Abstract
Introduction
Pressure injuries (PIs) arise from sustained pressure on tissue, leading to reduced blood flow to the affected area. In patients with spinal cord injuries (SCIs), these PIs can significantly diminish their independence and overall quality of life. This research sought to assess the frequency of surgical complications in treatment regimens for large sacral PIs involving the anus. Specifically, the study focused on the incorporation of stoma formation in patients with SCIs.
Methods
A retrospective review identified 25 SCI patients who had extensive sacral PIs. These patients underwent intestinal stoma formation as a preparatory step before plastic reconstructive surgery to address the wounds between 2015 and 2020.
Results
Successful wound closure was achieved in all instances. Notably, each patient had experienced a minimum of three unsuccessful reconstructive surgeries elsewhere before this intervention. The observed rate of surgical complications aligned with findings from previous analogous studies.
Conclusion
While often viewed as a treatment of last resort, an intestinal stoma might serve as a valuable strategy, particularly for SCI patients with extensive PIs near the anal region, to promote the healing of such injuries. Tailored decision-making is essential to ensure the best possible patient outcomes.
Keywords: spinal cord injury, pressure injuries, stoma formation, quality of life
Introduction
According to estimates from the World Health Organization, spinal cord injury (SCI) affects 40–80 cases per 1,000,000 population globally each year, remaining a significant challenge in the medical community and leading to between 250,000 and 500,000 new cases annually (Colquhoun et al., 2006; Waddell et al., 2020). One of the most debilitating complications arising from SCI is the development of pressure injuries (PIs), which can significantly impair a patient's quality of life and independence (Rahman et al., 2022). These complications not only escalate treatment expenses but also prolong hospital admissions (Biglari et al., 2014; Daniels et al., 2011; DeVivo & Farris, 2011; Filius et al., 2013). It's alarming that nearly 85% of individuals with paraplegia will experience at least one PI during their lifetime (Byrne & Salzberg, 1996). Furthermore, around 20% of paraplegic individuals suffer from one or multiple concurrent PIs. For those with quadriplegia, the incidence of PIs ranges between 26 and s60% (Barbenel, 1991; Wing, 2008). Notably, for patients who spend a significant amount of time lying supine, the sacral region becomes the most common site for these injuries (Gaab et al., 2014).
When addressing extensive PIs, specifically those classified as grade III–IV according to EPUAP guidelines (Black et al., 2007; European Pressure Ulcer Advisory Panel, EPUAP/NPIAP/PPPIA, 2019), surgical plastic reconstructive methods often become the treatment of choice. The decision to proceed with such interventions hinges on the injury's location and the overall health status of the patient (Barton, 2006; Safak et al., 1996). It's imperative that any surgical approach is meticulously strategized, ensuring that local conditions are optimized before the procedure. If a patient presents with signs of severe infection, compromised circulation, or deteriorating general health, it's paramount to first enhance the wound and the patient's overall condition before considering surgery. For injuries proximate to the anus or urogenital tract, contamination risks are heightened. In such scenarios, the formation of a stoma and the introduction of a trans-urethral or suprapubic bladder catheter become prudent measures (Gaab et al., 2014).
The unique tissue composition of sacral PIs, combined with their variable thickness and the ever-present risk of post-operative fecal contamination, drastically diminishes the likelihood of a successful surgical outcome. Consequently, the establishment of a stoma, occasionally paired with an anus amputation, becomes indispensable (Gray & Giuliano, 2018). To ensure thorough decontamination of the surgical site, employing a colostomy in conjunctions with a complete anus removal proves to be highly effective (Ambe et al., 2018).
Patients and Methods
Study Design
The study was set up at BG Trauma Centre Ludwigshafen, Department of Paraplegiology, Rhineland-Palatinate (Rheinland-Pfalz). All procedures were performed according to the Ethical Principles for Medical Research expressed in the Declaration of Helsinki in its current form (World Medical Association, 2013). Furthermore, the manuscript was composed according to the STROBE cohort checklist (Von Elm et al., 2008).
Ethics Approval
The present retrospective chart review of patients was conducted following the guidelines of the Declaration of Helsinki. It was approved on 2 February 2022 (number 2022-16366-retrospective) by the State Chamber of Physicians of Rheinland Palatinate Ethics Committee.
Patients
From 2015 to 2020, the Spinal Unit at BG Trauma Centre Ludwigshafen in Rhineland-Palatinate, Germany, retrospectively documented SCI patients who had grade III or IV PIs, as classified by EPUAP, and underwent stoma formation. Only individuals aged 18 or older who had these specific injuries, underwent stoma formation between January 2015 and December 2020, and later had reconstructive surgery were considered for the study. Those not meeting all these criteria were excluded. Out of 25 eligible patients, all were enrolled for evaluation.
Among potentially occurring complications 16 patients were identified with osteomyelitis. Our medical institution adopted a multi-disciplinary approach for their treatment. Initially, these patients received targeted antibiotic therapy based on culture and sensitivity results from bone biopsies. Severe cases required surgical debridement of the infected bone to remove necrotic tissue and reduce bacterial load. The treatment's progress was monitored through regular radiographic assessments and laboratory tests, such as C-reactive protein levels.
Following stoma surgery patients received antibiotics based on their antibiogram. They were placed on alternating pressure mattresses and frequently repositioned to alleviate pressure on the surgical sites. Three weeks after the surgery, skin closure materials were removed. Patients were also provided with a high-calorie diet. A patient was deemed ready for flap surgery once there were clear indications of the infection being under control. This was determined by a combination of factors: a consistent decrease in inflammatory markers, no signs of active infection on imaging, and a healthy granulating wound bed. Typically, a minimum of 6 weeks of antibiotic treatment was required before considering flap surgery, but this duration varied based on individual patient response.
The average time between ostomy formation and flap surgery was approximately 8 weeks. However, this duration was influenced by several factors, including the patient's overall health, the success of the osteomyelitis treatment, and the condition of the wound bed. Some patients required a longer preparatory period, especially if there were complications or if the osteomyelitis was particularly aggressive. Before undergoing flap surgery—a lengthy procedure ensuring vitality of all tissues, especially around the deep skin wound—each patient had to show no signs of osteomyelitis for several weeks. Flap surgery was only performed after successfully treating any complications that might impede the skin flap's healing.
Statistical Analysis
Statistical calculations were performed with R version 4.2.0 (R Core Team, 2023) in RStudio (Allaire, 2012), applying the tidyr (Wickham & Wickham, 2017) and dplyr (Wickham et al., 2015) packages. Figures were created using the ggplot2 package (Wickham, 2016). Where appropriate, images were created using BioRender (https://biorender.com/).
Results
The current study was designed retrospectively and aimed to explore clinical outcomes. Descriptive methods were employed for data analysis.
A total of 25 cases were examined. Among the participants, 4 females and 21 males were identified, with a median age of 61 years (ranging from 35 to 81 years). Complete paraplegia was observed in 23 patients, while incomplete paraplegia was found in 2, and PIs in the sacral region were seen in all. Thoracic SCIs were predominant. Colostomy was performed in 15 patients, while Sigmoidostomy was done in 10. Diabetes mellitus was identified as the most common comorbidity in 24.0% of the patients. The median hospital stay was recorded as 56 days, and the median wound size was measured at 46.0 cm2. Grade III or IV PIs were found in all patients.
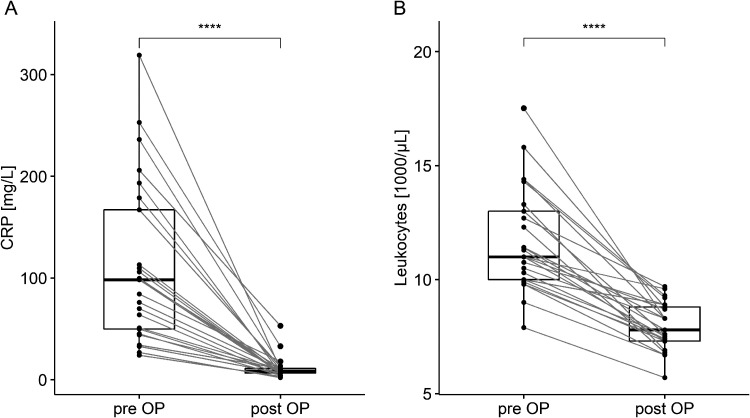
Table 1 provides the demographics, injury specifics, comorbidities, duration of hospital stay, and wound sizes of the participants. Routine pre- and post-operative inflammation tests, depicted in Figure 1, were conducted on the first day and around day 55, respectively.
Table 1.
Characteristics of the Study Population.
| Total (N = 25) | |
|---|---|
| Gender | |
| Female | 4 (16.0%) |
| Male | 21 (84.0%) |
| Age | |
| Median (IQR) | 61.0 (35.0, 81.0) |
| Type of plegia | |
| Complete | 23 (92.0%) |
| Incomplete | 2 (8.0%) |
| NLI | |
| Cervical | 1 (4.0%) |
| Thoracic | 18 (72.0%) |
| Lumbar | 6 (24.0%) |
| Comorbidities | |
| C2 abuse | 1 (4.0%) |
| COPD | 1 (4.0%) |
| DM | 5 (20.0%) |
| DM and KHK | 1 (4.0%) |
| Hydrocephalus | 1 (4.0%) |
| Length of hospital stay | |
| Median (IQR) | 56.0 (41.0, 120.0) |
| Area [cm2] | |
| Median (IQR) | 46.0 (20.0, 450.0) |
| Operation | |
| Colostoma | 15 (60.0%) |
| Sigmoidostoma | 10 (40.0%) |
| Complications | |
| Haematome | 2 (8.0%) |
| Infection | 1 (4.0%) |
| Suture dehiscence | 6 (24.0%) |
| Suture dehiscence and infection | 1 (4.0%) |
| None | 15 (60.0%) |
| Surgery ex domo [N] | |
| Median (IQR) | 4.0 (2.0, 6.0) |
| Osteomyelitis | |
| No | 9 (36.0%) |
| Yes | 16 (64.0%) |
| Flap | |
| Gluteus maximus Advancement flap | 23 (92.0%) |
| Total thigh flap | 2 (8.0%) |
Figure 1.
Pre- and post-operative routine lab diagnostics in CRP and leukocyte concentrations, respectively, on median day 1 “pre OP” (IQR 0-2), i.e., previously to the stoma formation, and day 55 “post OP” (IQR 40-119), namely following flap surgery. Abbreviations: CRP, C-reactive protein; pre OP, pre-operatively; post OP, post-operatively; p-values, calculated by comparing each blood parameter before and following surgery, <0.05 were considered as significant; *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
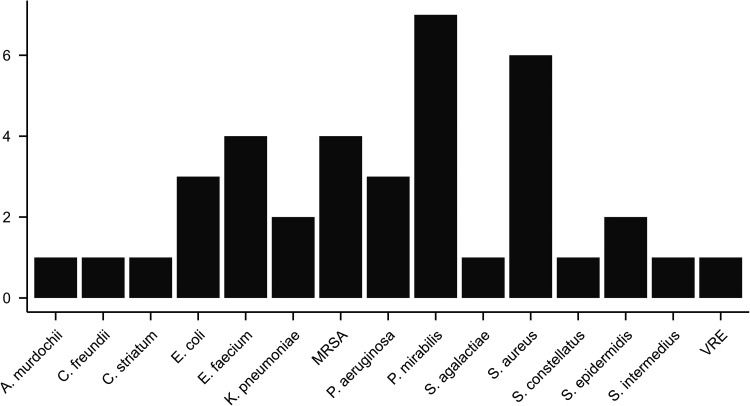
Microbiological tests were conducted on all patients during debridement to identify wound pathogens. Based on these results, targeted antibiotic treatments were administered. A stoma was surgically created in every patient initially, and reconstructive surgeries were later performed. The infectious agents identified are listed in Figure 2.
Figure 2.
Infective species of the study population. Among the 25 study patients the following pathogens infected one patient each: A. murdochii, C. freundii, C. striatum, S. agalactiae, S. constellatus, S. intermedius, and VRE. K. pneumoniae and S. epidermidis were isolated twice, E. coli and P. aeruginosa on three patients, E. faecium and MRSA on four, S. aureus on six and P. mirabilis on seven subjects. Some patients experienced multiple infections. Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant Enterococci.
Based on the PI's size and location, different surgical approaches were chosen: gluteus maximus flaps were given to 23 patients, total thigh flaps were given to 2. Table 2 Not only specifies the surgical method used, but also outlines the post-operative complications and wound sizes observed. Post-operative complications were experienced by 40% of the patients (10 out of 25), with suture dehiscence being the most commonly observed in seven cases. However, no complications were reported in 15 patients.
Table 2.
Frequency of Complications and Wound Area in Relation to the Type of Flap.
| Complications | Total | Gluteus maximus flap | Total thigh flap |
|---|---|---|---|
| Complications – all patients | |||
| Total | 25 (100.0) | 23 (100.0) | 2 (100.0) |
| None | 15 (60.0) | 15 (65.2) | 0 (0.0) |
| Yes (details right below) | 10 (40.0) | 8 (34.8) | 2 (100.0) |
| - Hematoma | 2 (8.0) | 2 (8.7) | 0 (0.0) |
| - Infection | 1 (4.0) | 1 (4.3) | 0 (0.0) |
| - Suture dehiscence | 6 (24.0) | 4 (17.4) | 2 (100.0) |
| - Suture dehiscence and infection | 1 (4.0) | 1 (4.3) | 0 (0.0) |
| Wound area [cm2] (Median IQR) | 46.0 (20.0–450.0) | 45.0 (20.0–92.0) | 330.0 (210.0–450.0) |
Abbreviations: IQR, interquartile range; p-values are calculated by comparing the flap type with each variable in the table.
In the group where gluteus maximus flaps were used (23 patients), a complication rate was 34.8% was observed. Specifically, suture dehiscence was found in four patients, hematomas in two, an infection in one, and both suture dehiscence and infection in one. Table 3 contrasts the complication rates of the gluteus maximus flap in this study with results from a previous study (Biglari et al., 2014).
Table 3.
Frequency of Gluteus Maximus Flap Complications: A Comparison between the Present Study and Biglari et al. in 2014.
| Gluteus maximus flaps (GMF) | Patients (present study) | Patients (Biglari et al.1) |
|---|---|---|
| Total (N) | 23 (100.0) | 82 (100.0) |
| Complications | ||
| None | 15 (65.2) | 64 (78.0) |
| Yes (details right below) | 8 (34.8) | 18 (22.0) |
| - Hematoma | 2 (8.7) | 4 (4.9) |
| - Suture dehiscence | 4 (17.4) | 4 (4.9) |
| - Infection | 1 (4.3) | 5 (6.1) |
| - Suture dehiscence and infection | 1 (4.3) | 0 (0.0) |
| - Necrosis | 0 (0.0) | 3 (3.6) |
| - Partial necrosis | 0 (0.0) | 2 (2.4) |
Discussion
This study conducted a retrospective analysis of stoma placement in paraplegic patients who presented with severe PIs, specifically those of grades III and IV according to the EPUAP guidelines. These PIs were either located at the anus or had anal involvement. PIs are a significant concern in individuals with paraplegia (Biglari et al., 2014; Byrne & Salzberg, 1996; Kreutztrager et al., 2018), with recurrence rates being alarmingly high, sometimes exceeding 40% (Bertheuil et al., 2013; Grassetti et al., 2014; Tavakoli et al., 1999; Thiessen et al., 2011). From our observations and experiences, it is pertinent to note that patients who are incontinent and have no prospects of regaining continence derive the most significant benefit from a permanent stoma. This is because in these situations, a stoma can play a pivotal role in consistently reducing the heightened contamination risks commonly linked to sacral and anal PIs. In contrast, patients who have experienced a trauma or are in a coma but maintain bowel continence can benefit from a temporary stoma. This temporary solution can later be revised once the wound has adequately healed. Additionally, as an alternative to the traditional stoma, modern stool tube systems are emerging as potential solutions (Waddell et al., 2020).
Bowel Management
Managing and emptying the bowel is a profound challenge that has a considerable negative impact on the lives of paraplegic patients (Glickman & Kamm, 1996; Levi et al., 1995). For many of these individuals, the process of bowel evacuation stands out as their most pressing issue (Hanson & Franklin, 1976). While the creation of a colostomy is often viewed as a last resort measure, for a significant number of patients, it can be a transformative method to address bowel dysfunction. This procedure can enhance their fecal continence, grant them a greater degree of independence, and substantially improve their overall quality of life (Bølling Hansen et al., 2016; Cooper et al., 2019; Kelly et al., 1999; Safadi et al., 2003; Stone et al., 1990). Elective colostomy, a specific type of stoma, is widely recognized as a viable method for bowel management in SCI patients (Swarnakar et al., 2022). A comprehensive review in 2020 by Waddell O. et al. emphasized the positive outcomes of stoma placement, noting improvements in patients’ quality of life, reduced time spent on post-operative bowel care, and high levels of patient satisfaction with their stomas (Waddell et al., 2020).
When patients develop large PIs, especially those in the sacral or ischial region that involve or are proximate to the anus, the clinical approach towards stoma indications shifts dramatically. The heightened risk of fecal contamination poses a significant threat to the surgical wounds, thereby increasing the chances of surgical flap loss.
Complications and Flap Types
Post-operative complications following flap surgery in paraplegic patients are unfortunately common, with complication rates varying between 20 and 60% (Biglari et al., 2014; Schryvers et al., 2000). In our study, we observed that all participants had multiple PIs and subsequently received targeted surgical interventions. The choice of flaps was determined based on individual patient needs, with some patients experiencing complications that were directly correlated to the size and severity of their PIs.
Regarding the choice of flaps for complications and wound areas (Table 2), two out of two patients becoming a full thigh flap surgical coverage presented suture dehiscence. A possible correlation to the very high complication rate in this subgroup might be related to the fact that the two largest PIs of 450.0 cm2 and 210.0 cm2 among the 25 subjects belong to the total thigh flap patients. A total thigh flap is often seen as a last resort option to cover relatively extended skin defects (Lazar et al., 2007), and it provides adequate blood supply and flap thickness where coverage of an extended PI is required (Shin et al., 2014).
In our previous study by Biglari et al. (2014), as reported in Table 3, we found a lower incidence of complications in gluteus maximus flap type surgeries among spinal cord-injured (SCI) patients with PIs grade I–IV. Specifically, out of a total of 82 gluteus maximus flap procedures, 18 (22.0%) resulted in complications, with the most common issues being infection (6.1%), suture dehiscence (4.9%), and hematoma (4.9%). It's important to note that these variations could be attributed to the fact that the current dataset primarily focuses on PIs grade III–IV, excluding less extensive grade I–II PIs, which tend to have lower complication rates (Biglari et al., 2014).
Review of Literature
Stoma construction has emerged as a potential solution, offering a safe procedure with a low rate of morbidity and mortality for patients grappling with medically intractable PIs. The rationale behind this approach is to divert the fecal stream away from the perineal area, thereby aiding the healing process of the PI by minimizing bacterial contamination and subsequently reducing the recurrence rate of the PI (Bock et al., 2021; Negosanti et al., 2020).
However, the evidence regarding the relationship between fecal contamination in patients with extensive PIs and the necessity for stoma placement remains inconclusive. Some studies, such as the 2021 retrospective cohort study (Pussin et al., 2021), suggest that colostomies might not always be the optimal solution; the authors reported that stoma patients required more time (an average of 77 days) to achieve complete healing of anus-near PIs compared to non-stoma patients (59 days). Given the physical and psychological challenges associated with stoma formation, the study's authors concluded that a colostomy might not be the standard solution for all patients with PIs near the anus. Instead, they advocate for more individualized approach, emphasizing the role of a specialized team in making these decisions. Another retrospective cohort study (Lichtenthaler et al., 2023), showed that by comparing a group of patients with a stoma to a matched group practicing supported natural defecation, there was no significant difference in the variety of pathogens found in the wound and the frequency of microbial colonization. The only exception was a reduced isolation of E. coli in the stoma subjects. In contrast, research, by de la Fuente et al. (2003) and Stone et al. (1990), underscores the importance of colostomies in treating paraplegic patients with PIs. De la Fuente et al. (2003), in their retrospective cohort study, discovered a significantly reduced rate of PI recurrence in patients who underwent a colostomy compared to those who did not. Additionally, a first case report suggests that the use of autologous platelet-rich fibrin membrane can enhance PI healing (Swarnakar et al., 2022).
Implications for Practice
The collected data and subsequent observations advocate for a patient-centric approach. We believe in the judicious use of stoma formation, especially as a last resort in challenging cases. The primary goal remains the successful and timely recovery post PI surgeries. Therefore, we champion the idea of considering stoma formation in cases of medically intractable PIs located in the anal region, especially before any reconstructive surgery. Further clinical studies are essential to refine the timing and necessity of stoma formation in these specific scenarios.
Multi-disciplinary teams, comprising doctors, nurses, and physical therapists, play a pivotal role in making informed decisions regarding SCI and stoma formation. These decisions are based on a combination of existing literature evidence (Allin et al., 2020; Benninger et al., 2022; Küçükakça Çelik & Taylan, 2023; McColl et al., 2012), their collective experience, and the preferences and wishes of the patients. However, not all SCI-affected individuals receive adequate information. In some instances, patients turn to the internet as their primary source of information, and while they are eager to consult healthcare professionals about their daily challenges, such as incontinence, constipation, bleeding, and the psychosocial difficulties associated with prolonged bowel emptying, they often feel unsupported. Many patients express the sentiment that only those who share their experiences, such as being wheelchair-bound, can truly understand their struggles, leading to feelings of abandonment by the healthcare system (Küçükakça Çelik & Taylan, 2023). The interdisciplinary approach throughout the entire treatment process, including the involvement of stoma care nurses, plays a pivotal role in ensuring patient safety and satisfaction. Furthermore, integrating clinical psychology and utilizing psychological assessment tools can offer additional support, ensuring that patients are mentally and emotionally prepared for surgery (Boucher et al., 2019). Telerehabilitation might be considered an additional treatment option to improve social isolation, satisfaction, diet, and exercise and support patients to not forget SCI management techniques (Allin et al., 2020; Solomon et al., 2022).
Strengths and Limitations
Limitations for this discussion are multifaceted and warrant careful consideration. One primary constraint is our reliance on a relatively limited body of published work in this specialized field. The existing literature often lacks uniformity in terms of research methodology, the criteria employed, and the analytical frameworks used. This inconsistency can lead to varied interpretations of results, which may, in some instances, be influenced by the author's biases or specific objectives (Boucher et al., 2019). Furthermore, the sample size, which focuses on a specific subgroup of TSCI patients with PIs, is arguably too modest to draw broad clinical conclusions. While our findings provide valuable insights, they should be viewed as preliminary observations that hint at potential directions for future therapeutic strategies rather than definitive conclusions. The nuanced and individualized nature of PIs in SCI patients further complicates the extrapolation of our results to a broader population. To truly understand and determine the most effective therapeutic approach for this patient group, a more comprehensive research approach is necessary. Ideally, this would involve a full-scale, randomized, prospective trial that can provide a more robust and generalizable set of findings. Such a trial would not only validate or challenge our current understanding but also pave the way for evidence-based interventions that can significantly improve patient outcomes.
Conclusion
Despite advancements in technology and enhanced preventive care, PIs remain prevalent among individuals with paraplegia. These PIs present challenges for patients and lead to significant burdens and costs for healthcare professionals. For extensive and deep PIs near the anus, a tailored approach to treatment is essential. Decisions should consider the patient's preferences, the size and location of the PI, any involvement of the anus, and specific surgical guidelines. Proper timing is crucial when managing bowel functions for those with SCI, especially for those recently injured who are undergoing both physical and emotional recovery. Elective colostomy is a reliable and safe method for bowel management in SCI patients. It is important to continually review the evidence that shapes professional training and practices and to question why colostomy isn't more routinely adopted. We advocate for fecal diversion in bedridden patients with resistant PIs. However, further research is essential to determine the ideal timing for stoma procedures in these scenarios.
Acknowledgements
We want to thank our patients for participating in this study. The presented results are part of the doctoral thesis of M.T.
Footnotes
Author Contributions: All authors have met authorship requirements. Conceptualization: R.A.H., A.M., and B.B.; methods: R.A.H., M.T., A.M., and B.B.; software: R.A.H. and M.T.; validation: B.B.; formal analysis: R.A.H. and M.T.; investigation: R.A.H., M.T., A.M., and B.B.; resources: R.A.H., M.T., A.M., and B.B.; data curation: R.A.H., M.T., and B.B.; writing—original draft preparation: R.A.H., M.T., and B.B.; writing—review and editing: J.G. and A.M.; visualization: R.A.H. and M.T.; supervision: R.A.H., A.M., and B.B.; project administration: R.A.H., A.M., and B.B. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement: The datasets are available from the corresponding author upon reasonable request.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Bundeswehr Hospital Berlin, Germany.
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
ORCID iD: Raban Heller https://orcid.org/0000-0001-8006-9742
References
- Allaire J. (2012). RStudio: Integrated development environment for R. Boston, MA, 770(394), 165–171. [Google Scholar]
- Allin S., Shepherd J., Thorson T., Tomasone J., Munce S., Linassi G., McBride C. B., Jiancaro T., Jaglal S. (2020). Web-based health coaching for spinal cord injury: Results from a mixed methods feasibility evaluation. JMIR Rehabilitation and Assistive Technologies, 7(2), e16351. 10.2196/16351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambe P. C., Kurz N. R., Nitschke C., Odeh S. F., Moslein G., Zirngibl H. (2018). Intestinal ostomy. Deutsches Ärzteblatt International, 115(11), 182–187. 10.3238/arztebl.2018.0182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbenel J. C. (1991). Pressure management. Prosthetics & Orthotics International, 15(3), 225–231. 10.3109/03093649109164292 [DOI] [PubMed] [Google Scholar]
- Barton A. A. (2006). The pathogenesis of skin wounds due to pressure. Journal of Tissue Viability, 16(3), 12–15. 10.1016/s0965-206x(06)63003-9 [DOI] [PubMed] [Google Scholar]
- Benninger I., Lampart P., Mueller G., Augutis M., Eriks-Hoogland I., Grunt S., Kelly E. H., Padden B., Scherer C., Shavit S., Taylor J., Rutz E., Scheel-Sailer A., Pepsci C. (2022). Needs and research priorities for young people with spinal cord lesion or spina bifida and their caregivers: A national survey in Switzerland within the PEPSCI collaboration. Children, 9(3), 318. 10.3390/children9030318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertheuil N., Huguier V., Aillet S., Beuzeboc M., Watier E. (2013). Biceps femoris flap for closure of ischial pressure ulcers. European Journal of Plastic Surgery, 36(10), 639–644. 10.1007/s00238-013-0862-z [DOI] [Google Scholar]
- Biglari B., Buchler A., Reitzel T., Swing T., Gerner H. J., Ferbert T., Moghaddam A. (2014). A retrospective study on flap complications after pressure ulcer surgery in spinal cord-injured patients. Spinal Cord, 52(1), 80–83. 10.1038/sc.2013.130 [DOI] [PubMed] [Google Scholar]
- Black J., Baharestani M. M., Cuddigan J., Dorner B., Edsberg L., Langemo D., Posthauer M. E., Ratliff C., Taler G., & The National Pressure Ulcer Advisory Panel (NPUAP). (2007). National Pressure Ulcer Advisory Panel's updated pressure ulcer staging system. Advances in Skin & Wound Care, 20(5), 269–274. 10.1097/01.ASW.0000269314.23015.e9 [DOI] [PubMed] [Google Scholar]
- Bock T., Heller R. A., Haubruck P., Raven T. F., Pilz M., Moghaddam A., Biglari B. (2021). Pursuing more aggressive timelines in the surgical treatment of traumatic spinal cord injury (TSCI): A retrospective cohort study with subgroup analysis. Journal of Clinical Medicine, 10(24), 5977. 10.3390/jcm10245977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bølling Hansen R., Staun M., Kalhauge A., Langholz E., Biering-Sørensen F. (2016). Bowel function and quality of life after colostomy in individuals with spinal cord injury. The Journal of Spinal Cord Medicine, 39(3), 281–289. 10.1179/2045772315y.0000000006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher M., Dukes S., Bryan S., Branagan G. (2019). Early colostomy formation can improve independence following spinal cord injury and increase acceptability of bowel management. Topics in Spinal Cord Injury Rehabilitation, 25(1), 23–30. 10.1310/sci18-00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne D. W., Salzberg C. A. (1996). Major risk factors for pressure ulcers in the spinal cord disabled: A literature review. Spinal Cord, 34(5), 255–263. 10.1038/sc.1996.46 [DOI] [PubMed] [Google Scholar]
- Colquhoun P., Kaiser R., Jr., Efron J., Weiss E. G., Nogueras J. J., Vernava A. M., 3rd, Wexner S. D. (2006). Is the quality of life better in patients with colostomy than patients with fecal incontience? World Journal of Surgery, 30(10), 1925–1928. 10.1007/s00268-006-0531-5 [DOI] [PubMed] [Google Scholar]
- Cooper E. A., Bonne Lee B., Muhlmann M. (2019). Outcomes following stoma formation in patients with spinal cord injury. Colorectal Disease, 21(12), 1415–1420. 10.1111/codi.14753 [DOI] [PubMed] [Google Scholar]
- Daniels R., van Rossum E., Metzelthin S., Sipers W., Habets H., Hobma S., van den Heuvel W., de Witte L. (2011). A disability prevention programme for community-dwelling frail older persons. Clinical Rehabilitation, 25(11), 963–974. 10.1177/0269215511410728 [DOI] [PubMed] [Google Scholar]
- de la Fuente S. G., Levin L. S., Reynolds J. D., Olivares C., Pappas T. N., Ludwig K. A., Mantyh C. R. (2003). Elective stoma construction improves outcomes in medically intractable pressure ulcers. Diseases of the Colon & Rectum, 46(11), 1525–1530. 10.1007/s10350-004-6808-6 [DOI] [PubMed] [Google Scholar]
- DeVivo M., Farris V. (2011). Causes and costs of unplanned hospitalizations among persons with spinal cord injury. Topics in Spinal Cord Injury Rehabilitation, 16(4), 53–61. 10.1310/sci1604-53 [DOI] [Google Scholar]
- Filius A., Damen T. H., Schuijer-Maaskant K. P., Polinder S., Hovius S. E., Walbeehm E. T. (2013). Cost analysis of surgically treated pressure sores stage III and IV. Journal of Plastic, Reconstructive & Aesthetic Surgery, 66(11), 1580–1586. 10.1016/j.bjps.2013.05.014 [DOI] [PubMed] [Google Scholar]
- Gaab J., Boyce M., Vogt P. M. (2014). Plastic surgery coverage of pressure ulcers of the trunk and pelvic region. Der Chirurg, 85(11), 1023–1038. 10.1007/s00104-013-2686-6. Plastisch-chirurgische Defektdeckung beim Dekubitus des Rumpfes und der Beckenregion. [DOI] [PubMed] [Google Scholar]
- Glickman S., Kamm M. A. (1996). Bowel dysfunction in spinal-cord-injury patients. The Lancet, 347(9016), 1651–1653. 10.1016/s0140-6736(96)91487-7 [DOI] [PubMed] [Google Scholar]
- Grassetti L., Scalise A., Lazzeri D., Carle F., Agostini T., Gesuita R., Di Benedetto G. (2014). Perforator flaps in late-stage pressure sore treatment: Outcome analysis of 11-year-long experience with 143 patients. Annals of Plastic Surgery, 73(6), 679–685. 10.1097/SAP.0b013e31828587a8 [DOI] [PubMed] [Google Scholar]
- Gray M., Giuliano K. K. (2018). Incontinence-associated dermatitis, characteristics and relationship to pressure injury: A multisite epidemiologic analysis. Journal of Wound, Ostomy & Continence Nursing, 45(1), 63–67. 10.1097/WON.0000000000000390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R. W., Franklin M. R. (1976). Sexual loss in relation to other functional losses for spinal cord injured males. Archives of Physical Medicine and Rehabilitation, 57(6), 291–293. https://www.ncbi.nlm.nih.gov/pubmed/1275682 [PubMed] [Google Scholar]
- Kelly S. R., Shashidharan M., Borwell B., Tromans A. M., Finnis D., Grundy D. J. (1999). The role of intestinal stoma in patients with spinal cord injury. Spinal Cord, 37(3), 211–214. 10.1038/sj.sc.3100764 [DOI] [PubMed] [Google Scholar]
- Kottner , J., Cuddigan, J., Carville, K., Balzer, K., Berlowitz , D., Law, S., Litchford, M., Mitchell, P., Moore, Z., Pittman, J., Sigaudo-Roussel, D., Yee Yee, C., Haesler, E. (2019). Prevention and treatment of pressure ulcers/injuries: The protocol for the second update of the international Clinical Practice Guideline 2019. Journal of Tissue Viability, 28(2), 51–58. [DOI] [PubMed] [Google Scholar]
- Kreutztrager M., Voss H., Scheel-Sailer A., Liebscher T. (2018). Outcome analyses of a multimodal treatment approach for deep pressure ulcers in spinal cord injuries: A retrospective cohort study. Spinal Cord, 56(6), 582–590. 10.1038/s41393-018-0065-3 [DOI] [PubMed] [Google Scholar]
- Küçükakça Çelik G., Taylan S. (2023). Colostomy may offer hope in improving quality of life: A phenomenological qualitative study with patients dependent on a wheelchair. Quality of Life Research, 32(7), 1981–1989. 10.1007/s11136-023-03368-3 [DOI] [PubMed] [Google Scholar]
- Lazar C. C., Auquit-Auckbur I., Milliez P. Y. (2007). La place du lambeau total de cuisse en chirurgie reconstructrice. Annales de Chirurgie Plastique Esthétique, 52(2), 144–147. 10.1016/j.anplas.2006.09.003 [DOI] [PubMed] [Google Scholar]
- Levi R., Hultling C., Nash M. S., Seiger A. (1995). The Stockholm spinal cord injury study: 1. Medical problems in a regional SCI population. Paraplegia, 33(6), 308–315. 10.1038/sc.1995.70 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler L. C., Pussin A. M., Aach M., Grasmucke D., Schildhauer T. A., Schmiegel W., Brechmann T. (2023). Minor microbial alterations after faecal diversion do not affect the healing process of anus-near pressure injuries in patients with spinal cord injury - results of a matched case-control study. Spinal Cord, 61(6), 352–358. 10.1038/s41393-023-00901-6 [DOI] [PubMed] [Google Scholar]
- McColl M. A., Aiken A., McColl A., Sakakibara B., Smith K. (2012). Primary care of people with spinal cord injury: Scoping review. Canadian Family Physician, 58(11), 1207–1216, e1626–1235. https://www.ncbi.nlm.nih.gov/pubmed/23152456 [PMC free article] [PubMed] [Google Scholar]
- Negosanti L., Sgarzani R., Linguerri R., Vetrone G., Liotta S., Bazzocchi G., Balloni M. (2020). “Imola-Montecatone” subtotal colectomy to improve bowel management in spinal cord injury patients. Retrospective analysis in 19 cases. Spinal Cord Series and Cases, 6(1), 59. 10.1038/s41394-020-0311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pussin A. M., Lichtenthäler L. C., Aach M., Schildhauer T. A., Brechmann T. (2021). Fecal diversion does not support healing of anus-near pressure ulcers in patients with spinal cord injury-results of a retrospective cohort study. Spinal Cord, 60(6), 477–483. 10.1038/s41393-021-00717-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M. A., Tharu N. S., Gustin S. M., Zheng Y. P., Alam M. (2022). Trans-spinal electrical stimulation therapy for functional rehabilitation after spinal cord injury: Review. Journal of Clinical Medicine, 11(6), 1550. 10.3390/jcm11061550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2023). R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria.
- Safadi B. Y., Rosito O., Nino-Murcia M., Wolfe V. A., Perkash I. (2003). Which stoma works better for colonic dysmotility in the spinal cord injured patient? The American Journal of Surgery, 186(5), 437–442. 10.1016/j.amjsurg.2003.07.007 [DOI] [PubMed] [Google Scholar]
- Safak T., Klebuc M. J., Kecik A., Shenaq S. M. (1996). The subcutaneous pedicle tensor fascia lata flap. Plastic & Reconstructive Surgery, 97(4), 765–774. 10.1097/00006534-199604000-00012 [DOI] [PubMed] [Google Scholar]
- Schryvers O. I., Stranc M. F., Nance P. W. (2000). Surgical treatment of pressure ulcers: 20-year experience. Archives of Physical Medicine and Rehabilitation, 81(12), 1556–1562. 10.1053/apmr.2000.17828 [DOI] [PubMed] [Google Scholar]
- Shin J. H., Hong I. P., Park C. G., Chung C. M. (2014). A modified total thigh flap in the reconstruction of decubitus ulcer. Archives of Plastic Surgery, 41(4), 440–442. 10.5999/aps.2014.41.4.440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon R. M., Dhakal R., Halpin S. J., Hariharan R., O'Connor R. J., Allsop M., Sivan M. (2022). Telerehabilitation for individuals with spinal cord injury in low-and middle-income countries: A systematic review of the literature. Spinal Cord, 60(5), 395–403. 10.1038/s41393-022-00797-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J. M., Wolfe V. A., Nino-Murcia M., Perkash I. (1990). Colostomy as treatment for complications of spinal cord injury. Archives of Physical Medicine and Rehabilitation, 71(7), 514–518. https://www.ncbi.nlm.nih.gov/pubmed/2350223 [PubMed] [Google Scholar]
- Swarnakar R., Rahman H., Venkataraman S. (2022). Platelet-Rich Fibrin Membrane-as a novel biomaterial for pressure injury healing in a person with spinal cord injury: A case report. Spinal Cord Series and Cases, 8(1), 75. 10.1038/s41394-022-00540-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakoli K., Rutkowski S., Cope C., Hassall M., Barnett R., Richards M., Vandervord J. (1999). Recurrence rates of ischial sores in para- and tetraplegics treated with hamstring flaps: An 8-year study. British Journal of Plastic Surgery, 52(6), 476–479. 10.1054/bjps.1999.3126 [DOI] [PubMed] [Google Scholar]
- Thiessen F. E., Andrades P., Blondeel P. N., Hamdi M., Roche N., Stillaert F., Van Landuyt K., Monstrey S. (2011). Flap surgery for pressure sores: Should the underlying muscle be transferred or not? Journal of Plastic, Reconstructive & Aesthetic Surgery, 64(1), 84–90. 10.1016/j.bjps.2010.03.049 [DOI] [PubMed] [Google Scholar]
- Von Elm E., Altman D. G., Egger M., Pocock S. J., Gøtzsche P. C., Vandenbroucke J. P. (2008). The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Journal of Clinical Epidemiology, 61(4), 344–349. 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- Waddell O., McCombie A., Frizelle F. (2020). Colostomy and quality of life after spinal cord injury: Systematic review. BJS Open, 4(6), 1054–1061. 10.1002/bjs5.50339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. (2016). Ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag. ISBN 978-3-319-24277-4. https://ggplot2.tidyverse.org [Google Scholar]
- Wickham H., Francois R., Henry L., Müller K. (2015). dplyr: A grammar of data manipulation. R package version 0.4, 3.
- Wickham H., Wickham M. H. (2017). Package ‘tidyr’. Easily Tidy Data with'spread'and'gather ()'Functions .
- Wing P. C. (2008). Early acute management in adults with spinal cord injury: A clinical practice guideline for health-care providers. Who should read it? The Journal of Spinal Cord Medicine, 31(4), 360. 10.1080/10790268.2008.11760737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Medical Association. (2013). World Medical Association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA, 310(20), 2191–2194. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]