ABSTRACT
Virus-neutralizing antibodies are often accepted as a correlate of protection against infection, though questions remain about which components of the immune response protect against SARS-CoV-2 infection. In this small observational study, we longitudinally measured spike receptor binding domain (RBD)-specific and nucleocapsid (NP)-specific serum IgG in a human cohort immunized with the Pfizer BNT162b2 vaccine. NP is not encoded in the vaccine, so an NP-specific response is serological evidence of natural infection. A greater than fourfold increase in NP-specific antibodies was used as the serological marker of infection. Using the RBD-specific IgG titers prior to seroconversion for NP, we calculated a protective threshold for RBD-specific IgG. On average, the RBD-specific IgG response wanes below the protective threshold 169 days following vaccination. Many participants without a history of a positive test result for SARS-CoV-2 infection seroconverted for NP-specific IgG. As a group, participants who seroconverted for NP-specific IgG had significantly higher levels of RBD-specific IgG following NP-seroconversion. RBD-specific IgG titers may serve as one correlate of protection against SARS-CoV-2 infection. These titers wane below the proposed protective threshold approximately six months following immunization. Based on serological evidence of infection, the frequency of breakthrough infections and consequently the level of SARS-CoV-2-specific immunity in the population may be higher than what is predicted based on the frequency of documented infections.
KEYWORDS: SARS-CoV-2, vaccine, antibody response, waning immunity, breakthrough infection
Introduction
Identifying laboratory correlates of immune protection for a given pathogen is typically essential to pre-clinical development of vaccines and highly desirable for the evaluation of clinical trials in humans. However, for SARS-CoV-2, the dire need for a vaccine following the virus’s emergence led to the accelerated formulation of many candidate vaccines based on generalities of what is known about the immune response to other viruses and other vaccines. Development and widespread distribution of the Pfizer and Moderna vaccines have been widely considered central to ending the SARS-CoV-2 pandemic. However, an incomplete understanding of the immune correlate of protection against SARS-CoV-2 has complicated communication and consistent policies about who should receive booster vaccinations and the best interval for booster immunizations. Understanding which laboratory component(s) of the immune response to vaccination correlate with protection against infection may ultimately be necessary for the development of informed vaccination recommendations.
NP-specific antibodies result from infection rather than vaccination. Consequently, the presence of NP-specific antibodies in vaccinated participants is serological evidence of breakthrough infections. We have estimated the threshold level of vaccine-elicited RBD-specific antibodies needed to prevent the seroconversion for NP-specific antibodies. We also measured the decay kinetics of RBD-specific antibodies in uninfected study participants. These data allowed us to calculate the average time required for RBD-specific antibodies to wane below the proposed protective threshold following immunization. This finding should be useful for informing policy and practice regarding the frequency and timing of booster vaccines.
Materials and methods
Study design and cohort
Samples analyzed in this study were collected from a cohort of employees of the University of Mississippi Medical Center (UMMC) (Figure 1). The initial intent of the study was to serologically document the natural history of antibody responses to the SARS-CoV-2 virus and to determine if occupational exposure to SARS-CoV-2-infected patients resulted in significantly greater rates of seroconversion than community exposure. This observational study was approved by the UMMC IRB (protocol 2020–0172), and informed consent was obtained from all participants. No interventions were performed as a component of this study. All participants received two doses of Pfizer BNT162b2 prior to their second study visit. Titers reported here are from samples collected from visits two, three, and four. Informed consent was obtained from all participants prior to enrollment in the study. Serum samples were collected in February and March of 2021 (Day ~50), July and August of 2021 (Day ~200), and March and April of 2022 (Day ~440). The Day ~ 200 time point occurred early in the Delta Peak in Mississippi, and the Day ~ 440 time point occurred late in the Omicron Peak. Widespread booster vaccinations were approved in October of 2021 and had been administered to greater than 95% of study participants by December 2021. Samples from participants who declined the primary SARS-CoV-2 vaccination course were not included in this analysis.
Figure 1.
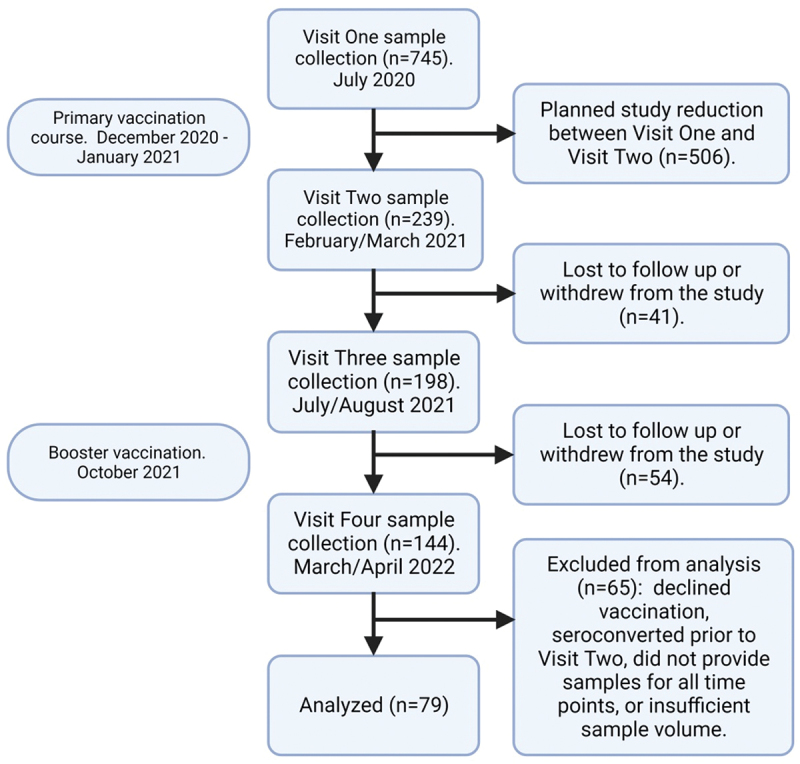
Consort diagram. Study participants were recruited from the University of Mississippi Medical Center employee population. Diagram created with BioRender.com.
Recombinant antigens and ELISA
Recombinant antigens bearing 6× histidine tags were expressed from constructs synthesized by Twist Biosciences. The SARS-CoV-2 RBD constructs encoded a secretion signal and amino acids 319–537 of the spike protein, and the NP construct encoded a secretion signal and amino acids 2–419 of the viral protein. Constructs also encoded a Gly-Ser linker and a 6× histidine tag on their carboxy termini. Proteins were expressed in Expi293 cells (ThermoFisher, A14635) following transient transfection of plasmids and then affinity purified via passage of HisTrap HP columns (Cytiva 29051021). Endpoint dilution titers for RBD-specific and NP-specific serum IgG were determined by ELISA as previously described.1 The endpoint dilution titers were defined as the inverse of the highest dilution that resulted in an absorbance value of 0.2 over that of naïve human sera plated at the same dilutions. Higher endpoint dilution titers represent higher concentration of antigen-specific antibody in the sera. Naïve sera were collected prior to the emergence of SARS-CoV-2.
Statistical methods
Scatterplots with appropriate y-scaling were utilized throughout the study. Kruskal-Wallis tests were used to compare titer levels. Receiver operating characteristics analysis was conducted according to standard methods to obtain sensitivities, specificities, positive predictive values, negative predictive values, and areas under the ROC curve. False negatives are defined as having an IgG titer greater than or equal to 100,000 and also having an NP fold greater than or equal to 4. Conversely, false positives are defined as IgG titer less than 100,000 while also having an NP fold less than 4. A logistic regression model was constructed to model the relationship between RBD-specific IgG titers (continuous) and an NP-specific IgG dichotomized at 4. Time-to-decay analyses were conducted with multilevel mixed models with a linear spline placed at 200 days. This model contained a random intercept for participants, and due to the skewed nature of the IgG outcome, used a gamma family and log link. IgG half-life was calculated as the average time (days) at which there was a 50% reduction in IgG at the participant level. All analyses were completed with Stata v17.1.
Results
Waning and rebounding of SARS-CoV-2 RBD-specific serum IgG
All participants in the study received two doses of Pfizer BNT162b2 prior to sample collection. Serum was collected ~50 days, ~200 days, and ~440 days following the second injection of the primary series. RBD-specific IgG titers waned significantly between day 50 and day 200 and increased significantly between day 200 and day 440 (Figure 2). The decrease in titer is characteristic of the normal vaccine-specific antibody decay2–4 and decline in neutralizing titers5 reported by other groups. The rebound in titers between day 200 (July/August, 2021) and 440 (March/April 2022) resulted largely from SARS-CoV-2 booster immunizations that received FDA approval in October, 2021. Sixty-eight of 79 participants received a booster vaccination between day ~ 200 and ~ 440.
Figure 2.
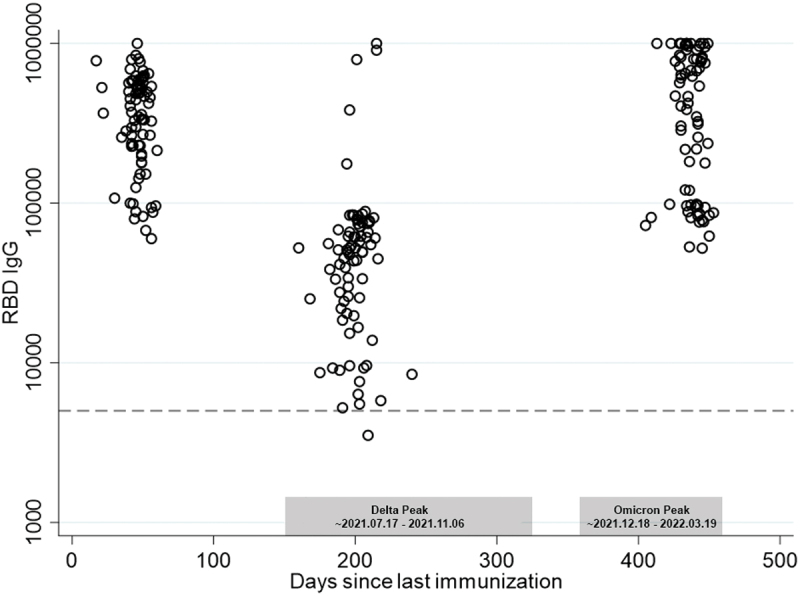
SARS-CoV-2 RBD-specific serum IgG endpoint dilution titers 50, 200, and 440 days following a second immunization with BNT162b2. Titers declined significantly between days 50 and 200 (p < .001) and rebounded significantly between days 200 and 440 (p < .001). Approximate time periods for the Delta and Omicron waves in Mississippi are shown in gray boxes.
NP-specific IgG titers are a serological marker of natural infection
To identify the study participants who experienced breakthrough infections with SARS-CoV-2, we measured serum NP-specific IgG at all three time points. NP serostatus has been used by other groups as an assessment of previous infection or breakthrough infection.6–8 Samples from individuals with detectable NP-specific antibodies on day ~ 50 were excluded from the analysis because these antibodies were evidence of infection before full vaccination. No samples included in the analysis had levels of NP-specific IgG with an endpoint dilution titer greater than 5,000 on Day 50. On day ~ 200, three participants had NP-specific IgG. By Day 440, 54% (43/79) of participants had detectable NP-specific IgG (Figure 3a). Similarly, using the standard of a 4× increase in titer as the marker for seroconversion, 54% (43/79) of study participants had seroconverted for NP-specific IgG by day 450 (Figure 3b), which is consistent with natural infection of these study participants. Interestingly, some study participants exhibited a several fold increase in RBD-specific IgG without seroconversion for NP-specific IgG, which likely resulted from a robust memory response to antigens first encountered at vaccination.
Figure 3.
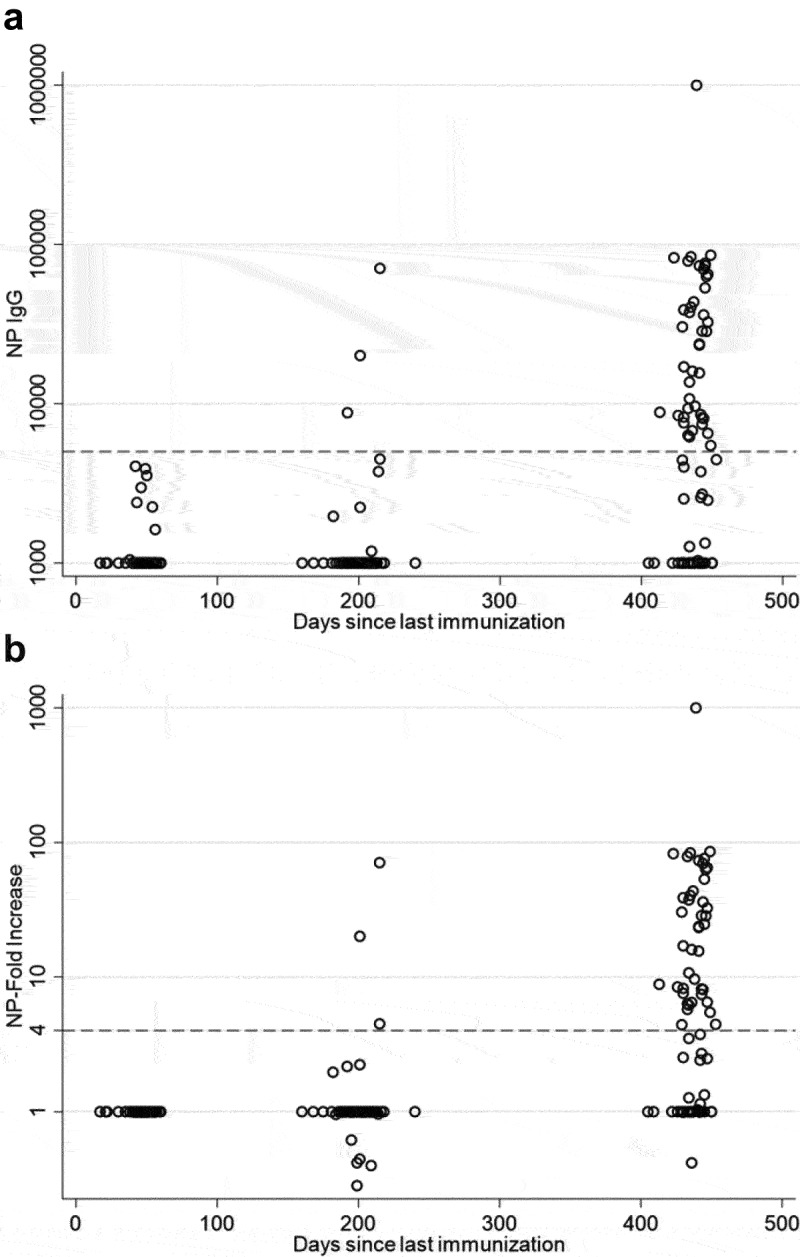
SARS-CoV-2 NP-specific serum antibodies 50, 200, and 440 days following vaccination with BNT162b2. (a) NP-specific serum IgG endpoint dilution titers were similar 50 and 200 days following vaccination but increased significantly by 440 days following vaccination (p < .001). (B) Rates of participant seroconversion, as determined by a > 4× increase in NP-specific titer also increased significantly between day 200 and 440 (p < .001).
Serum RBD-specific IgG titers are a correlate of protection against infection
We compared RBD-specific IgG from day ~ 50 and day ~ 200 to NP-specific IgG titers from day ~ 200 and day ~ 440 to determine if decay of RBD-specific IgG below a certain level was predictive of seroconversion for NP-specific IgG. We found that 85% of study participants who seroconverted for NP had RBD-specific IgG endpoint dilution titers of less than 100,000 at the previous study visit (Figure 4, upper quadrants), which suggests that this level of antibody may represent the threshold for protection against natural infection. Notably, many participants who did not seroconvert for NP also had RBD-specific titers less than 100,000 (Figure 4, lower left quadrant). Continuous value RBD-specific titers are shown in Figure 4. The AUC for this plot was 0.72 (Figure 5a), which supports the use of 100,000 as a good discriminant of protection. Increases in titer were observed to decrease the probability of seroconversion, especially after titer values of 100,000 (Figure 5b).
Figure 4.
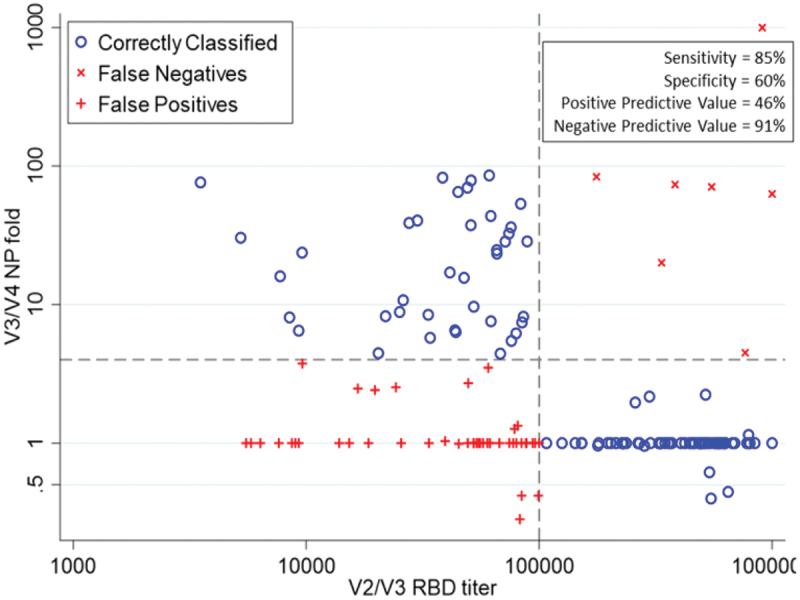
RBD-specific serum IgG titers from visits two and three plotted against fold change in NP-specific IgG for visits three and four to determine if waning RBD-specific IgG was predictive of seroconversion for NP. Sensitivity, specificity, positive predictive value, and negative predictive value are shown for predicting an NP fold of greater than or equal to 4 at an IgG titer of less than 100,000.
Figure 5.
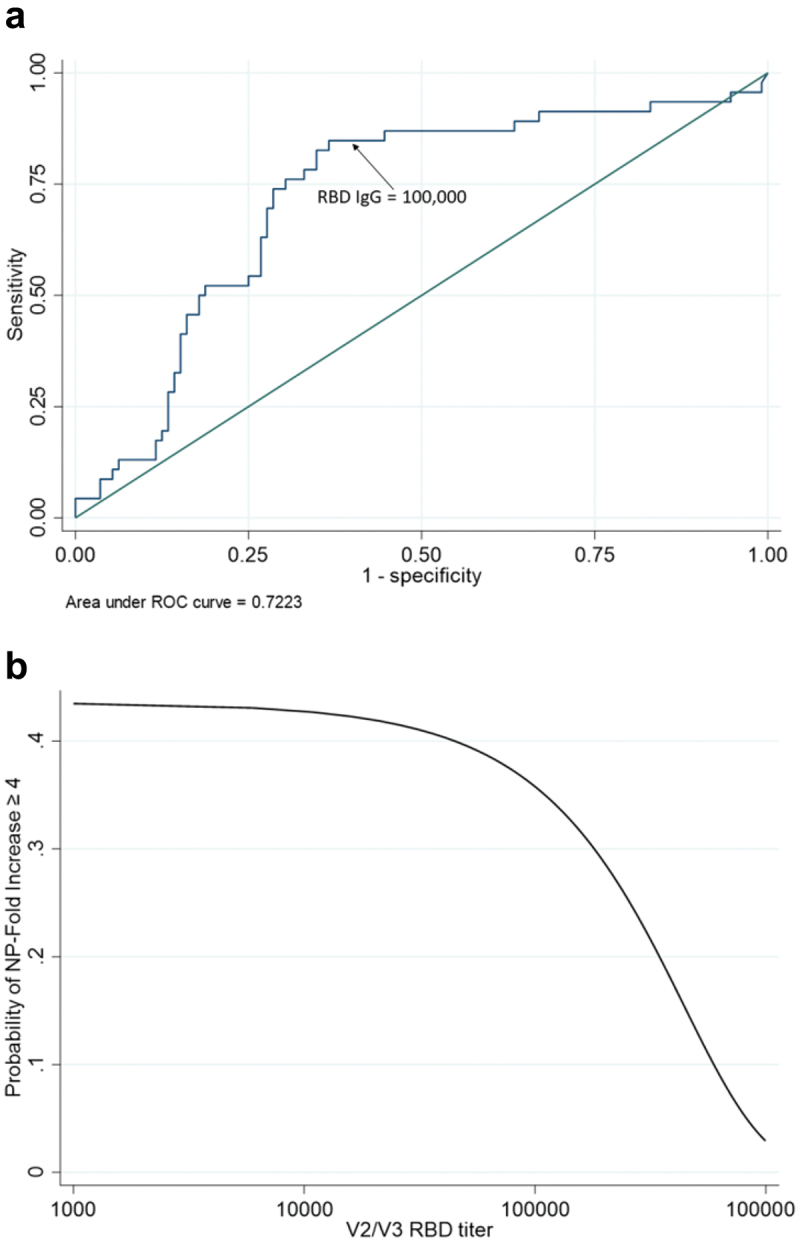
(a) receiver operator characteristics curve and (b) probability of seroconversion for NP-specific IgG as a function of continuous RBD-specific IgG titer. IgG titer shows good discriminant ability as a continuous predictor of seroconversion (a). The probability of seroconversion stays relatively unchanged until IgG titer approaches 100,000. At that point, the probability of seroconversion starts to decrease dramatically (b).
Calculated duration of vaccine-mediated protection
We used the data shown in Figure 1 to calculate the average rate of antibody decay in the vaccinated, uninfected population between day ~ 50 and ~ 200. The antigen-specific IgG decay rate calculation was based on the drop in endpoint titer dilution over time. We found that the average half life of vaccine-specific IgG was 54 days. This value agrees closely with a previously published estimate.9 On average, the RBD-specific endpoint dilution titer decayed to 100,000 at 169 days following immunization.
NP-converts more likely to report positive test
NP-specific IgG titers were measured, and those who showed a 4-fold increase in titers were considered to have serological evidence of infection. At the time of serum collection on day ~ 440, participants responded to a questionnaire asking if they had a positive test since the previous study visit (~day 200). Of the 43 seroconverts, 16 reported having tested positive for SARS-CoV-2, while only 3 out of the 36 of those who had no serological evidence of infection reported having a positive test (Fisher’s exact p = 0.003) (Table 1). NP-converts were 4.6× more likely to report having a positive test than NP-nonconverts. Rates of seroconversion were similar among different age groups (Table 2).
Table 1.
History of positive SARS-CoV-2 diagnosis in NP-Converts vs NP-Nonconverts.
| Reported Positive Test | Reported No Positive Test | |
|---|---|---|
| NP-Converts | 16 | 27 |
| NP-Nonconverts | 3 | 33 |
Table 2.
Cohort demographics. Total is shown for each group with the number of participants who seroconverted shown in parentheses.
| Sex |
Race |
|||||
|---|---|---|---|---|---|---|
| Age | F | M | Asian | Black | Hispanic | White |
| 23 to <40 | 32 (19) | 2 (2) | 1 (0) | 2 (1) | 1 (0) | 30 (21) |
| 40 to <60 | 34 (18) | 1 (0) | 1 (0) | 3 (2) | – | 31 (16) |
| 60 to <74 | 9 (5) | 1 (1) | – | 1 (0) | – | 9 (6) |
When compared to serological assessment of infection, participant-reported positive tests appear to significantly underestimate the frequency of infection, though this observation is not based on an organized testing program of study participants. Whether or not participants received a negative test result was not captured by the questionnaire. We hypothesized that if NP-seroconversion is a true marker of infection then individuals who seroconverted for NP-specific IgG would also have significantly higher levels for RBD-specific IgG. Increases in spike-specific responses have been previously reported following breakthrough infections.10 We measured RBD-specific serum IgG against the vaccine encoded RBD (WT), the Delta variant RBD, and the Omicron variant RBD. Responses to all three variants were analyzed because the Delta variant wave and the Omicron variant waves both peaked in Mississippi between visits three and four. We found that as a group, participants who seroconverted for NP-specific IgG at visit four did not have significantly higher WT and Omicron RBD-specific IgG at visit three prior to seroconversion (Figure 6). Curiously, Delta RBD-specific titers were higher prior to seroconversion for NP. At visit four, following seroconversion, all RBD-specific IgG titers were significantly higher among NP-seroconverters compared to participants who did not seroconvert. These increases in RBD-specific IgG titers are consistent with a boosted RBD-specific response following breakthrough infection.
Figure 6.
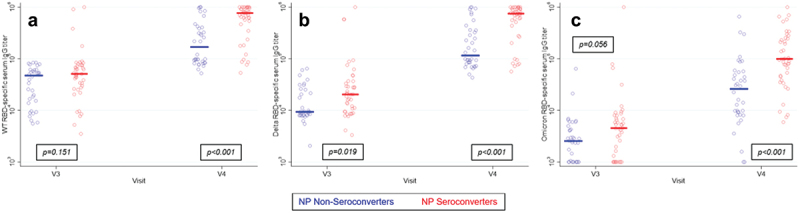
RBD-specific IgG titers for wild type (a), Delta (b), and Omicron (c) were compared at visit three, prior to seroconversion, and at visit four, following seroconversion, among participants who seroconverted for NP-specific IgG by visit 4 and participants who never seroconverted. RBD-specific IgG titers did not significantly differ between the two groups at visit 3, prior to seroconversion. By visit four, NP-specific IgG seroconverters had significantly higher RBD-specific IgG, which is consistent with an immune response to natural infection.
Discussion
Serological analysis of individuals without regard for a documented history of SARS-CoV-2 infection eliminates bias inherent in studies including only participants with a known history of infection. By analyzing the immune responses to vaccination (RBD-specific IgG) and to an antigen encountered only during natural infection (NP-specific IgG) in vaccinated individuals with and without documented histories of infection, we have been able to detect evidence of minor and subclinical infections in a defined study population. Other studies have also monitored vaccinated cohorts for seroconversion to NP,11,12 but to our knowledge, no one has analyzed NP-seroconversion in conjunction with RBD-specific antibody titers to estimate a serum IgG-based correlate of protective immunity.
Pathogen-neutralizing antibodies are generally accepted as a correlate of protection for most vaccine-preventable infections,13,14 and virus-neutralizing IgG has been shown to be protective against SARS-CoV-2 infection.15–17 Elicitation of protective levels of mucosal IgA by mRNA-based vaccines administered via injection has not been reported. Consequently, the protective mechanism underlying the mRNA-based SARS-CoV-2 vaccines may rely primarily on IgG-mediated protection of the respiratory mucosal surface. Though underappreciated, serum IgG is actively transported across the nasal epithelium to the surface of the mucosa,18 and IgG is also present on the luminal surfaces of the lower airways.19 A decrease in the level of virus-neutralizing antibodies present in circulation likely results in a decrease in the level of virus-neutralizing antibody on the luminal surface of the respiratory mucosa and a corresponding decline in immune protection against infection. Notably, the decay of virus-neutralizing titers following infection is reportedly slower than decay following vaccination,5 so individuals who experience breakthrough infections would likely have longer-lived immunity than what is conferred by vaccination alone.
Our data reveal important information regarding the ability of the SARS-CoV-2 vaccine to protect against infection. The participant-to-participant variation in antibody titers following vaccination is substantial. The difference between the best responder and worst responder varies by greater than an order of magnitude (Figure 2a). A small subset of healthy vaccinees did not generate a strong enough antibody response to exceed the protective threshold we observed for IgG. However, none of the individuals in this study suffered illness to the extent of requiring hospitalization. Many study participants with waning RBD-specific antibodies below a titer of 100,000 did not seroconvert for NP IgG. This outcome can likely be attributed to exposure and/or behavioral differences, e.g. social distancing, hand washing, etc., among participants.
Notably, many participants showed serological evidence of infection despite having not received a positive diagnostic test result or experiencing significant SARS-CoV-2 related illness. This observation has potentially special importance for two reasons. The first is that even though high antibody titers may be a correlate of protection against infection, infection without significant clinical symptoms appears to be widespread as titers wane. Early studies of infection of humans with 229-E, an alphacoronavirus and common cause of colds, showed that antibody titers were inversely related to symptomatic illness but that some individuals with very low antibody titers still did not present with symptomatic illness.20 SARS-CoV-2 hamster challenge studies have revealed that T-cell mediated responses can protect against severe illness even in the absence of spike-specific antibody responses,21 and our findings suggest a likely role for T cells in preventing illness in humans, even under circumstances of considerable viral antigenic variation. This possibility is currently under investigation by our group.
Secondly, direct measures of infection, as determined by RT-PCR or lateral flow tests, are possibly significant underestimates of the true infection rate. The majority of participants in our study who exhibited serological evidence of infection did not report a positive test result for SARS-CoV-2 infection. This observation could result from the limited sensitivity of at-home lateral flow test or from reluctance to seek testing even when symptomatic, either of which results in an underestimation of the community infection rate. Consequently, estimates of herd immunity may also be significantly underestimated.
As we transition from pandemic to endemic circulation of SARS-CoV-2, a better understanding of the immunological factors that affect breakthrough and repeat infections will be important for making informed policy decisions and counseling patients. For example, establishing an autumnal SARS-CoV-2 vaccination program in parallel with the annual influenza vaccination campaign would likely do little to prevent summer SARS-CoV-2 transmission peaks if, as we have observed in our cohort, vaccine-mediated immunity in the general population lasts only about six months. While the endemic seasonal peak of SARS-CoV-2 transmission will probably not be set for several years, analysis of other host immune factors such as the durability of T-cell memory and decay rates of antibody levels in the respiratory mucosa will ultimately provide us with knowledge to more effectively mitigate those peaks.
While the relatively small cohort size, limited demographic diversity in the cohort, and observational design are limitations to our study, these data provide useful information for interpreting the relationship between antibody titer and protection against infection. Additionally, our findings provide pilot data for design of future, prospective interventional trials that will allow actual prediction as well as evaluation of vaccine efficacy against current and future pandemic and endemic virus events.
Acknowledgments
We gratefully acknowledge Drs. Ritesh Tandon and Stephen Stray for helpful discussions and the UMMC Department of Pathology and the UMMC Biobank for assistance with sample collection.
Funding Statement
This work was supported by The Robert M. Hearin Foundation, The Bower Foundation, and James L. Barksdale. JTB received support through the UMMC Molecular Center for Health and Disease [P20GM144041].
Author contributions
JTB, STL, and GDM conceived and designed the study. APF, MAB, KBS, DRE, DDM, and GCB performed the experiments. JTB, STL, GDM, and ATP analyzed the data. JTB wrote the first draft of the manuscript. All authors co-edited the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Tandon R, Mitra D, Sharma P, McCandless MG, Stray SJ, Bates JT, Marshall GD.. Effective screening of SARS-CoV-2 neutralizing antibodies in patient serum using lentivirus particles pseudotyped with SARS-CoV-2 spike glycoprotein. Sci Rep. 2020;10(1):19076. doi: 10.1038/s41598-020-76135-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Congrave-Wilson Z, Cheng WA, Lee Y, Perez S, Turner L, Marentes Ruiz CJ, Mendieta S, Skura A, Jumarang J, Del Valle J. et al. Twelve-month longitudinal serology in SARS-CoV-2 Naïve and experienced vaccine recipients and unvaccinated COVID-19-Infected individuals. Vaccines (Basel). 2022;10(5):10. doi: 10.3390/vaccines10050813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dieckhaus KD, Kim MJ, Shen JB, Liang TS, Kleinberg MJ, Siedlarz KM, Banach DB, Metersky ML, Fuller RP, Mortensen EM. et al. SARS-CoV-2 antibody dynamics in healthcare workers after mRNA vaccination. Vaccines (Basel). 2023;11(2):11. doi: 10.3390/vaccines11020358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menon V, Shariff MA, Perez Gutierrez V, Carreno JM, Yu B, Jawed M, Gossai M, Valdez E, Pillai A, Venugopal U. et al. Longitudinal humoral antibody response to SARS-CoV-2 infection among healthcare workers in a New York City hospital. BMJ Open. 2021;11(10):e051045. doi: 10.1136/bmjopen-2021-051045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Planas D, Staropoli I, Porot F, Guivel-Benhassine F, Handala L, Prot M, Bolland WH, Puech J, Pere H, Veyer D. et al. Duration of BA.5 neutralization in sera and nasal swabs from SARS-CoV-2 vaccinated individuals, with or without omicron breakthrough infection. Med. 2022;3(12):838–47 e3. doi: 10.1016/j.medj.2022.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gromowski GD, Cincotta CM, Mayer S, King J, Swafford I, McCracken MK, Coleman D, Enoch J, Storme C, Darden J. et al. Humoral immune responses associated with control of SARS-CoV-2 breakthrough infections in a vaccinated US military population. EBioMedicine. 2023;94:104683. doi: 10.1016/j.ebiom.2023.104683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Boer J, Saade U, Granjon E, Trouillet-Assant S, Saade C, Pottel H, Zrein M, Ser Study g C, Nasrallah GK. A novel assessment method for COVID-19 humoral immunity duration using serial measurements in naturally infected and vaccinated subjects. PLoS One. 2022;17(9):e0274553. doi: 10.1371/journal.pone.0274553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobano C, Ramirez-Morros A, Alonso S, Ruiz-Olalla G, Rubio R, Vidal M, Prados de la Torre E, Jairoce C, Mitchell RA, Barrios D. et al. Eleven-month longitudinal study of antibodies in SARS-CoV-2 exposed and naïve primary health care workers upon COVID-19 vaccination. Immunology. 2022;167(4):528–7. doi: 10.1111/imm.13551. [DOI] [PubMed] [Google Scholar]
- 9.Arunachalam PS, Lai L, Samaha H, Feng Y, Hu M, Hui HS, Wali B, Ellis M, Davis-Gardner ME, Huerta C. et al. Durability of immune responses to mRNA booster vaccination against COVID-19. J Clin Invest. 2023;133(10):133. doi: 10.1172/JCI167955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kusunoki H, Ohkusa M, Iida R, Saito A, Kawahara M, Ekawa K, Kato N, Yamasaki K, Motone M, Shimizu H. Longitudinal changes in IgG-type SARS-CoV-2 antibody titers after COVID-19 vaccination and a prominent increase in antibody titers when infected after vaccination. Vaccines (Basel). 2023;11(4):11. doi: 10.3390/vaccines11040860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Follmann D, Janes HE, Buhule OD, Zhou H, Girard B, Marks K, Kotloff K, Desjardins M, Corey L, Neuzil KM. et al. Antinucleocapsid antibodies after SARS-CoV-2 infection in the blinded phase of the randomized, placebo-controlled mRNA-1273 COVID-19 vaccine efficacy clinical trial. Ann Intern Med. 2022;175(9):1258–65. doi: 10.7326/M22-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai YC, Lin YC, Ching LL, Tseng AC, Qin Y, Nerurkar VR, Wang WK. Identification of severe acute respiratory syndrome coronavirus 2 breakthrough infections by anti-nucleocapsid antibody among fully vaccinated non-healthcare workers during the transition from the delta to omicron wave. Front Med. 2022;9:1019490. doi: 10.3389/fmed.2022.1019490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17(7):1055–65. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plotkin SA. Recent updates on correlates of vaccine-induced protection. Front Immunol. 2022;13:1081107. doi: 10.3389/fimmu.2022.1081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, Subbarao K, Kent SJ, Triccas JA, Davenport MP. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–11. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 16.Fong Y, McDermott AB, Benkeser D, Roels S, Stieh DJ, Vandebosch A, Le Gars M, Van Roey GA, Houchens CR, Martins K. et al. Immune correlates analysis of the ENSEMBLE single Ad26.COV2.S dose vaccine efficacy clinical trial. Nat Microbiol. 2022;7:1996–2010. doi: 10.1038/s41564-022-01262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilbert PB, Montefiori DC, McDermott AB, Fong Y, Benkeser D, Deng W, Zhou H, Houchens CR, Martins K, Jayashankar L. et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375:43–50. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida M, Masuda A, Kuo TT, Kobayashi K, Claypool SM, Takagawa T, Kutsumi H, Azuma T, Lencer WI, Blumberg RS. IgG transport across mucosal barriers by neonatal Fc receptor for IgG and mucosal immunity. Springer Semin Immunopathol. 2006;28(4):397–403. doi: 10.1007/s00281-006-0054-z. [DOI] [PubMed] [Google Scholar]
- 19.Spiekermann GM, Finn PW, Ward ES, Dumont J, Dickinson BL, Blumberg RS, Lencer WI. Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: functional expression of FcRn in the mammalian lung. J Exp Med. 2002;196(3):303–10. doi: 10.1084/jem.20020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradburne AF, Bynoe ML, Tyrrell DA. Effects of a “new” human respiratory virus in volunteers. British Med J. 1967;3(5568):767–9. doi: 10.1136/bmj.3.5568.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arieta CM, Xie YJ, Rothenberg DA, Diao H, Harjanto D, Meda S, Marquart K, Koenitzer B, Sciuto TE, Lobo A. et al. The T-cell-directed vaccine BNT162b4 encoding conserved non-spike antigens protects animals from severe SARS-CoV-2 infection. Cell. 2023;186(11):2392–409 e21. doi: 10.1016/j.cell.2023.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


