. [DOI] [PubMed] [Google Scholar]
- 69.Gawne TJ, Ward AH, Norton TT. Long-wavelength (red) light produces hyperopia in juvenile and adolescent tree shrews. Vision Res. 2017;140:55–65. doi: 10.1016/j.visres.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu H, Chen W, Zhao F, Zhou Q, Reinach PS, Deng L, Ma L, Luo S, Srinivasalu N, Pan M, Hu Y. Scleral hypoxia is a target for myopia control. Proc Natl Acad Sci. 2018;115(30):E7091–E7100. doi: 10.1073/pnas.1721443115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fu A, Stapleton F, Wei L, Wang W, Zhao B, Watt K, Ji N, Lyu Y. Effect of low-dose atropine on myopia progression, pupil diameter and accommodative amplitude: low-dose atropine and myopia progression. Br J Ophthalmol. 2020;104(11):1535–1541. doi: 10.1136/bjophthalmol-2019-315440. [DOI] [PubMed] [Google Scholar]
- 72.He M, Xiang F, Zeng Y, Mai J, Chen Q, Zhang J, Smith W, Rose K, Morgan IG. Effect of time spent outdoors at school on the development of myopia among children in China: a randomized clinical trial. JAMA. 2015;314(11):1142–1148. doi: 10.1001/jama.2015.10803. [DOI] [PubMed] [Google Scholar]
- 73.Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR. Empirical assessment of effect of publication bias on meta-analyses. BMJ. 2000;320(7249):1574–1577. doi: 10.1136/bmj.320.7249.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. PRISMA 2020 Checklist.
Additional file 3. Table showing summary sheet of characteristic of the included studies.
Additional file 4. Table showing risk of bias summary and the details of the studies quality.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.