Abstract
Background:
Enteropathic spondyloarthritides (eSpAs) are chronic inflammatory joint diseases associated with inflammatory bowel disease (IBD). Limited data are available on the prevalence since arthritis in IBD patients may be underestimated because medications may hide disease activity with a possible diagnostic delay.
Objectives:
We aimed to evaluate diagnostic delay in eSpA and explore associated demographic, clinical, and radiographic characteristics.
Design:
Single-centre cross-sectional study conducted on consecutive out-patients referred to the combined Gi-Rhe clinic (November 2018–October 2019).
Methods:
We analysed eSpA patients for diagnostic delay, disease activity, inflammatory markers, conventional radiography (CR) and magnetic resonance images (MRI) of sacroiliac joints/spine.
Results:
A total of 190 eSpA patients [118 peripheral SpA, 72 axial (Ax) SpA including 44 non-radiographic (nr)-axSpA] were enrolled. axSpA patients had a higher prevalence of men sex, HLA-B27 positivity, uveitis and pancolitis compared with peripheral eSpA. Median diagnostic delay in eSpA was 48 months (IQR 6–77) with no difference between axial and peripheral patients. Radiographic-axial SpA (r-axSpA) patients displayed a higher diagnostic delay compared with nr-axSpA (median/IQR 36/17–129 versus 31/10–57 months, p = 0.03) and were older, with longer disease duration, low education status and high rate of employment than patients with nr-axSpA. r-axSpA patients with sclerosis, syndesmophytes and bridge at CR had higher diagnostic delay than those without lesions. Men showed higher prevalence of spine damage lesions than women as sclerosis, squaring, syndesmophytes and bridges. Longer disease duration was detected in patients with radiographic damage as bridge and sacroiliitis grade 3. On MRI, sacroiliac bone oedema was associated with reduced diagnostic delay, whereas bone erosions were associated with higher diagnostic delay compared with that in patients without these lesions. Patients with psoriasis displayed a higher diagnostic delay compared to those without skin involvement.
Conclusion:
Diagnostic delay was higher in r-axSpA compared with nr-axSpA despite the same treatment. Demographic, clinical features and radiological lesions were associated with diagnostic delay.
Keywords: axial SpA, diagnostic delay, enteropathic spondyloarthritis
Plain language summary
Diagnostic delay in patients affected by enteropathic spondyloarthritis
Enteropathic Spondyloarthritides (eSpA) are chronic inflammatory joint diseases associated with inflammatory bowel disease (IBD). Limited data are available on the prevalence since arthritis in IBD patients may be underestimated because medications may hide disease activity with a possible diagnostic delay. We aimed to evaluate diagnostic delay in eSpA and explore associated demographic, clinical and radiographic characteristics. We analysed eSpA patients for diagnostic delay, disease activity, inflammatory markers, conventional radiography and magnetic resonance images of sacroiliac joints/spine. 190 eSpA patients (118 peripheral SpA, 72 axial (Ax) SpA including 44 non-radiographic (nr)-axSpA)) were enrolled. Median diagnostic delay in eSpA was 48 months with no difference between axial and peripheral patients. Radiographic-axial SpA (r-axSpA) patients displayed a higher diagnostic delay compared with nr-axSpA and were older, with longer disease duration, low education status and high rate of employment than patients with nr-axSpA. Patients with psoriasis displayed a higher diagnostic delay compared to those without skin involvement. Diagnostic delay was higher in r-axSpA compared with nr-axSpA despite the same treatment. Demographic, clinical features and radiological lesions were associated with diagnostic delay.
Introduction
Enteropathic Spondyloarthritides (eSpAs) are chronic inflammatory joint diseases, that fall within the spectrum of spondyloarthritis (SpA). They are typically associated with inflammatory bowel disease (IBD) such as Crohn’s disease (CD) and ulcerative colitis (UC). 1 SpA primarily affect entheses, small and large joints, and the axial skeleton joints. 2 The Assessment of SpondyloArthritis International Society (ASAS) classification criteria for SpA distinguishes two clinical forms based on the predominant manifestation of the disease: axial SpA (axSpA) and peripheral SpA. 3 In accordance with ASAS criteria, axSpA is characterized by chronic back pain and encompasses radiographic axSpA (r-axSpA) as well as non-radiographic axSpA (nr-axSpA), which can lead to structural damage and disability. 4 Classically, patients affected by r-axSpA fulfill ASAS criteria and display sacroiliitis on radiographs in agreement with the modified New York (mNY) criteria. 5 In contrast, nr-axSpA patients can be identified by the presence of typical clinical features of SpA combined with either inflammatory sacroiliitis seen on the magnetic resonance imaging (MRI) scan (imaging arm) or HLA-B27 positivity (clinical arm). 6 Unlike radiography, MRI can detect inflammatory lesions typical of sacroiliitis in SpA patients, allowing clinicians for the recognition of patients in the non-radiographic stage of the disease much earlier than r-axSpA patients, defining chronic and acute lesions. 7 This earlier detection is crucial due to the diagnostic delay associated with the use of mNY criteria, as patients with r-axSpA often experience symptoms for several years before structural changes in the sacroiliac joints (SIJs) can be detected. 8 Despite this, the delay between symptom onset and axSpA diagnosis is still estimated to be 5–14 years. 9
The simultaneous presence of extra-articular manifestations may serve as an indication for the clinicians to consider the possibility of SpA. However, there are instances where this awareness is not immediate, and the presence of extra-articular manifestations may lead to a delayed referral of patients with back pain to a rheumatologist by general practitioners and other physicians. 10
Factors contributing to diagnostic delays include female gender, HLA-B27 negativity, onset of disease in juvenile versus adult age, the absence of a family history of SpA, lack of peripheral arthritis, occupational mechanical stress, misinterpretation of axSpA imaging features by specialists other than rheumatologists and the initiation of treatment for uveitis, psoriasis, or IBD, which may improve articular symptoms and consequently postpone referral to a specialist. 9
To address these challenges, recommendations for the referral of patients suspected of having axSpA by non-rheumatologists, such as gastroenterologists, have been developed. Additionally, guidance has been provided for the effective imaging of axSpA to assist both radiologists and rheumatologists in the appropriate and efficient use of imaging in clinical practice, with the aim of enhancing the early diagnosis of axSpA. 11
Prompt diagnosis of rheumatologic diseases in patients affected by IBD is necessary for optimal patient management, as eSpA may lead to permanent disability and structural damage. Moreover, delay in diagnosis postpones the introduction of appropriate disease-modifying treatment and may contribute to poor patient outcomes, including higher disease activity, worse physical function and more structural damage, compared to patients with an earlier diagnosis. 12
In eSpA patients, information regarding diagnostic delay across different phenotypes (peripheral versus r-axSpA and/or nr-axSpA) is currently unavailable. Quantifying the diagnostic delay in this particular population and identifying potential factors associated with this delay may help in building strategies to reduce it.
In this cross-sectional study, we aimed to evaluate diagnostic delay in eSpA patients, including peripheral SpA, axSpA and both r-axSpA and nr-axSpA. Our a priori hypothesis posited that individuals with r-axSpA would exhibit longer diagnostic delays compared to those with other phenotypes. We analysed potential factors associated with diagnostic delay, focusing on demographic, clinical and imaging findings.
Patients and methods
Single-centre cross-sectional study was conducted on consecutive outpatients referred from primary care physicians, gastroenterologists, dermatologists and ophthalmologists to the combined GastroIntestinal-RHEumatic (Gi-Rhe) clinic at the University of Rome Tor Vergata (Department of Medical Sciences) between 1 November 2018 and 31 October 2019. All patients with IBD experiencing musculoskeletal pain were referred to the Gi-Rhe clinic. To avoid selection bias, consecutive patients with a diagnosis of SpA and concomitant IBD were included in the study. The IBD diagnosis was performed by expert gastroenterologists, and the SpA diagnosis was carried out by expert rheumatologists. AxSpA patients were classified as affected by r-axSpA or nr-axSpA according to the ASAS criteria, 6 whereas patients with peripheral SpA fulfilled the ASAS criteria for peripheral involvement. 3 r-axSpA patients included those with SIJ involvement and/or those with syndesmophytes/ankylosis in radiographs of the spine. 13
Inclusion criteria were: (1) diagnosis of SpA with symptom duration ⩾ 3 months; (2) diagnosis of CD or UC; (3) age >18 years; (4) available demographic and clinical data and (5) availability of conventional radiography (CR) and/or MRI of SIJs and cervical/lumbosacral spine from patients with axSpA. Patients had to meet all of the above criteria. Exclusion criteria were (1) presence of a different rheumatologic diagnosis other than SpA; (2) lack of imaging to assess axial involvement.
Written informed consent was obtained from all enrolled patients. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, and the study was approved by the scientific ethic committee of the University of Rome Tor Vergata (N. 186/16), Rome, Italy.
Clinical assessment
At recruitment, comprehensive data on sociodemographic characteristics [age, gender, body mass index (BMI), current/ex/non-smoker, level of education], family history of psoriasis, human leucoocyte antigen (HLA)-B27 positivity, clinical data such as eSpA duration (time between recruitment and onset of symptoms), diagnostic delay (the total lag time from SpA-related symptom onset to the first rheumatological encounter), IBD location according to Montreal classification, 14 presence of extra-intestinal and extra-articular manifestations (i.e. uveitis diagnosed by the ophthalmologist through slit lamp exam, 15 erythema nodosum and psoriasis assessed by an expert dermatologist, 16 current therapy at the time of visit including corticosteroids, conventional synthetic and biologic disease-modifying antirheumatic drugs (cs- and bDMARDs) were obtained. The rheumatologic assessment included a physical examination with 68 tender and 66 swollen joint count, and evaluation of inflammatory spinal and buttock pain. Additional imaging studies, such as ultrasound or MRI, were employed as needed for a more detailed evaluation. The presence of enthesitis was evaluated using the Spondyloarthritis Research Consortium of Canada index 17 , whereas the presence of dactylitis was evaluated by Leeds Dactylitis Index. 18 Erythrocyte sedimentation rate (ESR, mm/h) and C-reactive protein (CRP, mg/dl) were measured. The disease activity was measured by Ankylosing Spondylitis Disease Activity Score (ASDAS, CRP-based), 19 Bath Ankylosing Spondylitis Disease Activity Index, 20 Bath Ankylosing Spondylitis Functional Index, 21 Health Assessment Questionnaire modified for SpA (HAQ-S), 22 Visual Analogue Scale (VAS) for pain and patient global (PG)-VAS, IBD activity evaluated with Harvey–Bradshaw Index and Mayo score. 23
Imaging assessment
CR and MR images were conducted at our institution by two radiologists specializing in musculoskeletal imaging. The radiologists were blinded to the patients’ clinical features during the assessment. Imaging procedures adhered to standard protocols either within our clinic or at other centres meeting quality standards. In particular, X-ray imaging of SIJs, including anteroposterior (AP) oblique view, as well as cervical and lumbosacral spine X-ray series in the standard AP and lateral views were evaluated. According to the mNY criteria, sacroiliitis was diagnosed if bilateral grade 2 inflammatory lesions, or minimum unilateral grade 3 lesions in SIJ were present.24,25 Joint space narrowing, subchondral sclerosis, ankylosis and erosions were evaluated. According to modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS), at spine level, presence of syndesmophytes, squaring, sclerosis, erosions and ankylosis was assessed. 26 SIJs and spine MRI examination, conducted with 1.5 or 3-T field strength machines with at least T1, T2, short tau inversion recovery/T2-fat sat sequences in oblique coronal plane for SIJs, and T1–T2 axial and coronal hip scan, were evaluated. According to ASAS definition, SIJs-MRI images were considered positive for sacroiliitis in presence of bone marrow oedema (BMO) lesions highly suggestive of SpA with ⩾1 BMO lesion on ⩾2 consecutive slices or several BMO lesions visible on a single slice. 4 The MRI inflammatory lesions in the spine were defined according to ASAS/Outcome Measures in Rheumatology Network criteria. 27 Apart from BMO, other inflammatory lesions (enthesitis and capsulitis) and structural damage lesions (sclerosis, erosions, fat metaplasia and ankylosis) were also detected by MRI.
Statistical analysis
Sample size calculation was based on national database of eSpA. 28 According to this reference, a sample of 72 subjects with axial involvement provides 98.6% power to detect a difference with p < 0.05 and an alpha error of 0.05. Due to the absence of unequivocal data on diagnostic delay in eSpA patients, as well as limited previous studies comparing nr- and r-axSpA in the eSpA population, the sample size for this subgroup was not precalculated. Consequently, the study was exploratory in nature. To test normality of datasets the D’Agostino and Pearson omnibus test was used. Normally distributed variables were presented using mean and standard deviation (SD), whereas nonnormally distributed variables were summarized with median and percentile ranges. Categorical variables were presented with absolute frequencies and percentages. Continuous variables were compared using the parametric unpaired t-est or the nonparametric Mann–Whitney U test when appropriate. Categorical variables were performed by Chi-squared test or Fisher’ exact test when appropriate. The one-way analysis of variance was used to estimate whether there is any significant difference between the means of two independent groups on a dependent variable (diagnostic delay). The significance of any correlation was determined by Spearman’s rank correlation coefficient. p values < 0.05 were considered significant. All statistical analyses were performed using GraphPad Prism version 6 (GraphPad software, San Diego, California, USA) and IBM SPSS version 24 (IBM Corp., Armonk, NY, USA).
Results
Study population
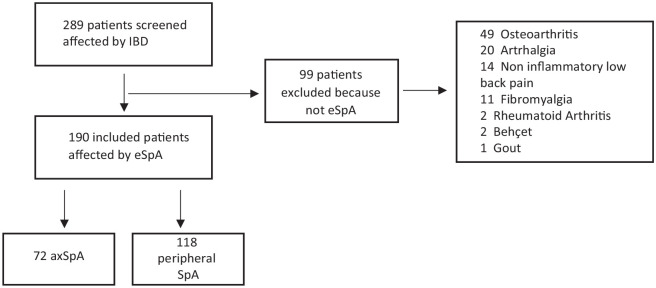
A total of 289 IBD patients with musculoskeletal pain were assessed at the combined GI-Rhe clinic of the University of Rome Tor Vergata. Of these IBD patients, 99 were excluded for not meeting SpA criteria (Figure 1). Therefore, our study included 190 eSpA patients (M 66, F 124, mean age 47.5 ± 12.8 years). eSpA patients displayed an axial involvement in 72 (37.9%) cases, whereas 118 (62.1%) patients had a peripheral involvement. Within the axial involvement group, 28 (38.9%) had r-axSpA and 44 (61.1%) had nr-axSpA (Figure 1). The median eSpA disease duration in all study population was 49.5 months (IQR 24–108) with no significant difference between axial and peripheral patients. Demographic and clinical characteristics of eSpA patients are described in Table 1. Peripheral eSpA showed a higher prevalence of females (70.3%) compared to the axial group (56.9%) (p < 0.0001). Polyarticular involvement was less frequent in patients with axSpA compared to those affected by peripheral SpA (29.1% versus 69.5%, p < 0.0001). Uveitis and HLA-B27 positivity were more common in axSpA patients than peripheral SpA (13.9% versus 4.2%, p = 0.016 and 22.2% versus 4.2%, p = 0.004, respectively). Among IBD, CD was more prevalent in axSpA patients, whereas UC was more prevalent in those with peripheral involvement (p = 0.021). Moreover, UC patients with peripheral involvement exhibited proctitis and left colitis more frequently than axSpA (p = 0.02 and 0.04, respectively), whereaspancolitis was prevalent in patients with axial involvement (p = 0.006).
Figure 1.
Flowchart of enrolment process.
Table 1.
Clinical and demographic characteristics of patients enrolled.
| Variables | Total eSpA N = 190 | Axial SpA N = 72 | Peripheral SpA N = 118 | p Value |
|---|---|---|---|---|
| Age (years) | 47.5 ± 12.8 | 47.9 ± 12.6 | 47.4 ± 13.1 | NS |
| Female sex (n/%) | 124/65.2 | 41/56.9 | 83/70.3**** | <0.0001 |
| BMI | 24.2 ± 3.7 | 24.3 ± 3.1 | 24.1 ± 4 | NS |
| Comorbidities (n/%) | 109/57.3 | 43/59.7 | 66/55.9 | NS |
| Low education status (n/%) | 61/32.8 | 23/31.9 | 32.2/38 | NS |
| Employment (n/%) | 134/70.5 | 50/69.4 | 84/71.8 | NS |
| Smoker (n/%) | 109/57.3 | 41/56.9 | 68/57.6 | NS |
| IBD surgery (n/%) | 52/27.3 | 20/27.7 | 32/27.1 | NS |
| Uveitis (n/%) | 15/7.8 | 10/13.9* | 5/4.2 | 0.016 |
| Personal or family history of psoriasis (n/%) | 58/30.5 | 21/29.1 | 37/31.3 | NS |
| SpA duration (months) | 49.5/24–108 | 52/24–107 | 48.5/26–108 | NS |
| SpA diagnostic delay (months) | 48/6–77 | 31.5/10–83 | 21.5/5–73 | NS |
| axial SpA | 72/37.9 | 72/100 | – | NA |
| r-axSpA (n/%) | 28/14.7 | 28/38.9 | 0/0 | NS |
| nr-axial SpA (n/%) | 44/23.1 | 44/61.1 | 0/0 | NS |
| Peripheral SpA | 118/62.1 | 40/55.5 | 118/100 | NA |
| HLA-B27 (n/%) a | 15/12 | 12/22.2** | 3/4.2 | 0.004 |
| CD (n/%) | 117/61.5 | 52/72.2* | 65/55 | 0.021 |
| UC (n/%) | 73/38.5 | 20/27.8 | 53/45* | 0.021 |
| CD localization (n/%) | ||||
| L1: Ileum | 61/52.1 | 23/44.2 | 37/56.9 | NS |
| L2: Colon | 14/11.9 | 5/9.6 | 9/13.8 | NS |
| L3: Ileum-colon | 41/35 | 23/44.2 | 18/27.7 | NS |
| L4: Upper | 9/7.69 | 5/9.6 | 4/6.1 | NS |
| CD behaviour (n/%) | ||||
| B1: nonstricturing | 59/50.4 | 29/55.8 | 30/46 | NS |
| B2: stricturing | 41/35 | 17/32.7 | 24/37 | NS |
| B3: penetrating | 16/13.6 | 5/9.6 | 11/17 | NS |
| P: perianal disease | 12/10.2 | 7/13.4 | 5/7.6 | NS |
| UC localization (n/%) | ||||
| 1: Proctitis | 15/20.5 | 3/15 | 12/22.6* | 0.02 |
| 2: Left colitis | 14/19.1 | 3/15 | 11/20.7* | 0.04 |
| 3: Pancolitis | 43/58.9 | 14/70** | 30/56.6 | 0.006 |
| ESR (mm/h) | 20.7 ± 19 | 25.6 ± 23.7* | 17.6 ± 14.5 | 0.006 |
| CRP (mg/dL) | 0.9 ± 2.3 | 1 ± 2.9 | 0.8 ± 1.8 | NS |
| ASDAS-CRP | 2.6 ± 1.1 | 2.9 ± 1.2* | 2.3 ± 0.9 | 0.001 |
| PG-VAS (0–100) | 43.8 ± 25.6 | 50 ± 26.4* | 40 ± 24.4 | 0.008 |
| VAS Pain (0–100) | 42 ± 27.8 | 48.3 ± 29* | 38.3 ± 26.5 | 0.019 |
| HAQ-S | 0.7 ± 0.64 | 0.8 ± 0.66 | 0.65 ± 0.6 | NS |
| Active IBD (n/%) | 60/31.5 | 24/33.3 | 40/33.9 | NS |
HLA-B27 missing data n = 65 (18 in ax SpA group and 47 in peripheral SpA group).
P value < 0.05 are indicated in bold; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; normally distributed variables are expressed as mean ± standard deviation (SD), whereas nonnormally distributed variables were presented using median and 25°–75° percentile ranges.
ASDAS, ankylosing spondylitis disease activity score; CD, Crohn’s disease; CRP, C-reactive protein; eSpA, enteropathic spondyloarthritis; ESR, erythrocyte sedimentation rate; HAQ-S, Health Assessment Questionnaire modified for SpA; HLA, human-leucocyte antigen; IBD, inflammatory bowel disease; PG-VAS, patient global-VAS; NA, not applicable; nr-axSpA, non-radiographic axial SpA; NS, not significative; r-axial SpA, radiographic axial SpA; UC, ulcerative colitis; VAS, Visual Analog Scale.
Demographic and clinical characteristics of axSpA patients, divided into r-axSpA and nr-axSpA, are described in Table 2. Patients with r-axSpA were older and had longer disease duration compared with nr-axSpA patients (p = 0.005 and p = 0.019, respectively). A lower education status and a higher rate of employment were found in r-axSpA compared with nr-axSpA (p = 0.003 and p = 0.03, respectively). No differences in gender, BMI, uveitis, family history of psoriasis, HLA-B27 positivity, IBD diagnosis and localization/behaviours were detected between r-axSpA and nr-axSpA.
Table 2.
Clinical and demographic characteristics of axial SpA patients.
| Variables | r-axial SpA N = 28 | nr-axial SpA N = 44 | p Value |
|---|---|---|---|
| Age (years) | 50.2 ± 12.7 | 44.7 ± 12.7** | 0.005 |
| Female sex (n/%) | 12/42.8 | 29/70 | NS |
| BMI | 24.5 ± 3.2 | 24.2 ± 2.5 | NS |
| Comorbidities (n/%) | 11/39.2 | 28/63.6 | NS |
| Low education status (n/%) | 20/71.4 | 11/25** | 0.003 |
| Employment (n/%) | 22/78.6 | 23/52.3* | 0.03 |
| Smoker (n/%) | 19/67.9 | 22/50 | NS |
| IBD surgery (n/%) | 9/32.1 | 11/25 | NS |
| Uveitis (n/%) | 5/17.9 | 5/11.3 | NS |
| Personal or family history of psoriasis (n/%) | 5/17.9 | 13/29.5 | NS |
| SpA duration (months) | 64/30–159* | 48/22–91 | 0.019 |
| Diagnostic delay of SpA (months) | 36/7–129* | 31/10–57 | 0.03 |
| HLA-B27 (n/%) a | 7/33 | 5/15 | NS |
| CD (n/%) | 21/75 | 31/70.4 | NS |
| UC (n/%) | 7/25 | 13/29.5 | NS |
| CD localization (n/%) | |||
| L1: Ileum | 8/38.3 | 15/48.3 | NS |
| L2: Colon | 1/4.7 | 4/13 | NS |
| L3: Ileum-colon | 11/52.3 | 11/38.7 | NS |
| L4: Upper | 4/19 | 4/13 | NS |
| CD behaviour (n/%) | |||
| B1: nonstricturing | 11/52.3 | 18/58 | NS |
| B2: stricturing | 9/42.8 | 8/25.8 | NS |
| B3: penetrating | 1/4.7 | 4/13 | NS |
| P: perianal disease | 3/14.2 | 4/13 | NS |
| UC localization (n/%) | |||
| 1: Proctitis | 0/0 | 3/23 | NS |
| 2: Left colitis | 0/0 | 3/23 | NS |
| 3: Pancolitis | 6/85.7 | 7/54 | NS |
| ESR (mm/h) | 32.7 ± 28.4* | 21 ± 19.2 | 0.04 |
| CRP (mg/dL) | 1.7 ± 4.5 | 0.6 ± 0.97 | NS |
| ASDAS-CRP | 3.1 ± 1.3 | 2.8 ± 1.1 | NS |
| PG-VAS (0–100) | 51.8 ± 27.5 | 49.2 ± 26 | NS |
| VAS Pain (0–100) | 47 ± 29.6 | 49.2 ± 29 | NS |
| BASDAI | 5.2 ± 2.6 | 5 ± 2.5 | NS |
| BASFI | 3 ± 2.6 | 2.4 ± 2.47 | NS |
| HAQ-S | 0.84 ± 0.7 | 0.77 ± 0.64 | NS |
| Active IBD (n/%) | 9/32.1 | 14/21.8 | NS |
HLA-B27 missing data n = 18 (7 r-axial SpA group and 11 nr-axial SpA group).
P value < 0.05 are indicated in bold; *: p < 0.05, **p < 0.01; normally distributed variables are expressed as mean ± standard deviation (SD), whereas nonnormally distributed variables were presented using median and 25°–75° percentile ranges.
ASDAS, ankylosing spondylitis disease activity score; BADSAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; CD, Crohn’s disease; CRP, C-reactive protein; eSpA, enteropathic spondyloarthritis; ESR, erythrocyte sedimentation rate; HAQ-S, Health Assessment Questionnaire modified for SpA; HLA, human-leucocyte antigen; IBD, inflammatory bowel disease; PG-VAS, patient global-VAS; NA, not applicable; nr-axSpA, non-radiographic axial SpA; NS, not significative; r-axial SpA, radiographic axial SpA; UC, ulcerative colitis; VAS, Visual Analog Scale.
Regarding disease activity indices, axSpA patients displayed higher ESR, ASDAS-CRP and VAS pain compared with peripheral SpA (p = 0.006, p = 0.001, p = 0.019, respectively). Likewise, r-axSpA patients showed a greater ESR than nr-axSpA (32.7 ± 28.4 versus 21 ± 19.2, p = 0.04). No differences in IBD disease activity were found in the two comparison groups (axial versus peripheral and r-axSpA versus nr-axSpA, Tables 1 and 2).
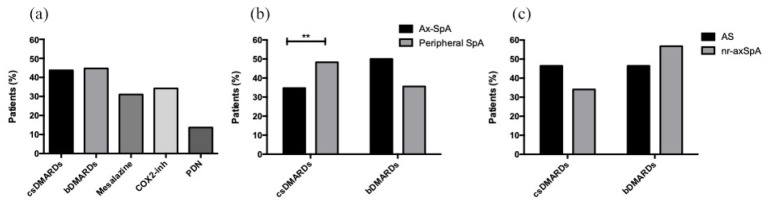
As regard to treatment, in the overall eSpA population, approximately half of the patients were on cs- or bDMARD (43.7%, n = 83 and 44.7%, n = 85, respectively). Among bDMARDs, adalimumab was the most used (51.7%, n = 44), followed by infliximab (20%, n = 17). A total of 44 patients (51.8%) were biologic naïve, whereas 41 patients (48.2%) were beyond the first line of treatment. A higher prevalence of csDMARDs was detected in peripheral SpA compared with axSpA (48.3% versus 34.7%, p = 0.002), whereas higher use of bDMARDs was observed in axSpA patients than peripheral ones (50% versus 35.6%, p = 0.05). The frequency of treatment with cs- and bDMARDs was similar in r-axSpA and nr-axSpA patients (46.4% versus 34.1% and 46.4% versus 56.8%, respectively) [Figure 2(a) to (c)].
Figure 2.
Treatment of eSpA patients.
Percentage of treatment in all eSpA patients (a). csDMARDs and bDMARDs treatments according to axial or peripheral involvement (b). csDMARDs and bDMARDs treatments in patients with ankylosing spondylitis and non-radiographic axial SpA (c).
**p < 0.01.
axSpA, axial spondyloarthritis; bDMARD, biological disease-modifying antirheumatic drug; COX2i, Cyclooxygenase-2 inhibitors; csDMARD, conventional synthetic disease-modifying antirheumatic drug; nr-axSpA, non-radiographic axial SpA; PDN, prednisone; r-axSpA, radiographic axial SpA.
Imaging findings
Imaging data were collected by 72 axSpA patients; in particular, we have analysed 28 spine and SIJ CR scans from r-axSpA patients, as well as 44 SIJ MRI scans and 36 spine MRI from nr-axSpA patients. Data of CR and MRI are summarized in Table 3.
Table 3.
Distribution of lesions evidenced CR and MRI at spine and SIJ in axSpA patients.
| Imaging | Lesions | N/% | r-ax SpA (n = 28) | nr-axSpA (n = 44) | Peripheral axSpA (n = 40) |
|---|---|---|---|---|---|
| CR SIJ | Joint space narrowing at right side | 27/96.4 | 24 | 3 | 10 |
| Joint space narrowing at left side | 26/92.8 | 26 | 0 | 13 | |
| Bone irregularity at right side | 25/89.2 | 22 | 3 | 12 | |
| Bone irregularity at left side | 26/92.8 | 26 | 0 | 14 | |
| Sclerosis or partial ankylosis at right side | 22/78.5 | 22 | 0 | 10 | |
| Sclerosis or partial ankylosis at left side | 23/82.1 | 23 | 0 | 11 | |
| Right ankylosis | 7/25 | 7 | 0 | 3 | |
| Left ankylosis | 8/28.5 | 8 | 0 | 3 | |
| CR Spine | Erosions of vertebral endplate | 8/28.5 | 5 | 3 | 9 |
| Sclerosis of vertebral endplate | 9/32.1 | 7 | 2 | 5 | |
| Squaring | 8/28.5 | 8 | 0 | 0 | |
| Syndesmophytes | 12/42.8 | 12 | 0 | 6 | |
| Bony bridging | 5/17.8 | 5 | 0 | 2 | |
| MRI SIJ inflammatory lesions | Bone marrow oedema | 38/86.3 | 3 | 35 | 29 |
| Capsulitis | 6/13.6 | 2 | 4 | 7 | |
| Enthesitis | 3/6.8 | 1 | 2 | 5 | |
| MRI SIJ chronic lesions | Sclerosis | 6/13.6 | 4 | 2 | 9 |
| Erosions | 6/13.6 | 2 | 4 | 7 | |
| Fat metaplasia | 11/25 | 5 | 6 | 9 | |
| MRI spine inflammatory lesions | Bone marrow oedema | 5/13.9 | 0 | 5 | 4 |
| Facet joint arthritis | 7/19.4 | 2 | 5 | 7 | |
| Spine ligaments enthesitis | 2/5.6 | 0 | 2 | 3 | |
| MRI spine chronic lesions | Fat metapalasia | 2/5.6 | 1 | 1 | 2 |
| Sclerosis | 1/2.8 | 1 | 0 | 2 | |
| Spine erosion | 1/2.8 | 1 | 0 | 4 |
axSpA, axial spondylarthritis; CR, Conventional radiography; MRI, magnetic resonance images; SIJs, sacroiliac joints.
Sacroiliitis at CR was detected in 13.7% (n = 26) of ESpA patients and in 92.9% of r-axSpA. Among r-axSpA patients, SIJ CR showed in 11.5% of cases a grade 2 of sacroiliitis, in 50% of cases a grade 3 of sacroiliitis and in 38.5% a grade 4 of sacroiliitis with total ankylosing of SIJ. From CR spine study, the most represented lesion was syndesmophyte, presented in 50% of patients, followed by sclerosis (32%), squaring (28%) and bridges (17.8%).
From SIJ-MRI of nr-axSpA patients, we have observed the presence of inflammatory lesions (BMO, enthesitis and capsulitis) in 41 patients (93.1%), especially BMO (n = 38, 86.3% of patients) and at least one chronic lesion (sclerosis, erosions, fat metaplasia and ankylosis) in 12 (34%) patients. The prevalence of inflammatory sacroiliitis was 20% (n = 38) in the entire eSpA cohort and specifically, it was 86.3% in the nr-axSpA group. Spine MRI showed inflammatory lesions as BMO, enthesitis of interspinous/supraspinous ligaments and arthritis of facet joint capsules in 14 cases (38.9%), whereas chronic lesions were presented in 4 (9%) patients.
Diagnostic delay in the study population
The analysis of diagnostic delay was conducted considering demographic and clinical characteristics in both the entire study population and the axSpA group (Tables 1 and 2). In the overall eSpA population, the median diagnostic delay was 48 months (IQR 6–77), with no significant differences between patients with axial and peripheral involvement. In the whole study, eSpA population positive associations emerged between diagnostic delay and age (p = 0.001, R = 0.2) and disease duration (p < 0.001, R = 0.9). No significant associations were found with the following demographic and clinical features sex, BMI, education level, employment, smoke habit, uveitis, psoriasis, type of IBD, IBD disease activity, HLA-B27 positivity, SpA disease activity, HAQ-S, ESR, CRP, number of cs and bDMARDs (data not shown).
From analysis of axSpA group, r-axSpA patients showed a longer diagnostic delay compared to those with nr-axSpA (median/IQR 36/17–129 versus 31/10–57 months, p = 0.03).
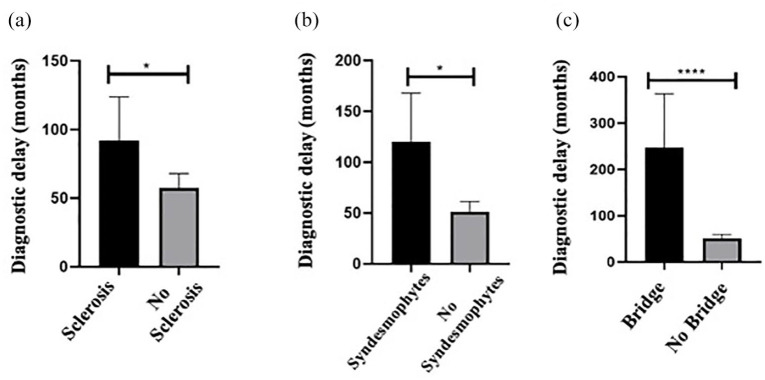
The presence of psoriasis was associated with a longer diagnostic delay in axSpA patients compared to those in patients without skin involvement (118.4 ± 152.3 versus 44.8 ± 57.7, p = 0.004). No significative differences were found in diagnostic delay based on gender, BMI and IBD/SpA activity. Diagnostic delay was significantly higher in patients with any radiographic damage according to mSASSS compared with patients without radiographic lesion at CR (mSASSS = 0) (p = 0.001). In particular, patients with spine sclerosis, syndesmophytes and bridge at CR had a higher diagnostic delay than those without lesions [129 ± 190 versus 52 ± 64.6 months, p = 0.03; 120 ± 180 versus 51.4 ± 62, p = 0.043; 246.8 ± 260 versus 51.1 ± 62, p < 0.0001, respectively, Figure 3(a) to (c)]. Moreover, longer disease duration was detected in those patients with bridge (272.2 ± 264.1 versus 72.9 ± 652, p < 0.0001) and sacroiliitis grade 3 (147.7 ± 197.1 versus 72.2 ± 67.5 months, p = 0.04) compared with patients without radiographic damage. Men showed a higher prevalence of spinal damage lesions than women as sclerosis (32.3% versus 4.9%, p = 0.002), squaring (25.8% versus 0%, p = 0.0006), syndesmophytes (35.5% versus 7.3%, p = 0.0028) and bridges (16.1% versus 0%, p = 0.007) at CR.
Figure 3.
Relationship between diagnostic delay and radiographic damage at spine. Diagnostic delay expressed in months in patients divided according to the presence or absence of radiographic spine lesions as sclerosis (a), syndesmophytes (b) and bridge (c).
*p < 0.05; ****p < 0.0001.
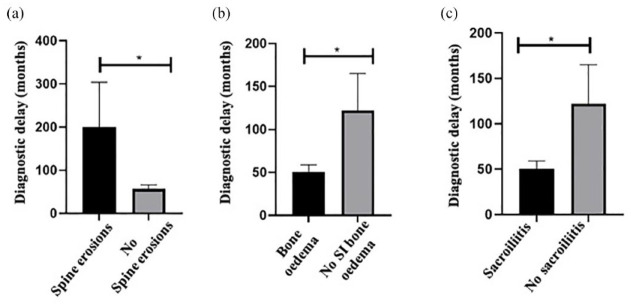
Diagnostic delay was lower in those patients that displayed on MRI the presence of BMO (52.1 ± 61.8 versus 112.4 ± 170.2 months, p = 0.04), and sacroiliitis (50.2 ± 61.9 versus 122 ± 171 months, p = 0.01) compared with that in patients without these lesions. Higher diagnostic delay was observed in patients with chronic lesion of spine bone erosions in MRI compared to that in patients without spine bone erosions (200.1 ± 255.1 versus 56.7 ± 66 months, p = 0.002) [Figure 4(a) to (c)].
Figure 4.
Relationship between diagnostic delay and damage at MRI in spine and SIJs. Relationship between diagnostic delay expressed in months in patients with or without MRI lesions as spine erosions (a), SIJ bone oedema (b) and sacroiliitis (c).
*p < 0.05
MRI, magnetic resonance imaging; SIJ, sacroiliac joint.
Of a note, AxSpA women had a higher prevalence of BMO of SIJ at MRI than men (73.2% versus 35.5%, p = 0.001).
Discussion
Reducing the diagnostic delay in SpA is a significant challenge in the clinical setting, as demonstrated by the extensive DANBIO registry, encompassing patients affected by rheumatoid arthritis, psoriatic arthritis or AS, suggesting the strong awareness of the importance of early diagnosis. 29 The available data on diagnostic delay in eSpA patients are inadequate, given the unique population where many confounding factors, such as the medications for IBD controlling SpA-related symptoms, may interfere with a proper diagnosis. 30 Delays can result from clinicians not consistently inquiring about joint complaints or patients themselves underreporting symptoms, often misinterpreted for nonspecific mechanical joint or back pain. Furthermore, diagnostic delay may indeed underestimate the global prevalence of SpA disease in IBD, ranging from 4% to 12%. 1 Our cohort revealed a diagnostic delay of 48 months, below the average delay in AS of 8–11 years, 31 and it is in line with prior findings in an eSpA cohort. 1 Notably, diagnostic delay was 21.5 months in peripheral eSpA patients and 31.5 months in axSpA. The high prevalence of peripheral eSpA in cohort corroborates data from literature.28–32 Female sex correlated with the peripheral involvement, whereas male sex correlated with the axial one, confirming gender-based differences in disease presentation in eSpA. 33 However, differently from the literature, gender did not impact diagnostic delay in our cohort, neither in the whole cohort nor in the peripheral or axial ones. Despite females exhibiting differences in disease presentation and more frequent inflammatory lesions detected by MRI compared with males, existing literature suggests a higher frequency of delayed SpA diagnosis in female, albeit potentially influenced by physician bias. 34 These observations need to be replicated in larger cohorts to avoid bias of selection and low prevalence of certain disease phenotypes. Nevertheless, in the axSpA group, patients affected by r-axSpA displayed a longer diagnostic delay compared with nr-axSpA ones. We attribute the seemingly better performance in detecting nr-axSpA to the specific diagnostic methods employed. MRI is known for its sensitivity in detecting early inflammatory changes. Conversely, radiographic evidence indicative of r-axSpA may manifest slowly, potentially prolonging the delay in diagnosis.35,36 Indeed, the presence of BMO and sacroiliitis in the MRI was associated with a reduced diagnostic delay. Conversely, the presence of chronic lesions in the spine, such as bone erosions, was associated with a higher diagnostic delay. Overall, in our cohort a higher prevalence of nr-axSpA than r-axSpA was observed and in line with data from literature patients with r-axSpA were older, with longer disease duration and higher diagnostic delay compared with nr-axSpA patients. Moreover, low education status and high employment rate were found in r-axSpA compared with nr-axSpA, as previously identified by Haroon et al. who demonstrated that PsA patients with low education status were significantly more likely to have a diagnostic delay of more than 2 years. Education status, income and employment status may affect the timing to referral, delay in diagnosis and outcome in terms of physical, functional and radiographic damage. 37 The identification of these factors might guide clinicians in a tailoring diagnostic approaches, as delayed axSpA diagnosis may lead to delayed treatment and irreversible structural damage. This in turn can result in significant disability and pain, which can have a negative impact on participation in employment, social activities and function.33,38–40
Among radiographic lesions at spine and SIJ, the presence of spine sclerosis, bridges and syndesmophytes was associated with diagnostic delay in r-axSpA, whereas only bridges and sacroiliitis grade 3 were correlated with long disease duration. Men sex was associated with the presence of sclerosis, squaring, syndesmophytes and bridges at CR, highlighting the burden of r-axSpA among male gender. These findings, in particular the presence of spine sclerosis, might represent red flags to identify early those patients at risk to develop r-axSpA, mainly in male patients. 41 However, these data warrant further exploration in prospective cohorts.
The presence of extra-articular manifestations, such as psoriasis, may postpone the rheumatologic diagnosis being the patient under the care of a dermatologist and gastroenterologist rather than a rheumatologist. In our cohort, psoriasis was associated with high diagnostic delay. In this context, a dedicated referral strategy with screening validated questionnaire and a multidisciplinary clinic are well-recognized working models designed to deliver patient-centred care by centralizing relevant practitioners at a single physical site improving efficiency, management and reducing diagnostic delay.1,42–44
This study encompasses some limitations such as the single-centre nature of the study which may create a bias of selection but at the same time avoid bias of inter-rater variability, the relatively low number of patients and imaging data when divided into different subgroups, the need for larger, well-designed studies using multivariable modelling to identify earlier patients affected by SpA and an axial involvement in IBD patients.
Strength of this study is to add a piece of knowledge in a particular population of eSpA patients regarding one of the major issues in clinical practice such as the magnitude of diagnostic delay in eSpA that may impact the quality of life of patients and the costs of a potential disability disease-related.
Conclusion
This clinical research investigates diagnostic delays in eSpA and explores the clinical and imaging factors contributing to this delay. Key considerations include the presence of psoriasis, spine bone erosions on MRI, and spine sclerosis, syndesmophytes, and bridging on CR. These factors act as crucial red flags, indicating the necessity for heightened clinical attention and expediting the diagnostic process. The presence of psoriasis serves as an early indicator, prompting timely consideration of enteropathic SpA in patients with back pain. Recognition of spine bone erosions on MRI and observation of imaging markers aid early diagnosis. Our research aims to deepen the understanding of diagnostic delays in eSpA, potentially informing recommendations and enhancing clinical guidelines. The identification of these red flags may lead to more efficient diagnostic strategies, promoting early referral and intervention, potentially improving patient outcomes and quality of life by addressing eSpA at its earliest stages.
Acknowledgments
None.
Footnotes
ORCID iDs: Paola Conigliaro  https://orcid.org/0000-0001-7905-8413
https://orcid.org/0000-0001-7905-8413
Arianna D’Antonio  https://orcid.org/0000-0003-1872-5299
https://orcid.org/0000-0003-1872-5299
Contributor Information
Paola Conigliaro, Rheumatology, Allergology and Clinical Immunology, Department of “Medicina dei Sistemi”, University of Rome Tor Vergata, Via Montpellier 1, Rome 00133, Italy.
Arianna D’Antonio, Rheumatology, Allergology and Clinical Immunology, Department of “Medicina dei Sistemi”, University of Rome Tor Vergata, Rome, Italy.
Andrea Wlderk, Department of Diagnostic, UOC of Diagnostic and Interventional Neuroradiology, San Camillo-Forlanini Hospital, Rome, Italy.
Federico Sabuzi, Department of Diagnostic Imaging and Interventional Radiology, University of Rome Tor Vergata, Rome, Italy.
Mario Ferraioli, Rheumatology, Allergology and Clinical Immunology, Department of “Medicina dei Sistemi”, University of Rome Tor Vergata, Rome, Italy.
Leonardo Sichi, Rheumatology Unit, Azienda Ospedaliero-Universitaria di Cagliari, Cagliari, Italy.
Valerio Da Ros, Department of Diagnostic Imaging and Interventional Radiology, University of Rome Tor Vergata, Rome, Italy.
Livia Biancone, Gastroenterology Unit, Department of “Medicina dei Sistemi”, University of Rome Tor Vergata, Rome, Italy.
Alberto Bergamini, Rheumatology, Allergology and Clinical Immunology, Department of “Medicina dei Sistemi”, University of Rome Tor Vergata, Rome, Italy.
Maria Sole Chimenti, Rheumatology, Allergology and Clinical Immunology, Department of “Medicina dei Sistemi”, University of Rome Tor Vergata, Rome, Italy.
Declarations
Ethics approval and consent to participate: Ethics approval was obtained by the scientific ethic committee the University of Rome Tor Vergata (N. 186/16), Rome, Italy. Written informed consent was obtained from all the patients enrolled in the study and has been waived by the Ethics Committee of the University of Rome Tor Vergata.
Consent for publication: Written informed consent for publication was provided by the participants.
Author contributions: Paola Conigliaro: Conceptualization; Data curation; Formal analysis; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Arianna D’Antonio: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing – original draft.
Andrea Wlderk: Conceptualization; Formal analysis; Investigation; Methodology; Writing – original draft.
Federico Sabuzi: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Software; Writing – original draft.
Mario Ferraioli: Conceptualization; Formal analysis; Investigation; Methodology; Writing – original draft.
Leonardo Sichi: Conceptualization; Formal analysis; Investigation; Methodology; Writing – original draft.
Valerio Da Ros: Conceptualization; Formal analysis; Investigation; Methodology; Supervision; Validation; Writing – review & editing.
Livia Biancone: Conceptualization; Formal analysis; Investigation; Methodology; Validation; Writing – review & editing.
Alberto Bergamini: Conceptualization; Formal analysis; Investigation; Methodology; Supervision; Validation; Writing – review & editing.
Maria Sole Chimenti: Conceptualization; Supervision; Validation; Visualization.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: The data underlying this article will be shared upon reasonable request to the corresponding author.
References
- 1. Conigliaro P, Chimenti MS, Ascolani M, et al. Impact of a multidisciplinary approach in enteropathic spondyloarthritis patients. Autoimmun Rev 2016; 15: 184–190. [DOI] [PubMed] [Google Scholar]
- 2. So J, De Craemer AS, Elewaut D, et al. Spondyloarthritis: how far are we from precision medicine? Front Med (Lausanne) 2022; 9: 988532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rudwaleit M, van der Heijde D, Landewé R, et al. The assessment of spondyloarthritis international society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis 2011; 70: 25–31. [DOI] [PubMed] [Google Scholar]
- 4. Lambert RG, Bakker PA, van der Heijde D, et al. Defining active sacroiliitis on MRI for classification of axial spondyloarthritis: update by the ASAS MRI working group. Ann Rheum Dis 2016; 75: 1958–1963. [DOI] [PubMed] [Google Scholar]
- 5. Deodhar A. Axial spondyloarthritis criteria and modified NY criteria: issues and controversies. Clin Rheumatol 2014; 33: 741–747. [DOI] [PubMed] [Google Scholar]
- 6. Rudwaleit M, van der Heijde D, Landewe R, et al. The development of assessment of spondyloArthritis international society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009; 68: 777–783. [DOI] [PubMed] [Google Scholar]
- 7. De Craemer AS, Łukasik Z, Carron P. Use of imaging in axial spondyloarthritis for diagnosis and assessment of disease remission in the year 2022. Curr Rheumatol Rep 2022; 24: 383–397. [DOI] [PubMed] [Google Scholar]
- 8. Poddubnyy D, Sieper J. Diagnostic delay in axial spondyloarthritis - a past or current problem? Curr Opin Rheumatol 2021; 33: 307–312. [DOI] [PubMed] [Google Scholar]
- 9. Hay CA, Packham J, Ryan S, et al. Diagnostic delay in axial spondyloarthritis: a systematic review. Clin Rheumatol 2022; 41: 1939–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chimenti MS, Triggianese P, De Martino E, et al. An update on pathogenesis of psoriatic arthritis and potential therapeutic targets. Expert Rev Clin Immunol 2019; 15: 823–836. [DOI] [PubMed] [Google Scholar]
- 11. Poddubnyy D, van Tubergen A, Landewé R, et al. Assessment of spondyloArthritis international Society (ASAS). development of an ASAS-endorsed recommendation for the early referral of patients with a suspicion of axial spondyloarthritis. Ann Rheum Dis 2015; 74: 1483–1487. [DOI] [PubMed] [Google Scholar]
- 12. Yi E, Ahuja A, Rajput T, et al. Clinical, economic, and humanistic burden associated with delayed diagnosis of axial spondyloarthritis: a systematic review. Rheumatol Ther 2020; 7: 65–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Regierer AC, Weiß A, Proft F, et al. Comparison of patients with axial PsA and patients with axSpA and concomitant psoriasis: an analysis of the German register RABBIT-SpA. RMD Open 2023; 9: e002837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006; 55: 749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jun A, Yuhan K. The role of primary care providers for uveitis. J Nurse Pract 2021; 17: 1194–1198. [Google Scholar]
- 16. Malik TF, Aurelio DM. Extraintestinal manifestations of inflammatory bowel disease . In: StatPearls [Internet]. Treasure Island, FL, USA: StatPearls Publishing, 2023. [PubMed] [Google Scholar]
- 17. Mease P. Enthesitis in psoriatic arthritis (Part 3): clinical assessment and management. Rheumatology (Oxford) 2020; 59(Suppl. 1): i21–i28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Helliwell PS, Firth J, Ibrahim GH, et al. Development of an assessment tool for dactylitis in patients with psoriatic arthritis. J Rheumatol 2005; 32: 1745–1750. [PubMed] [Google Scholar]
- 19. Lukas C, Landewé R, Sieper J, et al. Assessment of spondyloArthritis international society. Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis 2009; 68: 18–24. [DOI] [PubMed] [Google Scholar]
- 20. Garrett S, Jenkinson T, Kennedy LG, et al. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994; 21: 2286–2291. [PubMed] [Google Scholar]
- 21. Calin A, Garrett S, Whitelock H, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the bath ankylosing spondylitis functional index. J Rheumatol 1994; 21: 2281–2285. [PubMed] [Google Scholar]
- 22. Daltroy LH, Larson MG, Roberts NW, et al. A modification of the health assessment questionnaire for the spondyloarthropathies. J Rheumatol 1990; 17: 946–950. Erratum in: J Rheumatol 1991; 18: 305. [PubMed] [Google Scholar]
- 23. Maaser C, Sturm A, Vavricka SR, et al. European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR]. ECCO-ESGAR guideline for diagnostic assessment in IBD Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis 2019; 13: 144–164. [DOI] [PubMed] [Google Scholar]
- 24. Sudoł-Szopińska I, Urbanik A. Diagnostic imaging of sacroiliac joint and the spine in the course of spondyloarthropathies. Pol J Radiol 2013; 78: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lacout A, Rousselin B, Pelage J-P. CT and MRI of spine and sacroiliac involvement in spondyloartropathy. Am J Roentgenol 2008; 191: 1016–1023. [DOI] [PubMed] [Google Scholar]
- 26. van der Heijde D, Braun J, Deodhar A, et al. Modified stoke ankylosing spondylitis spinal score as an outcome measure to assess the impact of treatment on structural progression in ankylosing spondylitis. Rheumatol (Oxford) 2019; 58: 388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baraliakos X, Østergaard M, Lambert RG, et al. MRI lesions of the spine in patients with axial spondyloarthritis: an update of lesion definitions and validation by the ASAS MRI working group. Ann Rheum Dis 2022; 81: 1243–1251. [DOI] [PubMed] [Google Scholar]
- 28. Picchianti-Diamanti A, Lorenzetti R, Chimenti MS, et al. Enteropathic spondyloarthritis: results from a large nationwide database analysis. Autoimmun Rev 2020; 19: 102457. [DOI] [PubMed] [Google Scholar]
- 29. Sørensen J, Hetland ML; all departments of rheumatology in Denmark. Diagnostic delay in patients with rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis: results from the Danish nationwide DANBIO registry. Ann Rheum Dis 2015; 74: e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Evans J, Sapsford M, McDonald S, et al. Prevalence of axial spondyloarthritis in patients with inflammatory bowel disease using cross-sectional imaging: a systematic literature review. Ther Adv Musculoskelet Dis 2021; 13: 1759720X21996973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Braun J, Sieper J. Ankylosing spondylitis. Lancet 2007; 369: 1379–1390. [DOI] [PubMed] [Google Scholar]
- 32. Chimenti MS, Conigliaro P, Triggianese P, et al. Use of synthetic and biological DMARDs in patients with enteropathic spondyloarthritis: a combined gastro-rheumatological approach. Clin Exp Rheumatol 2019; 37: 723–730. [PubMed] [Google Scholar]
- 33. de Jong H, Paramarta JE, de Winter J, et al. Differences between females and males in axial spondyloarthritis: data from a real-life cross-sectional cohort. Scand J Rheumatol 2020; 49: 28–32. [DOI] [PubMed] [Google Scholar]
- 34. Jovaní V, Blasco-Blasco M, Ruiz-Cantero MT, et al. Understanding how the diagnostic delay of spondyloarthritis differs between women and men: a systematic review and metaanalysis. J Rheumatol 2017; 44: 174–183. [DOI] [PubMed] [Google Scholar]
- 35. Komsalova LY, Martínez Salinas MP, Jiménez JFG. Predictive values of inflammatory back pain, positive HLA B27 antigen and acute and chronic magnetic resonance changes in early diagnosis of Spondyloarthritis. A study of 133 patients. PLoS One 2020; 15: e0244184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Salvadorini G, Bandinelli F, Delle Sedie A, et al. Ankylosing spondylitis: how diagnostic and therapeutic delay have changed over the last six decades. Clin Exp Rheumatol 2012; 30: 561–565. [PubMed] [Google Scholar]
- 37. Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis 2015; 74: 1045–1050. [DOI] [PubMed] [Google Scholar]
- 38. Ward MM, Reveille JD, Learch TJ, et al. Impact of ankylosing spondylitis on work and family life: comparisons with the US population. Arthritis Rheum 2008; 59: 497–503. [DOI] [PubMed] [Google Scholar]
- 39. Healey EL, Haywood KL, Jordan KP, et al. Ankylosing spondylitis and its impact on sexual relationships. Rheumatology (Oxford) 2009; 48: 1378–1381. [DOI] [PubMed] [Google Scholar]
- 40. Fallahi S, Jamshidi AR. Diagnostic delay in ankylosing spondylitis: related factors and prognostic outcomes. Arch Rheumatol 2015; 31: 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Felice C, Leccese P, Scudeller L, et al.; Italian SpA-IBD Expert Panel Group. Red flags for appropriate referral to the gastroenterologist and the rheumatologist of patients with inflammatory bowel disease and spondyloarthritis. Clin Exp Immunol 2019; 196: 123–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Benfaremo D, Luchetti MM, Di Carlo M, et al. Multicenter validation of the DETAIL questionnaire for the screening of spondyloarthritis in patients with inflammatory bowel diseases. J Rheumatol 2021; 48: 179–187. [DOI] [PubMed] [Google Scholar]
- 43. Li J, Xu Y, Chen Y, et al. A multidisciplinary clinic approach to improve physician-related diagnostic delay for patients with axial spondyloarthritis: a retrospective study. J Int Med Res 2019; 47: 2483–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Variola A, Zanolin ME, Cipriano G, et al. The IBIS-Q [IBd Identification of Spondyloarthritis Questionnaire]: a novel tool to detect both axial and peripheral arthritis in inflammatory bowel disease patients. J Crohns Colitis 2020; 14: 1680–1686. [DOI] [PubMed] [Google Scholar]