Our classical perception of microorganisms as unicellular life forms is almost entirely based on the pure-culture mode of growth; since microbial suspensions can be diluted to a single cell and studied in liquid culture, this mode of growth has traditionally predominated in the study of microbial physiology and pathogenesis in the research laboratory. However, many microbes in their natural habitats are found in biofilm ecosystems attached to surfaces and not as free-floating (planktonic) organisms (20, 21, 27). Thus, biofilms are defined as structured microbial communities that are attached to a surface and encased in a matrix of exopolymeric material. This is of particular significance since it is now estimated that a significant proportion of all human microbial infections involve biofilm formation (20, 22, 25, 26, 29, 48).
Candida species are frequently found in the normal microbiota of humans, which facilitates their encounter with most implanted biomaterials and host surfaces. Devices such as stents, shunts, prostheses, implants, endotracheal tubes, pacemakers, and various types of catheters, to name a few, have all been shown to support colonization and biofilm formation by Candida (50). Candida albicans remains the fungal species most commonly associated with biofilm formation (28, 29, 56), and the increase in Candida infections in the last decades has almost paralleled the increase and widespread use of a broad range of medical implant devices, mainly in populations with impaired host defenses. Strikingly, fungi (mainly C. albicans) are the third leading cause of catheter-related infections, representing the second highest colonization-to-infection rate and the overall highest crude mortality (23). The formation of Candida biofilms carries important clinical repercussions because of their increased resistance to antifungal therapy and the ability of cells within biofilms to withstand host immune defenses. Also, biofilm formation on medical devices can negatively impact the host by causing the failure of the device and by serving as a reservoir or source for future continuing infections (29, 50). The net effect is that Candida biofilms adversely impact the health of these patients with increasing frequency and severity and with soaring economic sequelae (11, 86, 87, 89).
FORMATION AND CHARACTERISTICS OF C. ALBICANS BIOFILMS
Most information on the structural characteristics associated with C. albicans biofilms comes from in vitro experiments in which a variety of biofilm models were developed by different groups of investigators. These model systems comprise catheter disks, sheets and tubing made from different materials, glass slides, a perfused biofilm fermentor, cylindrical cellulose filters, acrylic strips and disks, germanium substratum, microtiter plates, and tissue culture flasks, among other systems, and include biofilms formed under both static and flowthrough conditions (6-10, 17, 18, 35, 37, 38, 57, 66, 71, 72, 76, 79, 84). Recently, two different animal models of catheter-associated Candida infections have been described, and visualizations of the resulting in vivo-formed biofilms indicate structural features similar to those formed in vitro (3, 83). Biofilms have also been recovered directly from clinical samples and visualized using different microscopy techniques (17, 74, 82). Again, these data suggest that in vitro model systems closely mimic in vivo events and therefore that the observations made may be clinically relevant.
To colonize any surface, fungal cells must first adhere to biomaterial surfaces. The initial attachment of Candida cells to biomaterials is mediated by both nonspecific factors (cell surface hydrophobicity and electrostatic forces) and by specific adhesins on the fungal surface recognizing ligands in the conditioning films, such as serum proteins (fibrinogen and fibronectin) and salivary factors (reviewed in reference 16). Importantly, biofilm formation correlates with cell surface hydrophobicity (63). Recent studies suggest that specific adherence events may also be mediated by cell surface proteins such as those encoded by members of the ALS family of adhesin-producing genes and EAP1 (33, 62). Additionally, Candida cells can also coaggregate and/or bind to bacteria (14, 43, 66, 67). The initial focal attachment of individual cells to a substratum is closely followed by cell division, proliferation, and biofilm development. Mature Candida biofilms exhibit a complex three-dimensional structure and display extensive spatial heterogeneity (6, 17, 18, 28, 37, 38, 53, 56, 75, 78, 79). This structural complexity is thought to represent the optimal spatial arrangement to facilitate the influx of nutrients, the disposal of waste products, and the establishment of microniches throughout the biofilm. The overall architecture of the biofilm may vary depending on the substrate on which it is formed and its growth conditions (17, 30, 84). Moreover, different strains of C. albicans and different Candida spp. differ in their capacities to form biofilms (38, 53, 63, 75).
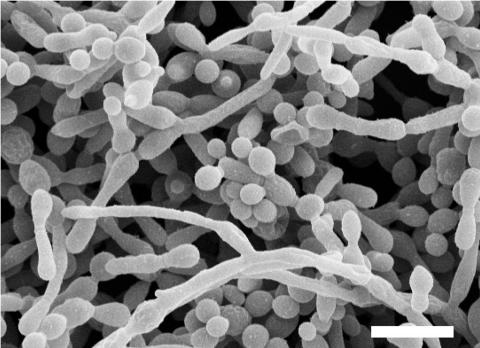
In seminal work by Hawser and Douglas (38), the structure of C. albicans biofilms formed on catheter disks was first examined by scanning electron microscopy (SEM). The initial attachment of yeast cells was followed by germ tube formation within 3 to 6 h. After 24 to 48 h of incubation, the fully mature C. albicans biofilms consisted of a dense network of yeasts, hyphae, and pseudohyphae, and extracellular polymeric material was visible on the surfaces of some of these morphological forms. Interestingly, the growth conditions used by these investigators do not normally lead to filamentation under planktonic conditions, suggesting that conditions or factors within the biofilm induce filament formation. Similar observations were made for C. albicans biofilms formed on acrylic material (17, 79). Figure 1 shows a SEM image of a mature C. albicans biofilm. In contrast to SEM techniques, the nondestructive nature of confocal scanning laser microscopy allows the visualization of fully hydrated living biofilms. The use of this technique has revealed the complex three-dimensional structure of mature C. albicans biofilms, in particular the spatial heterogeneity and architecture of microcolonies with ramifying water channels, and shown that biofilms can range in thickness from 25 to more than 450 μm (17, 79). A movie showing a rotating three-dimensional reconstruction using confocal microscopy of a mature C. albicans biofilm is available (see Video S1 in the supplemental material). In this case, a 24-h-old biofilm formed by C. albicans 3153A on a plastic coverslip was stained using the FUN 1 component of the LIVE/DEAD yeast viability kit (Molecular Probes, Eugene, OR).
FIG. 1.
SEM image of a mature (48 h) C. albicans biofilm. Note that biofilms are composed of yeast, hyphal, and pseudohyphal elements. Please note also that most of the exopolymeric material (matrix) is lost due to dehydration during SEM procedures. Bar is 10 μm.
ROLE OF MORPHOGENETIC CONVERSIONS IN C. ALBICANS BIOFILM FORMATION
Undoubtedly, C. albicans morphogenetic conversions (the ability to reversibly switch between yeast and filamentous forms) are important for multiple aspects of C. albicans biology and pathogenicity and have remained the focus of much investigation throughout the years (12, 13, 32). It is clear that morphogenesis plays a pivotal role in C. albicans biofilm development; Baillie and Douglas (10) demonstrated that hyphae are essential elements for providing the structural integrity and multilayered architecture characteristic of mature/fully developed biofilms. To begin to dissect the molecular pathways governing filamentation that are involved in biofilm formation, Ramage et al. (78) used a series of genetically defined C. albicans mutant strains characterized by their inability to filament under different environmental conditions and tested their capacity to form biofilms. Of the several mutant strains tested, the single Δefg1 and double Δcph1/Δefg1 deletion mutants were unable to filament and formed rather poor biofilms lacking in three-dimensional structure and composed mainly of sparse monolayers of elongated cells (78). The Δcph1/Δefg1 double mutant was also profoundly deficient in colonizing plasma-coated polyurethane catheters (61) but was still able to form a thin biofilm on glass, again composed principally of blastospores (30). Together, these results demonstrate that the Efg1 regulator protein is a key factor in the formation and subsequent development of mature C. albicans biofilms on most biological and artificial surfaces, although this role could be a consequence of its filamentation defect, since strains with mutations in several other genes involved in this process also display defects in their biofilm-forming abilities (30, 47, 51). It remains a possibility, however, that dimorphism per se may not be an absolute prerequisite for biofilm formation, since substantial yeast-only biofilms have been described (8, 10), but might be necessary for the development of the spatially organized structure seen in mature, highly structured biofilms.
QUORUM SENSING IN C. ALBICANS BIOFILMS
Cell-cell signaling, in particular quorum sensing, is fundamental to microbial biofilm formation (27, 49, 65, 70). This strategy of cell-cell communication benefits the biofilms' well-being by preventing unnecessary overpopulation and controlling competition for nutrients and has important implications in the infectious process, particularly for dissemination and for the establishment of distal sites of infection. It has been shown that farnesol acts as a quorum-sensing molecule that inhibits filamentation in C. albicans (44). Preincubation of C. albicans cells with high concentrations of farnesol almost completely inhibited biofilm formation. Furthermore, supernatants recovered from mature biofilms inhibited the filamentation of planktonic C. albicans, indicating that a morphogenetic autoregulatory compound, most likely farnesol, is produced in situ in biofilms (73). In a recent study, Cao et al. used a partial C. albicans cDNA microarray to analyze changes in the gene expression in C. albicans biofilms grown in the presence of farnesol (15). As expected, some hyphal-formation-associated genes were differentially expressed in farnesol-treated biofilms, including TUP1 (up-regulated). Other differentially expressed genes included some involved in drug resistance as well as genes encoding proteins with roles in cell wall maintenance (namely chitinases), iron transport, and heat shock/stress response. Also, CSH1, which codes for a protein that has been associated with cell surface hydrophobicity, was down-regulated in farnesol-treated biofilms. Another quorum-sensing/autoregulatory molecule with a role in the growth and morphogenesis of C. albicans is tyrosol, which is found in conditioned medium from high-density cultures (19); tyrosol abolishes the delay of growth after dilution and stimulates filamentation under conditions permissive for germ tube formation, but its role in biofilms has not been investigated. A recent study has shown that both quorum sensing and biofilm formation in C. albicans are regulated by the two-component signal transduction protein Chk1p (52). Another interesting set of experiments comes from studies on the interactions between C. albicans and the bacterium Pseudomonas aeruginosa. P. aeruginosa forms a dense biofilm on C. albicans filaments and kills the fungus, but the bacteria neither bind to nor kill yeast forms of C. albicans (41). Furthermore, C. albicans cells were shown to be capable of suppressing filamentation in response/upon exposure to a P. aeruginosa quorum-sensing molecule (3-oxo-C12 homoserine lactone) and structurally related compounds (42). Together, these results indicate that morphogenesis in C. albicans is under complex control by environmental conditions.
MOLECULAR BASIS OF RESISTANCE IN CANDIDA BIOFILMS
Several groups have demonstrated that the Candida biofilm lifestyle leads to dramatically increased levels of resistance to the most commonly used antifungal agents (6, 18, 36, 39, 54, 58, 60, 75-77, 79, 80). However, newer antifungal agents, such as the echinocandins and liposomal formulations of amphotericin B, show increased activity against Candida biofilms (4, 5, 54, 77). It is important to note that NCCLS broth-dilution techniques for antifungal susceptibility testing use planktonic populations and will not enable the prediction of antifungal efficacy against Candida biofilms (69). Thus, this may be one of the main reasons for the lack of correlation between results of antifungal susceptibility testing as determined by NCCLS guidelines and clinical outcomes in patients suffering from these types of infections (31, 81). However, the determination of the effectiveness of different antifungal agents against biofilms in this setting has important clinical implications in that it may guide therapeutic decisions that potentially affect the outcomes of patients suffering from these difficult-to-treat infections. A recently developed microtiter-plate-based biofilm model that is compatible with the 96-well platform technology has proven valuable for the determination and standardization of antifungal susceptibility testing in Candida biofilms (76). The rationale for this system was to provide a rapid and reproducible methodology to test an array of clinical and laboratory strains as well as a battery of antifungal compounds. In this system, biofilms are formed in 96-well microtiter plates and then challenged with antifungal drugs over a selected time period. The assay relies on the measurement of the metabolic activities of the sessile cells growing within the biofilm and is based on the reduction of 2,3-bis(2-methoxy-4-nitro-5-sulfo-phenyl)-2H-tetrazolium-5-carboxanilide (XTT) to yield a water-soluble formazan-colored product that can then be measured in a microtiter plate reader (40, 85).
As yet, there appears to be no one specific resistance factor responsible for the increased recalcitrance to antimicrobial agents exhibited by biofilms. Instead, biofilm resistance is a complex multifactorial phenomenon which still remains to be fully elucidated and understood. Different mechanisms may be responsible for the intrinsic resistance of Candida biofilms. These include the following: (i) the high density of cells within the biofilm; (ii) the effects of the biofilm matrix; (iii) decreased growth rate and nutrient limitation; (iv) the expression of resistance genes, particularly those encoding efflux pumps; and (v) the presence of “persister” cells (7-9, 46, 55, 59, 64, 68, 72, 80). Some of these are discussed below in relation to Candida biofilm resistance.
The matrix or exopolymeric substance (EPS) is produced by and envelops sessile communities of cells, and this could act as a barrier to the diffusion of antibiotics and/or as an ion-exchange resin to bind charged antibiotic molecules (34, 45). In C. albicans biofilms, matrix production was seen to increase dramatically when developing biofilms were grown under liquid flow compared to static conditions; however, both types of biofilms were equally resistant to antifungal drugs (9, 37). Subsequent experiments with resuspended sessile cells recovered from mature biofilms indicated that when tested as free-floating cells, they were also resistant to fluconazole and amphotericin B challenge, but not to the same level as that found in the mature biofilms from which they were derived (7, 8, 72). In mixed biofilms, the EPS produced by bacterial cells was shown to retard the diffusion of the antifungal agents, but poor penetration did not account for the drug resistance of Candida biofilm cells (1, 2). Overall, these results seem to indicate that the EPS plays a partial role in sessile resistance, but other factors are also likely involved.
Several studies have examined the effects of growth rate and nutrient limitation in relation to drug resistance in C. albicans biofilms. Baillie and Douglas (7, 8) demonstrated that mature biofilms were resistant to amphotericin B at all growth rates tested and also at different levels of nutrient limitation. In addition, Chandra and colleagues reported that a progression of drug resistance was associated with an increase in the metabolic activity of the developing biofilm and not with a lower growth rate, which clearly indicates that drug resistance develops over time, coincident with biofilm maturation (17, 18).
Under planktonic conditions, one of the main mechanisms through which azole resistance develops in C. albicans is the active efflux of these drugs mediated by ABC transporter and major facilitator proteins (88). The expression of genes encoding both types of efflux pumps was found to be up-regulated during the different phases of biofilm development, both in vitro and in vivo (3, 64, 68, 72). Interestingly, however, biofilms formed by mutant strains deleted for genes encoding several of the efflux pumps retained their drug-resistant phenotype, although they were more susceptible during the early adherence phase of biofilm formation (64, 68, 72). Notwithstanding this observation of continued resistance in their absence, the overexpression of genes encoding efflux pumps by cells within a biofilm likely represents a normal physiological phenomenon, since they are likely employed primarily as a means of both nutrient uptake and cellular detoxification (24, 72). Finally, sterol analyses have revealed that ergosterol levels are significantly decreased in the intermediate and mature phases of biofilm growth compared to those in the early phases of development (68). Since sterol metabolism is the primary cellular process affected by the most widely employed antifungal drugs, the diminished levels of ergosterol present in sessile C. albicans may reflect a physiological state more conducive to resistance in these cells. Taken together, all of these observations reinforce the notion that biofilm resistance is a multifactorial phenomenon.
CONCLUSIONS AND FUTURE PERSPECTIVES
The ability to form biofilms is intimately associated with the ability to cause infection and as such should be considered an important virulence determinant during candidiasis. The biofilm lifestyle results in antifungal drug resistance and protection from host defenses, both of which carry important clinical repercussions. Molecular studies on biofilm formation have begun to shed light on the driving forces behind the transition to the biofilm mode of existence, including quorum sensing, which in the future may offer a potential therapeutic avenue. The application of powerful DNA microarray and proteomic technologies should facilitate a more detailed analysis of the biofilm lifestyle. Future studies should focus on in vivo-grown biofilms, mixed bacterial-fungal biofilms, and the determination of the biofilm-forming capacity of other Candida species and also investigate the use of new materials and other preventive strategies that could be employed to inhibit biofilm formation.
Supplementary Material
Acknowledgments
We gratefully acknowledge the support of the Texas Higher Education Coordinating Board and the National Institutes of Health. J.L.L.-R. is the recipient of a New Investigator Award in molecular pathogenic mycology from the Burroughs Wellcome Fund.
Footnotes
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Adam, B., G. S. Baillie, and L. J. Douglas. 2002. Mixed species biofilms of Candida albicans and Staphylococcus epidermidis. J. Med. Microbiol. 51:344-349. [DOI] [PubMed] [Google Scholar]
- 2.Al-Fattani, M. A., and L. J. Douglas. 2004. Penetration of Candida biofilms by antifungal agents. Antimicrob. Agents Chemother. 48:3291-3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andes, D., J. Nett, P. Oschel, R. Albrecht, K. Marchillo, and A. Pitula. 2004. Development and characterization of an in vivo central venous catheter Candida albicans biofilm model. Infect. Immun. 72:6023-6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachmann, S. P., G. Ramage, K. VandeWalle, T. F. Patterson, B. L. Wickes, and J. L. Lopez-Ribot. 2003. Antifungal combinations against Candida albicans biofilms in vitro. Antimicrob. Agents Chemother. 47:3657-3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachmann, S. P., K. VandeWalle, G. Ramage, T. F. Patterson, B. L. Wickes, J. R. Graybill, and J. L. Lopez-Ribot. 2002. In vitro activity of caspofungin against Candida albicans biofilms. Antimicrob. Agents Chemother. 46:3591-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baillie, G. S., and L. J. Douglas. 1999. Candida biofilms and their susceptibility to antifungal agents. Methods Enzymol. 310:644-656. [DOI] [PubMed] [Google Scholar]
- 7.Baillie, G. S., and L. J. Douglas. 1998. Effect of growth rate on resistance of Candida albicans biofilms to antifungal agents. Antimicrob. Agents Chemother. 42:1900-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baillie, G. S., and L. J. Douglas. 1998. Iron-limited biofilms of Candida albicans and their susceptibility to amphotericin B. Antimicrob. Agents Chemother. 42:2146-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baillie, G. S., and L. J. Douglas. 2000. Matrix polymers of Candida biofilms and their possible role in biofilm resistance to antifungal agents. J. Antimicrob. Chemother. 46:397-403. [DOI] [PubMed] [Google Scholar]
- 10.Baillie, G. S., and L. J. Douglas. 1999. Role of dimorphism in the development of Candida albicans biofilms. J. Med. Microbiol. 48:671-679. [DOI] [PubMed] [Google Scholar]
- 11.Beck-Sague, C., and W. R. Jarvis. 1993. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980-1990. National nosocomial infections surveillance system. J. Infect. Dis. 167:1247-1251. [DOI] [PubMed] [Google Scholar]
- 12.Brown, A. J., and N. A. Gow. 1999. Regulatory networks controlling Candida albicans morphogenesis. Trends Microbiol. 7:333-338. [DOI] [PubMed] [Google Scholar]
- 13.Brown, A. J. P. 2002. Morphogenetic signaling pathways in Candida albicans, p. 95-106. In R. A. Calderone (ed.), Candida and candidiasis. ASM Press, Washington, D.C.
- 14.Cannon, R. D., and W. L. Chaffin. 1999. Oral colonization by Candida albicans. Crit. Rev. Oral Biol. Med. 10:359-383. [DOI] [PubMed] [Google Scholar]
- 15.Cao, Y. Y., Y. B. Cao, Z. Xu, K. Ying, Y. Li, Y. Xie, Z. Y. Zhu, W. S. Chen, and Y. Y. Jiang. 2005. cDNA microarray analysis of differential gene expression in Candida albicans biofilm exposed to farnesol. Antimicrob. Agents Chemother. 49:584-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaffin, W. L., J. L. Lopez-Ribot, M. Casanova, D. Gozalbo, and J. P. Martinez. 1998. Cell wall and secreted proteins of Candida albicans: identification, function, and expression. Microbiol. Mol. Biol. Rev. 62:130-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandra, J., D. M. Kuhn, P. K. Mukherjee, L. L. Hoyer, T. McCormick, and M. A. Ghannoum. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 183:5385-5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandra, J., P. K. Mukherjee, S. D. Leidich, F. F. Faddoul, L. L. Hoyer, L. J. Douglas, and M. A. Ghannoum. 2001. Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J. Dent. Res. 80:903-908. [DOI] [PubMed] [Google Scholar]
- 19.Chen, H., M. Fujita, Q. Feng, J. Clardy, and G. R. Fink. 2004. Tyrosol is a quorum-sensing molecule in Candida albicans. Proc. Natl. Acad. Sci. USA 101:5048-5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costerton, J. W., K. J. Cheng, G. G. Geesey, T. I. Ladd, J. C. Nickel, M. Dasgupta, and T. J. Marrie. 1987. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 41:435-464. [DOI] [PubMed] [Google Scholar]
- 21.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711-745. [DOI] [PubMed] [Google Scholar]
- 22.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 23.Crump, J. A., and P. J. Collignon. 2000. Intravascular catheter-associated infections. Eur. J. Clin. Microbiol. Infect. Dis. 19:1-8. [DOI] [PubMed] [Google Scholar]
- 24.Del Sorbo, G., H. Schoonbeek, and M. A. De Waard. 2000. Fungal transporters involved in efflux of natural toxic compounds and fungicides. Fungal Genet. Biol. 30:1-15. [DOI] [PubMed] [Google Scholar]
- 25.Donlan, R. M. 2001. Biofilm formation: a clinically relevant microbiological process. Clin. Infect. Dis. 33:1387-1392. [DOI] [PubMed] [Google Scholar]
- 26.Donlan, R. M. 2001. Biofilms and device-associated infections. Emerg. Infect. Dis. 7:277-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donlan, R. M. 2002. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 8:881-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Douglas, L. J. 2003. Candida biofilms and their role in infection. Trends Microbiol. 11:30-36. [DOI] [PubMed] [Google Scholar]
- 29.Douglas, L. J. 2002. Medical importance of biofilms in Candida infections. Rev. Iberoam. Micol. 19:139-143. [PubMed] [Google Scholar]
- 30.Garcia-Sanchez, S., S. Aubert, I. Iraqui, G. Janbon, J. M. Ghigo, and C. d'Enfert. 2004. Candida albicans biofilms: a developmental state associated with specific and stable gene expression patterns. Eukaryot. Cell 3:536-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghannoum, M. A. 1997. Susceptibility testing of fungi and correlation with clinical outcome. J. Chemother. 9(Suppl. 1):19-24. [PubMed] [Google Scholar]
- 32.Gow, N. A., A. J. Brown, and F. C. Odds. 2002. Fungal morphogenesis and host invasion. Curr. Opin. Microbiol. 5:366-371. [DOI] [PubMed] [Google Scholar]
- 33.Green, C. B., G. Cheng, J. Chandra, P. Mukherjee, M. A. Ghannoum, and L. L. Hoyer. 2004. RT-PCR detection of Candida albicans ALS gene expression in the reconstituted human epithelium (RHE) model of oral candidiasis and in model biofilms. Microbiology 150:267-275. [DOI] [PubMed] [Google Scholar]
- 34.Gristina, A. G., Y. Shibata, G. Giridhar, A. Kreger, and Q. N. Myrvik. 1994. The glycocalyx, biofilm, microbes, and resistant infection. Semin. Arthroplasty 5:160-170. [PubMed] [Google Scholar]
- 35.Hawser, S. 1996. Adhesion of different Candida spp. to plastic: XTT formazan determinations. J. Med. Vet. Mycol. 34:407-410. [PubMed] [Google Scholar]
- 36.Hawser, S. 1996. Comparisons of the susceptibilities of planktonic and adherent Candida albicans to antifungal agents: a modified XTT tetrazolium assay using synchronised C. albicans cells. J. Med. Vet. Mycol. 34:149-152. [PubMed] [Google Scholar]
- 37.Hawser, S. P., G. S. Baillie, and L. J. Douglas. 1998. Production of extracellular matrix by Candida albicans biofilms. J. Med. Microbiol. 47:253-256. [DOI] [PubMed] [Google Scholar]
- 38.Hawser, S. P., and L. J. Douglas. 1994. Biofilm formation by Candida species on the surface of catheter materials in vitro. Infect. Immun. 62:915-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hawser, S. P., and L. J. Douglas. 1995. Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 39:2128-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hawser, S. P., H. Norris, C. J. Jessup, and M. A. Ghannoum. 1998. Comparison of a 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) colorimetric method with the standardized National Committee for Clinical Laboratory Standards method of testing clinical yeast isolates for susceptibility to antifungal agents. J. Clin. Microbiol. 36:1450-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hogan, D. A., and R. Kolter. 2002. Pseudomonas-Candida interactions: an ecological role for virulence factors. Science 296:2229-2232. [DOI] [PubMed] [Google Scholar]
- 42.Hogan, D. A., A. Vik, and R. Kolter. 2004. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol. Microbiol. 54:1212-1223. [DOI] [PubMed] [Google Scholar]
- 43.Holmes, A. R., R. D. Cannon, and H. F. Jenkinson. 1995. Interactions of Candida albicans with bacteria and salivary molecules in oral biofilms. J. Ind. Microbiol. 15:208-213. [DOI] [PubMed] [Google Scholar]
- 44.Hornby, J. M., E. C. Jensen, A. D. Lisec, J. J. Tasto, B. Jahnke, R. Shoemaker, P. Dussault, and K. W. Nickerson. 2001. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 67:2982-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoyle, B. D., J. Jass, and J. W. Costerton. 1990. The biofilm glycocalyx as a resistance factor. J. Antimicrob. Chemother. 26:1-5. [DOI] [PubMed] [Google Scholar]
- 46.Jabra-Rizk, M. A., W. A. Falkler, and T. F. Meiller. 2004. Fungal biofilms and drug resistance. Emerg. Infect. Dis. 10:14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelly, M. T., D. M. MacCallum, S. D. Clancy, F. C. Odds, A. J. Brown, and G. Butler. 2004. The Candida albicans CaACE2 gene affects morphogenesis, adherence and virulence. Mol. Microbiol. 53:969-983. [DOI] [PubMed] [Google Scholar]
- 48.Khardori, N., and M. Yassien. 1995. Biofilms in device-related infections. J. Ind. Microbiol. 15:141-147. [DOI] [PubMed] [Google Scholar]
- 49.Kjelleberg, S., S. Molin, M. B. Miller, and B. L. Bassler. 2002. Is there a role for quorum sensing signals in bacterial biofilms? Quorum sensing in bacteria. Curr. Opin. Microbiol. 5:254-258. [DOI] [PubMed] [Google Scholar]
- 50.Kojic, E. M., and R. O. Darouiche. 2004. Candida infections of medical devices. Clin. Microbiol. Rev. 17:255-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krueger, K. E., A. K. Ghosh, B. P. Krom, and R. L. Cihlar. 2004. Deletion of the NOT4 gene impairs hyphal development and pathogenicity in Candida albicans. Microbiology 150:229-240. [DOI] [PubMed] [Google Scholar]
- 52.Kruppa, M., B. P. Krom, N. Chauhan, A. V. Bambach, R. L. Cihlar, and R. A. Calderone. 2004. The two-component signal transduction protein Chk1p regulates quorum sensing in Candida albicans. Eukaryot. Cell 3:1062-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuhn, D. M., J. Chandra, P. K. Mukherjee, and M. A. Ghannoum. 2002. Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect. Immun. 70:878-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuhn, D. M., T. George, J. Chandra, P. K. Mukherjee, and M. A. Ghannoum. 2002. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob. Agents Chemother. 46:1773-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuhn, D. M., and M. A. Ghannoum. 2004. Candida biofilms: antifungal resistance and emerging therapeutic options. Curr. Opin. Investig. Drugs 5:186-197. [PubMed] [Google Scholar]
- 56.Kumamoto, C. A. 2002. Candida biofilms. Curr. Opin. Microbiol. 5:608-611. [DOI] [PubMed] [Google Scholar]
- 57.Lamfon, H., S. R. Porter, M. McCullough, and J. Pratten. 2003. Formation of Candida albicans biofilms on non-shedding oral surfaces. Eur. J. Oral Sci. 111:465-471. [DOI] [PubMed] [Google Scholar]
- 58.Lamfon, H., S. R. Porter, M. McCullough, and J. Pratten. 2004. Susceptibility of Candida albicans biofilms grown in a constant depth film fermentor to chlorhexidine, fluconazole and miconazole: a longitudinal study. J. Antimicrob Chemother. 53:383-385. [Online.] [DOI] [PubMed] [Google Scholar]
- 59.Lewis, K. 2001. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 45:999-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lewis, R. E., D. P. Kontoyiannis, R. O. Darouiche, I. I. Raad, and R. A. Prince. 2002. Antifungal activity of amphotericin B, fluconazole, and voriconazole in an in vitro model of Candida catheter-related bloodstream infection. Antimicrob. Agents Chemother. 46:3499-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lewis, R. E., H. J. Lo, I. I. Raad, and D. P. Kontoyiannis. 2002. Lack of catheter infection by the efg1/efg1 cph1/cph1 double-null mutant, a Candida albicans strain that is defective in filamentous growth. Antimicrob. Agents Chemother. 46:1153-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li, F., and S. P. Palecek. 2003. EAP1, a Candida albicans gene involved in binding human epithelial cells. Eukaryot. Cell 2:1266-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li, X., Z. Yan, and J. Xu. 2003. Quantitative variation of biofilms among strains in natural populations of Candida albicans. Microbiology 149:353-362. [DOI] [PubMed] [Google Scholar]
- 64.Mateus, C., S. A. Crow, Jr., and D. G. Ahearn. 2004. Adherence of Candida albicans to silicone induces immediate enhanced tolerance to fluconazole. Antimicrob. Agents Chemother. 48:3358-3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 66.Millsap, K. W., R. Bos, H. C. van der Mei, and H. J. Busscher. 1999. Adhesion and surface-aggregation of Candida albicans from saliva on acrylic surfaces with adhering bacteria as studied in a parallel plate flow chamber. Antonie Leeuwenhoek 75:351-359. [DOI] [PubMed] [Google Scholar]
- 67.Millsap, K. W., R. Bos, H. C. van der Mei, and H. J. Busscher. 2001. Adhesive interactions between voice prosthetic yeast and bacteria on silicone rubber in the absence and presence of saliva. Antonie Leeuwenhoek 79:337-343. [DOI] [PubMed] [Google Scholar]
- 68.Mukherjee, P. K., J. Chandra, D. M. Kuhn, and M. A. Ghannoum. 2003. Mechanism of fluconazole resistance in Candida albicans biofilms: phase-specific role of efflux pumps and membrane sterols. Infect. Immun. 71:4333-4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard. NCCLS document M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 70.O'Toole, G., H. B. Kaplan, and R. Kolter. 2000. Biofilm formation as microbial development. Annu. Rev. Microbiol. 54:49-79. [DOI] [PubMed] [Google Scholar]
- 71.Raad, I., I. Chatzinikolaou, G. Chaiban, H. Hanna, R. Hachem, T. Dvorak, G. Cook, and W. Costerton. 2003. In vitro and ex vivo activities of minocycline and EDTA against microorganisms embedded in biofilm on catheter surfaces. Antimicrob. Agents Chemother. 47:3580-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramage, G., S. Bachmann, T. F. Patterson, B. L. Wickes, and J. L. Lopez-Ribot. 2002. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J. Antimicrob. Chemother. 49:973-980. [DOI] [PubMed] [Google Scholar]
- 73.Ramage, G., S. P. Saville, B. L. Wickes, and J. L. Lopez-Ribot. 2002. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl. Environ. Microbiol. 68:5459-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramage, G., K. Tomsett, B. L. Wickes, J. L. Lopez-Ribot, and S. W. Redding. 2004. Denture stomatitis: a role for Candida biofilms. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 98:53-59. [DOI] [PubMed] [Google Scholar]
- 75.Ramage, G., K. Vande Walle, B. L. Wickes, and J. L. Lopez-Ribot. 2001. Biofilm formation by Candida dubliniensis. J. Clin. Microbiol. 39:3234-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ramage, G., K. Vande Walle, B. L. Wickes, and J. L. Lopez-Ribot. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 45:2475-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ramage, G., K. VandeWalle, S. P. Bachmann, B. L. Wickes, and J. L. Lopez-Ribot. 2002. In vitro pharmacodynamic properties of three antifungal agents against preformed Candida albicans biofilms determined by time-kill studies. Antimicrob. Agents Chemother. 46:3634-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ramage, G., K. VandeWalle, J. L. Lopez-Ribot, and B. L. Wickes. 2002. The filamentation pathway controlled by the Efg1 regulator protein is required for normal biofilm formation and development in Candida albicans. FEMS Microbiol. Lett. 214:95-100. [DOI] [PubMed] [Google Scholar]
- 79.Ramage, G., K. VandeWalle, B. L. Wickes, and J. L. Lopez-Ribot. 2001. Characteristics of biofilm formation by Candida albicans. Rev. Iberoam. Micol. 18:163-170. [PubMed] [Google Scholar]
- 80.Ramage, G., B. L. Wickes, and J. L. Lopez-Ribot. 2001. Biofilms of Candida albicans and their associated resistance to antifungal agents. Am. Clin. Lab. 20:42-44. [PubMed] [Google Scholar]
- 81.Rex, J. H., M. A. Pfaller, J. N. Galgiani, M. S. Bartlett, A. Espinel-Ingroff, M. A. Ghannoum, M. Lancaster, F. C. Odds, M. G. Rinaldi, T. J. Walsh, A. L. Barry, et al. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin. Infect. Dis. 24:235-247. [DOI] [PubMed] [Google Scholar]
- 82.Sbarbati, A., V. Fanos, P. Bernardi, and L. Tato. 2001. Rapid diagnosis of fungal infection of intravascular catheters in newborns by scanning electron microscopy. Scanning 23:376-378. [DOI] [PubMed] [Google Scholar]
- 83.Schinabeck, M. K., L. A. Long, M. A. Hossain, J. Chandra, P. K. Mukherjee, S. Mohamed, and M. A. Ghannoum. 2004. Rabbit model of Candida albicans biofilm infection: liposomal amphotericin B antifungal lock therapy. Antimicrob. Agents Chemother. 48:1727-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Suci, P. A., G. G. Geesey, and B. J. Tyler. 2001. Integration of Raman microscopy, differential interference contrast microscopy, and attenuated total reflection Fourier transform infrared spectroscopy to investigate chlorhexidine spatial and temporal distribution in Candida albicans biofilms. J. Microbiol. Methods 46:193-208. [DOI] [PubMed] [Google Scholar]
- 85.Tellier, R., M. Krajden, G. A. Grigoriew, and I. Campbell. 1992. Innovative endpoint determination system for antifungal susceptibility testing of yeasts. Antimicrob. Agents Chemother. 36:1619-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Viudes, A., J. Peman, E. Canton, P. Ubeda, J. L. Lopez-Ribot, and M. Gobernado. 2002. Candidemia at a tertiary-care hospital: epidemiology, treatment, clinical outcome and risk factors for death. Eur. J. Clin. Microbiol. Infect. Dis. 21:767-774. [DOI] [PubMed] [Google Scholar]
- 87.Wey, S. B., M. Mori, M. A. Pfaller, R. F. Woolson, and R. P. Wenzel. 1988. Hospital-acquired candidemia. The attributable mortality and excess length of stay. Arch. Intern. Med. 148:2642-2645. [DOI] [PubMed] [Google Scholar]
- 88.White, T. C., K. A. Marr, and R. A. Bowden. 1998. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 11:382-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilson, L. S., C. M. Reyes, M. Stolpman, J. Speckman, K. Allen, and J. Beney. 2002. The direct cost and incidence of systemic fungal infections. Value Health 5:26-34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.