Assessment of measurable residual disease (MRD) through flow cytometric analysis is an important prognostic and clinically relevant factor and is currently implemented in clinical treatment protocols for patients with B cell non‐Hodgkin lymphomas (NHL), similar to follow‐up in acute hematologic malignancies. 1 , 2 , 3 , 4 This approach provides significant post‐therapy risk stratification and guides risk‐adapted therapeutic approaches, along with the evolution of immunophenotypic and molecular diagnostic techniques. 5 , 6 , 7 Patients with NHL can be treated with B cell depletion therapy. One example of such a drug is tafasitamab, a crystallizable fragment (Fc)‐enhanced humanized monoclonal antibody (mAb) targeting CD19, a surface antigen extensively expressed on both malignant and non‐malignant B cells. 3 , 4 Tafasitamab has recently been approved by the European Medicines Agency for the treatment of adult patients with diffuse large B cell lymphoma (DLBCL) who are ineligible for autologous stem cell transplantation after having had at least two failed lines of therapy. Therefore, tafasitamab is a valuable new therapeutic tool for patients with DLBCL, in whom refractory disease is often encountered. 3 , 8 In addition to its applicability in DLBCL, tafasitamab is currently under investigation in numerous clinical trials to explore its potential efficacy in treating non‐DLBCL subtypes of NHL. 9 , 10 , 11
Flow cytometric assessment of MRD in patients with B‐NHL often involves evaluating CD19 and CD20 expression, together with the assessment of clonality through the analysis of surface immunoglobulin (sIg) kappa/lambda expression and examination of the co‐expression of aberrant markers (e.g., CD5 and/or CD10). 12 In patients who are treated with B cell depletion therapy (e.g., tafasitamab), an absence of B cells is suspected within 1–2 weeks after first administration, in which case we, logically, expect to see no remaining B cells on flow cytometric follow‐up analysis. 13
In this letter, we demonstrate the flow cytometric interference of tafasitamab during MRD analysis, which can spuriously indicate the presence of residual lymphoma cells, and thus, treatment failure. Therefore, it is crucial for clinical laboratory practitioners to be mindful of this interference and exercise caution. Failure to do so may result in unnecessary escalation or alteration of the treatment regimen.
Suspicion of interference was aroused through the evaluation of two patients with NHL who were under observation at the Ghent University Hospital in Belgium. Both patients were enrolled in a clinical trial and underwent treatment with tafasitamab in conjunction with rituximab and lenalidomide. The first patient was an 85‐year‐old man who was diagnosed with a marginal zone lymphoma characterized by infiltration of both blood and bone marrow. At diagnosis, immunophenotyping revealed a malignant B cell population with CD19, CD20, and monotypic sIg lambda expression, and partial CD5 positivity. Over 4 years, the patient received different treatment regimens: rituximab monotherapy; rituximab in association with cyclophosphamide, vincristine, and prednisone; and rituximab in association with bendamustine and ibrutinib. Owing to progressive disease, the patient was included in the aforementioned clinical trial and was subsequently treated with rituximab in combination with lenalidomide and tafasitamab. The second patient, a 74‐year‐old woman, was diagnosed with a DLBCL transformed from a previously treated follicular lymphoma. Due to disease progression, rituximab and lenalidomide were initiated. During this treatment phase, flow cytometric analysis revealed the absence of B cells. Subsequently, the patient relapsed again (phenotype: sIg lambda/CD20+/CD5−/CD10+), after which tafasitamab was introduced into the treatment regimen. Remarkably, in both patients, following the incorporation of tafasitamab, MRD analysis identified a distinct cell population characterized by sIg kappa restriction and diminished CD19 expression, but lacking CD10 and CD5 expression. In addition, these cells did not express CD20, which can be attributed to the treatment with anti‐CD20 monoclonal antibodies (e.g., rituximab). The occurrence of these ‘monoclonal cell populations’ during MRD analysis was unexpected and raised the question of potential relapse. However, in both patients, the light chain expression of this new population differed from that in the initial clonal population, and thus a false positive result was suspected (Figure 1A,B).
Figure 1.
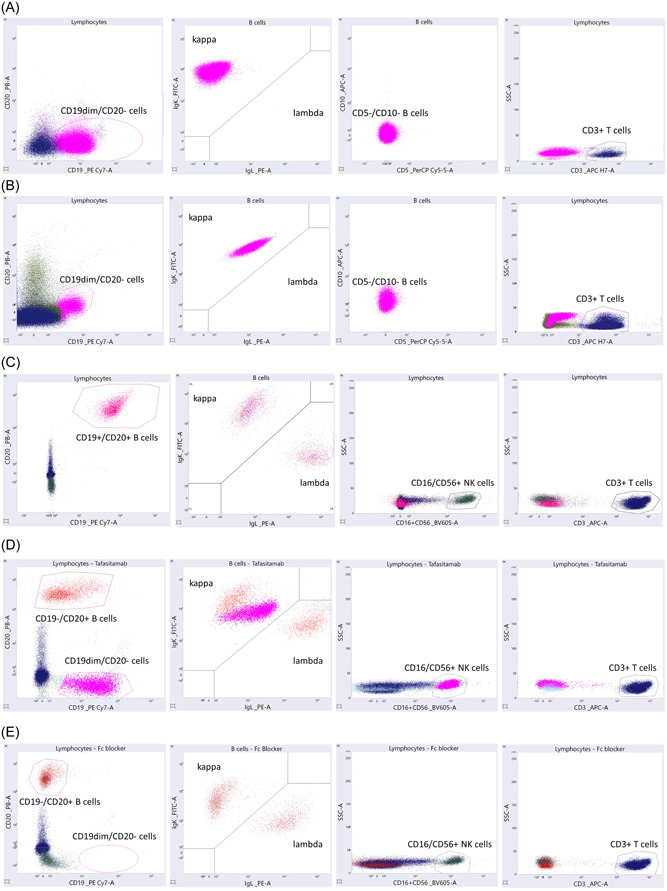
In vitro treatment of healthy volunteers with tafasitamab. (A) Measurable minimal disease (MRD) analysis of patient 1, after enrollment in the clinical trial (rituximab, lenalidomide, and tafasitamab), showing the presence of CD19dim/CD20− events with a monoclonal sIg kappa expression and no CD5 or CD10 expression (pink) next to CD3+ T cells (blue). (B) MRD analysis of patient 2, after enrollment in the clinical trial (rituximab, lenalidomide, and tafasitamab), showing the presence of CD19dim/CD20− events with a monoclonal sIg kappa expression and no CD5 or CD10 expression (pink) next to CD3+ T cells (blue). (C) Flow cytometric analysis of a native sample showing normal CD19+/CD20+ B cells with a polyclonal distribution of sIg kappa and lambda (pink), the presence of CD16 and/or CD56+ NK cells (green) and CD3+ T cells (blue). (D) Flow cytometric analysis of a sample spiked with tafasitamab (200 µg/mL) showing the presence of CD19−/CD20+ B cells with a polyclonal distribution of sIg kappa and lambda (red), and a population of CD19dim/CD20− events with strong monoclonal sIg kappa expression and CD16 and/or CD56 expression (pink). Normal CD3+ T cells (blue). (E) Flow cytometric analysis of a sample spiked with tafasitamab (200 µg/mL) after the addition of an Fc blocking agent showing CD19−/CD20+ B cells with a polyclonal distribution of sIg kappa and lambda (red), the presence of CD16 and/or CD56+ NK cells (green), and CD3+ T cells (blue).
We hypothesized that this unexpected cell population could be attributed to the interference of tafasitamab. To test this hypothesis, an experiment was conducted wherein EDTA‐anticoagulated blood samples were collected from three healthy volunteers. Subsequently, the samples were spiked with tafasitamab (Boehringer Ingelheim Pharma GmbH & Co KG) at three different concentrations: 20, 200, and 2000 µg/mL (30 min incubation at room temperature in the dark). These concentrations were selected in accordance with the recommended intravenous infusion dosage of tafasitamab (12 mg/kg). 14 It is expected that the therapeutic range in the bloodstream, when administered weekly, will typically fall within 200 µg/mL. Additionally, 10‐fold higher and lower concentrations were included to encompass a wide range of tafasitamab dosages. All native and spiked samples were analyzed by flow cytometry using a template similar to that used for MRD analysis in NHL. All analyses were performed on a FACSLyric™ system (BD Biosciences), and the following panel was used: 5 µL CD20 PacB (Biolegend, no. 302 320), 5 µL CD19 PC7 (Beckman Coulter, no. IM3628), 10 µL Igκ FITC + Igλ PE (Dako no. FR48150‐2), CD3 APC (BD, no. 345 767), 2,5 µL CD16 BV605 (BD, no. 563 172), 2,5 µL CD56 BV605 (BD, no. 562 780), and 5 µL CD45 PacO (MHCD4530; Life Technologies).
Flow cytometric evaluation of the native samples confirmed the presence of natural killer (NK) cells, T lymphocytes (T cells), and B lymphocytes (B cells) expressing both CD19 and CD20 antigens, with a polyclonal distribution of sIg kappa and lambda, thereby showing no evidence of disease (Figure 1C). Furthermore, analysis of samples spiked with tafasitamab revealed the presence of a population of polyclonal B cells expressing CD20 without CD19 expression at all three concentrations tested (Figure 1D). The loss of CD19 positivity was expected due to the presence of anti‐CD19 antibodies (i.e., tafasitamab) occupying the available binding sites. Additionally, a substantial cellular population without CD20 expression but with diminished CD19 positivity was detected in all spiked samples. The population also demonstrated strong monoclonal sIg kappa expression and was positive for CD16 and/or CD56. These monoclonal cells were not identified as B cells because they did not demonstrate CD20 expression, but were assumed to be natural killer (NK) cells due to their CD16 and/or CD56 expression. 15 We hypothesized that the apparent sIg kappa monoclonality is caused by the binding of Fc receptors on NK cells with the Fc fraction of tafasitamab, which is an immunoglobulin (Ig)G1‐IgG2 antibody with two identical kappa light chains.
To validate this hypothesis, a second experiment was conducted by adding an Fc receptor blocking agent (1 µL/300 µL sample, 30 min incubation at room temperature in the dark) (Miltenyi, no. 130‐059‐901) to the blood samples of healthy volunteers before spiking with tafasitamab. Subsequent flow cytometric analysis demonstrated the presence of polyclonal CD20‐positive B cells without CD19 expression, as these antigens were occupied by tafasitamab (Figure 1E). However, no cellular population positive for CD16 and/or CD56 with sIg kappa restriction was identified. These findings support our hypothesis that tafasitamab binds to NK cells through the interaction of the Fc region of the antibody with the Fc receptor on NK cells, resulting in a monoclonal sIg kappa‐restricted population in flow cytometric analysis.
Finally, a conclusive experiment was performed to investigate whether the interference is B cell‐ and/or NK cell‐dependent (data not shown). A sample lacking CD19+ CD20+ B cells was analyzed before and after tafasitamab addition. The native sample showed neither B cells (CD19+/CD20+) nor a dim CD19+ population. Upon the addition of tafasitamab (200 µg/mL), a notable population of events lacking CD20 expression yet possessing diminished CD19 positivity was observed. This population, consistent with prior experiments, exhibited strong monoclonal sIg kappa expression and positivity for CD16 and/or CD56. This shows how the interference persists despite the absence of CD19+/CD20+ B cells, confirming its independence from B cells. To investigate NK cell involvement, we replicated the experiment using a sample from a patient with an almost absence of NK cells. After tafasitamab addition, the observed interference did not occur, illustrating the role of NK cells in the emergence of the interfering dim CD19+ κ+ population.
A possible explanation for this phenomenon is the mode of action of tafasitamab. The anti‐lymphoma effect of tafasitamab is mostly related to the interaction of its two antigen‐binding fragments with CD19 on B cells, and the binding of its Fc site to Fc receptors on the surface of immune effector cells. The latter interaction enhances the cytotoxic potency through three different pathways (Figure 2A). The first pathway is antibody‐dependent cell‐mediated cytotoxicity (ADCC), wherein the binding of a monoclonal antibody to CD19 on B cells and subsequent activation of NK cells and gamma delta T cells results in the release of cytotoxic enzymes. Second, tafasitamab stimulates antibody‐dependent cellular phagocytosis (ADCP), as monoclonal antibodies bound on the surface of B cells can bind with their Fc region to Fc receptors on macrophages, resulting in the phagocytic destruction of B cells. Lastly, tafasitamab is responsible for crosslinking CD19 receptors on the cell surface, potentiating caspase‐induced apoptosis. 16 , 17
Figure 2.
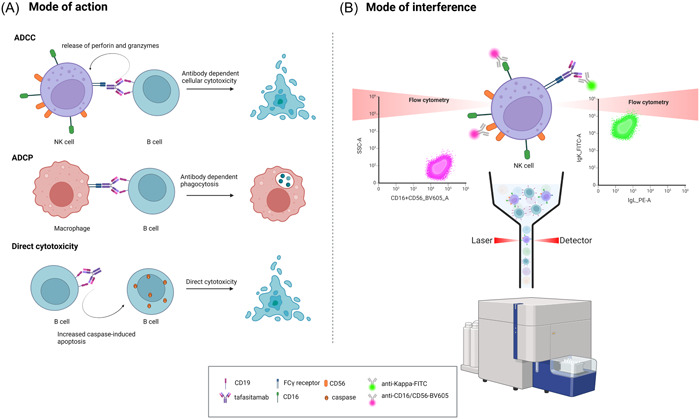
Tafasitamab's mode of action and potential interference mechanism. (A) Mode of action: Antibody‐dependent cell‐mediated cytotoxicity (ADCC) by initiating cytotoxic enzyme release by activated natural killer (NK) cells and gamma delta T cells after binding to CD19 on B cells. Antibody‐dependent cellular phagocytosis (ADCP) by enhancing the interaction with macrophages resulting in B cell phagocytosis. Direct cytotoxicity by enhancing caspase‐induced apoptosis via CD19 receptor crosslinking. (B) Hypothesis of interference: The interaction of tafasitamab and NK cells during the process of ADCC potentially causes sIg kappa monoclonality due to the binding of Fc receptors on NK cells with the Fc portion of tafasitamab, an (Ig)G1‐IgG2 κ antibody. Flow cytometric positivity for CD16 and/or CD56 illustrates the role of NK cells in the observed interference.
The emergence of the interfering population is postulated to stem from the formation of tafasitamab‐NK cell complexes within the ADCC pathway, resulting from the binding of Fc receptors on NK cells with the Fc portion of tafasitamab, presenting itself as a sIg kappa monoclonal population. The concomitant expression of CD16 and/or CD56 can be elucidated by the binding of fluorescent anti‐CD16/CD56 detection antibodies to CD16 and/or CD56 antigens on the NK cell's surface within this complex (Figure 2B). Furthermore, given the fact that no other B cell markers were expressed (e.g., CD20 in the spiked samples, CD5 for patient 1, and CD10 for patient 2) and that our experiments have shown that the interference is B cell‐independent, the diminished CD19 expression is likely attributed to nonspecific staining of the NK cell‐tafasitamab complex.
As clinical trials that include tafasitamab in their therapeutic regimen for NHL show encouraging results, it can be expected that the antibody will be used more regularly in the future. Consequently, the described interference could be encountered more frequently and should be considered when performing flow cytometric analysis to avoid an erroneous diagnosis of the remaining lymphoma cells. To differentiate between monoclonal sIg kappa B cells and interference due to the binding of tafasitamab to NK cells, it is essential to implement strategies aimed at circumventing flow cytometric interference. One potential approach is to explore the possibility of targeting alternative antigens, such as CD20, in addition to CD19 to determine whether the flow cytometric events in question are malignant B cells or the result of interference. However, the use of CD20 alone as a distinguishing marker may be inadequate in cases where patients concurrently receive anti‐CD20 monoclonal antibodies, as exemplified in the two cases. The targeted antigens should preferably be expressed in all stages of B cells (e.g., cytoplasmic CD22). 18
In conclusion, this letter highlights the risk of false positive results during flow cytometric MRD analysis on patient samples treated with tafasitamab, and thus the risk of falsely reporting residual lymphoma cells and treatment failure. Awareness of this interference is crucial for clinical laboratory practitioners to ensure the accurate interpretation of MRD analysis and appropriate clinical decision‐making.
AUTHOR CONTRIBUTIONS
Lisa Proost, Stijn Lambrecht, Mattias Hofmans, and Sander De Bruyne contributed to the study design, data collection, analysis, and interpretation of the data. Lisa Proost and Sander De Bruyne did figure preparation and wrote the manuscript. All authors revised the content and gave final approval of the submitted version.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING
This research received no funding.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Kumar A, Eyre TA, Lewis KL, Thompson MC, Cheah CY. New directions for mantle cell lymphoma in 2022. Am Soc Clin Oncol Educ Book. 2022;42:614‐628. [DOI] [PubMed] [Google Scholar]
- 2. Galimberti S, Luminari S, Ciabatti E, et al. Minimal residual disease after conventional treatment significantly impacts on progression‐free survival of patients with follicular lymphoma: the FIL FOLL05 trial. Clin Cancer Res. 2014;20(24):6398‐6405. [DOI] [PubMed] [Google Scholar]
- 3. Salles G, Długosz‐Danecka M, Ghesquières H, Jurczak W. Tafasitamab for the treatment of relapsed or refractory diffuse large B‐cell lymphoma. Expert Opin Biol Ther. 2021;21(4):455‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Her JH, Pretscher D, Patra‐Kneuer M, et al. Tafasitamab mediates killing of B‐cell non‐Hodgkin's lymphoma in combination with γδ T cell or allogeneic NK cell therapy. Cancer Immunol Immunother. 2022;71(11):2829‐2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gajra A, Rathish YS, Jeune‐Smith Y, Klink AJ, Feinberg B. Assessment of measurable residual disease (MRD) in chronic lymphocytic leukemia (CLL) and multiple myeloma (MM) among US community hematologists/oncologists (cH/O). Blood. 2020;136:42‐43. [Google Scholar]
- 6. Riva G, Nasillo V, Ottomano AM, et al. Multiparametric flow cytometry for MRD monitoring in hematologic malignancies: clinical applications and new challenges. Cancers. 2021;13(18):4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Galimberti S, Genuardi E, Mazziotta F, et al. The minimal residual disease in non‐Hodgkin's lymphomas: from the laboratory to the clinical practice. Front Oncol. 2019;9:528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Delgado J, Papadouli I, Sarac SB, et al. The European Medicines Agency review of tafasitamab in combination with lenalidomide for the treatment of adult patients with relapsed/refractory diffuse large B‐cell lymphoma. HemaSphere. 2021;5(12):e666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Laurie HS, Christian WS, Stefano L, et al. A phase 3 study to evaluate the efficacy and safety of tafasitamab plus lenalidomide and rituximab versus placebo plus lenalidomide and rituximab in patients with relapsed/refractory (R/R) follicular lymphoma (FL) or marginal zone lymphoma (MZL). J Clin Oncol. 2021;39(15_suppl):TPS7568. [Google Scholar]
- 10. Staber PB, Jurczak W, Greil R, et al. Tafasitamab combined with idelalisib or venetoclax in patients with CLL previously treated with a BTK inhibitor. Leuk Lymphoma. 2021;62(14):3440‐3451. [DOI] [PubMed] [Google Scholar]
- 11. Makita S, Tobinai K. Antibody therapy targeting CD19 for B‐cell non‐Hodgkin's lymphoma. Ann Oncol. 2018;29(5):1086‐1089. [DOI] [PubMed] [Google Scholar]
- 12. Seegmiller AC, Hsi ED, Craig FE. The current role of clinical flow cytometry in the evaluation of mature B‐cell neoplasms. Cytom B Clin Cytom. 2019;96(1):20‐29. [DOI] [PubMed] [Google Scholar]
- 13. Klisovic RB, Leung WH, Brugger W, et al. A phase 2a, single‐arm, open‐label study of tafasitamab, a humanized, Fc‐modified, anti‐CD19 antibody, in patients with relapsed/refractory B‐precursor cell acute lymphoblastic leukemia. Cancer. 2021;127(22):4190‐4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nedved A, Maddocks K, Nowakowski GS. Clinical Treatment guidelines for tafasitamab plus lenalidomide in patients with relapsed or refractory diffuse large B‐cell lymphoma. Oncologist. 2023;28(3):199‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Poli A, Michel T, Thérésine M, Andrès E, Hentges F, Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. 2009;126(4):458‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gonzalez‐Rodriguez AP, Villa‐Álvarez M, Sordo‐Bahamonde C, Lorenzo‐Herrero S, Gonzalez S. NK cells in the treatment of hematological malignancies. J Clin Med. 2019;8(10):1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zinzani PL, Minotti G. Anti‐CD19 monoclonal antibodies for the treatment of relapsed or refractory B‐cell malignancies: a narrative review with focus on diffuse large B‐cell lymphoma. J Cancer Res Clin Oncol. 2022;148(1):177‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. DiGiuseppe JA, Wood BL. Applications of flow cytometric immunophenotyping in the diagnosis and posttreatment monitoring of B and T lymphoblastic leukemia/lymphoma. Cytom B Clin Cytom. 2019;96(4):256‐265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


