Abstract
Background:
The medicinal herb Centella asiatica has been long been used for its neuroprotective and cognitive enhancing effects. We have previously shown that two weeks of treatment with a water extract of Centella asiatica (CAW) improves cognition and activates the endogenous antioxidant response pathway without altering amyloid-β (Aβ) plaque burden.
Objective:
Here, we assess the effect of long-term treatment of CAW in the 5xFAD mouse model of Aβ accumulation.
Methods:
Four-month-old 5xFAD mice were treated with CAW in their drinking water (2g/L) for three months at which point they underwent cognitive testing as well as analysis of Aβ plaque levels and antioxidant and synaptic gene expression. In order to confirm the involvement of the antioxidant regulatory transcription factor NRF2 on the effects of CAW on synaptic plasticity, neurons isolated from 5xFAD mice were also treated with CAW and the targeted inhibitor ML385.
Results:
Three months of treatment with CAW improved spatial and contextual memory as well as executive function in 5xFAD mice. This improvement was accompanied by increased antioxidant gene expression and a decrease in Aβ plaque burden relative to untreated 5xFAD animals. In isolated neurons, treatment with ML385 blocked the effects of CAW on dendritic arborization and synaptic gene expression.
Conclusion:
These results suggest that prolonged CAW exposure could be beneficial in Alzheimer’s disease and that these effects likely involve NRF2 activation. Moreover, these findings suggest that targeting NRF2 itself may be a relevant therapeutic strategy for improving synaptic plasticity and cognitive function in Alzheimer’s disease.
Keywords: Alzheimer’s disease, amyloid-β, centella asiatica, cognition, NRF2
INTRODUCTION
Alzheimer’s disease (AD) is an irreversible progressive form of dementia that affects 44 million people worldwide [1]. Currently there are no disease modifying therapies available owing in part to the incomplete understanding of the biology underlying AD. The pathological hallmarks of AD are the formation of amyloid-β (Aβ) plaques and neurofibrillary tangles which lead to synaptic degeneration, neuronal loss, and severe cognitive impairments, including profound deficits in memory and executive function.
Increased oxidative stress is also widespread in the AD brain [2] and considered to be an early event contributing to cognitive decline [3]. Oxidative stress occurs when there is an excess of free radicals which can cause oxidative damage to cellular macromolecules. Markers of increased oxidative stress are evident in AD patients [4–6] as well as in experimental models of AD where increased oxidative stress results in increased Aβ production, synaptic dysfunction, neural death, and cognitive impairment [7–11]. The endogenous antioxidant response pathway is regulated by the transcription factor NRF2 (nuclear factor erythroid 2-related factor 2, also called NFE2L2) and activation of NRF2 has been shown mitigate oxidative damage and cognitive deficits in models of AD and other neurological diseases [12–16].
Centella asiatica (L.) Urban (Apiaceae) is an herb used in Ayurvedic and Chinese traditional medicine to boost memory and enhance cognitive function [17]. This traditional usage has been supported by several human trials demonstrating cognitive enhancing effects in both healthy and impaired populations with no reported adverse events [18, 19]. This excellent safety profile supports the classification of Centella asiatica as a Class 1 herb (one that can be safely consumed when used appropriately) by the Botanical Safety Handbook [20] and has made it an attractive candidate for wider clinical use. Extracts of Centella asiatica have been shown to have neuroprotective effects in models of neurodegenerative conditions including AD [21, 22]. We and other have shown that the water extract of Centella asiatica (CAW) can protect against Aβ cytotoxity, activate NRF2 and reduce oxidative damage as well as improve mitochondrial function in both cell and animal models of AD [23–29]. CAW has been shown to possess cognitive enhancing effects. Our laboratory has previously shown that two weeks of treatment with CAW can activate NRF2, increase synaptic density, and improve memory and executive function in models of Aβ accumulation and also healthy aging [23, 24, 27, 30, 31]. In Aβ overexpressing mice, the cognitive enhancing effects of 3–5 weeks of CAW treatment were not consistently associated with decreases in plaque burden [23, 24, 31]. However, the effects of prolonged exposure to CAW on these endpoints remain unknown. This study aims to address this issue by evaluating the effects of prolonged CAW treatment in the 5xFAD mouse model of Aβ accumulation, including cognitive testing as well as evaluations of effects on the expression of NRF2 and its target genes. Since it is impractical to chronically inhibit NRF2 in vivo, these studies go on to evaluate the role of NRF2 in CAW’s neuroprotectant effects by using isolated primary neurons from these animals.
MATERIALSANDMETHODS
CAW extract
CAW was prepared as previously described [24, 26, 28]. Briefly, Centella asiatica was obtained from Oregon’s Wild Harvest (Redmond, OR) and the water extract was prepared by refluxing 1200 g of the raw plant material with 15 L of water for about 1.5 h, in several batches. This extract was filtered and lyophilized to a powder. A representative sample of both the raw Centella asiatica and the CAW is retained by our laboratory at −20°C. CAW prepared from this plant material contained 4.4% w/w of triterpenes and 1.7% w/w mono and dicaffeoyquinic acids [32] determined by targeted liquid chromatography-high resolution tandem mass spectrometry analysis [33]. A full description of the chemical composition can be found in our recent publication [32].
Animals
5xFAD animals were generated from a founder breeding pair from The Jackson Laboratory (cat# 006554). The background strain for these mice, and the strain of the non-transgenic WT littermates, is B6SJLF1/J. Litters were kept in a climate-controlled environment with a 12 h light/dark cycle and provided with water and diet ad libitum until aged to 4 months old. All procedures were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the Portland VA Healthcare System (IACUC #4255-18). At 4 months of age (Fig. 1), 5xFAD and WT littermates were given CAW (2 g/L) in their drinking water or untreated water calculated to deliver approximately 200 mg/kg body weight/day. Water was replaced multiple times a week and treatment given for a total of four months. The amount of water consumed for each cage was recorded and estimates of the CAW water consumed for each group can be found in Supplementary Table 1. The concentration of CAW used in this study was chosen to match the concentration used in previous studies [24, 27, 30, 31, 34] to facilitate a direct comparison of the results. Behavioral testing began at 7 months of age, following 3 months of CAW exposure and continued during testing. Animals were euthanized and tissue was collected after completion of behavioral testing at 8 months of age. The number of animals tested in each group was WT control female n = 6, WT CAW female n = 6, 5xFAD control female n = 4, 5xFAD CAW female n = 9, WT control male n = 5, WT CAW male n = 6, 5xFAD control male n = 5, 5xFAD CAW male n = 6.
Fig. 1.
Timeline of CAW treatment and behavioral assessment. Mice were treated with CAW (2 g/L) three months prior to the beginning of behavioral testing and treatment continued throughout the experiment. After testing, animals were sacrificed and tissue was harvested. CAW treatment lasted a total of 4 months.
Behavioral tests
Odor discrimination reversal learning (ODRL)
This test of learning and executive function occurs in three stages: shaping, acquisition, and shift. Animals were food restricted the night before testing and then given access to food in the afternoon once testing for the day was complete. All stages occurred in an opaque 8”×7”×12” chamber bisected by a removable partition.
In the shaping phase, animals were introduced to the testing chamber and trained to dig for food rewards in bedding material that smells of lavender. Individual mice were exposed to a bowl containing the food reward (Supreme Mini Treat pellet, BioServ, Flemington, NJ) that had been filled to progressively higher levels with the bedding: 0%, 25%, 50%, 75%, and 100%. Once the animal successfully retrieved the food reward 5 times in succession, it proceeded to the subsequent training step.
The acquisition phase followed immediately after the animals completed the shaping phase. In the acquisition phase, mice were presented with two cups, one that contained dried beans, and one that contained string. In each trial, one material had a mint odor and the other had a vanilla odor. Each material/scent pair was alternated randomly in every trial but balanced throughout the acquisition phase such that all mice were exposed to roughly equal combinations of each odor and digging material. In the acquisition phase, the cup with the mint scent was always baited with the reward, regardless of the digging material and whether the baited cup was presented on the right or the left side of the chamber was likewise balanced throughout testing. Each trial began when a partition separating the mouse from the bowls was raised. The number of trials required for each mouse to achieve 8 correct digs of any session of 10 was recorded. Once a mouse successfully reached this criterion, it immediately moved to the shift phase.
As in the acquisition phase, in the shift phase the mouse was again presented with two cups containing the same digging materials and odors with the Odor + digging material pairings and left/right location balanced throughout the trial. In the shift phase, however, the reward was always found in the cup with dried beans, regardless of odor. Again, the criteria was defined as 8 correct trials out of any session of 10 and the number of trials to reach criteria was recorded. Example pairings for each phase of the ODRL are given in Table 1.
Table 1.
Examples of test pairings for Odor Discrimination Reversal Learning (ODRL test). Representative combinations of odor and digging material pairings during each phase of the ODRL. D1, dried bean; D2, string; O1, vanilla; O2, mint. Bold indicates correct trial
| Right position | Left position | |
|---|---|---|
|
| ||
| Acquisition phase | D1 +O1 | D2 + O2 |
| D1 + O2 | D2 + O1 | |
| D2 + O1 | D1 + O2 | |
| D2 + O2 | D1 + O1 | |
| Shift phase | D1 + O1 | D2 + O2 |
| D1 + O2 | D2 + O1 | |
| D2 + O1 | D1 + O2 | |
| D2 + O2 | D1 + O1 | |
While acquisition phase of the ODRL reflects learning and is influenced by multiple brain regions, including both the hippocampus and cortex [35], the shift phase specifically probes the cognitive flexibility domain of executive function and is very tightly controlled by the prefrontal cortex [36].
Object Location Memory (OLM) test
The OLM test of spatial memory and is mediated by the hippocampus [37]. It has three phases: habituation, training, and testing. In the habituation phase, a mouse was exposed to an empty chamber (38 × 38 × 64 cm, made of a white acrylonitrile butadiene styrene) for two 10 min sessions on two consecutive days. Next animals were moved to the training phase, where they were exposed to the same chamber with two identical objects located in the front of the chamber for 10 min once an hour over three hours. Two hours after the training phase, animals were moved to the testing phase, where they were exposed for 5 min to the same objects as the training phase, but one of the objects had been moved to a new location in the chamber. The amount of time the mouse spent interacting the with the object in the novel location as well as the object in the familiar location is recorded via the ANYmaze video tracking system (Stoelting Co, Wood Dale, IL).
Conditioned Fear Response (CFR) test
The CFR test evaluates contextual memory and has been shown to be affected by inputs from the hippocampus, cortex, and amygdala [38]. It has three phases: habituation, conditioning, and testing. In the habituation phase, an animal was exposed to a 16 × 16 × 12 inch chamber with a wire floor for 5 min. The conditioning phase occurred immediately following habituation, where the animal was exposed to three 1 s shocks (0.7A) randomly distributed over a 3 min period with no more than one shock per minute. The test phase occurred 24 h after the conditioning phase, where the animal was reintroduced once more to the same chamber but this time not exposed to any shocks. The amount of time spent freezing over a 5 min period is recorded. Freezing time is represented as the change in freezing time from the habituation phase to the test phase in order to account for any baseline differences in overall activity.
Immunohistochemistry
After euthanasia, the brain of each animal was carefully dissected, and each hemisphere was separated. One hemisphere was fixed in 4% paraformaldehyde. This hemisphere was incubated sequentially in a sucrose gradient and frozen. Forty-micron coronal sections were taken on a freezing microtome and incubated in blocking buffer (100 mM TBS, pH 8.0, 2 mg/ml bovine serum albumin, 2% horse serum, 0.5% triton X-100) for 2 h. These sections were then incubated overnight in primary antibody directed against Aβ (44–136, Invitrogen, Carlsbad, CA), then exposed to biotinylated secondary antibody (1:200, Vector Labs, Burlingame, CA) for 2 h, then to an avidin-linked peroxidase complex (ABC, Vector Labs) for 2 h and finally developed with diaminobenzidine (DAB, Sigma) in PBS. Sections were then washed, mounted in Permount (Fisher Scientific, Pittsburg, PA) on slides, coverslipped and scanned using a PrimeHisto XE as per the manual with settings of 10,000 dpi, 8-bit black and white images with an exposure of 1 minute (Pacific Image Electronics, Torrance, CA). The protein expression was quantified in at least three coronal sections per mouse: anterior, middle, and posterior hippocampus and cortex. Hippocampal and cortical areas were traced using a computerized stage and stereo investigator software (Image J, Wayne Rasband, NIH, USA). Aβ levels were expressed as percentage of hippocampus or cortex occupied by these plaques.
Gene expression
The hemisphere that was not fixed and used for immunohistochemistry was sub-dissected and frozen at −20°C. Portions of the frontal cortex were homogenized, and RNA was extracted using Tri-Reagent (Molecular Research Center) using the protocol provided by the manufacturer. RNA was reverse transcribed with the Superscript III First Strand Synthesis kit (Invitrogen) to generate cDNA as per the manufacturer’s instructions. Relative gene expression was determined using TaqMan Gene Expression Master Mix (Invitrogen) and commercially available TaqMan primers (Invitrogen) for synaptophysin (Mm0043685_m1), post-synaptic density protein 95 (PSD95; Mm00492193_m1), NRF2 (Mm00477784_m1), NAD(P) H dehydrogenase-quinone oxidoreductase 1 (NQO1; Mm01253561_m1), glutamatecysteine ligase catalytic subunit (GCLC; Mm00802655_m1), heme oxygenase 1 (HMOX1; Mm00516005_m1), and glyceraldehyde-3 phosphate dehydrogenase (GAPDH; Hs02758991_g1). Quantitative PCR (qPCR) was performed on a StepOne Plus Machine (Applied Biosystems) and analyzed using the delta-delta Ct method.
Primary neurons
Embryos from a 5xFAD X WT cross were harvested to generate primary neurons. Hippocampal and cortical neurons were isolated as described in Kaech and Banker [39]. Briefly embryos were harvested at embryonic day 18 and genotyped via PCR from tail samples taken during dissection. Hippocampi were sub-dissected from embryos, gently minced and trypsinized to generate suspensions of dispersed neurons. These neurons were plated on poly-l-lysine coated plates.
Neuronal gene expression
Hippocampal neurons were plated on poly-l-lysine coated 12-well Tissue Culture plates at a density of 250,000 cells per well. Cells were cultured at 37°C and 5% CO2 for 7 days and then treated with DMSO, 50 μg/ml CAW, 10 μM ML385, or CAW + ML385 and allowed to continue to grow for 2 days, then media was removed and RNA was extracted, cDNA generated and relative gene expression determined as described above for the mouse tissue.
Reactive oxygen species
Hippocampal neurons were plated on poly-L-lysine coated 96-well tissue culture plates at a density of 20,000 cells per well. Cells were cultured at 37°C and 5% CO2 for 7 days and then treated with DMSO, 50 μg/ml CAW, 10 μM ML385, or CAW + ML385 and allowed to continue to grow for 2 days. Then media was removed, and reactive oxygen species (ROS) levels were determined by a DCFDA assay kit (enQuire Bioreagents). On day 7, in vitro cells were incubated with the fluorogenic probe for 45 min at 37°C prior to measurement. Values were normalized to protein content determined by a bicinchoninic acid (BCA) protein assay as per the manufacturer’s instructions (Pierce Biotechnology).
Dendritic arborization
Hippocampal neurons were plated on poly-l-lysine coated glass coverslips at a density of 130,000 cells per 60 mm dish containing 4 coverslips in MEM medium (Life Technologies), 5% FBS (Atlanta Biologicals), and 0.6% glucose (Sigma-Aldrich). After 4 h, coverslips were flipped cell side down into 60 mm dishes containing neural stem cell-derived glial cells (provided by Dr. Gary Banker, Jungers Center, OHSU) and maintained in Neurobasal Medium supplemented with 1x GlutaMAX (Life Technologies) and 1x B-27Plus (Life Technologies). Dishes were fed every week by removing 1 ml of the culture medium and adding 1 ml fresh Neurobasal media containing GlutaMAX and B-27 Plus, with the first feed (at 5 days in vitro) containing 6 μM cytosine β-D-arabinofuranoside hydrochloride (AraC; Sigma-Aldrich). At 12 days in vitro, the cell culture feeding contained either CAW (50 μg/ml final), ML385 (10 μM final), CAW + ML385, or DMSO. At 19 days in vitro, coverslips were fixed in 4% paraformaldehyde, rinsed in PBST and stained with Anti-MAP2B (Sigma-Aldrich #M4403; 3.3 μg/ml) and Goat anti-mouse IgG1-Cy3 (Jackson ImmunoResearch #115-165-205; 1.5 μg/ml). Stained neurons were imaged using a Zeiss ApoTome2 microscope, blinded and analyzed for morphology via the Sholl method by using Fiji software [40]. We measured 30 non-overlapping, easily isolatable neurons per coverslip.
Statistics
All bar graphs have error bars indicating standard error of the mean. Statistical significance was determined by the General Linear Model (GLM) for all in vivo experiments except OLM and Aβ plaque area quantification. GLM was utilized to account for unequal group sizes. Post-hoc pairwise comparisons were tested using Tukey and the lsmeans statement in SAS. In each of these figures the F values and model statistical significance is presented on the graph. For the OLM analysis, preference for the novel location was determined within each group by t-test. The quantification of Aβ plaque area was likewise determined by t-test for each sex. Statistical significance for the in vitro assays was determined by ANOVA followed by Bonferroni post-hoc pairwise comparisons. Significance was defined as p ≤ 0.05. Analyses were performed using Excel, GraphPad Prism 6, and SAS 9.4.
RESULTS
Long-term CAW treatment attenuates memory deficits in 5xFAD mice
To assess the effects of prolonged CAW treatment we evaluated memory in 5xFAD mice and their WT littermates following 3 months of CAW exposure in their drinking water using CFR and OLM tests. In the CFR test of contextual memory, the animal is allowed to freely explore a chamber after which it is exposed to a mild foot shock. The following day the animal is reintroduced to that same chamber and the amount of time that the animal spends frozen is recorded. If the mouse remembers the association between the painful stimuli and the chamber, the amount of time frozen will be higher. Performance in the CFR has been shown to be mediated by inputs from the cortex, hippocampus, and amygdala [38]. A deficit in freezing time was observed in male and female 5xFAD mice compared to CAW-treated WT mice (Fig. 2A, B). A similar trend was observed when compared to control-treated WT mice, although it did not reach statistical significance. Male and female 5xFAD mice exposed to prolonged CAW treatment exhibited freezing behavior that was indistinguishable from either control or CAW treated WT mice (Fig. 2A, B).
Fig. 2.

CAW improves memory in 5xFAD mice. Deficits in CFR performance, represented by the change in time freezing in the test phase relative to the habituation phase, were observed in control-treated female (A) and male (B) 5xFAD mice relative to CAW-treated WT mice (2g/L in the drinking water). A similar trend was seen as compared to WT controls, but it did not reach statistical significance in either sex. CAW attenuated these deficits in male and female mice such that the mice were not significantly different from either WT group. CAW treatment also restored the novel location preference in female 5xFAD mice (C). A similar but non-significant trend was also observed in male 5xFAD mice (D). n = 4–9, *p < 0.05, **p < 0.01, ***p < 0.001.
The OLM test was used to evaluate spatial memory and has been shown to be mediated by the hippocampus [37]. In this test, the mouse is exposed to two identical objects in fixed locations throughout training. Two hours after the final training session, one object is moved to a novel location and the amount of time the mouse spends exploring each object is measured. Because of the exploratory nature of rodents, if the animal remembers the location of the object during training it will spend more time exploring the new location. We found that unlike WT animals, 5xFAD mice did not display any preference for the novel location, but CAW treatment restored a significant preference in female 5xFAD mice (Fig. 2C) and a nearly significant (p = 0.06) preference in male 5xFAD male (Fig. 2D). CAW treatment did not impact WT performance in this test in either female (Fig. 2C) or male (Fig. 2D) mice.
CAW treatment improves learning and executive function in 5xFAD mice
The ODRL test was utilized to study learning and executive function. Learning is assessed in the acquisition phase of this test while the shift phase evaluates cognitive flexibility domain of executive function. While the learning phase involved inputs from both the hippocampus and cortex, the cognitive flexibility part of the ODRL has been shown to be mediated by the prefrontal cortex [35, 36]. We found that CAW-treatment significantly reduced the number of trials necessary to reach criteria in the acquisition phase for female 5xFAD mice (Fig. 3A). A similar, but non-significant, pattern of response to CAW in the shift phase (Fig. 3B). CAW treatment had no effect on the performance of WT female mice in either phase of this test (Fig. 3A, B). There was also a reduction the number of trials required to reach criteria in both the acquisition and shift phase of this test in CAW-treated male 5xFAD mice (Fig. 2C, D). Again, as with the females, CAW treatment did not affect the performance of WT male mice.
Fig. 3.
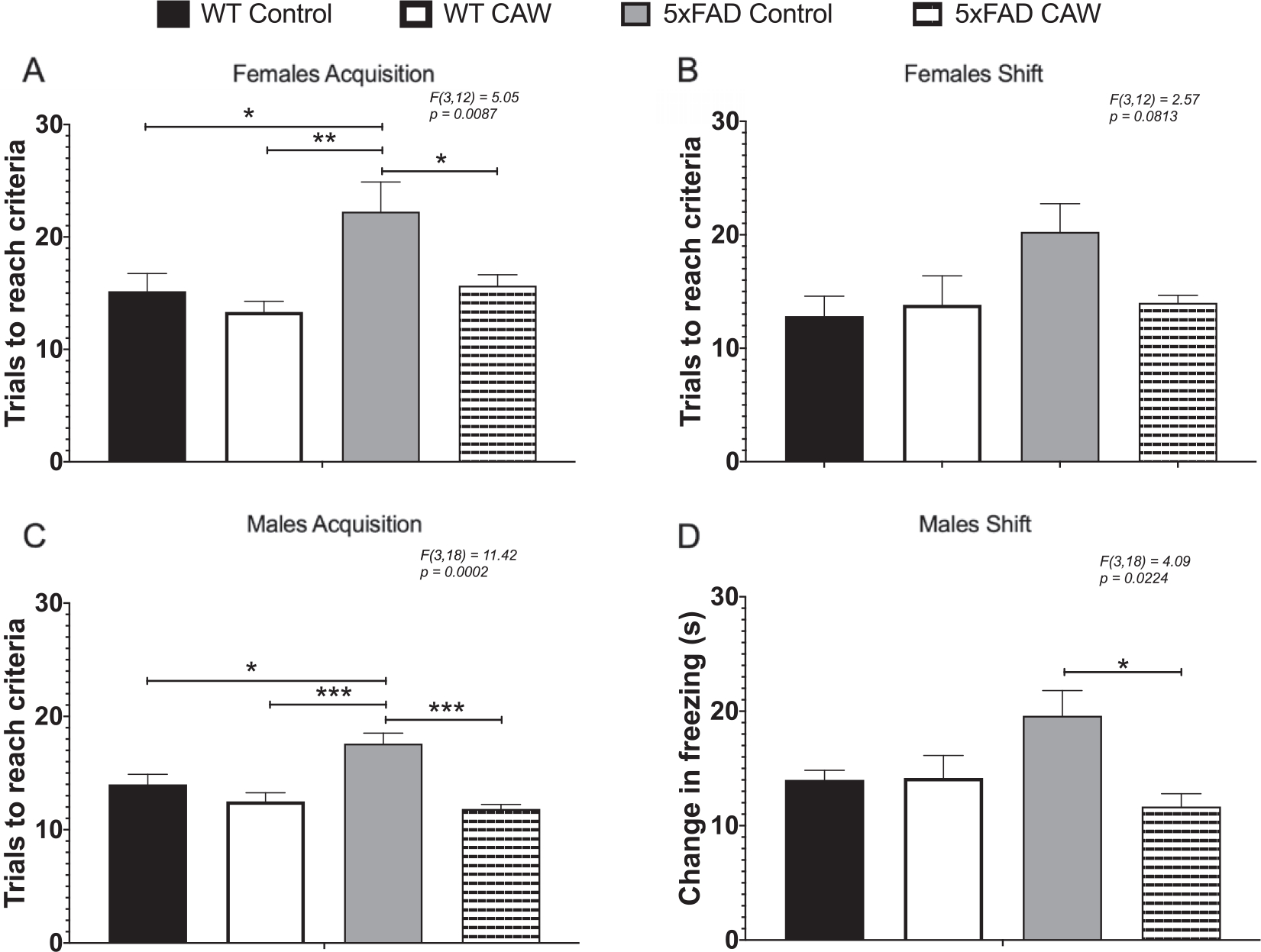
CAW improves learning and cognitive flexibility in 5xFAD but not WT mice. CAW treatment (2 g/L in the drinking water) improved impairments in ODRL performance in the acquisition phase of the ODRL in female (A) 5xFAD mice. A similar improvement was also seen in the shift phase for female CAW-treated 5xFAD mice (B) although this did not reach statistical significance. In male 5xFAD mice CAW also improved performance in both the acquisition (C) and shift (D) phases. n = 4–9, *p < 0.05, **p < 0.01, ***p < 0.001.
Long-term CAW treatment reduces Aβ plaque burden in 5xFAD mice
Aβ plaque area was significantly reduced in the cortex of both male and female 5xFAD animals treated with CAW (Fig. 4A). CAW treatment likewise reduced the hippocampal plaque burden in 5xFAD female mice but, notably, had no effect on hippocampal plaque area in male mice (Fig. 4B).
Fig. 4.
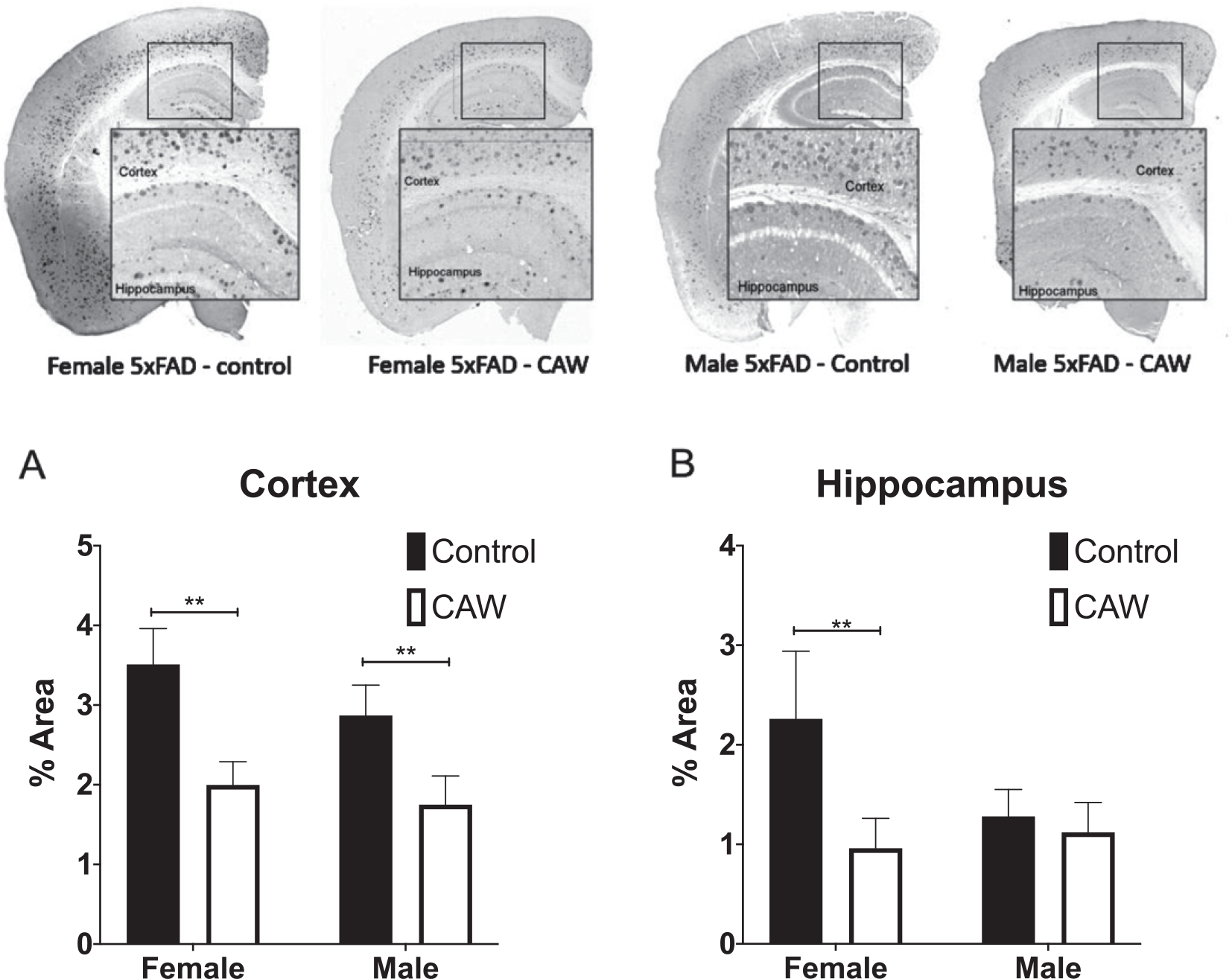
CAW reduced cortical Aβ plaque burden in female and male 5xFAD mice and hippocampal plaque burden in female 5xFAD. Representative images are shown. CAW treatment (2 g/L in the drinking water) significantly reduced A plaque area in both male and female mice (A) but only reduced hippocampal plaque area in female mice (B). n = 4–9, **p < 0.01.
CAW modulates cortical gene expression in 5XFAD mice
A trend toward decreased gene expression in synaptophysin and PSD95 was observed in the frontal cortex of female 5xFAD mice relative to WT animals (Fig. 5A, B) and a trend toward restored expression with CAW treatment; however, the only group difference to reach statistical significance was between the synaptophysin expression of the CAW-treated WT mice and the control treated 5xFAD mice (Fig. 5A). In male mice, the pattern of expression of these synaptic genes was similar although none of the group differences reached statistical significance (Fig. 5C, D).
Fig. 5.

CAW does not significantly increase synaptic gene expression in the cortex of 5xFAD mice. CAW treatment (2 g/L in the drinking water) did not robustly alter the expression of synaptophysin (A) or PS95 (B) in the cortex female mice. There was similarly no significant effect of CAW treatment in male mice for either synaptophysin (C) or PSD95 (D). n = 4–9, *p < 0.05, **p < 0.01, ***p < 0.001.
CAW treatment did robustly increase the expression of NRF2 and its antioxidant target genes HMOX1 and NQO1 in the cortex of female 5xFAD mice relative to WT control mice (Fig. 6A, C, D). A similar pattern was also observed with the antioxidant target gene GCLC, but it did not reach statistical significance (Fig. 6B). For male 5xFAD mice, long-term CAW treatment increased the expression of NRF2 and all three of the target genes relative to WT control mice (Fig. 6E–H).
Fig. 6.

CAW increases antioxidant gene expression in the cortex of 5xFAD mice. There was a trend towards increased expression of NRF2 regulated antioxidant genes following CAW treatment (2 g/L in the drinking water) in the cortex in female 5xFAD amice relative to WT. This increase reached statistical significance NRF2 (A), HMOX1 (B), and NQO1 (D). The same pattern was evident in male mice with significant induction in 5xFAD mice observable for all antioxidant genes (E, F, G, H). n = 4–9, *p < 0.05, **p < 0.01, ***p < 0.001.
Antioxidant effects of CAW are blocked by NRF2 inhibition in primary neurons
CAW treatment increased the expression of NRF2 and its antioxidant response element (ARE) containing target genes in cortical neurons isolated from both 5xFAD and WT mice (Fig. 7A–D). ARE-like elements are also present in the NRF2 promoter suggesting possible autoregulation [41], an idea supported by associations between changes in NRF2 transcript and both cytoplasmic and nuclear NRF2 protein levels [41, 42]. The induction observed by CAW treatment was blocked by co-treatment with ML385, a NRF2 inhibitor [43]. Similarly, the ability of CAW to attenuate increased intracellular ROS seen in 5xFAD neurons was likewise prevented by co-treatment with ML385 (Fig. 8). Treatment with ML385 alone exacerbated this increase in ROS in 5xFAD neurons.
Fig. 7.
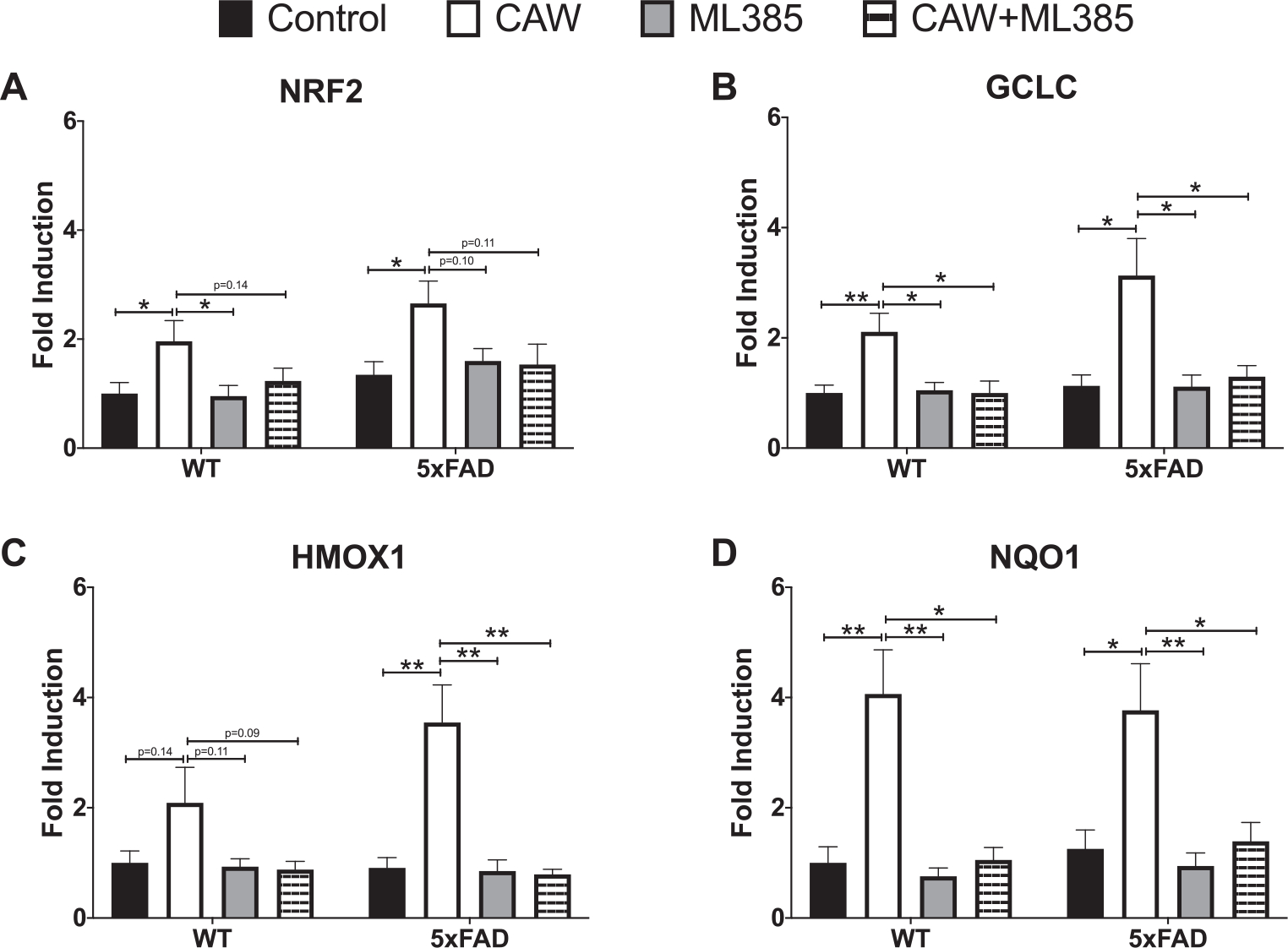
CAW (50 μg/mL) increases gene expression of NRF2 and its antioxidant targets in cortical neurons isolated from 5xFAD and WT animals. Co-treatment with ML385 (10 μM) blocks this effect. n = 8–12, *p < 0.05, **p < 0.01.
Fig. 8.
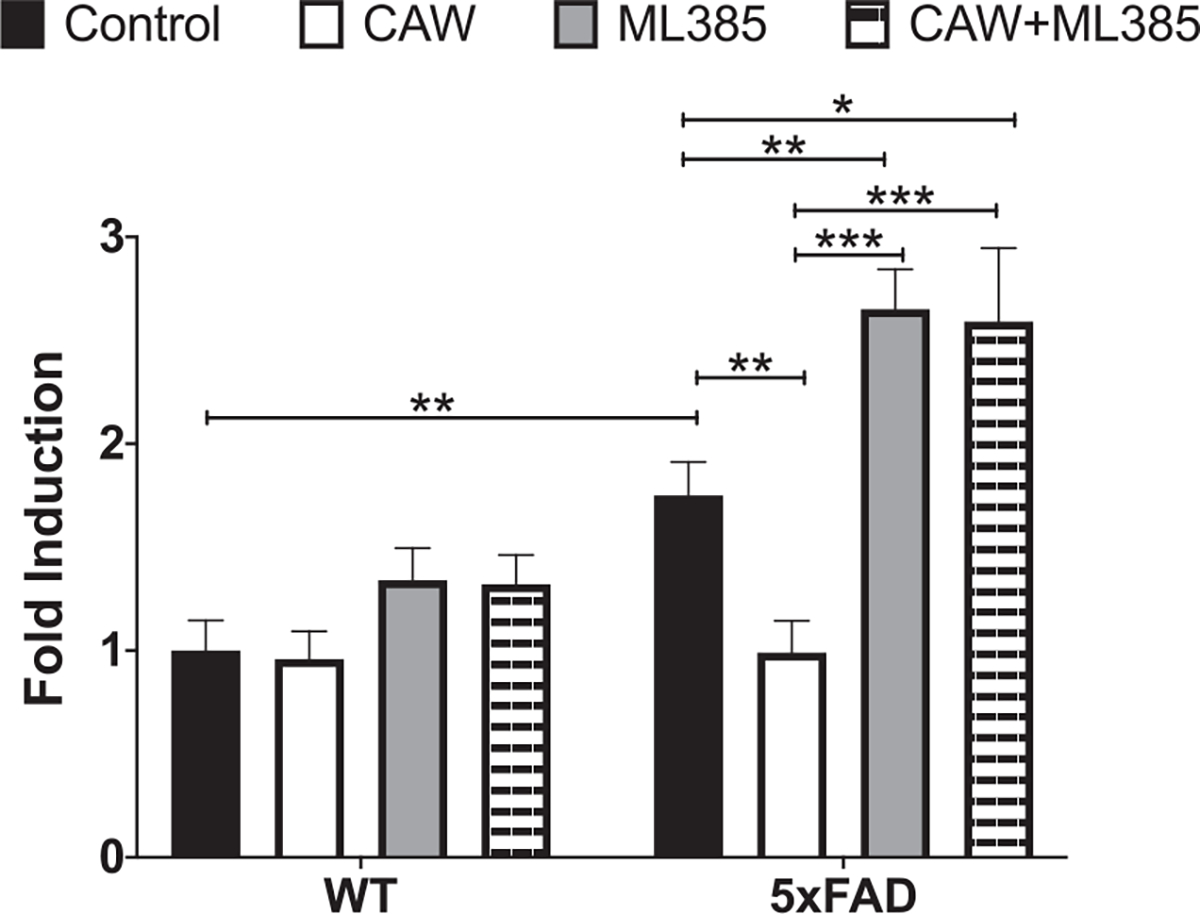
CAW attenuates increased ROS in 5xFAD neurons. Treatment with ML285 (10 μM) alone and co-treatment with CAW (50 μg/mL) results in further elevation of intracellular ROS. n = 8–12, *p < 0.05, **p < 0.01, ***p < 0.001.
NRF2 inhibition blocks effects of CAW on synaptic plasticity
We and others have previously reported that Aβ overexpressing neurons grown in culture for several weeks display a dystrophic phenotype characterized by reduced dendritic arborization [26, 44, 45]. Consistent with these previous findings we found that the degree of arborization in hippocampal 5xFAD neurons was markedly reduced relative to WT neurons after 19 days in vitro (Fig. 9A–C). This reduction was ameliorated by CAW treatment. In contrast, treatment with the NRF2 inhibitor ML385 alone exacerbated this deficit in arborization and co-treatment with CAW prevented the arborization-inducing effects of the extract.
Fig. 9.

NRF2 inhibition prevents effects of CAW on dendritic arborization and synaptic gene expression. Co-treatment with ML385 (10 μM) blocks CAW-induced (50 μg/mL) increases dendritic arborization in WT (A) and 5xFAD (B) hippocampal neurons (data is quantified by sholl analysis curves (A and B) as well as area under the curve (C), n = 200–300 cells per condition) as well as CAW-induced increases in the expression of synaptophysin (C) and PSD95 (D) in 5xFAD and WT cortical neurons. n = 8–12, *p < 0.05, **p < 0.01, ***p < 0.001.
Because we have previously observed a robust effect of CAW on the expression of the synaptic genes synaptophysin and PSD95 [26, 46], we therefore decided to investigate it as an endpoint in ML385 and CAW co-treated neurons despite not observing an effect with prolonged treatment in vivo. Expression of both synaptophysin (Fig. 9D) and PSD95 (Fig. 9E) was significantly reduced in 5xFAD neurons relative to WT neurons and CAW increased this expression in neurons of both genotypes. This effect was lost in neurons that were co-treated with both CAW and ML385. Interestingly ML385 treatment alone did not alter synaptic gene expression in either genotype.
DISCUSSION
The medicinal herb Centella asiatica has been shown to have a cognitive-enhancing effect in aging and neurodegenerative disease in both animal models as well as human populations [23, 24, 30, 31, 47–51]. Previously our laboratory has shown that two to three weeks of treatment with the water extract of the plant, CAW, significantly improved spatial memory, recognition memory, and executive function in the 5xFAD mouse model of Aβ accumulation, and these improvements were accompanied by increased NRF2-regulated antioxidant signaling [23, 24]. In this study, we investigate the cognitive and antioxidant effects of prolonged CAW treatment in 5xFAD mice and to begin to elucidate the role of NRF2 in mediating those effects using primary neurons from these animals.
We found that three months of CAW treatment had significant cognitive effects in 5xFAD mice. Improvements were observed in learning, memory, and executive function tasks. The magnitude of these changes was quite similar to what we have previously reported in 5xFAD mice following a two-week CAW treatment [24], suggesting that a tolerance to CAW does not develop over time and the cognitive effects are not diminished with prolonged exposure. Prolonged CAW treatment did not affect cognitive performance in WT animals. This again is similar to what we have reported in the past with shorter durations of this concentration of CAW [23, 24]. It is possible that prolonged treatment with higher concentrations of CAW would elicit a cognitive improvement in WT mice. This is in fact what we have seen with short term exposure to higher concentrations of CAW [23].
In addition, in contrast to our prior studies with short term CAW treatment, prolonged CAW treatment reduced the Aβ plaque burden in the cortex of 5xFAD mice. Although our previous studies have shown that two weeks of CAW treatment does not affect cortical Aβ accumulation [23, 24, 31], others have reported an Aβ-reducing effect of long-term Centella asiatica treatment in the PSAPP mouse model of Aβ accumulation [52]. In that study, Dhanasekaran et al. showed that 8 months of treatment with an ethanol extract of Centella asiatica administered in the chow selectively reduced Aβ accumulation in the hippocampus but not cortex of female animals. Interestingly in the present study, utilizing a water extract of the plant, administered for 4 months in a different mouse model of Aβ accumulation, we observed a decrease in Aβ accumulation in both the hippocampus and cortex of female mice but just the cortex of male mice. This sex difference could be in part because of the fact that control treated male 5xFAD mice had substantially lower hippocampal Aβ plaque area than their female counterparts which perhaps could affect the detection of a further reduction. We have observed this reduced plaque load in male 5xFAD mice in our previous studies as well [23]. Importantly, however, these effects on plaque burden do not appear to be necessary for cognitive enhancement as we saw similar improvements in both sexes with CAW treatment in both hippocampal and cortically mediated tasks.
In addition to these histological sex differences, variability in the magnitude of response to CAW was also evident in some of the behavioral and gene expression results. This is in line with our previous work with CAW in models of aging and AD which likewise showed differing magnitudes of effect in male and female animals [23, 25, 27]. It is unclear what precisely accounts for these differing responses. One possibility is that differential expression of NRF2 or the 5xFAD transgenes, both of which have been reported between male and female animals [53–55], affected the response to CAW. An alternative hypothesis is that although the male and female animals consumed roughly the same volume of CAW (Supplementary Table 1) due to the size differences between male and female mice that may actually be a different level of exposure per gram body weight. Studies are underway in our laboratory to quantify the amounts of active compounds in blood and tissue of treated animals to see if this could explain the sex differences.
The cognitive changes that we observed in 5xFAD mice treated with CAW were accompanied by an increase in NRF2-regulated antioxidant response genes in the frontal cortex. This is consistent with our previous reports following the shorter CAW treatment paradigm [23, 24]. There is significant evidence in the literature of a link between antioxidant response and cognitive function in aging and AD. In fact, increased oxidative stress is considered to be an early event in AD brains [2] and diminished antioxidant capacity along with increased markers of oxidative stress are evident in both the blood and brains of AD patients [4–6]. These same alterations in ROS levels, and cognitive function, are seen in mouse models of AD [56–60]. Rodent studies also have demonstrated a relationship between antioxidant capacity and memory. In mice, aging-related cognitive decline is associated with increased oxidative damage [61], and decreased brain and plasma antioxidants [62]. Moreover, overexpressing antioxidant enzymes has been shown to improve memory in rodents [63].
Our own work suggests that NRF2 specifically plays an important role in maintaining cognitive function. We have previously reported that aged NRF2 knockout (NRF2KO) mice show even greater impairments in synaptic gene expression and cognitive function than their aged WT counterparts [46]. Additionally, NRF2 activation by various compounds has been shown to improve cognitive function in rodent models of aging as well as in older adults [64–70]. To further explore the direct role NRF2 may be playing in mediating the effects of CAW in the context of Aβ accumulation, we co-treated primary neurons from 5XFAD mice with CAW and the NRF2 inhibitory compound ML385. We found that NRF2 inhibition blocked the antioxidant and synaptic effects of CAW. These results indicate that NRF2 activation is required for the beneficial neuronal effects of CAW in vitro and suggest a role for NRF2 in synaptic plasticity in primary neurons. This is consistent with our previous work with isolated NRF2KO neurons which also display impaired arborization and reduced synaptic gene expression [46] as well as studies from other groups that have shown a relationship between NRF2 activation and markers of synaptic plasticity, including dendritic spine density [71] and neurite outgrowth [72]. We have also recently reported that loss of NRF2 prevents the cognitive enhancing effects of CAW in a mouse model of aging which further supports a role for the transcription factor in the mechanism of action of CAW in healthy aging [34]. However, it remains to be seen whether NRF2 is similarly required for the in vivo effects of CAW in AD models. Our laboratory is currently generating 5xFAD mice in which NRF2 has been knocked out in order to better understand the specific role the transcription factor plays in maintaining synaptic density and cognitive health in the context of in vivo Aβ accumulation.
It remains unknown which of the compounds within the CAW extract is responsible for its neuroprotective and cognitive enhancing action. There is evidence that the triterpenes compounds within the plant (asiatic acid, asiaticoside, madecassic acid, and madecassoside) have beneficial effects on cognition [73–78]; however, our own group has shown that caffeoylquinic acids (CQAs) within CAW also participate in its neuroprotective and cognitive enhancing effects in models of Aβ accumulation [29, 32]. Triterpenes and CQAs have both been reported to activate NRF2 [79–84] and thus could be responsible for NRF2-mediated effects of CAW. However, our chemical analysis has shown that CAW is an extremely complex mixture containing hundreds of distinct chemical compounds [33] including many phenolic compounds, like hydroxycinnamic acids and flavonoids that have been reported to activate NRF2 in other biological systems [85–87]. Future studies that focus on individual compounds from the extract, alone or in combination, or that utilize bioassay guided fractionation to identify relevant compounds are necessary to determine which compounds are responsible for each specific effects of CAW. This will be a critical step in standardizing the extract in order to move to clinical testing in humans.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by NIH-NCCIH grant R00 AT008831(Gray), NIH-NCCIHgrantT32AT002688 (Wright), NIH-NCCIH grant R01AT008099 (Soumyanath), and a Department of Veterans Affairs Merit Review grant (Quinn).
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/21-0271r2).
Footnotes
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-210271.
REFERENCES
- [1].Mendiola-Precoma J, Padilla K, Rodriguez-Cruz A, Berumen LC, Miledi R, Garcia-Alcocer G (2017) Theobromine-induced changes in A1 purinergic receptor gene expression and distribution in a rat brain Alzheimer’s disease model. J Alzheimers Dis 55, 1273–1283. [DOI] [PubMed] [Google Scholar]
- [2].Lovell M, Markesbery WR (2007) Oxidative damage in mild cognitive impairment and early Alzheimer’s disease. J Neurosci Res 85, 3036–3040. [DOI] [PubMed] [Google Scholar]
- [3].Yao J IR, Zhao L, Nilsen J, Hamilton RT, Brinton RD (2009) Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A 106, 14670–14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gubandru M, Margina D, Tsitsimpikou C, Goutzourelas N, Tsarouhas K, Ilie M, Tsatsakis AM, Kouretas D (2013) Alzheimer’s disease treated patients showed different patterns for oxidative stress and inflammation markers. Food Chem Toxic 61, 209–214. [DOI] [PubMed] [Google Scholar]
- [5].Schrag M, Mueller C, Zabel M, Crofton A, Kirsch WM, Ghribi O, Squitti R, Perry G (2013) Oxidative stress in blood in Alzheimer’s disease and mild cognitive impairment: A meta-analysis. Neurobiol Dis 59, 100–110. [DOI] [PubMed] [Google Scholar]
- [6].Zabel M, Nackenoff A, Kirsch WM, Harrison FE, Perry G, Schrag M (2018) Markers of oxidative damage to lipids, nucleic acids and proteins and antioxidant enzymes activities in Alzheimer’s disease brain: A meta-analysis in human pathological specimens. Free Radic Biol Med 115, 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Butterfield DA, Boyd-Kimball D (2018) Oxidative stress, amyloid-beta peptide, and altered key molecular pathways in the pathogenesis and progression of Alzheimer’s disease. J Alzheimers Dis 62, 1345–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Butterfield DA, Castegna A, Lauderback CM, Drake J (2002) Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer’s disease brain contribute to neuronal death. Neurobiol Aging 23, 655–664. [DOI] [PubMed] [Google Scholar]
- [9].Tonnies E, Trushina E (2017) Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzheimers Dis 57, 1105–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sultana R, Mecocci P, Mangialasche F, Cecchetti R, Baglioni M, Butterfield DA (2011) Increased protein and lipid oxidative damage in mitochondria isolated from lymphocytes from patients with Alzheimer’s disease: Insights into the role of oxidative stress in Alzheimer’s disease and initial investigations into a potential biomarker for this dementing disorder. J Alzheimers Dis 24, 77–84. [DOI] [PubMed] [Google Scholar]
- [11].Markesbery WR (1997) Oxidative stress hypothesis in Alzheimer’s disease. Free Radic Biol Med 23, 134–147. [DOI] [PubMed] [Google Scholar]
- [12].Bahn G, Park JS, Yun UJ, Lee YJ, Choi Y, Park JS, Baek SH, Choi BY, Cho YS, Kim HK, Han J, Sul JH, Baik SH, Lim J, Wakabayashi N, Bae SH, Han JW, Arumugam TV, Mattson MP, Jo DG (2019) NRF2/ARE pathway negatively regulates BACE1 expression and ameliorates cognitive deficits in mouse Alzheimer’s models. Proc Natl Acad Sci U S A 116, 12516–12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dysken MW, Sano M, Asthana S, Vertrees JE, Pallaki M, Llorente M, Love S, Schellenberg GD, McCarten JR, Malphurs J, Prieto S, Chen P, Loreck DJ, Trapp G, Bakshi RS, Mintzer JE, Heidebrink JL, Vidal-Cardona A, Arroyo LM, Cruz AR, Zachariah S, Kowall NW, Chopra MP, Craft S, Thielke S, Turvey CL, Woodman C, Monnell KA, Gordon K, Tomaska J, Segal Y, Peduzzi PN, Guarino PD (2014) Effect of vitamin E and memantine on functional decline in Alzheimer disease: The TEAM-AD VA cooperative randomized trial. JAMA 311, 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sancheti H, Kanamori K, Patil I, Diaz Brinton R, Ross BD, Cadenas E (2014) Reversal of metabolic deficits by lipoic acid in a triple transgenic mouse model of Alzheimer’s disease: A 13C NMR study. J Cereb Blood Flow Metab 34, 288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Joshi G, Gan KA, Johnson DA, Johnson JA (2015) Increased Alzheimer’s disease-like pathology in the APP/PS1 DeltaE9 mouse model lacking Nrf2 through modulation of autophagy. Neurobiol Aging 36, 664–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhu X, Liu J, Huang S, Zhu W, Wang Y, Chen O, Xue J (2019) Neuroprotective effects of isoliquiritigenin against cognitive impairment via suppression of synaptic dysfunction, neuronal injury, and neuroinflammation in rats with kainic acid-induced seizures. Int Immunopharmacol 72, 358–366. [DOI] [PubMed] [Google Scholar]
- [17].Uddin MS, Al Mamun A, Kabir MT, Jakaria M, Mathew B, Barreto GE, Ashraf GM (2019) Nootropic and anti-Alzheimer’s actions of medicinal plants: Molecular insight into therapeutic potential to alleviate Alzheimer’s neuropathology. Mol Neurobiol 56, 4925–4944. [DOI] [PubMed] [Google Scholar]
- [18].Shinomol GK, Muralidhara, Bharath MM (2011) Exploring the role of “Brahmi” (Bacopa monnieri and Centella asiatica) in brain function and therapy. Recent Pat Endocr Metab Immune Drug Discov 5, 33–49. [DOI] [PubMed] [Google Scholar]
- [19].Gray NE, Alcazar Magana A, Lak P, Wright KM, Quinn J, Stevens JF, Maier CS, Soumyanath A (2018) Centella asiatica - Phytochemistry and mechanisms of neuroprotection and cognitive enhancement. Phytochem Rev 17, 161–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].American Herbal Products Association (1997) Botanical Safety Handbook, CRC Press, Boca Raton, USA. [Google Scholar]
- [21].Hafiz ZZ, Amin MM, Johari James RM, Teh LK, Salleh MZ, Adenan MI (2020) Inhibitory effects of raw-extract Centella asiatica (RECA) on acetylcholinesterase, inflammations, and oxidative stress activities via in vitro and in vivo. Molecules 25, 892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tabassum R, Vaibhav K, Shrivastava P, Khan A, Ejaz Ahmed M, Javed H, Islam F, Ahmad S, Saeed Siddiqui M, Safhi MM, Islam F (2013) Centella asiatica attenuates the neurobehavioral, neurochemical and histological changes in transient focal middle cerebral artery occlusion rats. Neurol Sci 34, 925–933. [DOI] [PubMed] [Google Scholar]
- [23].Matthews DG, Caruso M, Murchison CF, Zhu JY, Wright KM, Harris CJ, Gray NE, Quinn JF, Soumyanath A (2019) Centella asiatica improves memory and promotes antioxidative signaling in 5XFAD mice. Antioxidants (Basel) 8, 630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gray NE, Zweig JA, Caruso M, Zhu JY, Wright KM, Quinn JF, Soumyanath A (2018) Centella asiatica attenuates hippocampal mitochondrial dysfunction and improves memory and executive function in beta-amyloid overexpressing mice. Mol Cell Neurosci 93, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gray NE, Zweig JA, Matthews DG, Caruso M, Quinn JF, Soumyanath A (2017) Centella asiatica attenuates mitochondrial dysfunction and oxidative stress in Abeta-exposed hippocampal neurons. Oxid Med Cell Longev 2017, 7023091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gray NE, Zweig JA, Murchison C, Caruso M, Matthews DG, Kawamoto C, Harris CJ, Quinn JF, Soumyanath A (2017) Centella asiatica attenuates Abeta-induced neurodegenerative spine loss and dendritic simplification. Neurosci Lett 646, 24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gray NE, Harris CJ, Quinn JF, Soumyanath A (2016) Centella asiatica modulates antioxidant and mitochondrial pathways and improves cognitive function in mice. J Ethnopharmacol 180, 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gray NE, Sampath H, Zweig JA, Quinn JF, Soumyanath A (2015) Centella asiatica attenuates amyloid-beta-induced oxidative stress and mitochondrial dysfunction. J Alzheimers Dis 45, 933–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gray NE, Morre J, Kelley J, Maier CS, Stevens JF, Quinn JF, Soumyanath A (2014) Caffeoylquinic acids in Centella asiatica protect against amyloid-beta toxicity. J Alzheimers Dis 40, 359–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gray NE, Zweig JA, Caruso M, Martin MD, Zhu JY, Quinn JF, Soumyanath A (2018) Centella asiatica increases hippocampal synaptic density and improves memory and executive function in aged mice. Brain Behav 8, e01024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Soumyanath A, Zhong YP, Henson E, Wadsworth T, Bishop J, Gold BG, Quinn JF (2012) Centella asiatica extract improves behavioral deficits in a mouse model of Alzheimer’s disease: Investigation of a possible mechanism of action. Int J Alzheimers Dis 2012, 381974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Matthews DG, Caruso M, Alcazar Magana A, Wright KM, Maier CS, Stevens JF, Gray NE, Quinn JF, Soumyanath A (2020) Caffeoylquinic acids in Centella asiatica reverse cognitive deficits in male 5XFAD Alzheimer’s disease model mice. Nutrients 12, 3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Alcazar Magana A, Wright K, Vaswani A, Caruso M, Reed RL, Bailey CF, Nguyen T, Gray NE, Soumyanath A, Quinn J, Stevens JF, Maier CS (2020) Integration of mass spectral fingerprinting analysis with precursor ion (MS1) quantification for the characterisation of botanical extracts: Application to extracts of Centella asiatica (L.) Urban. Phytochem Anal 31, 722–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zweig JA, Brandes MS, Brumbach BH, Caruso M, Wright KM, Quinn JF, Soumyanath A, Gray NE (2021) Loss of NRF2 accelerates cognitive decline, exacerbates mitochondrial dysfunction, and is required for the cognitive enhancing effects of Centella asiatica during aging. Neurobiol Aging 100, 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kaut KP, Bunsey MD (2001) The effects of lesions to the rat hippocampus or rhinal cortex on olfactory and spatial memory: Retrograde and anterograde findings. Cogn Affect Behav Neurosci 1, 270–286. [DOI] [PubMed] [Google Scholar]
- [36].Dalley JW, Cardinal RN, Robbins TW (2004) Prefrontal executive and cognitive functions in rodents: Neural and neurochemical substrates. Neurosci Biobehav Rev 28, 771–784. [DOI] [PubMed] [Google Scholar]
- [37].Assini FL, Duzzioni M, Takahashi RN (2009) Object location memory in mice: Pharmacological validation and further evidence of hippocampal CA1 participation. Behav Brain Res 204, 206–211. [DOI] [PubMed] [Google Scholar]
- [38].Moustafa AA, Gilbertson MW, Orr SP, Herzallah MM, Servatius RJ, Myers CE (2013) A model of amygdala-hippocampal-prefrontal interaction in fear conditioning and extinction in animals. Brain Cogn 81, 29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kaech S, Banker G (2006) Culturing hippocampal neurons. Nat Protoc 1, 2406–2415. [DOI] [PubMed] [Google Scholar]
- [40].Ferreira TA, Blackman AV, Oyrer J, Jayabal S, Chung AJ, Watt AJ, Sjostrom PJ, van Meyel DJ (2014) Neuronal morphometry directly from bitmap images. Nat Methods 11, 982–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kwak MK IK, Yamamoto M, Kensler TW (2002) Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: Role of antioxidant response element-like sequences in the nrf2 promoter. Mol Cell Biol 22, 2883–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Suzuki T ST, Takaya K, Shiraishi K, Kohno T, Kunitoh H, Tsuta K, Furuta K, Goto K, Hosoda F, Sakamoto H, Motohashi H, Yamamotoa M (2013) Regulatory nexus of synthesis and degradation deciphers cellular Nrf2 expression levels. Mol Cell Biol 33, 2402–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Singh A, Venkannagari S, Oh KH, Zhang YQ, Rohde JM, Liu L, Nimmagadda S, Sudini K, Brimacombe KR, Gajghate S, Ma J, Wang A, Xu X, Shahane SA, Xia M, Woo J, Mensah GA, Wang Z, Ferrer M, Gabrielson E, Li Z, Rastinejad F, Shen M, Boxer MB, Biswal S (2016) Small molecule inhibitor of NRF2 selectively intervenes therapeutic resistance in KEAP1-deficient NSCLC tumors. ACS Chem Biol 11, 3214–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gray NE, Zweig JA, Kawamoto C, Quinn JF, Copenhaver PF (2016) STX, a novel membrane estrogen receptor ligand, protects against amyloid-beta toxicity. J Alzheimers Dis 51, 391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wu HY, Hudry E, Hashimoto T, Kuchibhotla K, Rozkalne A, Fan Z, Spires-Jones T, Xie H, Arbel-Ornath M, Grosskreutz CL, Bacskai BJ, Hyman BT (2010) Amyloid beta induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J Neurosci 30, 2636–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zweig JA, Caruso M, Brandes MS, Gray NE (2020) Loss of NRF2 leads to impaired mitochondrial function, decreased synaptic density and exacerbated age-related cognitive deficits. Exp Gerontol 131, 110767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Chiroma SM, Hidayat Baharuldin MT, Mat Taib CN, Amom Z, Jagadeesan S, Adenan MI, Mohd Moklas MA (2019) Protective effect of Centella asiatica against D-galactose and aluminium chloride induced rats: Behavioral and ultrastructural approaches. Biomed Pharmacother 109, 853–864. [DOI] [PubMed] [Google Scholar]
- [48].Farhana KM, Malueka RG, Wibowo S, Gofir A (2016) Effectiveness of gotu kola extract 750 mg and 1000 mg compared with folic acid 3 mg in improving vascular cognitive impairment after stroke. Evid Based Complement Alternat Med 2016, 2795915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Tiwari S, Singh S, Patwardhan K, Ghlot S, Gambhir I (2008) Effect of Centella asiatica on mild cognitive impairment (MCI) and other commonage-related clinical problems. Dig J Nanomater Biostruct 3, 215–220. [Google Scholar]
- [50].Veerendra Kumar MH, Gupta YK (2003) Effect of Centella asiatica on cognition and oxidative stress in an intracere-broventricular streptozotocin model of Alzheimer’s disease in rats. Clin Exp Pharmacol Physiol 30, 336–342. [DOI] [PubMed] [Google Scholar]
- [51].Wattanathorn J, Mator L, Muchimapura S, Tongun T, Pasuri-wong O, Piyawatkul N, Yimtae K, Sripanidkulchai B, Singkhoraard J (2008) Positive modulation of cognition and mood in the healthy elderly volunteer following the administration of Centella asiatica. J Ethnopharmacol 116, 325–332. [DOI] [PubMed] [Google Scholar]
- [52].Dhanasekaran M, Holcomb LA, Hitt AR, Tharakan B, Porter JW, Young KA, Manyam BV (2009) Centella asiatica extract selectively decreases amyloid beta levels in hippocampus of Alzheimer’s disease animal model. Phytother Res 23, 14–19. [DOI] [PubMed] [Google Scholar]
- [53].Rohrer PR, Rudraiah S, Goedken MJ, Manautou JE (2014) Is nuclear factor erythroid 2-related factor 2 responsible for sex differences in susceptibility to acetaminophen-induced hepatotoxicity in mice? Drug Metab Dispos 42, 1663–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Xu SF, Ji LL, Wu Q, Li J, Liu J (2018) Ontogeny and aging of Nrf2 pathway genes in livers of rats. Life Sci 203, 99–104. [DOI] [PubMed] [Google Scholar]
- [55].Bundy JL, Vied C, Badger C, Nowakowski RS (2019) Sex-biased hippocampal pathology in the 5XFAD mouse model of Alzheimer’s disease: A multi-omic analysis. J Comp Neurol 527, 462–475. [DOI] [PubMed] [Google Scholar]
- [56].McLellan ME KS, Hyman BT, Bacskai BJ. (2003) In vivo imaging of reactive oxygen species specifically associated with thioflavine S-positive amyloid plaques by multiphoton microscopy. J Neurosci 23, 2212–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wang L, Guo L, Lu L, Sun H, Shao M, Beck SJ, Li L, Ramachandran J, Du Y, Du H. (2016) Synaptosomal mitochondrial dysfunction in 5xFAD mouse model of Alzheimer’s disease. PLoS One 11, e0150441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Cheng Y, Bai F (2018) The association of tau with mitochondrial dysfunction in Alzheimer’s disease. Front Neurosci 12, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].López-González I, Aso E, Carmona M, Armand-Ugon M, Blanco R, Naudí A, Cabré R, Portero-Otin M, Pamplona R, Ferrer I (2015) Neuroinflammatory gene regulation, mitochondrial function, oxidative stress, and brain lipid modifications with disease progression in tau P301S transgenic mice as a model of frontotemporal lobar degeneration-tau. J Neuropathol Exp Neurol 74, 975–999. [DOI] [PubMed] [Google Scholar]
- [60].Melov S, Adlard PA, Morten K, Johnson F, Golden TR, Hinerfeld D, Schilling B, Mavros C, Masters CL, Volitakis I, Li QX, Laughton K, Hubbard A, Cherny RA, Gibson B, Bush AI (2007) Mitochondrial oxidative stress causes hyperphosphorylation of tau. PLoS One 2, e536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Forster M, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS (1996) Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci U S A 93, 4765–4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Perrig W, Perrig P, Stähelin HB (1997) The relation between antioxidants and memory performance in the old and very old. J Am Geriatr Soc 45, 718–724. [DOI] [PubMed] [Google Scholar]
- [63].Olsen RH JL, Zuloaga DG, Limoli CL, Raber J (2013) Enhanced hippocampus-dependent memory and reduced anxiety in mice over-expressing human catalase in mitochondria. J Neurochem 125, 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Anton S, Ebner N, Dzierzewski JM, Zlatar ZZ, Gurka MJ, Dotson VM, Kirton J, Mankowski RT, Marsiske M, Manini TM (2018) Effects of 90 days of resveratrol supplementation on cognitive function in elders: A pilot study. J Altern Complement Med 24, 725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Whyte A, Cheng N, Fromentin E, Williams CM. (2018) A randomized, double-blinded, placebo-controlled study to compare the safety and efficacy of low dose enhanced wild blueberry powder and wild blueberry extract (ThinkBlue™) in maintenance of episodic and working memory in older adults. Nutrients 10, E660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].McNamara R, Kalt W, Shidler MD, McDonald J, Summer SS, Stein AL, Stover AN, Krikorian R. (2018) Cognitive response to fish oil, blueberry, and combined supplementation in older adults with subjective cognitive impairment. Neurobiol Aging 64, 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Poulose S, Miller MG, Scott T, Shukitt-Hale B (2017) Nutritional factors affecting adult neurogenesis and cognitive function. Adv Nutr 8, 804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bensalem J, Dudonné S, Gaudout D, Servant L, Calon F, Desjardins Y, Layé S, Lafenetre P, Pallet V (2018) Polyphenol-rich extract from grape and blueberry attenuates cognitive decline and improves neuronal function in aged mice. J Nutr Sci 7, e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Shih P, Chan YC, Liao JW, Wang MF, Yen GC (2010) Antioxidant and cognitive promotion effects of anthocyanin-rich mulberry (Morus atropurpurea L.) on senescence-accelerated mice and prevention of Alzheimer’s disease. J Nutr Biochem 21, 598–605. [DOI] [PubMed] [Google Scholar]
- [70].Souza L, Antunes MS, Filho CB, Del Fabbro L, de Gomes MG, Goes AT, Donato F, Prigol M, Boeira SP, Jesse CR (2015) Flavonoid Chrysin prevents age-related cognitive decline via attenuation of oxidative stress and modulation of BDNF levels in aged mouse brain. Pharmacol Biochem Behav 134, 22–30. [DOI] [PubMed] [Google Scholar]
- [71].Gureev AP, Popov VN, Starkov AA (2020) Crosstalk between the mTOR and Nrf2/ARE signaling pathways as a target in the improvement of long-term potentiation. Exp Neurol 328, 113285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Liang Y, Zou Y, Niu C, Niu Y (2019) Astragaloside IV and ferulic acid synergistically promote neurite outgrowth through Nrf2 activation. Mech Ageing Dev 180, 70–81. [DOI] [PubMed] [Google Scholar]
- [73].Chaisawang P, Sirichoat A, Chaijaroonkhanarak W, Pannangrong W, Sripanidkulchai B, Wigmore P, Welbat JU (2017) Asiatic acid protects against cognitive deficits and reductions in cell proliferation and survival in the rat hippocampus caused by 5-fluorouracil chemotherapy. PLoS One 12, e0180650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Sirichoat A, Chaijaroonkhanarak W, Prachaney P, Pannangrong W, Leksomboon R, Chaichun A, Wigmore P, Welbat JU (2015) Effects of asiatic acid on spatial working memory and cell proliferation in the adult rat hippocampus. Nutrients 7, 8413–8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Welbat JU, Chaisawang P, Pannangrong W, Wigmore P (2018) Neuroprotective properties of asiatic acid against 5-fluorouracil chemotherapy in the hippocampus in an adult rat model. Nutrients 10, 1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Lin X, Huang R, Zhang S, Wei L, Zhuo L, Wu X, Tang A, Huang Q (2013) Beneficial effects of asiaticoside on cognitive deficits in senescence-accelerated mice. Fitoterapia 87, 69–77. [DOI] [PubMed] [Google Scholar]
- [77].Hamid K, Ng I, Tallapragada VJ, Varadi L, Hibbs DE, Hanrahan J, Groundwater PW (2016) An investigation of the differential effects of ursane triterpenoids from Centella asiatica, and their semisynthetic analogues, on GABAA receptors. Chem Biol Drug Des 88, 386–397. [DOI] [PubMed] [Google Scholar]
- [78].Lin X, Zhang S, Huang R, Wei L, Tan S, Liang C, Lv S, Chen Y, Liang S, Tian Y, Lu Z, Huang Q (2014) Protective effect of madecassoside against cognitive impairment induced by D-galactose in mice. Pharmacol Biochem Behav 124, 434–442. [DOI] [PubMed] [Google Scholar]
- [79].Liang N, Dupuis JH, Yada RY, Kitts DD (2019) Chlorogenic acid isomers directly interact with Keap 1-Nrf2 signaling in Caco-2 cells. Mol Cell Biochem 457, 105–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Fan J, Chen Q, Wei L, Zhou X, Wang R, Zhang H (2018) Asiatic acid ameliorates CCl4-induced liver fibrosis in rats: Involvement of Nrf2/ARE, NF-kappaB/IkappaBalpha, and JAK1/STAT3 signaling pathways. Drug Des Devel Ther 12, 3595–3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kamble SM, Patil CR (2018) Asiatic acid ameliorates doxorubicin-induced cardiac and hepato-renal toxicities with Nrf2 transcriptional factor activation in rats. Cardiovasc Toxicol 18, 131–141. [DOI] [PubMed] [Google Scholar]
- [82].Lv H, Qi Z, Wang S, Feng H, Deng X, Ci X (2017) Asiatic acid exhibits anti-inflammatory and antioxidant activities against lipopolysaccharide and d-galactosamine-induced fulminant hepatic failure. Front Immunol 8, 785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Qi Z, Ci X, Huang J, Liu Q, Yu Q, Zhou J, Deng X (2017) Asiatic acid enhances Nrf2 signaling to protect HepG2 cells from oxidative damage through Akt and ERK activation. Biomed Pharmacother 88, 252–259. [DOI] [PubMed] [Google Scholar]
- [84].Liang N, Kitts DD (2018) Amelioration of oxidative stress in caco-2 cells treated with pro-inflammatory proteins by chlorogenic acid isomers via activation of the Nrf2-Keap1-ARE-signaling pathway. J Agric Food Chem 66, 11008–11017. [DOI] [PubMed] [Google Scholar]
- [85].Hussain T, Tan B, Liu G, Murtaza G, Rahu N, Saleem M, Yin Y (2017) Modulatory mechanism of polyphenols and Nrf2 signaling pathway in LPS challenged pregnancy disorders. Oxid Med Cell Longev 2017, 8254289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Nabavi S, Barber AJ, Spagnuolo C, Russo GL, Daglia M, Nabavi SM, Sobarzo-Sánchez E. (2016) Nrf2 as molecular target for polyphenols: A novel therapeutic strategy in diabetic retinopathy. Crit Rev Clin Lab Sci 53, 293–312. [DOI] [PubMed] [Google Scholar]
- [87].Pallauf K, Duckstein N, Hasler M, Klotz LO, Rimbach G (2017) Flavonoids as putative inducers of the transcription factors Nrf2, FoxO, and PPARγ. Oxid Med Cell Longev 2017, 4397340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


