In 2020, 18,796 allo‐stem cell transplants (SCT) were performed in European countries 1 and approximately 2000 in France alone. 2 Although the toxicity‐related mortality (TRM) had decreased, infections remained a significant cause of TRM. 3 , 4 Of note, the current use of either ex vivo or in vivo T‐cell depletion prevents alloreactivity (graft rejection and graft‐versus‐host disease [GVHD]) but enables viral infections/reactivations due to a delayed immune reconstitution. The incidence and severity of viral infections/reactivations has decreased thanks to the implementation of pre‐emptive antiviral chemotherapies, but they still represent a life‐threatening complication of allo‐SCT. 5 Indeed, a relevant number of patients still experience therapy‐refractory infections in the absence of concomitant immune reconstitution. 6 , 7 , 8 A recent observational study reported that in the first 100 days posttransplant, the cumulative incidence of death caused by infections is 2.3% (0.25% from viral origin). 4 Antiviral therapies are associated with consistent side effects such as acute kidney injury or hematotoxicity that might worsen the overall prognosis. 6 , 7 , 8 , 9 For adenovirus (AdV), 10 , 11 , 12 cytomegalovirus (CMV), 13 , 14 and Epstein Barr virus (EBV), virus‐specific T‐cells (VST) have been developed as an alternate therapy, 15 , 16 since the pioneering work of Pr Riddell et al. in 1992 and international colleagues afterward. 17 , 18 , 19 , 20 , 21 , 22
Since 2016, France approved AdV‐VST, EBV‐VST, or CMV‐VST production in the Cell Therapy Unit of the University Hospital of Nancy (UTCT) as Advanced Therapy Medicinal Products (ATMP). Twenty‐nine consecutive patients with AdV, EBV, or CMV replications/infections, persisting despite an optimized antiviral therapy, were infused. We retrospectively analyzed the data in terms of safety and efficacy, considering VST were in more than half cases prepared from third‐party haploidentical donors. The protocol was approved by the French Society of Bone Marrow Transplantation and Cellular Therapy (SFGM‐TC) research committee and was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice Guideline of the International Conference on Harmonisation. All patients provided written informed consent, including legal guardians for patients under 18 years old.
Eligible patients were defined as: (1) allo‐SCT patients (2) with viral replication and/or tissue infection with CMV, AdV, or EBV refractory to standard therapy; (3) standard therapy was defined by at least 14 days of full‐treatment dose of ganciclovir or foscarnet or cidofovir for CMV, or cidofovir or brincidofovir for AdV (off‐label use) and stable disease (SD) or progressive disease (PD) observed under weekly perfusion of rituximab for EBV replication, or SD or PD observed under cytotoxic chemotherapy with or without rituximab for EBV‐related posttransplant lymphoproliferative disorder (PTLD). Patients were considered ineligible when they presented uncontrolled aGVHD > grade II 23 or uncontrolled chronic GVHD > NIH 2. 24 All immunosuppressive drugs were allowed and corticosteroids were <1 mg/kg/day in most cases. UTCT of Nancy is the only laboratory in France authorized by the French regulatory agency Agence Nationale de Sécurité du Médicament (ANSM) to produce AdV‐specific T‐cells as an ATMP under hospital exemption (MTI‐PP‐009) and to deliver EBV‐VST or CMV‐VST for compassionate use after nominative ANSM “non opposition” with a prospective monitoring requirement. The definition of replication or disease followed the guidelines of the SFGM‐TC. Conditioning regimens were divided into myeloablative (MAC), reduced‐intensity conditioning (RIC), and nonmyeloablative (NMA) as the established definitions by Bacigalupo et al., 25 applicable to both adult and pediatric patients. Serious adverse events (SAE) were defined according to the Common Terminology Criteria for Adverse Events (CTCAE version 5.0). Complete response (CR) was defined as complete clearance of the virus, and partial response (PR) was defined as a viral load decrease ≥1 log. The immune reconstitution monitoring was assessed by each center using an IFN‐γ‐positive ELIspot assay or a proliferative test when the technique was locally available.
The SFGM‐TC group received eighty referral procedures: fifty obtained a favorable opinion, and finally, twenty‐nine consecutive patients were infused in 13 different centers (Supporting Information: Figure 1). In the entire cohort, the median follow‐up was 74 days (from 8 to 555 days) (see Table 1). Seventeen children (<18 years old) and 12 adults (≥18 years old) were treated. Of note, five patients received two infusions (four patients against CMV and one patient against AdV) and one patient received one VST infusion prepared against both AdV and CMV. Briefly, 51.7% were transplanted for primary immunodeficiency or nonmalignant hematological diseases, and the majority of patients (n = 28) received an in vivo T‐cell depletion; the median time between allo‐SCT and the viral replication was 46 days (range: 0–1372 days). Infused VST were mainly derived from a related intrafamilial third‐party donor (n = 16, 55.2%). Patients were heavily pretreated with a median time of 50 days (range: 21–300 days) of antiviral therapy before the initial VST infusion.
Table 1.
Patients' characteristics.
| AdV | CMV | EBV | ADV+CMV | Total | |
|---|---|---|---|---|---|
| Patients | 13 | 7 | 8 | 1 | 29 |
| Adults | 7 | 3 | 2 | 0 | 12 (41%) |
| Children (<18 years) | 6 | 4 | 6 | 1 | 17 (59%) |
| Sex (F/M) | 13/0 | 3/4 | 5/2 | 0/1 | 21/8 |
| Age (years) [min–max] | 32 [1–67] | 2 [0.6–68] | 13 [1–40] | 5 | 14 [0.6–68] |
| Initial disease (n) | |||||
| Primary immunodeficiency | 4 | 3 | 3 | 0 | 11 |
| Blackfan‐Diamond anemia | 0 | 0 | 0 | 1 | 1 |
| Dyskeratosis congenita | 0 | 1 | 0 | 0 | 1 |
| Idiopathic aplastic anemia | 0 | 0 | 1 | 0 | 1 |
| Sickle cell disease | 1 | 0 | 0 | 0 | 1 |
| Acute myeloid leukemia | 3 | 1 | 1 | 0 | 5 |
| Acute lymphoid leukemia | 1 | 1 | 1 | 0 | 3 |
| Myelodysplastic syndrome | 1 | 1 | 1 | 0 | 3 |
| Chronic myeloid leukemia | 1 | 0 | 0 | 0 | 1 |
| Richter's syndrome | 1 | 0 | 0 | 0 | 1 |
| Adult T‐cell lymphoma | 1 | 0 | 0 | 0 | 1 |
| Graft source | |||||
| BM | 7 | 2 | 2 | 1 | 12 (41.4%) |
| PBSC | 5 | 4 | 5 | 0 | 14 (48.3%) |
| CBU | 1 | 1 | 1 | 0 | 3 (10.3%) |
| Graft donor | |||||
| MSD | 1 | 1 | 1 | 0 | 3 (10%) |
| Unrelated donors | 6 | 2 | 4 | 1 | 14 (48%) |
| MUD 10/10 | 5 | 2 | 4 | 1 | 12 |
| MMUD 9/10 | 1 | 0 | 0 | 0 | 1 |
| MMUD (≤8/10) | 0 | 0 | 1 | 0 | 1 |
| Haploidentical donors | 5 | 3 | 1 | 0 | 9 (31%) |
| CBU | 1 | 1 | 1 | 0 | 3 (10%) |
| Conditioning regimen | |||||
| Myeloablative | 2 | 2 | 2 | 0 | 6 (21%) |
| Reduced‐intensity conditioning | 9 | 1 | 4 | 0 | 14 (48%) |
| Nonmyeloablative | 2 | 3 | 2 | 1 | 8 (28%) |
| T‐cell depletion ex vivo (T‐depleted graft) | 0 | 1 | 1 | 0 | 2 |
| T‐cell depletion in vivoa | |||||
| Anti‐thymocyte globulin (ATG) | 8 | 4 | 5 | 0 | 17 (59%) |
| Cyclophosphamide | 5 | 2 | 0 | 0 | 7 |
| Alemtuzumab | 2 | 1 | 3 | 1 | 7 |
| Median time between allo‐SCT and infection (days) [min‐max] | 46 [3–1372] | 31 [0–130] | 64.5 [13–163] | 18 | 50 [21–300] |
| GVH disease prophylaxis | |||||
| Ciclosporin A+MMF | 7 | 5 | 5 | 0 | 17 (59%) |
| Ciclosporin alone | 6 | 1 | 2 | 1 | 10 |
| None | 0 | 1 | 1 | 0 | 2 |
| GVHD before VST | 8 | 2 | 1 | 0 | 11 (38%) |
| Acute GVHD grade I–II | 1 | 1 | 0 | 0 | 2 |
| Acute GVHD grade III–IV | 7 | 1 | 1 | 0 | 9 |
| Ruxolitinib before VST | 2 | 1 | 1 | 0 | 4 |
| Median lines of antiviral therapy before VST (range)b | 2 (1–3) | 3 (2–4) | 1 (0–5) | 2 | 2 (0–5) |
| Viral status (n. of replication/disease) | 10/3 | 2/5 | 4/4 | 1/0 | 17/12 |
| Mean viral load at D0 (log) (±SD) | 4.88 (±1.79) | 3.64 (±1.14) | 4.06 (±1.07) | ADV 5.05/CMV 3.99 | 4.34 (±1.47) |
| VST origin | |||||
| Graft donor | 8 | 3 | 2 | 0 | 13 (45%) |
| Third‐party donor | 5 | 4 | 6 | 1 | 16 (55%) |
| Median time between infection and VST infusion (range) | 39 (10‐89) | 85 (19‐216) | 52 (21‐310) | 53 | 51,5 (10‐310) |
| Mean infused CD3+T‐cells (104/kg) (±SD)c | 0.42 ± 0.35 | 3.29 ± 5.06 | 0.84 ± 0.43 | 0.5 ± 0.02 | 1.43 (±3.06) |
Abbreviations: AdV, adenovirus; BM, bone marrow; CBU, cord blood unit; CMV, cytomegalovirus; EBV, Epstein Barr virus; F/M, female/male; MMF, mycophenolate mofetil; MMUD, mismatched unrelated donor; MSD, matched sibling donor; MUD, matched unrelated donor; PBSC, peripheral blood stem cells, SD, standard deviation.
28 patients received a T‐depletion post‐allo‐SCT (patients 6 and 7 received an ex vivo T‐depleted graft). Among these 28 patients, patient 6 (AdV‐VST) received both alemtuzumab and cyclophosphamide (haploidentical donor), patient 3 (AdV‐VST) and patient 18 (CMV‐VST) received both ATG and cyclophosphamide because of a FLAMSA‐RIC conditioning regimen and a haploidentical donor, respectively. Before infusion, 11 patients were treated for aGVHD (grade I–II: n = 2, grade III–IV: n = 9) with corticosteroids and patients received ruxolitinib.
One patient (patient 25) received an allo‐SCT as a curative treatment for a chronic Aichi virus infection with renal failure secondary to Bruton's agammaglobulinemia. He was not treated with rituximab before EBV‐VST because the B‐cell depletion for EBV replication would have delayed the immune reconstitution necessary to control the Aichi virus.
Taking into account 34 infusions.
VST were produced from leukapheresis obtained from the graft donor for 13 patients (44.8%) and from third‐party haplo‐identical relatives in 16 patients (55.2%), according to interferon gamma (IFN‐γ) immunomagnetic isolation, as previously described by Qian et al. 26 The mean dose of CD3+ IFN‐γ+ T‐cells administered to the patient was 0.82 × 104 ± 0.89 × 104 T‐cells/kg with a mean enrichment of 72% of CD4+ IFN‐γ+ T‐cells (SD: ±17%) and 80% of CD8+ IFN‐γ+ T‐cells (SD: ±16%). VST were released according to flow cytometry control results. The absence of microbiologic contamination was confirmed after 10 days, except for one production positive for Cutibacterium acnes. Functional and proliferative assays performed a few weeks after infusion demonstrated a low alloreactivity and a high cytotoxicity against specific virus.
Within 3 months post‐VST infusion, there was no de novo aGVHD. Unfortunately, two patients experienced a recurrent grade III–IV aGVHD. Patients 3 and 12 developed recurrent aGVHD attributed to rapid corticosteroid taper (patient 3 had a recurrence at D38 from the day of VST infusion (D0) when corticosteroids were lower than 0.3 mg/kg/day, and patient 12 had a recurrence at D27, 10 days after corticosteroid discontinuation). One patient developed a de novo pulmonary cGVHD at D45. Of note, all the patients infused with VST were considered in remission or at least with controlled GVHD at the time of infusion. The first cause of death within the 3 months post‐VST was the initial virus‐related replication/disease (n = 9) followed by TRM (n = 3), one from severe progressive grade III (hepatic aGVHD), one from hepatic failure secondary to hepatorenal syndrome with refractory ascites, and one from pulmonary alveolar hemorrhage. After 3 months, the causes of death were allo‐SCT TRM (n = 5).
Overall response rate (ORR) at 1‐month postinfusion was 56%. The ORR at 3 months was 62%. At 3 months, six patients were still nonresponders: four of them had advanced viral disease at D0 and died (patients 2, 3, 7, and 13) and two maintained a stable viral load (patients 20 and 24). Patient 10 died at D78 after achieving PR at D30 and CR at D75 (Figure 1A). Patients who received an AdV‐VST infusion were significantly better responders than those who received CMV‐VST and EBV‐VST (p < 0.01) (Figure 1B). There was no significant difference in ORR between an HLA‐matched original graft donor versus a third‐party haploidentical donor (p = 0.415) (Figure 1C). Finally, the median time to the best virological response (CR) was 18 days (range: 10–75 days). In the entire cohort, OS post‐VST‐infusion was 56% and 29% at 3 and 12 months postinfusion, respectively (Figure 1D). Interestingly, OS was significantly higher when the viral load at infusion was <5 log (p < 0.001), regardless of the implicated virus (Figure 1E).
Figure 1.
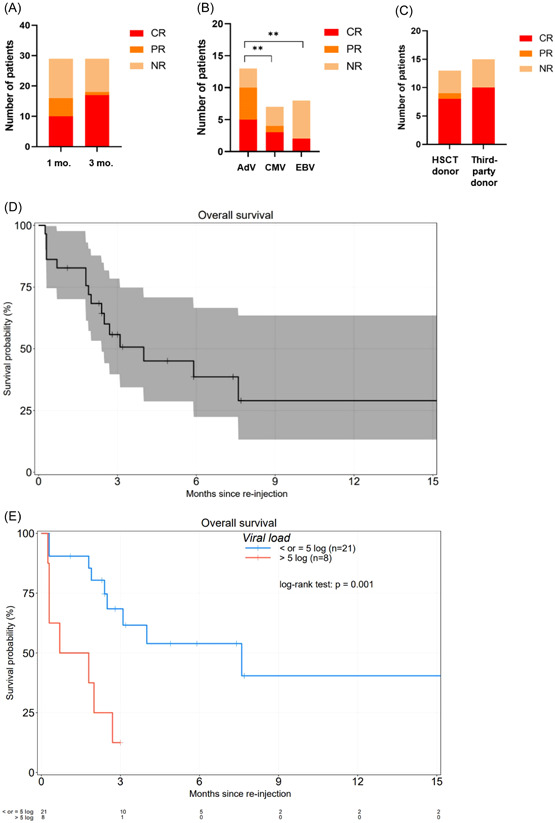
Patients' outcome. (A) ORR at 1 month and 3 months in the entire cohort. ORR at 1 month postinfusion was observed for 16 patients (10 patients with a CR and six patients with a PR). Thirteen patients were NR: nine patients had SD and four patients died of progressive disease at a median time of 12.5 days (range: 8–16 days). ORR at 3 months was observed for 18 patients (CR = 17, 10 at 1 month plus two patients who were initially NR plus five patients initially with PR, and PR = 1). At 3 months, six patients were still NR: four of them had advanced viral disease at D0 and died (patients 2, 3, 7, 13) and two maintained a stable viral load (patients 20 and 24). (B) ORR at 1 month according to the virus (AdV, CMV, EBV). (C) ORR at 1 month according to the VST‐donor. (D) OS in the entire cohort. (E) OS according to viral load. AdV, adenovirus; CMV, cytomegalovirus; CR, complete response; EBV, Epstein Barr virus; NR, non responder; ORR, overall response rate; PR, partial response.
We performed univariate analysis to assess the following possible risk factors for response, and these are our findings. The ORR was not significantly different according to the following immunological parameters: the previous in vivo T‐cell depletion (ATG vs. alemtuzumab vs. PTCy, p = 1.000), the continuation of corticosteroids at a dose <1 mg/kg/day at the time of VST transfer (n = 12) (p = 0.677), and adapted immunosuppression (n = 8) or not (n = 21) (p = 0.626) (see Supporting Information: Tables 1 and 2).
Immune reconstitution was investigated in 11 patients, with 7 of them experiencing specific immune reconstitution assessed by proliferative assay or ELIspot IFN‐γ assay. Among patients treated with corticosteroids at the time of VST infusion, immune reconstitution was documented for patients 3, 5, 11, 14, and 22, and 4 out of these 5 patients cleared the virus (see Supporting Information: Figures 1 and 2).
In conclusion, this is a real‐life study reporting a cohort of 29 patients treated with VST. It is also the largest cohort of patients receiving VST generated by IFN‐γ immunomagnetic isolation from a haploidentical related donor different from the allo‐SCT donor. We report encouraging results but also highlight the fact that results were probably impaired due to the significant delay between infection and VST infusion.
AUTHOR CONTRIBUTIONS
Esther Hazane Leroyer, Nadine Petitpain, Maud D'Aveni, and Danièle Bensoussan collected all data. Esther Hazane Leroyer, Maud D'Aveni, and Danièle Bensoussan wrote the main manuscript text. Esther Hazane Leroyer prepared all figures and Maud D'Aveni and Danièle Bensoussan reviewed them. Sébastien Maury performed statistical analysis. All authors reviewed the manuscript. Maud D'Aveni and Danièle Bensoussan contributed equally to the work.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING
This research received no funding.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
The authors warmly thank all the technical staff of the cell therapy unit from the Nancy University Hospital for VST production, including Jessica Morello and Mathilde Ollinger, for performing functional quality controls. They also thank all the technical staff of the Cytometry Platform of the Nancy University Hospital. Finally, they thank the MTI‐PP group (Dr. Karin Bilger, Dr. Laurence Clément, Pr. Jean‐Hugues Dalle, Pr. Véronique Decot, Dr. Jérôme Larghero, Dr. Anne Legendre, Dr. Nadine Petitpain, Dr. Cécile Pochon, and Dr. Hélène Rouard).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Passweg JR, Baldomero H, Chabannon C, et al. Impact of the SARS‐CoV‐2 pandemic on hematopoietic cell transplantation and cellular therapies in Europe 2020: a report from the EBMT activity survey. Bone Marrow Transplant. 2022;57(5):742‐752. 10.1038/s41409-022-01604-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agence de la Biomédecine. Le Rapport Annuel Médical et Scientifique 2017. Accessed October 18, 2023. https://www.agence-biomedecine.fr/annexes/bilan2017/donnees/cellules/04-national/synthese.html
- 3. Passweg JR, Baldomero H, Chabannon C, et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years. Bone Marrow Transplant. 2021;56(7):1651‐1664. 10.1038/s41409-021-01227-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Styczyński J, Tridello G, Koster L, et al. Death after hematopoietic stem cell transplantation: changes over calendar year time, infections and associated factors. Bone Marrow Transplant. 2020;55(1):126‐136. 10.1038/s41409-019-0624-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Illiaquer M, Imbert‐Marcille BM, Guillaume T, et al. Impact of stem cell graft on early viral infections and immune reconstitution after allogeneic transplantation in adults. J Clin Virol. 2017;93:30‐36. 10.1016/j.jcv.2017.05.019 [DOI] [PubMed] [Google Scholar]
- 6. González‐Vicent M, Verna M, Pochon C, et al. Current practices in the management of adenovirus infection in allogeneic hematopoietic stem cell transplant recipients in Europe: the AdVance study. Eur J Haematol. 2019;102(3):210‐217. 10.1111/ejh.13194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Matthes‐Martin S, Feuchtinger T, Shaw PJ, et al. European guidelines for diagnosis and treatment of adenovirus infection in leukemia and stem cell transplantation: summary of ECIL‐4. Tranpl Infect Dis. 2012;14(6):555‐563. 10.1111/tid.12022 [DOI] [PubMed] [Google Scholar]
- 8. Hiwarkar P, Kosulin K, Cesaro S, et al. Management of adenovirus infection in patients after haematopoietic stem cell transplantation: state‐of‐the‐art and real‐life current approach. Rev Med Virol. 2018;28(3):e1980. 10.1002/rmv.1980 [DOI] [PubMed] [Google Scholar]
- 9. Hiwarkar P, Amrolia P, Sivaprakasam P, et al. Brincidofovir is highly efficacious in controlling adenoviremia in pediatric recipients of hematopoietic cell transplant. Blood. 2017;129(14):2033‐2037. 10.1182/blood-2016-11-749721 [DOI] [PubMed] [Google Scholar]
- 10. Chakrabarti S, Collingham K, Fegan C, Pillay D, Milligan D. Adenovirus infections following haematopoietic cell transplantation: is there a role for adoptive immunotherapy? Bone Marrow Transplant. 2000;26(3):305‐307. 10.1038/sj.bmt.1702508 [DOI] [PubMed] [Google Scholar]
- 11. van Tol MJD, Kroes ACM, Schinkel J, et al. Adenovirus infection in paediatric stem cell transplant recipients: increased risk in young children with a delayed immune recovery. Bone Marrow Transplant. 2005;36(1):39‐50. 10.1038/sj.bmt.1705003 [DOI] [PubMed] [Google Scholar]
- 12. Feuchtinger T, Lang P, Handgretinger R. Adenovirus infection after allogeneic stem cell transplantation. Leuk Lymphoma. 2007;48(2):244‐255. 10.1080/10428190600881157 [DOI] [PubMed] [Google Scholar]
- 13. Barron MA, Gao D, Springer KL, et al. Relationship of reconstituted adaptive and innate cytomegalovirus (CMV)‐specific immune responses with CMV viremia in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2009;49(12):1777‐1783. 10.1086/648423 [DOI] [PubMed] [Google Scholar]
- 14. Blyth E, Withers B, Clancy L, Gottlieb D. CMV‐specific immune reconstitution following allogeneic stem cell transplantation. Virulence. 2016;7(8):967‐980. 10.1080/21505594.2016.1221022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Al Hamed R, Bazarbachi AH, Mohty M. Epstein‐Barr virus‐related post‐transplant lymphoproliferative disease (EBV‐PTLD) in the setting of allogeneic stem cell transplantation: a comprehensive review from pathogenesis to forthcoming treatment modalities. Bone Marrow Transplant. 2020;55(1):25‐39. 10.1038/s41409-019-0548-7 [DOI] [PubMed] [Google Scholar]
- 16. Styczynski J, van der Velden W, Fox CP, et al. Management of Epstein‐Barr Virus infections and post‐transplant lymphoproliferative disorders in patients after allogeneic hematopoietic stem cell transplantation: Sixth European Conference on Infections in Leukemia (ECIL‐6) guidelines. Haematologica. 2016;101(7):803‐811. 10.3324/haematol.2016.144428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leen AM, Bollard CM, Mendizabal AM, et al. Multicenter study of banked third‐party virus‐specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood. 2013;121(26):5113‐5123. 10.1182/blood-2013-02-486324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Withers B, Blyth E, Clancy LE, et al. Long‐term control of recurrent or refractory viral infections after allogeneic HSCT with third‐party virus‐specific T cells. Blood Adv. 2017;1(24):2193‐2205. 10.1182/bloodadvances.2017010223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tzannou I, Papadopoulou A, Naik S, et al. Off‐the‐shelf virus‐specific T cells to treat BK virus, human herpesvirus 6, cytomegalovirus, Epstein‐Barr virus, and adenovirus infections after allogeneic hematopoietic stem‐cell transplantation. J Clin Oncol. 2017;35(31):3547‐3557. 10.1200/JCO.2017.73.0655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prockop S, Doubrovina E, Suser S, et al. Off‐the‐shelf EBV‐specific T cell immunotherapy for rituximab‐refractory EBV‐associated lymphoma following transplantation. J Clin Invest. 2020;130(2):733‐747. 10.1172/JCI121127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jiang W, Clancy LE, Avdic S, et al. Third‐party CMV‐ and EBV‐specific T‐cells for first viral reactivation after allogeneic stem cell transplant. Blood Adv. 2022;6(17):4949‐4966. 10.1182/bloodadvances.2022007103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pfeiffer T, Tzannou I, Wu M, et al. Posoleucel, an allogeneic, off‐the‐shelf multivirus‐specific T‐cell therapy, for the treatment of refractory viral infections in the post‐HCT setting. Clin Cancer Res. 2023;29(2):324‐330. 10.1158/1078-0432.CCR-22-2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harris AC, Young R, Devine S, et al. International, multicenter standardization of acute graft‐versus‐host disease clinical data collection: a report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant. 2016;22(1):4‐10. 10.1016/j.bbmt.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft‐versus‐host disease: I. The 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant. 2015;21(3):389‐401.e1. 10.1016/j.bbmt.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628‐1633. 10.1016/j.bbmt.2009.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qian C, Campidelli A, Wang Y, et al. Curative or pre‐emptive adenovirus‐specific T cell transfer from matched unrelated or third party haploidentical donors after HSCT, including UCB transplantations: a successful phase I/II multicenter clinical trial. J Hematol Oncol. 2017;10(1):102. 10.1186/s13045-017-0469-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


