Summary
Static high magnetic fields (MFs) from 7 T to 9 T can elicit behavioral responses in rodents such as suppression of rearing, locomotor circling, and acquisition of a conditioned taste aversion (CTA). MF exposure also induces c-Fos expression in the visceral and vestibular nuclei of the brainstem, suggesting the stimulation of some sensory pathways. It is not clear, however, if the effects of the MF are caused by exposure to the uniform maximal field at the center of the magnet, or by exposure to the steep field gradients along the bore of the magnet during the rat’s placement. In addition, the site of action within the rat is unknown. In an attempt to limit MF exposure to rostral or caudal portions of the rats’ body, we exposed male and female rats at different positions within the bore of a 14.1 T superconducting magnet ranging from 2 cm (1.6 T at the head) to 155 cm (0.05 T at the head), with the center of the bore at 65 cm (14.1 T across the whole body). This approach also allowed us to expose rats to the maximal field strength (14.1 T) vs. the maximal field gradients (54 T/m). To assess both immediate and delayed behavioral effects, locomotor and CTA responses were recorded. A small but significant CTA was seen after exposure of the head to the lowest MF tested (0.05 T at 155 cm). Graded effects were seen, however, with greater circling and CTA acquisition as the MF strength increased at the rostral end of the rat. This suggests a cephalic site of action. Furthermore, maximal circling and CTA was induced after exposure to the uniform center field, and not after exposure to high field gradients on either side of the center. This suggests that the behavioral responses seen after MF exposure are a consequence of the uniform static field at the center of the magnet, and are not caused by passage through, or exposure to, the vertical field gradients. Female rats responded similarily to male rats, although magnet-induced CTA appeared resistant to extinction in female rats.
Keywords: vestibular system, magnetic resonance imaging, conditioned taste aversion, circling, rearing
Introduction
Although the static high magnetic fields (MFs) of magnetic resonance imaging (MRI) machines are usually considered benign and undetectable to mammals, there is some evidence that they have transient sensory effects on humans. A survey of MRI engineers found statistically significant reports of vertigo and nausea experienced while working on 4 tesla (T) magnets [1]. More recently, investigators using an experimental 8.4 T MRI machine reported vertigo after rapid head movements within the bore of the magnet [2].
We have accumulated evidence in rodents that high MFs exert behavioral and neural effects. At field strengths of 7 T and above, three phenomena have been observed: 1) After restraint within the MF, rats walked in tight circles for 1–2 minutes; the direction of circling is dependent on the rats orientation within the MF, such that they circle counterclockwise after facing the south pole and clockwise after facing the north pole [3]. 2) When consumption of a novel taste solution (e.g. a glucose+saccharin solution) is paired with exposure to the MF, rats acquired a conditioned taste aversion (CTA) and avoided consumption of the taste in subsequent 2-bottle tests [3, 4]. 3) During or after 30-min exposure to the MF, neurons within visceral and vestibular relays of the brain stem were activated, as revealed by the induction of c-Fos immunoreactivity [5]. Furthermore, we have evidence that rats are able to detect and avoid voluntary entry into high MFs. Rats were trained to climb a vertical “ladder” of mesh tubing to reach a palatable food reward. When the ladder was inserted through the bore of a 14.1 T magnet, the rats would traverse the ladder through 14.1 T at most one time, and subsequently refused to climb into the bore of the magnet [6].
These results demonstrate both immediate (circling, avoiding the field) and delayed effects (CTA, c-Fos) of high strength static MFs on rats. Because circling and CTA are also induced by vestibular perturbations such as unilateral labyrinthectomy or whole-body rotation, the high strength MF may interact with the vestibular system of the rat. The induction of c-Fos in vestibular relays of the brainstem such as the medial vestibular nucleus and prepositus nucleus supports this hypothesis [5].
While these findings suggest that high MFs interact with the vestibular system, the peripheral sites of interaction or detection are unknown. Typically, the analysis of receptive sites for a stimulus would include the focal stimulation of specific parts of the body. Site-specific stimulation can define the sites sufficient for the production of behavioral responses. If stimulation of a particular site evokes an optimal response, then that location may house part or all of the receptive apparatus or mechanism.
Focused or site-specific application is straightforward for many categories of sensory stimuli and has proved informative in many systems. For example, during the investigation of the detection of ionizing radiation by mammals, it was possible to limit irradiation of rats or monkeys to either the abdomen or the head using focal x-ray machines or employing lead shielding to limit exposure. Ultimately, 3 sites were identified that mediated 3 different behavioral responses to ionizing radiation: 1) in rats, the abdomen mediated delayed acquisition of conditioned taste aversions via radiation-induced histamine release[7, 8]; 2) in both rats [9] and monkeys [10], the olfactory epithelium mediated an olfactory-based response, possibly due to the induction of ozone in the nasal passages adjacent to the olfactory receptors; and 3) in monkeys, the retina mediated a visual response generated by the action of ionizing radiation on photoreceptors in dark-adapted animals [11]. The necessary roles of the abdomen, olfactory system, and retina were subsequently confirmed by pharmacological blockade of histamine in rats [7] or by oblation studies in rats [9], monkeys [11] and other species [12].
Thus, in the analysis of MF effects it would be helpful to limit exposure to specific somatic regions, e.g. the abdomen vs. the head. Unfortunately, it is impossible to shield against MFs in the higher range typical of MRI machines. There is no substance that is opaque to these higher MFs as exists for electromagnetic radiation (e.g. lead for x-rays), nor are there ways to limit MFs as exist for interfering electric fields (e.g. a Faraday cage). The fringe of the high MFs generated by NMR or MRI machines typically falls off across meters, rather than the centimeters needed for localization in rodents. Indeed, it may be this gradient of the MF that imposes a differential field across a region of the rat’s body and thereby induces the responses of circling and CTA reported above.
In order to approximate site-specific exposure to the high MF, we placed rats at different positions along the bore of a superconducting magnet with a fixed field strength of 14.1 T used for biochemical nuclear magnetic resonance (NMR) studies (see Figure 1A). It can be seen that the magnet has a uniform central field in the center of the bore, with a steep field gradient extending along the vertical axis. While all rats were exposed to MFs well above earth-strength, exposure of the body and head was roughly limited to one or both of the two salient components of the MF: the uniform center of constant 14.1 T or the steep gradient above and below the maximum MF. By varying the vertical position within the bore, rats could be exposed such that a) both the head and the body would be exposed to the uniform, maximal MF at the center; b) the head would be exposed in the center to 14.1 T while the body would be in the steep gradient, or vice versa; or c) both the head and the body would be in the steep gradient above or below the maximal MF at the center.
Figure 1.
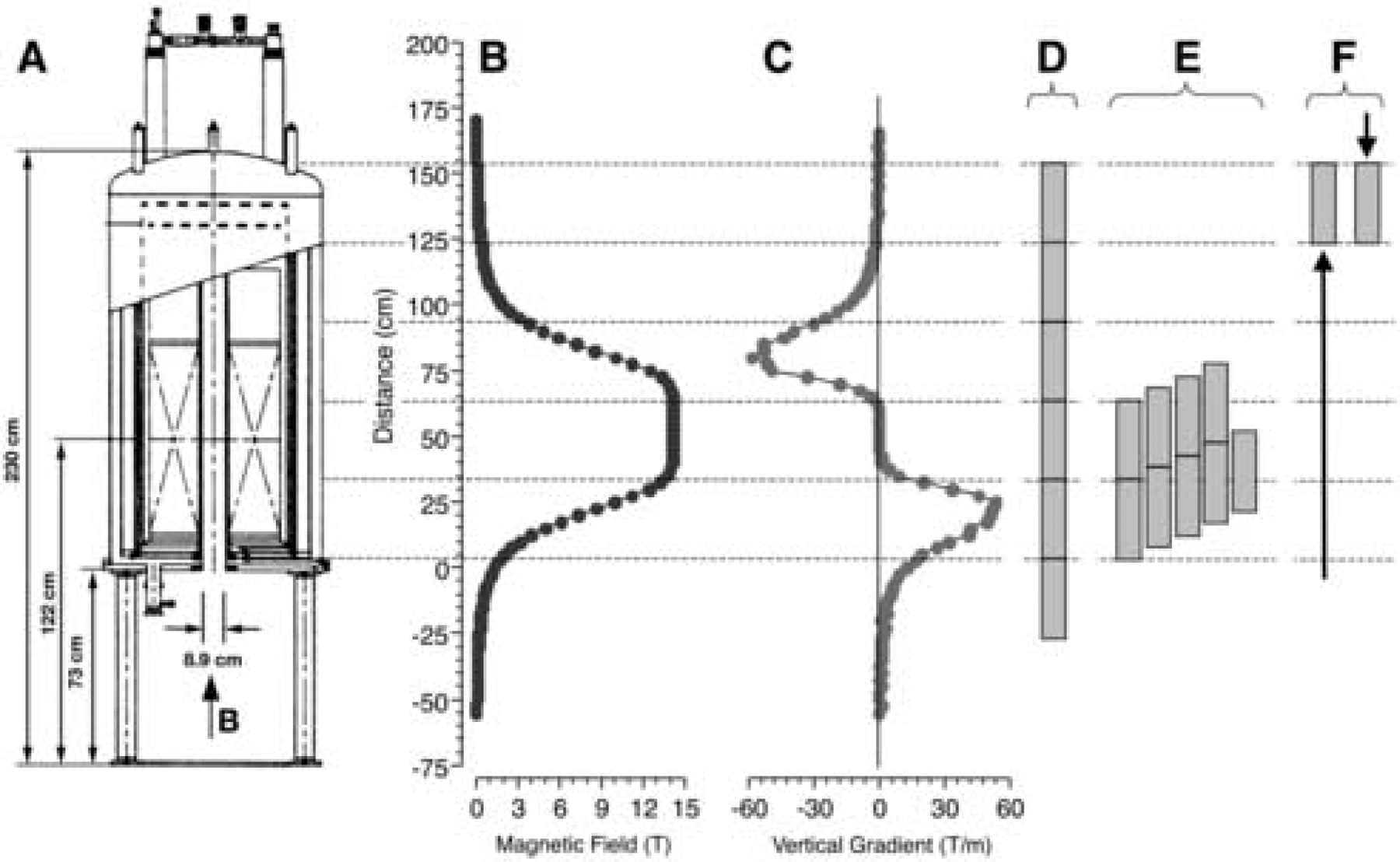
A. Cross sectional schematic of the 14.1 T superconducting NMR magnet. B. The magnetic field measured in the center of the magnet’s bore. The opening to the magnet’s bore is set at 0 mm. The maximum central field (B0) is 14.1 T. C. The field gradient along the vertical axis of the magnet (dB/dz) derived from the measured magnetic field. To expose rats to different MF strengths and gradients, rats were restrained in Plexiglas tubes and placed at different intervals with the vertical bore of the magnet. The positions of the rats and their alignment with the measured magentic field are shown for Experiments 1 (D), 2 (E), and 3 (F). All rats were the raised from the bottom of the bore into the magnetic field, except for the one group of rats in Experiment 3 that were lowered from the top of the bore.
In total, rats were tested at 13 positions within the field. The behavioral response to exposure was measured by recording acute locomotor activity (circling and rearing) and by testing the acquisition and extinction of CTA with 2-bottle tests.
In addition, both male and female rats were exposed to the MF to determine if there were any significant sex differences in behavioral responses. In addition to intrinsic interest in the comparison, there was a practical reason for verifying that the effect of MFs on females was similar to the effect on males. The small 89mm bore of the NMR superconducting magnet can only accommodate a male Sprague-Dawley rat for a brief developmental period before he grows too large to fit in the restraint tube. This limits the duration and timing of experiments with the superconducting magnet. In contrast, female Sprague-Dawley rats remain small enough to fit in the restraint tube and the bore of the magnet for several months. Thus it would increase the flexibility and duration of possible experiments if female rats could be employed. In order to validate the use of female rats, the behavioral responses of male and female rats to MF exposure were assessed in parallel.
Methods
Animals
Male and female Sprague-Dawley rats (175–200 g; Charles River) were housed individually in polycarbonate cages in a temperature-controlled colony room at the National High Magnetic Field Laboratory at The Florida State University. The light/dark cycle was 12:12 with lights on at 0700 hours. All conditioning trials were conducted during the light cycle. The rats had free access to pelleted Purina Rat Chow 5001 and deionized-distilled water except as specified otherwise. All procedures were approved by the Institutional Animal Care and Use Committee of Florida State University.
Magnet
Rats were placed at different positions along the bore of a superconducting magnet in order to approximate site-specific application of the high MF. A 600 MHz Magnex Cryo magnet with an 89 mm bore and a fixed central field strength (B0) of 14.1 T was used (see Figure 1A). The magnet contained shim magnets extending along the magnet’s bore for approximately ± 15 cm from the magnet core, which were used to stabilize the MF and to give a central core field of uniform strength. The MF was orientated vertically so that the positive pole was at the top of the magnet. The magnet was operated without radiofrequency pulses, so rats were exposed to a static MF only.
The gradient of the MF along the bore of the magnet was mapped by pulling a copper coil through the magnet at a constant speed [6]. The position of the coil as it ascended through the bore of the magnet was recorded to the nearest 1 mm and the voltage generated by the coil as it traversed the MF was recorded at a rate of 1000 times per second. The voltage measurements were integrated, producing the field strength of the magnet at any position. The resulting curve was calibrated, knowing that the peak field strength (B0) of the magnet was 14.1 T (see Figure 1B). The approximate field gradient along the vertical axis (dB/dz) was derived from the measured MF (dB/dT).
Conditioning
Eight days prior to the conditioning day, the rats were placed on a water restriction schedule under which they received daily water access in one drinking session, during which a water bottle was presented simultaneously with an empty bottle to accustom the rats to a 2-bottle choice. The first daily session was 3 h in length and the session times were diminished each day so that for two days before conditioning the rats received water access in a single 10-min session.
On the conditioning day, rats were given access to 0.125% sodium saccharin solution (saccharin) for 10 min. Immediately following saccharin access, rats were placed in restraint tubes for sham- or magnet-exposure. The restraint tubes were 30- cm in length with an inside diameter of 5.6 cm and an outside diameter of 6.4 cm. A plug was inserted into the rostral end of the tube and held in position by nylon screws. The inside of this rostral plug was fabricated in a cone shape to accommodate the head of the rat. A 1- cm hole was bored in this plug at the apex of this cone to allow fresh breathing air. A second plug was inserted into the caudal end of the tube and could be adjusted to restrain the movement of the rat. A hole in the center of this plug accommodated the rat’s tail. When in the tube, the rat was almost completely immobilized.
Restrained rats were transported from the animal facility to the 14.1T magnet in approximately 30 seconds. Rats exposed to the MF were inserted into the bore of the magnet for 30 min. All rats were inserted into position in less than 10 seconds. As controls for the effects of restraint, some rats were “sham-exposed” by placing them in the restraint tubes and inserting them into an opaque PVC pipe placed in the same room as the magnet but beyond the 5-gauss line of the MF.
Behavioral Scoring
After 30-min sham-exposure or exposure within the bore of the magnet, the rostral plug of the restraining tube was removed and each rat was released into an open polycarbonate cage (37 cm wide by 47 cm long by 20 cm high) with cob bedding. The locomotor behavior of each rat was recorded on videotape for two minutes after release into the cage. (Most rats exhibited locomotor effects of the MF for less than 1 minute; thus, 2 minutes of recording captured most of the phenomena of interest.) The rat was then returned to its home cage and ad libitum water was returned. The videotapes were scored later by an observer blind to the rats’ treatment. Instances of tight-circling behavior were quantified. Rats were scored as “circling” if they moved continuously around a full circle with diameter less than the length of the rat’s body. Partial circles or circles interrupted by stationary pauses were not counted. Rearing behavior (both forepaws off the floor of the cage and one or both forepaws on the side of the cage) was also scored at this time.
The strength of the CTA induced by the magnet was measured with daily 24-h, 2-bottle preference tests that were initiated the day after conditioning. Two bottles were placed on the cages, one containing saccharin and the other distilled water. Fluid consumption was measured every 24 h and a preference score was calculated as the ratio of saccharin to total fluid consumption:
The preference tests were continued for up to 14 post-conditioning test days. The left/right position of saccharin and water bottles on the rats’ cages was reversed each day. Because saccharin access during the preference tests was not paired with any treatment, the preference tests constituted extinction trials. The CTA of an experimental group was considered extinguished when the average saccharin preference was not different from the average preference of rats exposed to 0 T. Preference for saccharin measured during the first 24-h, 2-bottle test was analyzed as the magnitude of CTA; changes in preference across repeated 2-bottle tests were analyzed for extinction rate.
Statistics
Comparisons between groups on single-day data were analyzed with appropriate ANOVA’s or t-tests (Statistica). Because differences in observations of locomotor circling were recorded as frequency data, they were analyzed by χ2 tests. Results collected over multiple 2-bottle preference test days were analyzed by 2-way ANOVA, with groups as one factor and test days as the second factor, which consisted of repeated sampling of the same subjects across test days (Statistica). Post-hoc comparisons were made with the Tukey test. Data are presented as mean ± standard error of the mean.
Experiment 1. Male and Female Exposure at 5 Positions
The first experiment compared the behavioral responses of male and female rats to MF exposure. Five groups each of male and female rats were exposed at different vertical placements within the bore of the magnet to determine the most effective positions within the MF. Male (n= 35) and female rats (n= 32) were placed on a water restriction schedule as above. On the day of conditioning, rats in groups of 5 were given 10-min access to 0.125% saccharin. Immediately after saccharin access, the rats were placed in 5 individual restraint tubes. The tubes were inserted into the bore of the magnet so that they were stacked vertically within the bore, and held in place by a PVC support resting on the ground. A separate group of male rats was given 10 min access to 0.12% saccharin and then exposed with their head positioned at 2 cm above the opening of the magnet’s bore.
As each restraint tube was 30 cm in length, the position of the heads of rats was approximately 2, 35, 65, 95, 125, or 155 cm from the opening of the bore (n= 5–7 male or female rats were exposed at each position; see Figure 1D for the position of the top five tubes). The uniform maximum of the MF at 14.1 T extended from 40 to 65 cm. Table 1 shows the approximate MF intensity and the vertical field gradient at the level of the head and the abdomen (15 cm below the head) for each of the 6 positions. Note that when rats were raised into the 3 highest positions, they first traversed an increasing MF to 14.1 T and then passed through a decreasing MF to reach their final positions.
Table 1.
Intensity of the magnetic field and the vertical field gradient experienced by rats in Experiment 1 exposed at different positions from the entrance of the bore of the 14.1 T magnet.
| Position of head (cm) | Magnetic field at head (T) | Gradient at head (T/m) | Magnetic field at abdomen (T) | Gradient at abdomen (T/m) |
|---|---|---|---|---|
| 2 | 1.6 | 12.8 | .50 | 2.7 |
| 35 | 13.7 | 20.1 | 7.3 | 49.36 |
| 65 | 14.1 | −0.9 | 14.1 | 0 |
| 95 | 3.0 | −30.2 | 9.9 | −51.2 |
| 125 | 0.32 | −1.8 | 0.85 | −7.3 |
| 155 | 0.05 | −0.9 | 0.14 | −0.9 |
Rats were exposed to the magnet field for 30 minutes. Upon removal from the magnet, rats in the lower 4 positions (with heads positioned at 2, 35, 65, and 95 cm) were released from restraint into the locomotor test cage and videotaped for 2 min. Because of time and equipment constraints, rats in the upper 2 positions (125 and 155 cm) were not videotaped.
As a control for the effects of restraint, a single group of male rats (n=7) was given 10 min access to saccharin and then sham-exposed as described above. After 30 minutes of sham-exposure, the rats were released into the locomotor test cage and videotaped for 2 min. After exposure and videotaping, all rats were returned to their home cages and given ad libitum access to water overnight. The day after conditioning the 24-h, 2-bottle preference tests were begun and continued for 14 days.
Experiment 2: Effects of Exposure at 35–81 cm
The results of Experiment 1 demonstrated that the greatest behavioral response to the MF occurred after exposure with the head positioned between 35 cm and 95 cm, and the measured maximum occurred at 65 cm. To determine if even more pronounced effects could be detected at intermediate positions, male rats were exposed with their heads positioned at 6 cm intervals between 35 cm and 95 cm from the opening of the bore.
Male rats (n=61) were placed on a water restriction schedule as above. On the day of conditioning, rats in groups of 1 or 2 were given 10-min access to 0.125% saccharin. Immediately after saccharin access, rats were placed in individual restraint tubes. The tubes were inserted into the bore of the magnet singley or in pairs so that they were stacked vertically within the bore. Each tube or pair of tubes was elevated within the bore by a PVC support so that the rats’ heads were positioned at 5–7 cm intervals between 35 and 81 cm from the opening of the magnet’s bore (see Figure 1E for the positions of the tubes). Altogether, nine groups of rats (n= 6–8 /group) were exposed to the MF while positioned at 35, 40, 47, 52, 58, 65, 69, 76, or 81 cm from the opening of the magnet’s bore. Table 2 shows the approximate MF intensity and vertical field gradient at the level of the head and the abdomen (15 cm below the head) for each of the 9 positions.
Table 2.
Intensity of the magnetic field and the vertical field gradient experienced by rats in Experiment 2 exposed at different positions from the entrance of the bore of the 14.1 T magnet.
| Position of head (cm) | Magnetic field at head (T/m) | Gradient at head (T/m) | Magnetic field at abdomen (T) | Gradient at abdomen (T/m) |
|---|---|---|---|---|
| 35 | 13.7 | 20.1 | 7.3 | 49.4 |
| 40 | 14.1 | 4.6 | 9.9 | 52.1 |
| 47 | 14.1 | 0 | 13.2 | 33.8 |
| 52 | 14.1 | 0 | 13.9 | 9.1 |
| 58 | 14.1 | 0 | 14.1 | 0.9 |
| 65 | 14.1 | −0.9 | 14.1 | 0 |
| 69 | 13.9 | −9.1 | 14.1 | 0 |
| 76 | 11.9 | −49.4 | 14.1 | 0 |
| 81 | 9.2 | −54.1 | 14.0 | −1.9 |
Immediately after the 30-min exposure, the rats were released into locomotor test cage and videotaped for 2 min. Rats were then returned to their home cages and given ad libitum water overnight. The day after conditioning, the 24-h, 2-bottle preference tests were begun and continued for 9 days.
In order to provide a point of reference between the results of Experiment 1 and Experiment 2, the data from the sham-exposed male rats (n= 7) from Experiment 1 were used for statistical comparisons. (We have consistently found that sham-exposed rats do not circle and reliably display a high preference for saccharin in subsequent 2-bottle tests [3, 4, 13, 14], and so the sham exposed group of Experiment 1 provides representative control data.)
Experiment 3: Effects of Low-Field Exposure
The results of Experiment 1 demonstrated a small but significant effect on locomotor activity and CTA when rats were exposed with their heads positioned at 125 and 155 cm from the opening of the bore of the magnet. Therefore it is possible that exposure to the relatively low MF at these positions (< 0.32 T at the head) is sufficient to affect locomotion and induce CTA. In order to position rats at 125 and 155 cm, however, the rats in Experiment 1 were raised through an ascending MF, through the uniform and maximum MF at the center, and then through the descending gradient in the upper part of the magnet’s bore to their final position. Therefore, an alternative possibility is that acute movement through the bore of the magnetic is sufficient to produce behavioral effects. In order to distinguish these possibilities, two groups of rats were exposed for 30 min to 0.05 T with their heads positioned at 155 cm. One group of rats was raised from the bottom of the magnet, and thus experiencedthe ascending gradient, the central 14.1 T MF, and the descending gradient above the magnet’s center. The second group was lowered from the top of the magnet to the same position, experiencing only the low MF and the shallower gradient at the top of the magnet.
Male rats (n=24) were placed on a water restriction schedule as above. On the day of conditioning, rats were given 10-min access to 0.125% saccharin. Immediately after saccharin access, the rats were placed in individual restraint tubes. Two groups of rats (n=8 each) were exposed for 30 min in the magnet by the two methods described above (see Figure 1F for the positions of the tubes). A third group of rats (n=8) was sham-exposed outside the magnet.
Immediately after the 30-min exposure, the rats were released into the locomotor test cage and videotaped for 2 min. Rats were then returned to their home cages and given ad libitum water overnight. The day after conditioning, the 24-h 2-bottle, preference tests were begun and continued for 7 days.
Results
Experiment 1: Exposure at 5 Positions within 14.1 T Magnet
Locomotor Effects
Exposure for 30 min at 35 and 65 cm within the bore of the 14.1 T magnet induced locomotor circling in some but not all male rats; no circling was observed in male rats exposed at 2 cm or 95 cm within the magnet (see Figure 2A). This difference in circling between groups was significant by chi-squared analysis [χ2 (4) = 11.93, p < 0.05].
Figure 2.
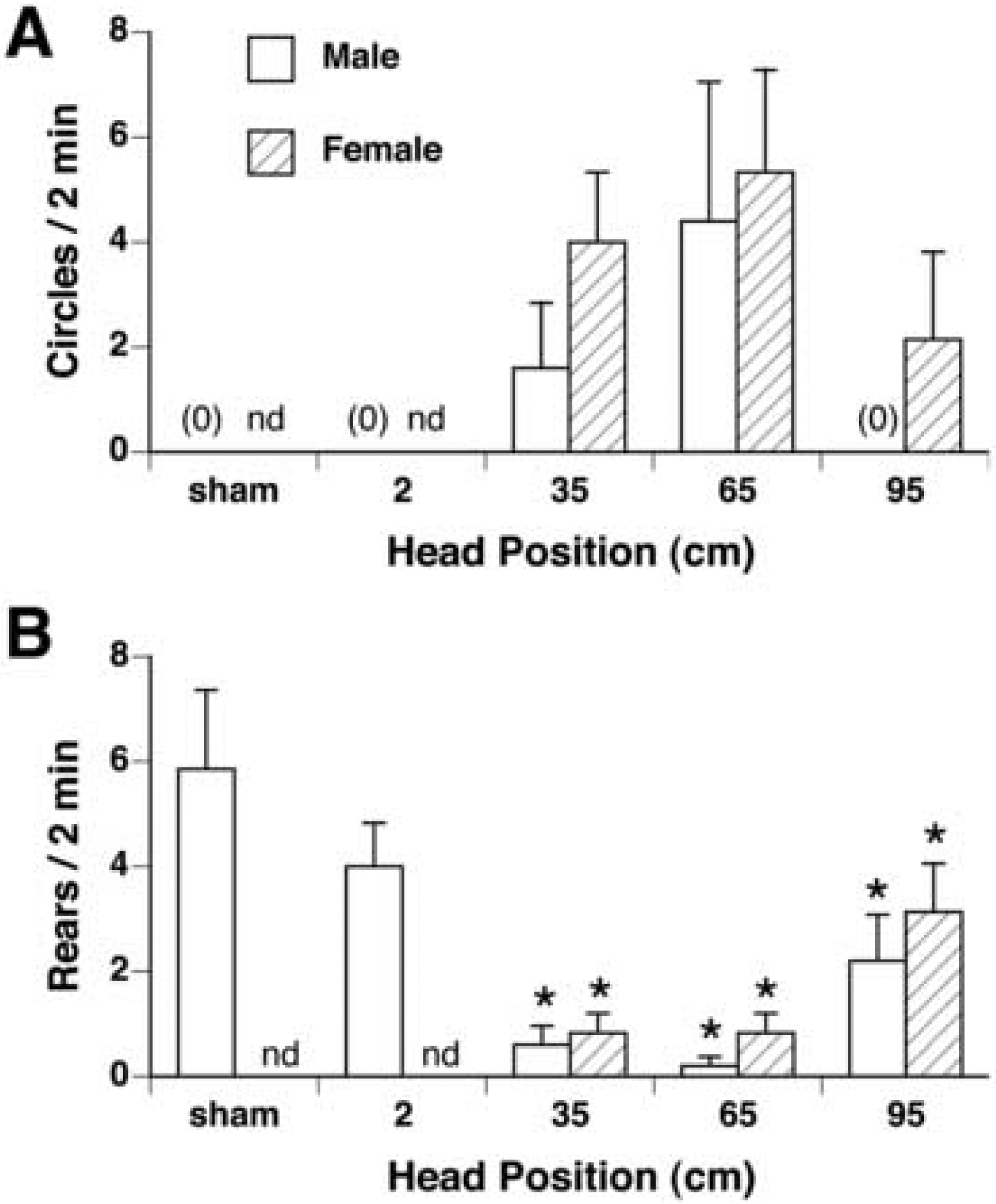
Quantification (mean ± s.e.m.) of locomotor circling (A) and rearing (B) in male (white bars) and female rats (hatched bars) after 30-min exposure within the 14.1 T magnet at the given head positions (n=5–7/group). A. Significant circling was found by χ2 in male rats after 35 and 65 cm exposure, and in female rats after exposure at 35, 65, and 95cm. B. Rearing was significantly suppressed in both male and fermale rats after exposure at 35, 65, and 95cm. nd, not determined for female rats. * p < 0.05 vs sham-exposed rats.
Circling was also induced in female rats exposed at 35, 65, and 95 cm [χ2 (4) = 8.41, p < 0.05], with no differences between the 3 groups (see Figure 2A). There was no sex difference in circling at any head position.
Compared to rearing in sham-exposed rats, rearing was suppressed in male and female rats exposed with heads at positions 35, 65, and 95 cm from the bottom of the magnet [males: F(3,20) = 7.6, p< 0.005; females: F(3,23) = 6.57, p < 0.005] (see Figure 2B). There was no difference in rearing among the 3 magnet-exposed groups at 35, 65 and 95 cm, however, nor was any sex difference found between groups.
Conditioned Taste Aversion
On conditioning day prior to magnet- or sham-exposure, rats drank an average of 6.1 ± 0.3 g of saccharin; there was no difference in intake among groups of the same sex, and no difference between sexes.
Male rats exposed within the MF at all positions except 2 cm expressed a significant CTA on the first day of 2-bottle testing [by one-way ANOVA, F(6,35)=6.42, p < 0.0001], such that all had lower preferences for saccharin compared to sham-exposed rats (See Figure 3A). (The preference scores of rats exposed at 2 cm were not significantly different from sham rats at p = 0.06.) Male rats exposed with heads at 35 and 65 cm had stronger aversions than other groups, however.
Figure 3.
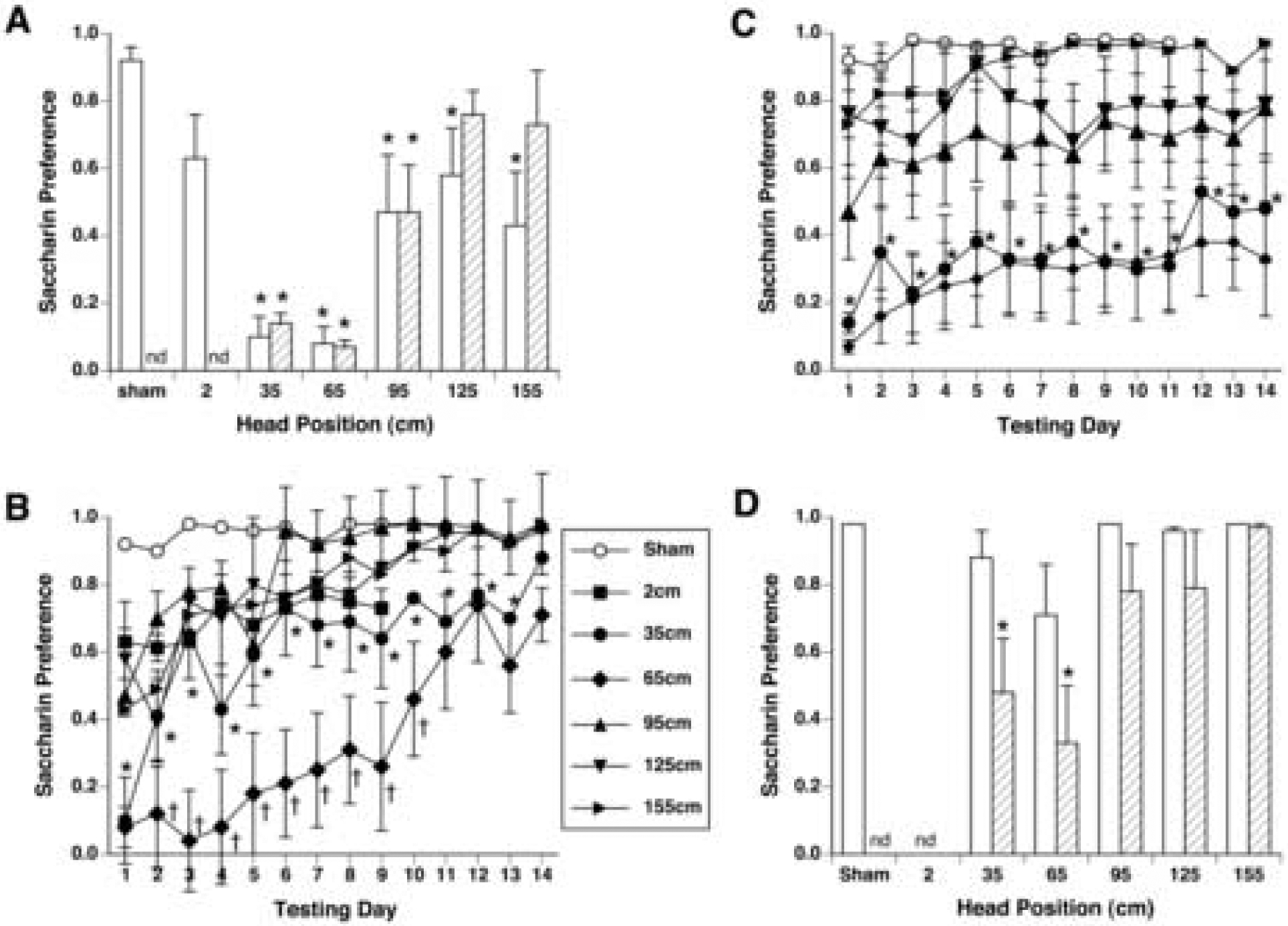
A. Initial magnitude of conditioned taste aversion (CTA) measured by saccharin preference (mean ± s.e.m.) on the first day of 2-bottle preference testing in male (white bars) and female rats (hatched bars.) B. Time course of CTA extinction across 14 days of 2-bottle preference tests in male rats. * p <0.05 35-cm group vs. sham-exposed rats; † p < 0.05 65-cm group vs. 35-cm group. C. Time course of CTA extinction in female rats. Saccharin preference in female rats exposed at 35 and 65 cm was significantly lower than sham-exposed rats on all 14 days. * p < 0.05 35-cm and 65-cm groups vs. sham-exposed rats. Other groups were not different from sham-exposed rats. nd, not determined for female rats. D. Saccharin preference on the last day of 2-bottle preference testing (day 14). The CTA of male rats (white bars) extinguished by day 14, while the saccharin preference of female rats (hatched bars) exposed at 35 cm and 65 cm were still significantly lower than sham-exposed rats. * p < 0.05 vs sham-exposed rats.
Across 14 days of 2-bottle testing, two-way ANOVA revealed a significant interaction of groups and test days in males (F[5,65] = 2.28, p < 0.001; see Figure 3B). There was no significant difference between male rats exposed at 95, 125 and 155 cm on almost all days; rats in all 3 groups were significantly different from sham-exposed rats until days 6–8. Male rats exposed at 35 and 65 cm had stronger CTAs that took longer to extinguish. Rats exposed at 35 cm maintained significantly lower preferences than sham rats until day 14. Rats exposed at 65 cm had significantly lower preferences than rats exposed at 35 cm on days 2–10, and significantly lower preferences than sham rats on all 14 days.
Female rats exposed at 35, 65, and 95 cm within the magnet field expressed a significant CTA on the first day of 2-bottle testing [by one-way ANOVA, F(5)=14.2, p < 0.001], such that these 3 groups had lower preferences for saccharin compared to sham-exposed rats (see Figure 3A). Female rats exposed with heads at 35 and 65 cm had stronger aversions than rats exposed at 95 cm.
In female rats across 14 days of 2-bottle testing, two-way ANOVA revealed a significant effect between groups [F(5) = 6.53, p < 0.001] and across days [F(13) 5.20, p < 0.001], but no interaction, reflecting the similar extinction pattern of all groups (see Figure 3C). Interestingly, the CTA expressed by female rats appeared resistant to extinction. Thus the preferences of rats exposed to the MF at 35 and 65 cm were not different from each other (except on day 2) and were significantly lower than the preferences of all other groups across all 14 days. Female rats exposed at 95 cm had an intermediate CTA, with preferences remaining significantly lower than the preferences of sham-exposed rats but higher than the 35 and 65 cm groups across all 14 days. The preferences of female rats exposed at 125 and 155 cm were not different from the preferences of sham-exposed rats on any day.
Comparison of Magnetic Field-Induced CTA in Males and Females
The rank ordering of CTA strength induced by position in the magnet as measured by preference on the first day of 2-bottle testing was broadly similar in both sexes: 35 = 65 > 95 = 125 = 155 cm > sham in male rats, and 35 = 65 > 95 > 125 = 155 cm = sham in female rats. Two-way ANOVA comparison of male and female preferences on the first day of testing revealed a significant effect of position [F(4, 49) = 11.19, p < 0.001], but no significant effect of sex and no interaction (see Figure 3A).
The time course of extinction, however, appeared to be different between male and female rats. Two-way ANOVA comparison of male and female preferences on Day 14, the last day of extinction testing, revealed a significant effect of position [F(4, 49) = 4.32, p < 0.005] and a significant effect of sex [F(1, 49) = 8.40, p < 0.01], although there was no interaction (see Figure 3D). Thus both male and female rats initially acquired similar CTAs hat were dependent on exposure position within the MF. While the CTA in male rats extinguished over 14 days, however, CTA in female rats was resistant to extinction.
Experiment 2: Effects of Exposure at 35–81 cm
The effect of exposing male rats within the 14.1 T magnet at 5 cm intervals from 35 cm to 81 cm were broadly similar to the results of Experiment 1.
Circling was observed in some but not all rats after magnet exposure. A significant difference in circling between groups was found by chi-squared analysis [χ2 (9) = 19.68, p < 0.05], such that all magnet-exposed groups circled more than sham-exposed rats (See Figure 4A).
Figure 4.
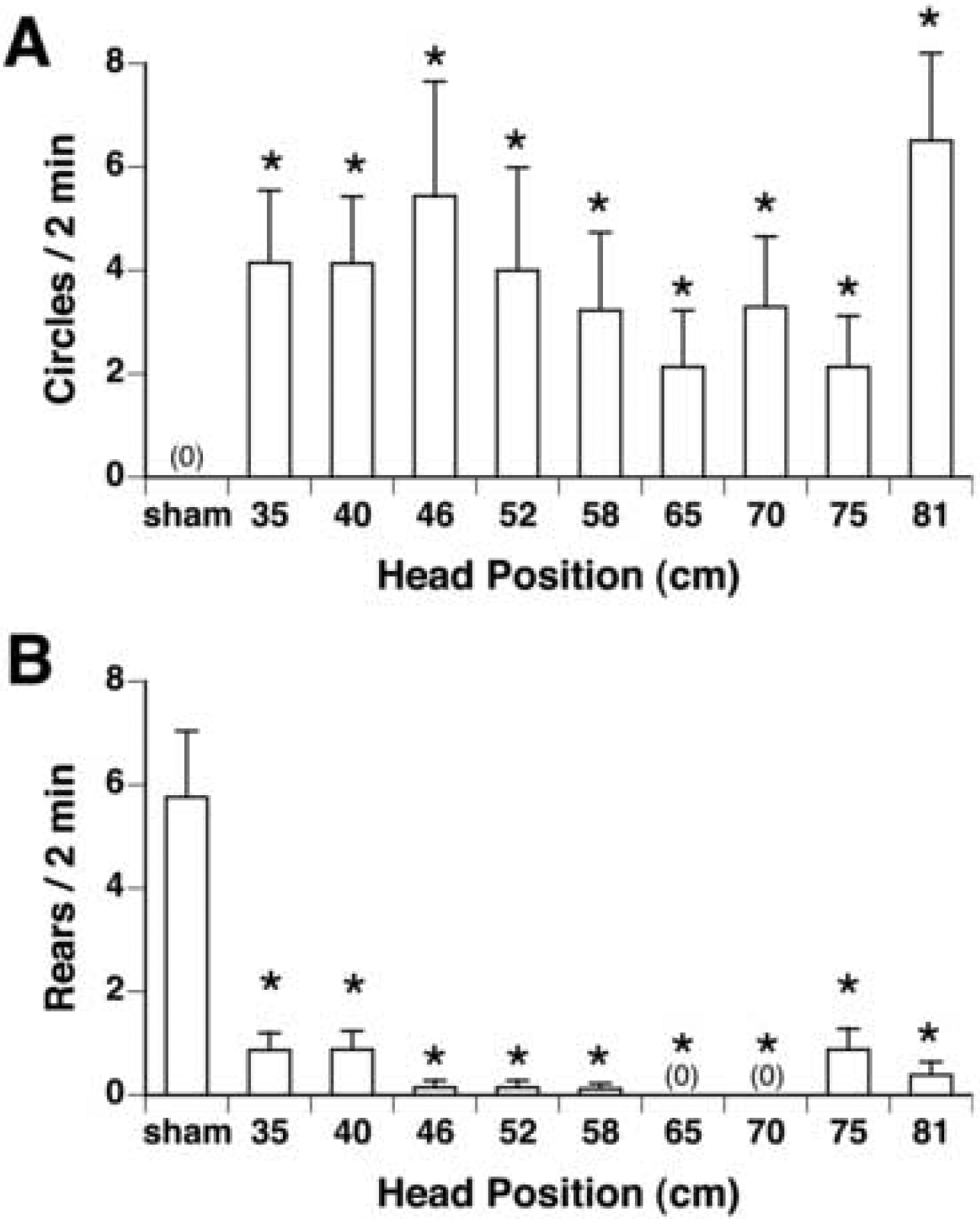
Quantification (mean ± s.e.m.) of locomotor circling (A) and rearing (B) in male rats after 30-min exposure within the 14.1 T magnet at intervals within the bore of the magnet ranging from 35 cm to 81 cm (n=6–8/group). A. Significant circling was found by χ2 in all magnet-exposed groups. B. Rearing was significantly suppressed in all magnet-exposed groups. * p < 0.05 vs sham-exposed rats.
Magnet exposure also suppressed rearing in all magnet-exposed rats [by ANOVA, F(9,67)=13.57, p < 0.0001] such that all magnet-exposed groups reared less than the sham-exposed group; there was no difference among the magnet-exposed groups (See Figure 4B).
On conditioning day, rats drank an average of 8.5 ± 0.4 g of saccharin prior to magnet- or sham-exposure; there was no difference in intake among groups. As in Experiment 1, magnet exposure also induced a significant CTA as measured on the first day of 2-bottle testing [by one-way ANOVA, F(9,58) = 5.26, p < .001]. All magnet-exposed groups showed a significantly lower preference than the sham-exposed group, and there was no difference in preference among the magnet-exposed groups (see Figure 5A).
Figure 5.
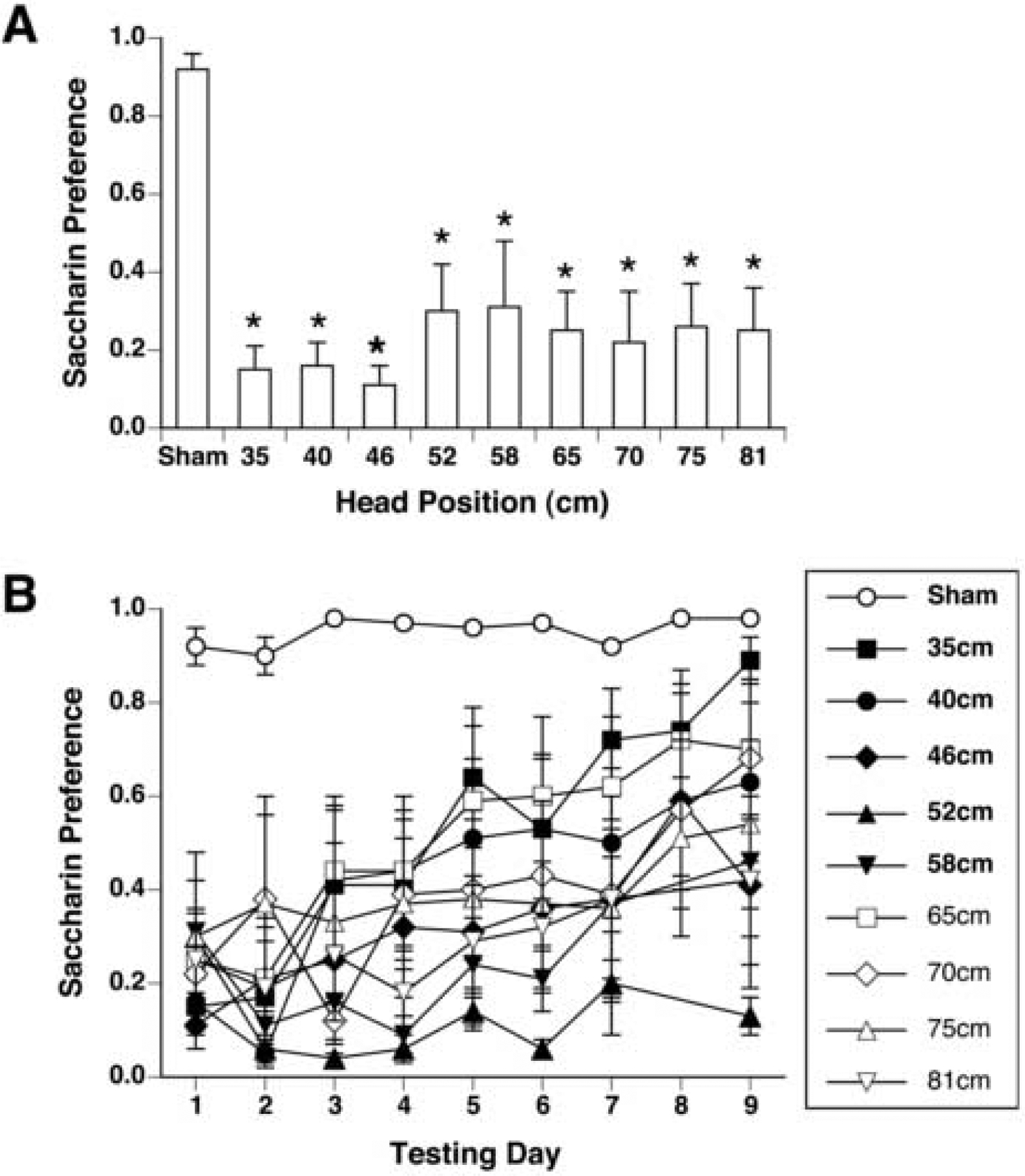
Initial magnitude (A) and extinction (B) of CTA measured by saccharin preference (mean ± s.e.m.) in male rats exposed at intervals within the bore of the magnet ranging from 35 cm to 81 cm. All magnet-exposed rats acquired a CTA. *p <0.05 vs sham-exposed rats. For clarity, significant differences are not indicated in panel B.
Across the 9 days of 2-bottle testing, a significant interaction of groups and days was seen by two-way ANOVA [F(9,90) = 1.83, p< 0.001]. Post-hoc testing confirmed that all magnet-exposed groups had preferences lower than sham-exposed rats for the first 8 days of extinction (see Figure 5B). The first group to extinguish was the group positioned at 35 cm on day 9, while the group exposed at 52 cm maintained the lowest preference of all groups across all 9 days. All other groups showed significant CTAs that were partially but not completely extinguished within the testing period.
In summary, MF exposures with the position of the head ranging from 35 to 81 cm inside the bore of the magnet had very similar effects, although rats exposed at 52 cm may acquired the most persistent CTA, while rats exposed at 35 cm acquired the weakest CTA.
Experiment 3: Effects of Low-Field Exposure
On conditioning day, rats consumed on average 11.9 ± 0.7 g of saccharin; there was no significant difference in intake among groups. Exposure to the low MFs at 155 cm had no effect on locomotion and induced only a weak CTA. Following exposure, no locomotor circling was observed in either the rats that were raised to 155 cm through the core of the magnet, nor in the rats that were lowered to 155 cm from the top of the magnet. There was no difference in amount of rearing among rats raised to 155 cm (4.1 ± 1.2 rears in 2 min), lowered to 155 cm (5.7 ± 0.8 rears in 2 min), or sham-exposed (5.7 ± 1.0 rears in 2 min).
There was no significant difference in preference scores between rats raised to 155 cm (first day preference = 0.8 ± 0.1) and rats lowered to 155 cm (first day preference = 0.6 ± 0.1), so data from both groups were combined and compared to the sham-exposed group.
Taken together, rats exposed at 155 cm above the opening of the magnet expressed a small but significant CTA on the first day of 2-bottle testing [t(20) = −2.2, p < 0.05; see Figure 6A]. Across 9 days of 2-bottle testing, two-way ANOVA revealed a significant effect of group [F(1,160) = 4.566, p < 0.05; see Figure 6B]. On almost all days of the 2-bottle preference tests, rats exposed at 155 cm were significantly different from sham-exposed rats.
Figure 6.
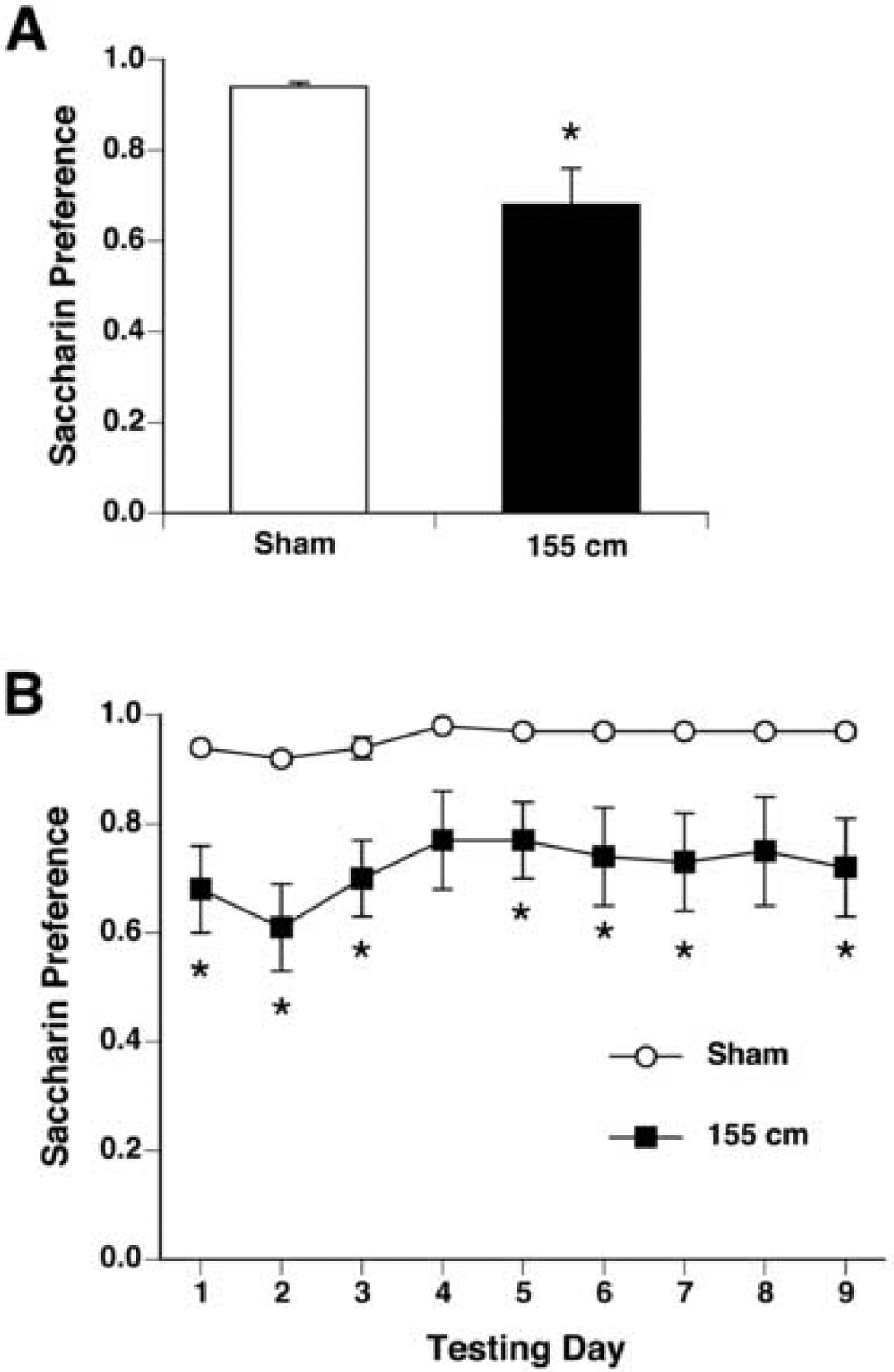
Initial magnitude (A) and extinction (B) of CTA measured by saccharin preference (mean ± s.e.m.) in males exposed at 155 cm (black bar and squares; n = 16) or in sham-exposed males (white bar and circles; n = 8). At this point the magnetic field was 0.05 T at the rats’ head, and a small but significant CTA was acquired. * p < 0.05 vs sham-exposed group.
Discussion
This study found that MF exposure had a maximal effect on rat behavior when the head and body of rats were exposed to the maximal, static MF. The effect of the magnet on circling, rearing, and CTA appeared sensitive to the absolute magnitude of the MF (B) applied to the head, and not to either the vertical gradient of the magnetic field (dB/dz) or motion through the field (dB/dt). The induction of circling and the suppression of rearing were seen after head exposure to 3 T and above (Experiment 1). Conditioned taste aversion was maximal after pairing saccharin with 14.1 T exposure of the head and body (Experiment 2). The threshold for acquisition of a robust CTA was between 3 and 7 T, although a small but significant CTA was observed after exposure to only 0.05 T (Experiment 3). Although no difference was seen in initial CTA between male and female rats, the CTA of most male rats extinguished within 10 days, while female rats did not extinguish for at least 14 days.
These experiments have localized the most sensitive site of action of high strength MFs to the rostral body and head of the rat. A subtractive approach was used, in which we compared the response of rats positioned at different field intensities within the same superconducting 14.1 T magnet. This approach was necessary, as it is practically impossible to expose one part of the rat to high strength static MFs while shielding the rest of the body. The results indicated that exposure of the head is necessary for maximal effects of the MF. For example, rats exposed just below the peak MF intensity (at 35 cm, with caudal body at 7 T and head at 14 T) showed robust circling and CTA acquisition, while rats exposed just above the peak MF intensity (at 95 cm, with caudal body at 10 T and head at 3 T) showed much weaker responses.
Significantly, the MF effects appeared unrelated to the vertical gradient of the MF experienced by the rats. In the preceding example, both groups of rats positioned at 35 cm and 95 cm within the bore of the magnet experienced large rostral-caudal gradients (20.1 T/m and-30.2T, respectively), yet a much greater response was seen in rats exposed at 35 cm.
Likewise, the response of rats appeared unrelated to transient passage through the core of the magnet. Movement of a conductor such as rat tissue through MFs has the potential to generate electric currents that could stimulate the tissue [15]. However, rats that moved through the 14.1 T core of the magnet during loading to positions above the core did not show stronger responses than rats that never passed through the core (either positioned below the core or lowered into position above the core from the top of the magnet). The effects of continuous motion into or within high strength static MFs have not been evaluated, however.
Thus, the response to the MF depended on prolonged exposure to a high magnitude MF (e.g. 30 min), as opposed to transient passage through the field or exposure to severe magnetic gradients. This is consistent with our previous findings that behavioral responses to 14.1 T in the core of the magnet are proportional to exposure duration (0 – 30 min) [3]. The dependence on a static uniform field is surprising, however, because translational force (i.e. a pull towards the magnet) is imposed on magnetic objects only when the object is within a field gradient (i.e. outside of the core of the magnet). Within the uniform MF at the core of the magnet, no net translational force will be experienced [15]. Although translational force would not be experienced within the core, torque would be applied to magnetic substrates within the rat that were not parallel with the uniform MF [15]. Alternatively, small motions of the rat’s head could generate perceptible forces within receptive organs. For example, Schenck has proposed that movement of the inner ear could generate a magnetohydrodynamic force on the charged endolymph of the semicircular canal, thus stimulating the vestibular system and inducing motion sickness [16].
Surprisingly, we found that pairing saccharin with 30-min exposure to a MF as low as 0.05 T at the very top of the magnet was sufficient to produce a small CTA (i.e. a statistically significant decrease in saccharin preference). This effect was small and only detected with a large group of rats (n= 16). We [6] and Weiss et al. [17] have shown that rats are capable of detecting relative small static MFs. Weiss et al. reported that rats would not voluntarily walk into the horizontal bore of a 2 T MRI magnet, and turned back when they entered a 1 T MF. More recently, we have trained rats to climb a vertical “ladder” to reach a food reward. Rats were trained outside the magnet, and then tested with the ladder extending through the 14.1 T magnet used in the present study. On the first trial with the ladder extended through the magnet, rats would readily climb the ladder and enter the bore far enough to be exposed to 14.1T. On subsequent trials, however, rats would begin to ascend the ladder but then stop, reverse direction, and return to the bottom of the ladder. The median MF at the reversal point was 2 T or less. Together, these results suggest that rats are in fact capable of detecting static MFs in the range of 0.05 to 2T.
Sex Differences
A practical aspect of this study was to confirm that the effects of high MFs are qualitatively similar in both male and female rats. Our previous studies employed male rats. Male Sprague-Dawley rats grow so fast, however, that there is only a narrow time window when they are able to fit within the 89mm bore of the 14.1 T magnet. Thus the smaller body size of female rats is advantageous in that it allows longer and more flexible experimental protocols. In fact, while females rat did respond qualitatively similar to male rats, there appeared to be quantitative differences. Female rats appeared more sensitive than male rats as measured by the theshold MF that induced circling (3 T in females vs. 7 T in males). Also, CTA in female rats extinguished much slower than CTA in male rats.
We have recently completed a more formal analysis of sex differences in responses to MF exposure [18]. In that study, female rats showed more circling than male rats after exposure to 14.1 T for 30 min. The degree of circling was dependent on phase of the estrous cycle, with greatest sensitivity on the day of estrus (when endogenous ovarian steroid levels are lowest.) Ovariectomized female rats also circled more than male rats, and chronic estrogen replacement attenuated the circling response. As in the present study, male and female rats acquired CTA after the pairing of saccharin and 14.1 T exposure, but while the CTA extinguished in males the CTA in female rats persisted without extinction for 9–14 days. The resistance to extinction in females was unaffected by ovariectomy or steroid replacement, suggesting that the sex difference in CTA extinction rate is organized developmentally rather than controlled by adult hormone levels. At this time, however, we cannot determine if the divergence in extinction rates represents a sexual dimorphism in central processing or learning, or if it is secondary to other well-known sex differences such as daily fluid intake and innate taste preferences.
Conclusion
We have now demonstrated an intensity-response function for magnetic fields and rat behavior in 3 ways: first, by exposing rats to the core of 3 different superconducting magnets (7, 9.4, and 14.1 T) [3]; second, by exposing rats in a resistive electromagnet at various current levels (4, 7, 9, 11, 14, 17, and 19.8 T) [14]; and, third in the present study by exposing rats at different positions within the magnetic field of a 14.1 T magnet (0.05 – 14.1 T). The thresholds for behavioral effects are consistent across these 3 studies: at 3–4 T and above, circling was induced and rearing was suppressed; acquisition of a maximal CTA required exposure to at least 7T. The consistent replication of stimulus thresholds across different experimental preparations and different magnets eliminates the possibility that the observed effects are artifacts of procedure or equipment. Finally, the observation that head exposure, but not caudal body exposure, is sufficient for maximal effects of the high magnetic field is consistent with a cephalic site of action, such as the vestibular apparatus of the inner ear.
Acknowledgements
This work was supported by National Institute on Deafness and Other Communication Disorders Grants RO1DC4607 (TAH, JCS), F31DC6521 (AMC), and T32DC0044 (AMC, GJG); and an FSU Research Foundation Program Enhancement Grant. We thank Drs. Timothy Cross and Zhehong Gan of the United States National Magnetic Field Laboratory for providing access to the magnets.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schenck JF;Dumoulin CL;Redington RW;Kressel HY;Elliot RT, and McDougall IL, Human exposure to 4.0-Tesla magnetic fields in a whole body scanner. Med. Phys, 1992. 19: 1089–1098. [DOI] [PubMed] [Google Scholar]
- 2.Kangarlu A;Burgess RE;Zhu H;Nakayama T;Hamlin RL;Abduljalil AM, and Robitaille PML, Cognitive, cardiac, and physiological safety studies in ultra high field magnetic resonance imaging. Magn. Reson. Imag, 1999. 17: 1407–1416. [DOI] [PubMed] [Google Scholar]
- 3.Houpt TA;Pittman DM;Barranco JM;Brooks EH, and Smith JC, Behavioral effects of high strength magnetic fields on rats. J. Neurosci, 2003. 23: 1498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nolte CM;Pittman DW;Kalevitch B;Henderson R, and Smith JC, Magnetic field conditioned taste aversion in rats. Physiol. Behav, 1998. 63: 683–688. [DOI] [PubMed] [Google Scholar]
- 5.Snyder D;Jahng JW;Smith JC, and Houpt TA, c-Fos induction in visceral and vestibular nuclei of the rat brainstem by a 9.4 T magnetic field. NeuroReport, 2000. 11: 1681–5. [DOI] [PubMed] [Google Scholar]
- 6.Houpt TA;Cassell JA;Riccardi C;DenBleyker MD;Hood A, and Smith JC, Avoidance by rats of high magnetic fields depends on an intact vestibular system. Physiol. Behav, 2005. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy CS;Carroll ME;Smith JC, and Hofer KG, Antihistamines block radiation induced taste aversions. Science, 1974. 186: 1044–1046. [DOI] [PubMed] [Google Scholar]
- 8.Smith JC;Hollander GR, and Spector AC, Taste aversions conditioned with partial body radiation exposures. Physiol. Behav, 1981. 27: 903–913. [DOI] [PubMed] [Google Scholar]
- 9.Dinc HI and Smith JC, Role of the olfactory bulbs in the detection of ioninizing radiation by the rat. Physiol. Behav, 1966. 1: 139–144. [Google Scholar]
- 10.Taylor HL;Smith JC;Wall AH, and Chaddock B, Role of the olfactory system in the detection of X-rays by the rhesus monkey. Phsyiol. Behav, 1968. 3: 929–933. [Google Scholar]
- 11.Chaddock TE, Visual detection of x-ray by the rhesus monkey. J. Comp. Physiol. Psychol, 1972. 78: 190–201. [DOI] [PubMed] [Google Scholar]
- 12.Smith JC, Radiation: Its detection and its effect on taste preferences, in Progress in Physiological Psychology IV, Stellar E and Sprague J, Editors. 1971, Academic Press: NY. p. 53–118. [Google Scholar]
- 13.Cason AM;DenBleyker MD;Ferrance K;Smith JC, and Houpt TA, Sex and estrous cycle differences in the behavioral effects of high-strength static magnetic fields: role of ovarian steriods. Am. J. Physiol, 2006. 290: R659–67. [DOI] [PubMed] [Google Scholar]
- 14.Houpt TA;Pittman DW;Riccardi C;Cassell JA;Lockwood DR;Barranco JM;Kwon BS, and Smith JC, Behavioral effects on rats of high strength magnetic fields generated by a resistive electromagnet. Physiol. Behav, 2005. 86: 379–89. [DOI] [PubMed] [Google Scholar]
- 15.Halliday D and Resnick R, Fundamentals of Physics. 2 ed. 1986, NY: John Wiley & Sons. 625. [Google Scholar]
- 16.Schenck JF, Health and physiological effects of human exposure to whole-body four-tesla magnetic fields during MRI. Ann. NY Acad. Sci, 1992. 649: 285–301. [DOI] [PubMed] [Google Scholar]
- 17.Weiss J;Herrick RC;Taber KH;Contant C, and Plishker GA, Bio-effects of high magnetic fields: a study using a simple animal model. Magnetic Resonance Imag., 1992. 10: 689–694. [DOI] [PubMed] [Google Scholar]
- 18.Cason AM;DenBleyker MD;Ferrance K;Smith JC, and Houpt TA, Sex and estrous cycle differences in the behavioral effects of high-strength static magnetic fields: role of ovarian steriods. Am. J. Physiol, 2005. 290: R659–67. [DOI] [PubMed] [Google Scholar]


