ABSTRACT
Herpes simplex virus-1 (HSV-1) establishes a latent infection in peripheral neurons and periodically reactivates to permit transmission, which can result in clinical manifestations. Viral transactivators required for lytic infection are largely absent during latent infection, and therefore, HSV-1 relies on the co-option of neuronal host signaling pathways to initiate its gene expression. The activation of the neuronal c-Jun N-terminal kinase (JNK) cell stress pathway is central to initiating biphasic reactivation in response to multiple stimuli. However, how host factors work with JNK to stimulate the initial wave of gene expression (known as Phase I) or the progression to full Phase II reactivation remains unclear. Here, we found that c-Jun, the primary target downstream of neuronal JNK cell stress signaling, functions during reactivation but not during the JNK-mediated initiation of Phase I gene expression. Instead, c-Jun was required to transition from Phase I to full HSV-1 reactivation and was detected in viral replication compartments of reactivating neurons. Interestingly, we also identified a role for both c-Jun and enhanced neuronal stress during initial neuronal infection in promoting a more reactivation-competent form of HSV-1 latency. Therefore, c-Jun functions at multiple stages during the HSV latent infection of neurons to promote reactivation but not during the initial JNK-dependent Phase I. Importantly, by demonstrating that initial infection conditions can contribute to later reactivation abilities, this study highlights the potential for latently infected neurons to maintain a molecular scar of previous exposure to neuronal stressors.
IMPORTANCE
The molecular mechanisms that regulate the reactivation of herpes simplex virus-1 (HSV-1) from latent infection are unknown. The host transcription and pioneer factor c-Jun is the main target of the JNK cell stress pathway that is known to be important in exit of HSV from latency. Surprisingly, we found that c-Jun does not act with JNK during exit from latency but instead promotes the transition to full reactivation. Moreover, c-Jun and enhanced neuronal stress during initial neuronal infection promoted a more reactivation-competent form of HSV-1 latency. c-Jun, therefore, functions at multiple stages during HSV-1 latent infection of neurons to promote reactivation. Importantly, this study contributes to a growing body of evidence that de novo HSV-1 infection conditions can modulate latent infection and impact future reactivation events, raising important questions on the clinical impact of stress during initial HSV-1 acquisition on future reactivation events and consequences.
KEYWORDS: herpes simplex virus, c-Jun, reactivation, stress response
INTRODUCTION
Long-term viral infection can be regulated at multiple steps by the activation of host cell signaling pathways. The herpesviruses establish lifelong latent infections within specific host cells and periodically reactivate to permit transmission. During latent infection, the double-stranded DNA herpesvirus genomes reside in an epigenetically silent state, coated with host histone proteins within host nuclei (1–6). The transcription of viral lytic mRNAs is largely repressed, and therefore, viral proteins that mediate lytic gene transactivation during productive infection are largely absent. To reactivate, repressed herpesviruses rely on host signaling pathways that appear to play important physiological roles in their respective latent cell type. For example, the Gammaherpesvirus Epstein–Barr virus (EBV) establishes latency in memory B cells, and reactivation can be initiated following B cell receptor ligation and activation (7). Likewise, the Betaherpesvirus human cytomegalovirus (HCMV) establishes a latent infection in hematopoietic progenitor cells, and reactivation can be initiated through cytokine signaling, including TNFɑ, which mediates differentiation (8–11). Following the activation of these signaling pathways, host transcriptional factors activated downstream of signaling pathways can bind viral genomes to initiate lytic gene expression (12). Understanding the contribution of host cell proteins to the initiation of reactivation can help uncover the mechanisms of viral gene expression and ultimately identify targets for therapeutics that can prevent the occurrence of reactivation.
The Alphaherpesvirus, herpes simplex virus-1 (HSV-1), establishes a latent infection in post-mitotic neurons, during which lytic promoters are assembled into heterochromatin, as defined by the enrichment of trimethylated histone H3 lysine 27 (H3K27me3) and di- and tri-methylated lysine 9 (H3K9me2/3) (13–18). Periodically, following common physiological stressors like fever, UV exposure, and psychological stress, full reactivation, as characterized by new infectious virus production, can occur. At the neuronal level, the activation of certain signaling pathways has been found to induce reactivation [reviewed in reference (19)]. These include the loss of neurotrophic factor support (20–27), neuronal hyperexcitability (21, 28), corticosteroid signaling (29, 30), and perturbation of the DNA damage response and axonal damage (31, 32). However, unlike the studies of EBV and HCMV, the host transcription factors that act downstream of a reactivation stimulus are largely unknown.
Transcriptionally, the full reactivation of HSV-1 mirrors de novo acute replication, which takes place in neuronal and non-neuronal cells; viral immediate early (IE) gene transcription precedes and is essential to early (E) gene expression, which is required for viral DNA replication, and subsequent late (L) gene transcription. Efficient IE gene expression and, therefore, the entire downstream lytic transcriptional cascade during acute infection or full reactivation require viral transactivator VP16 (33–37). VP16 complexes have cellular factors involved in transcriptional activation, including general transcription factors, ATP-dependent chromatin remodelers, RNA polymerase, and histone-modifying enzymes that may remove repressive H3K9me2/3 and add euchromatin-associated modifications (38–43). In the context of acute infection, VP16 is delivered to the host cell nuclei with the incoming virus tegument. However, during a latent infection, transcription of the gene encoding VP16 (UL48) is restricted, and therefore, viral gene expression must initiate in the absence of VP16 protein by alternative host or viral factors.
There is accumulating evidence that reactivation is a biphasic process. Phase I gene expression precedes “full reactivation” (also referred to as “Phase II”) as a transcriptional burst of all classes of lytic viral genes, with late gene expression being uncoupled from viral DNA replication. This Phase I gene expression phenomenon has been observed in both in vitro and ex vivo models of HSV-1 reactivation (21, 25, 28, 33, 44, 45). The use of in vitro model systems has enabled the molecular mechanisms of Phase I and Phase II reactivation to be further teased apart. In these models, Phase I reactivation does not require VP16 nor activation of the host histone demethylase enzymes that remove H3K27me3 and H3K9me2 (21, 25, 28, 33, 44). However, Phase I reactivation, as well as the progression to full Phase II, requires the activation of cellular c-Jun N-terminal kinase (JNK), which is specifically redirected to perform a neuronal stress response by mixed lineage kinase protein dual leucine kinase (DLK) and the JNK scaffold protein, JNK-interacting protein-3. The contribution of this neuronal stress signaling pathway was first demonstrated during reactivation using small molecule inhibitor LY294002, which inhibits the activation of the PI3-kinase and AKT-pathways that occur downstream of nerve growth factor (NGF) signaling (25). Since this discovery, the requirement of DLK/JNK for viral reactivation has been demonstrated using multiple systems and triggers converging upon diverse cellular pathways. HSV-1 can co-opt an innate immune signaling pathway mediated by IL-1, which induces neuronal hyperexcitation and subsequent reactivation (28). HSV-1 reactivation is also elicited via the disruption of neuronal DNA damage or repair pathways, for example, through the addition of DNA-damaging agents or the inhibition of the ATM-dependent repair pathway by AKT inhibition (31, 32). In response to a combinatorial stimulus of an NGF deprivation mimic (LY294002), neuronal hyperexcitability (forskolin), and heat shock, reactivation can be induced from a very silent form of latency established in vitro (21). DLK is integral to reactivation using all these stimuli, and JNK has also been demonstrated to be required for reactivation when tested in these systems.
JNK activation during Phase I of HSV-1 reactivation results in the phosphorylation of the serine (S10) neighboring repressive mark H3K9me3, and possibly H3K27me3 (S28), on the viral genome during Phase I (25). This phospho/methyl switch is known to result in the eviction of repressive reader proteins and, therefore, likely permits a chromatin environment that is conducive to transcription (46–48). However, additional host proteins, including transcription and pioneer factors, that directly promote viral gene expression would also be required for Phase I reactivation. Moreover, JNK lacks DNA-binding capabilities, which suggests that an additional DNA-binding protein mediates JNK recruitment to viral chromatin.
DLK-mediated activation of JNK both up-regulates and phosphorylates its primary transcriptional factor target c-Jun, which can mediate neuronal cell death and axon pruning following the loss of nerve growth factor signaling (49–54). We previously observed c-Jun activation in neuronal models used for HSV-1 latency following PI3-kinase inhibition (25) and forskolin treatment (28). Unlike traditional transcription factors, c-Jun can maneuver or pioneer through heterochromatin to modulate genome accessibility in a broad range of host cells, including neurons (55, 56). We, therefore, set out to investigate whether c-Jun is critical for HSV-1 reactivation, with the hypothesis that c-Jun up-regulation and phosphorylation by JNK acts to induce Phase I gene expression. Interestingly, we found that c-Jun protein was required for reactivation during Phase II but does not function directly during Phase I. In carrying out this study, we also found that the activation of c-Jun via neuronal stress during de novo infection resulted in enhanced reactivation. Therefore, we show that cell stress and potentially other signals that could activate c-Jun during initial neuronal infection can have a long-term impact on either the neuron or viral chromatin to regulate the propensity of HSV-1 to reactivate.
RESULTS
c-Jun depletion prior to HSV-1 latency establishment restricts both Phase I gene expression and full reactivation
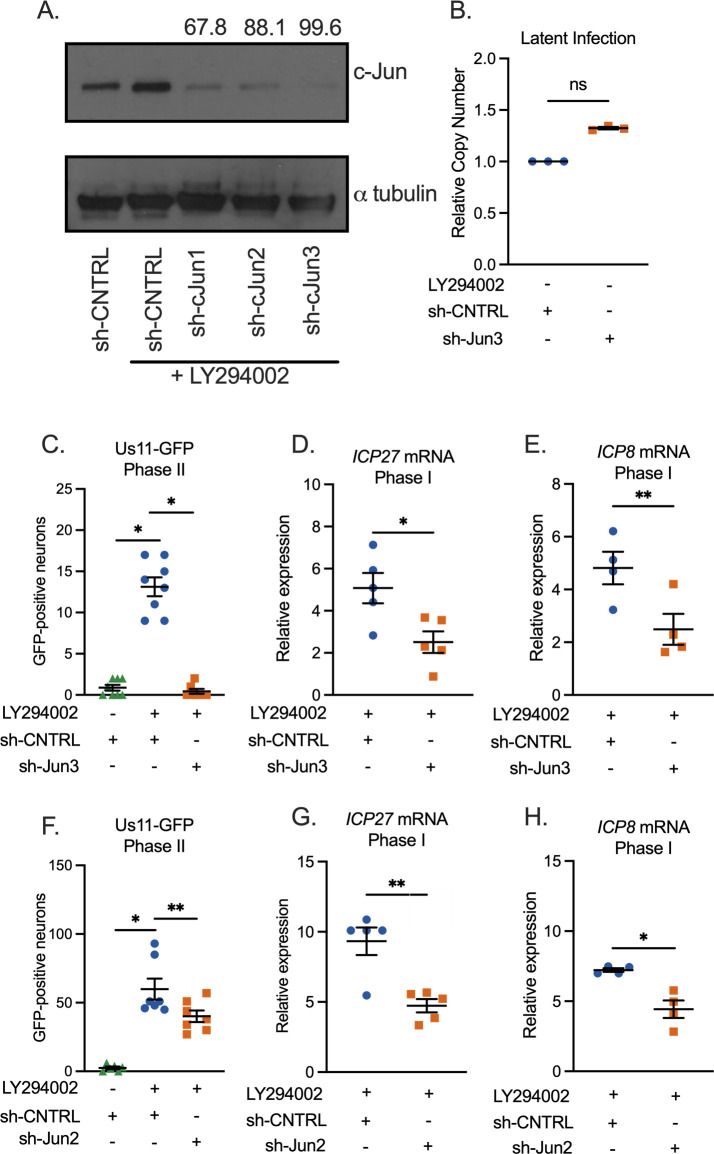
To Investigate the contribution of c-Jun to HSV-1 latency and reactivation, we used an in vitro primary neuronal model because this permits the easy manipulation of c-Jun at different times during the latency/reactivation cycle. In addition, robust reactivation can be achieved in intact neurons using this system. Latent infection was established in sympathetic neurons isolated from the superior cervical ganglia (SCG) of newborn mice as described previously (21, 25, 28, 57). Neurons were infected with a gH-null virus containing Us11-GFP (Stayput-GFP), which permits the quantification of individual reactivating neurons (21). The depletion of c-Jun protein was validated in SCG neurons using three independent shRNA lentiviruses (Fig. 1A). Neurons were also treated with the PI3-kinase inhibitor, LY294002 (20 µM), which is a trigger for HSV reactivation and also induces c-Jun up-regulation (25). c-Jun was depleted from primary neurons using the two most effective lentiviruses (sh-cJun2 and sh-cJun3) and subsequently infected with HSV-1 Stayput-GFP at an of 7.5 PFU/cell in the presence of acyclovir (ACV; 50 µM) for 6 days, at which point ACV was washed from cultures. Two days post-removal of the acyclovir, the infected neurons were reactivated with LY294002 (20 µM), and the number of Us11-GFP-positive neurons was quantified at 48 hours post-treatment, which is indicative of Phase II reactivation, in addition to Phase I lytic gene expression analysis at 18 hours post-treatment. Despite similar viral DNA loads during latency (Fig. 1B), both full reactivation (Fig. 1C and F) and Phase I gene expression (Fig. 1D, E, G, and H) were significantly reduced in c-Jun-depleted cultures. Therefore, the presence of c-Jun protein during HSV-1 latent infection was required for the initial exit from latency and Phase I gene expression.
Fig 1.
c-Jun depletion prior to latency establishment limits both Phase I gene expression and full reactivation. (A) Neurons were transduced with a non-targeting shRNA control lentivirus or one of three independent lentiviruses expressing shRNAs that target c-Jun (sh-cJun1, sh-cJun2, and sh-cJun3). Five days post-transduction, LY294002 was added to neurons for 18 hours, and western blotting for c-Jun or α-tubulin was performed. The percentage knockdown of c-Jun normalized to α-tubulin is shown. (B–H) Following c-Jun depletion with sh-cJun3 (B–E) or sh-cJun2 (F–H), primary neurons were infected with Stayput-GFP at a multiplicity of infection (MOI) of 7.5 PFU/cell in the presence of ACV (50 µM) for 6 days and then reactivated 2 days after the removal of acyclovir with LY294002 (20 µM). (B) Quantification of relative latent viral DNA load at 8 days post-infection. Biological replicates from three separate dissections. (C and F) Quantification of the number of GFP-positive neurons at 48 hours post-stimulus. Individual biological replicates from at least three individual dissections. (D, E, G, and H) Relative viral gene expression at 18 hours post-stimulus compared to latent samples quantified by reverse transcription–quantitative PCR (RT-qPCR) for ICP27 (D, G) and ICP8 (E, H) normalized to cellular control mGAPDH. Statistical comparisons were made using a normal or non-normal (Wilcoxon, B, C, and F) paired t-test. Biological replicates from at least three individual dissections. Individual biological replicates along with the means and SEMs are represented. *P < 0.05; **P < 0.01.
c-Jun depletion following latency establishment does not prevent entry into Phase I
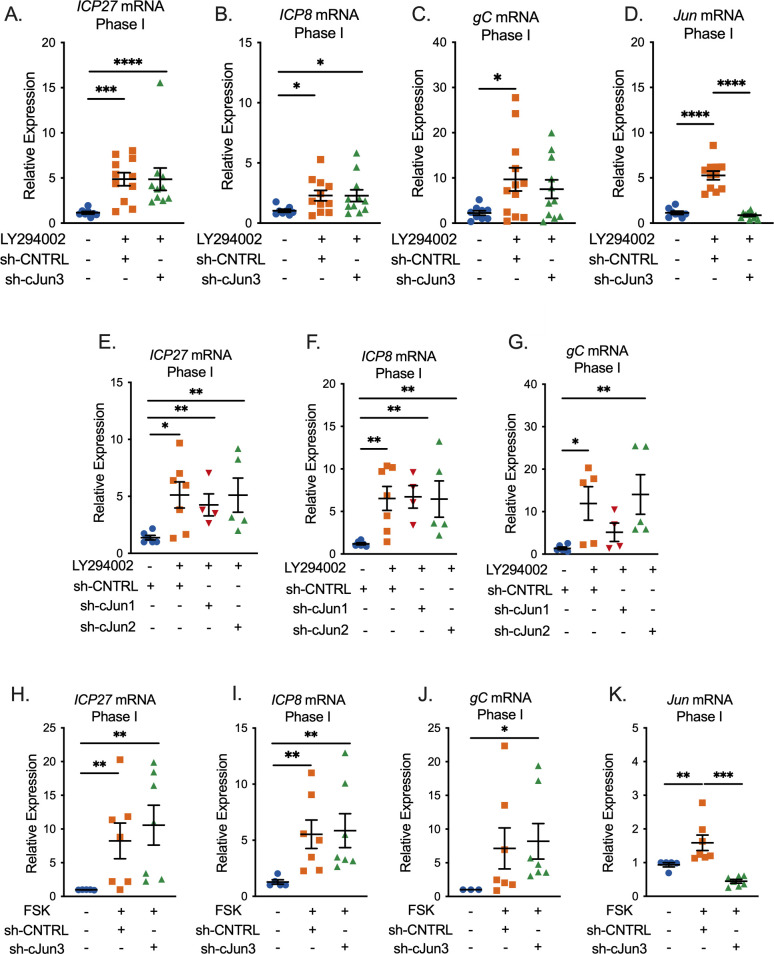
The data shown in Fig. 1 support our hypothesis that c-Jun mediates Phase I gene expression and ultimately full reactivation downstream of the DLK/JNK signaling cascade. However, these experiments come with the caveat that c-Jun was depleted prior to HSV-1 infection, and therefore, we cannot rule out an indirect role of c-Jun in latency establishment that ultimately perturbs reactivation. To test the role of c-Jun solely in Phase I reactivation, the time point where both DLK and JNK act to promote exit from latency and, therefore, reactivation, we depleted c-Jun following HSV-1 infection and latency establishment and analyzed Phase I gene expression. In contrast to what we observed following the depletion of c-Jun prior to infection, Phase I gene expression was not perturbed following c-Jun depletion after latency establishment (Fig. 2A through C). Importantly, these experiments were performed with an shRNA that resulted in approximately 99% knockdown of c-Jun protein following PI3-kinase inhibition (Fig. 1A), and we verified knockdown at the RNA level for each replicate (Fig. 2D). This phenotype was not limited to a single shRNA clone as Phase I gene expression remained unchanged with the additional two independent c-Jun lentiviruses (Fig. 2E through G) that were previously validated (Fig. 1A), although the degree of knockdown of these two shRNAs is less than that for c-Jun shRNA3, used in Fig. 2A through C. However, to further support this phenotype, we found that c-Jun depletion did not impact Phase I gene expression when reactivation was induced by forskolin (Fig. 2H through K). In contrast, c-Jun-depleted cultures displayed unexpectedly enhanced Phase I gene expression following treatment with forskolin. Therefore, our data indicate that c-Jun does not play a direct role alongside DLK and JNK in supporting Phase I gene expression during reactivation. In addition, these data acquired from experiments where c-Jun was depleted post-latency establishment contrasted with what was observed for the impact of c-Jun depletion pre-infection on entry into Phase I gene expression.
Fig 2.
c-Jun is not necessary for Phase I gene expression. (A–K) Latently infected neurons were transduced with a non-targeting shRNA lentivirus or sh-cJun3, sh-cJun2, or sh-cJun1 at 6 days post-infection and reactivated 5 days later. In (A–G), neurons were reactivated with LY294002 and in (H–K) with forskolin. RT-qPCR was carried out at 18 hours post-reactivation; ICP27 (A, E, and H), ICP8 (B, F, and I), gC (C, G, and J), and cellular Jun (D and K) at 18 hours post-stimulus are represented. N = 6 biological replicates from at least three independent dissections. Statistical comparisons were made using a non-normal (Mann–Whitney) t-test. Individual biological replicates along with the means and SEMs are represented. *P < 0.05; **P < 0.01.
Depletion of c-Jun restricts late gene expression during full (Phase II) reactivation
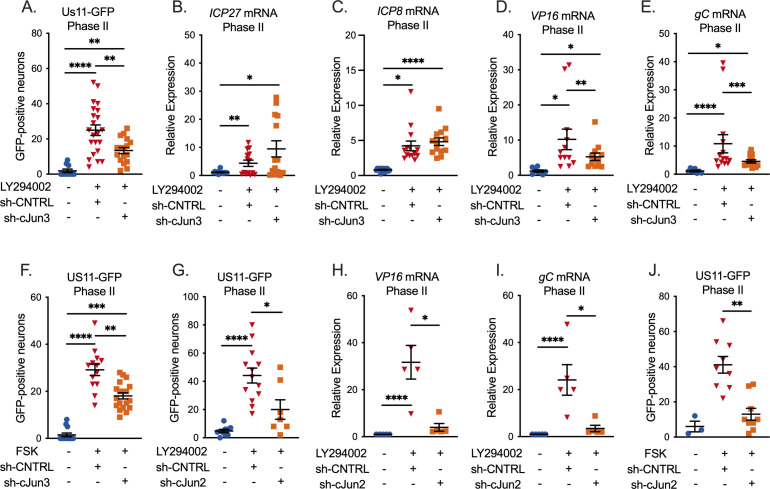
Although we did not detect a role for c-Jun in entry into Phase I gene expression, we went on to examine whether it played any role during HSV-1 reactivation. We, therefore, again depleted c-Jun prior to reactivation and quantified entry into full Phase II reactivation. We now did detect a role for c-Jun during Phase II/full reactivation, as the numbers of Us11-GFP-positive neurons were significantly decreased following c-Jun depletion (Fig. 3A). This was verified using a second c-Jun shRNA lentiviral clone (Fig. 3G). This result indicated that c-Jun was required for Us11 expression. Because Us11 is a true late (TL) gene, we additionally quantified immediate early (Fig. 3B), early (Fig. 3C), and late (Fig. 3D and E) viral transcripts during Phase II. Importantly, our data indicate that c-Jun was specifically required for the expression of the late genes tested and not the immediate early gene ICP27 nor the early gene ICP8. The impact of c-Jun depletion on late gene expression was validated using a second c-Jun shRNA clone (Fig. 3H and I). We also quantified the impact of c-Jun depletion on the progression to full reactivation induced by forskolin. Consistent with the data for PI3-kinase-induced reactivation, the numbers of Us11-GFP-positive neurons were decreased in the c-Jun-depleted neurons, and this was verified using two independent shRNA clones (Fig. 3F and J). Therefore, these data indicate that c-Jun is directly required for full Phase II HSV reactivation and specifically affects late viral transcripts.
Fig 3.
c-Jun is necessary for full HSV-1 reactivation. (A–J) Neurons were infected with HSV-1 and transduced 6 days post-infection with a non-targeting shRNA lentivirus or sh-cJun3 (A–F) or sh-cJun2 (G–J) and reactivated 5 days later. Acyclovir was added for the first 6 days post-infection. (A, G) Quantification of Us11-GFP-positive neurons following reactivation with LY294002. (B–E, H, and I) RT-qPCR for viral mRNA transcripts ICP27 (B), ICP8 (C), VP16 (D, H), and gC (E, I) at 48 hours post-reactivation with LY294002. (F, J) Quantification of Us11-GFP-positive neurons following reactivation with forskolin. Individual replicates from at least four separate dissections are shown. Statistics determined by a normal or non-normal (Wilcoxon test, A, F–J) paired t-test. Individual biological replicates along with the means and SEMs are represented. *P < 0.05; **P < 0.01. ns, not significant.
c-Jun is required for full lytic replication in neurons
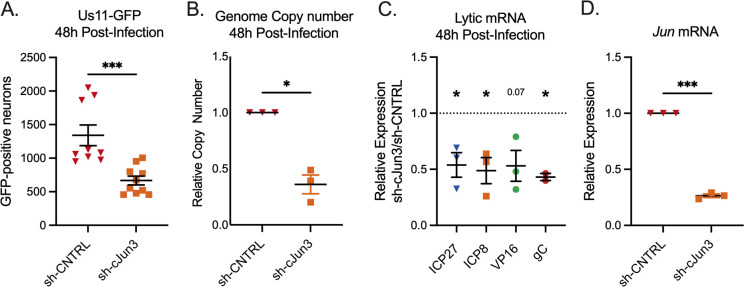
Phase II reactivation has previously been demonstrated to transcriptionally mirror HSV-1 de novo lytic infection in non-neuronal and neuronal cells, which contrasts with Phase I. Therefore, we investigated the contribution of c-Jun to de novo lytic infection in sympathetic neurons. Following c-Jun depletion and infection with HSV-1 Stayput-GFP, we quantified the numbers of Us11-GFP-positive neurons at 48 hours post-infection. This time point was chosen as it is when we previously detected the maximum number of GFP-positive neurons following de novo infection with Stayput-GFP (21). Consistent with our observation that c-Jun is required for full HSV-1 reactivation, the number of GFP-positive neurons indicative of de novo lytic infection events in this model system was robustly reduced following infection in c-Jun-depleted cultures (Fig. 4A). In addition, viral DNA replication (Fig. 4B) and transcription of all three classes of lytic genes, IE ICP27, E ICP8, L VP16, and TL gC were robustly decreased in c-Jun-depleted cells (Fig. 4C), demonstrating that c-Jun is required for de novo lytic infection in neurons. These data suggest that c-Jun is a critical mediator of HSV-1 reactivation and lytic infection in neurons. However, these data also indicate that the mechanism of action may differ in reactivation versus de novo infection as c-Jun was required for the expression of all three classes of viral genes during de novo infection but not during reactivation.
Fig 4.
c-Jun is necessary for maximum de novo lytic infection in neurons. (A–H) Neurons were transduced with a non-targeting shRNA lentivirus or sh-cJun3 and infected with HSV-1 Stayput-GFP in the absence of viral DNA replication inhibitors 5 days post-transduction at an MOI of 5 PFU/cell for 48 hours. (A) Quantification of the numbers of GFP-positive neurons. Replicates from three separate dissections are shown; unpaired t-test. (B) qPCR for viral DNA copy number. (C) RT-qPCR for viral mRNAs ICP27, ICP8, VP16, and gC. (D) RT-qPCR for Jun. Replicates from three separate dissections are shown; paired t-test. Individual biological replicates along with the means and SEMs are represented. *P < 0.05; **P < 0.01.
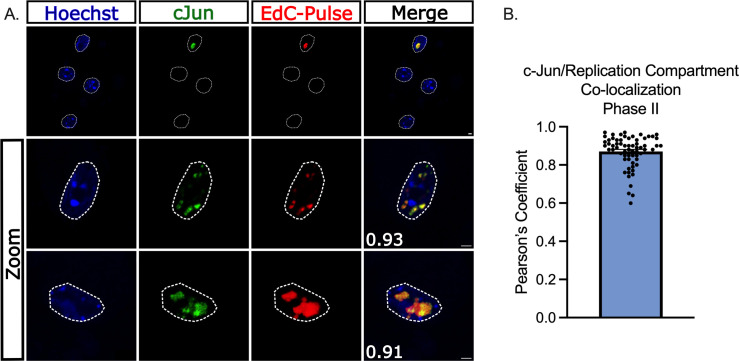
c-Jun is present in HSV-1 replication compartments during full reactivation
As a DNA-binding protein, we proposed that c-Jun could modify viral gene expression during reactivation either directly on the viral genome or indirectly by altering a host cell factor. We, therefore, employed a single-cell imaging approach where the co-localization of c-Jun with individual reactivating neurons could be analyzed. As anticipated, c-Jun was not co-localized with latent viral genomes prior to the addition of the reactivation stimulus LY294002 (data not shown). To quantify c-Jun co-localization with viral genomes during Phase II reactivation, neurons were pulsed with 5-ethynyl-2′-deoxycytidine (EdC; 10 µM) for 1 hour prior to carrying out click chemistry to visualize viral genomes, along with immunofluorescence for c-Jun. We observed a robust up-regulation of c-Jun only in reactivating neurons (Fig. 5A). Importantly, c-Jun robustly co-localized with replicating viral genomes during Phase II of reactivation (Fig. 5A). This co-localization was quantified using Pearson’s coefficient between c-Jun and EdC-Pulse (Fig. 5B). Therefore, c-Jun is both up-regulated specifically in reactivating neurons and recruited into viral replication compartments.
Fig 5.
c-Jun co-localizes with replicating viral DNA during HSV-1 reactivation. (A, B) Latently infected neurons were reactivated with LY294002 and pulsed with 10 µM EdC for 1 hour to label viral DNA replication compartments. Nuclear stain Hoechst is shown in blue, and immunofluorescence was performed to visualize c-Jun in green. EdC-Pulse was visualized using click chemistry (shown in red). (A) Representative images of reactivating neurons. Scale bar = 10 µm. Pearson’s coefficient between c-Jun and EdC-Pulse featured in bottom left corner of merge. (B) Compiled Pearson’s coefficients. Thirty-five individual neurons; two biological replicates.
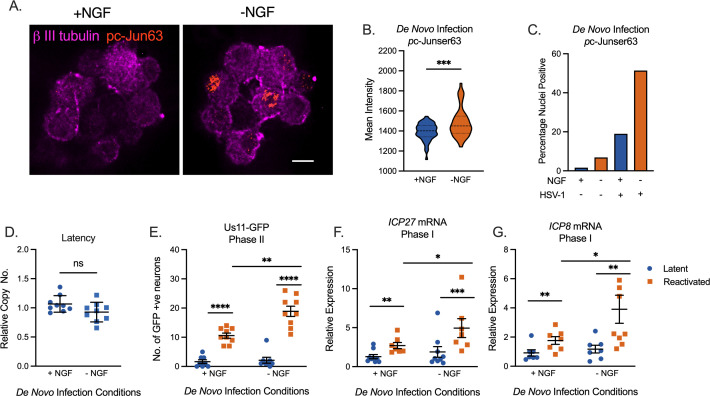
Activation of neuronal stress signaling during latency establishment enhances future reactivation
So far, our data point to a role for c-Jun specifically in stimulating late gene expression during Phase II reactivation. However, an additional observation was the differential phenotypes when c-Jun was depleted before infection versus before reactivation. This differential impact suggested that c-Jun signaling during initial infection promoted a form of viral latency that was more amenable to reactivation. As c-Jun is activated in response to neuronal stress signaling, we investigated how the active manipulation of cell stress pathways and, therefore, enhanced c-Jun activation during initial infection impacted the later ability of the virus to reactivate. An inoculum with or without HSV-1 Stayput-Us11-GFP was added to neonatal sympathetic neurons in the presence or absence of NGF for 3.5 hours. Immediately following the removal of this inoculum, the neurons were fixed and assayed for the presence of nuclear-localized and phosphorylated c-Jun (ser63) as an indication of c-Jun activation. The percentage of neurons under each condition demonstrating nuclear-localized phosphorylated c-Jun was quantified from multiple fields of view, along with the mean intensity of the nuclear staining of each neuron. Using this approach, we were able to verify that NGF deprivation during the inoculation period is sufficient to elicit c-Jun activation, as anticipated (Fig. 6A and B). Interestingly, the neurons in the cultures infected with HSV-1 had enhanced c-Jun phosphorylation in both NGF-positive and -negative conditions as measured by the percentage of p-c-Jun-positive neurons (Fig. 6C). Therefore, HSV-1 de novo infection can also promote neuronal stress signaling as indicated by c-Jun phosphorylation, which was also consistent with c-Jun activation during Phase II reactivation.
Fig 6.
Stress signaling events during de novo HSV-1 infection. (A–C) Neonatal sympathetic cultures were infected with Stayput-GFP in inoculation media with or without NGF for 3.5 hours and subsequently fixed and stained for neuronal marker B III tubulin (magenta) or phosphorylated c-Jun (orange). (A) The representative image of nuclear phosphorylated c-Jun is demonstrated. Scale bar = 10 µm. (B) Mean signal intensity for phosphorylated c-Jun in the nucleus following infection. N = 100 from one biological replicate. (C) Quantification of proportion of neurons with pc-Jun-positive nuclei pooled from several fields of view. (D–G) Neuronal cultures were latently infected. NGF was either included or omitted during the 3.5-hour inoculation period. Cultures were later reactivated with LY294002 20 µM. (D) Latent viral DNA load. Replicates from three dissections shown. The peak number of GFP-positive neurons 48 hours post-stimulus (E) and relative expression of ICP27 (F) or ICP8 (G) transcripts 18 hours post-stimulus were quantified to analyze the full reactivation and Phase I gene expression, respectively. Replicates from three dissections shown. Statistical comparisons were made using a normal or non-normal (Mann–Whitney) (E) t-test. Individual biological replicates along with the means and SEMs are represented. *P < 0.05; **P < 0.01.
Following our validation that c-Jun phosphorylation could be enhanced during initial infection by omitting NGF from the inoculum, we investigated the impact on HSV-1 reactivation. In agreement with the pre-latency establishment c-Jun depletion experiments (Fig. 1), the perturbation of the NGF signaling pathway during the initial infection did not alter latent viral DNA load (Fig. 6D), which is also consistent with transient loss of NGF signaling not impacting neuronal survival (58). However, in cultures where NGF had been deprived during the inoculation period and where c-Jun activation was enhanced, we observed enhanced reactivation stimulated with LY294002, based on the quantification of Us11-GFP-positive neurons at 48 hours post-treatment (Fig. 6E). Importantly, Phase I gene expression, as analyzed through ICP27 and ICP8 expression 18 hours post-stimulus, was also enhanced in the cultures infected under NGF deprivation conditions (Fig. 6F and G). Therefore, these data indicate that enhanced neuronal stress mediated by reduced NGF signaling and enhanced c-Jun phosphorylation results in an enhanced ability of HSV-1 to ultimately undergo reactivation without impacting the latent viral load.
DISCUSSION
c-Jun is both a transcription and pioneer factor that is known to regulate host gene expression in neurons following cell stress. The up-regulation and phosphorylation of c-Jun following DLK-mediated activation of JNK is a key step in both neuronal apoptosis and axon pruning following the interruption of nerve growth factor signaling (51, 59, 60). The up-regulation of c-Jun-dependent genes is consistent with the kinetics of HSV-1 Phase I gene expression (61), hence our initial hypothesis that c-Jun plays a direct role in HSV-1 Phase I gene expression. However, our data indicate that c-Jun functions to promote reactivation but not during the JNK-dependent Phase I wave of lytic gene expression. In carrying out this study, we also observed that cell stress conditions during de novo infection have a long-term impact on either neurons themselves or the viral genome, resulting in an enhanced ability of the virus to reactivate. This has important implications for how neurons have a memory of previous cellular stresses and interpretations of HSV reactivation studies.
Although not required during reactivation for Phase I gene expression, we did identify a role for c-Jun in promoting late gene expression during full Phase II reactivation. Notably, this finding was also distinct from the role of c-Jun in promoting the expression of all three classes of viral genes during de novo infection. The exact mechanism of action and explanation for this difference between de novo infection and reactivation are unclear. One possible difference is the nature of the viral chromatin during reactivation versus de novo infection. The starting point for reactivation is a genome with a regularly spaced nucleosomal structure (62) and associated with heterochromatin (13, 18), whereas the viral genome during de novo infection is highly accessible and lacks a regular nucleosomal structure (1, 38, 63–66). Although the nature of the nucleosomal structure and overall accessibility of the viral genome during Phase II are currently unknown, it is possible that prior to viral DNA replication, potential c-Jun binding sites are inaccessible. Additional proteins may also be activated in response to reactivation triggers that act during Phase I and on IE and E genes during Phase II as discussed below.
The inability to detect a role for c-Jun during Phase I gene expression and IE/E gene expression during Phase II suggests that additional undetermined host factors instead play a role. Additional candidates known to be activated or induced in response to reactivation triggers include other bZIP proteins such as Fos, JunD, ATF3, and Ddit3 (CHOP), along with other transcription factors: NF-Y, Gadd45α, Gadd45γ, and FOXO (61). In a previous study, the depletion of Gadd45α had no impact on HSV-1 reactivation (67). An intriguing candidate for promoting Phase I gene expression is the NF-Y complex because it has been implicated in the recruitment of JNK to chromatin during neuronal differentiation (68). NF-Y has also been identified as potentially stimulating transcription of the IE gene ICP0 in response to heat stress (69), making NF-Y a viable candidate for driving initial reactivation events. Additional pioneer factors that may have a role in stimulating viral gene expression during reactivation are the Krüppel-like transcription factor (KLF) proteins, particularly KLF15 and KLF4 (70). KLF family members are up-regulated in corticosteroid-mediated reactivation of the related bovine herpesvirus-1 and may transactivate viral immediate–early promoters (71–73). KLF4 is a well-known pioneer factor that activates previously silenced genes, a role that has been well characterized during cellular reprogramming (74, 75). The potential role of these proteins in regulating the transcription of HSV-1 genes from silenced heterochromatin during the different stages of HSV-1 reactivation, therefore, warrants further investigation.
c-Jun functions through either homo- or hetero-dimerization. Whether c-Jun binds to an additional bZIP protein to promote late gene expression is unknown. c-Jun can dimerize with Fos, Jun, CREB, or ATF family members. Recently, we found that JNK/DLK-dependent reactivation mediated by forskolin required CREB, as the addition of CREB inhibitor 666-15 restricted the full reactivation as quantified by the number of GFP-positive neurons (28), although these data come with the caveat that a role for CREB has not been validated using genetic approaches. As observed here for c-Jun, CREB activity was not required for Phase I gene expression. Further, mapping the exact binding sites on the viral genome will help identify underlying sequence motifs bound by c-Jun, which can vary depending on the interacting protein (76). We did attempt to perform cleavage under target and release under nuclease (CUT&RUN) for c-Jun during Phase II reactivation. However, we found that the background signal for viral genomes from the non-specific control antibody was much higher on Phase II reactivating neurons than during latent infection (data not shown). For reasons that are not clear, ongoing viral DNA replication may result in substantial background in the CUT&RUN reaction, and therefore, resolving c-Jun interacting sites on replicating viral genomes is currently problematic.
We also report a role for c-Jun for maximal de novo lytic infection in neurons as indicated by the decreased expression of all classes of viral lytic genes, viral DNA replication, and late viral protein synthesis. Consistent with previous reports investigating lytic replication in non-neuronal cells, we found that HSV-1 infection and reactivation induced c-Jun activation (77, 78). However, a direct role for c-Jun during lytic replication has not previously been reported. Interestingly, a previous study from our lab found that JNK inhibition during de novo infection in neurons did not impact immediate early viral gene expression, although viral replication and the expression of other later classes of viral genes were not explored in this study (25). Therefore, it remains possible that during de novo infection, c-Jun may be activated through an alternative pathway.
Environmental stressors have long been reported to correlate with HSV-1 reactivation and clinical disease (79–82), and the addition of stress mediated by synthetic corticosteroids during reactivation enhances viral shedding in certain models (30, 83). Fewer studies have explored the impact on HSV-1 and clinical outcomes when stress occurs during initial inoculation. Evidence from mouse models suggests that psychological stress during inoculation with HSV-1 enhances acute infection, as measured by infectious titer and pathology (84, 85). Complementary evidence from primary autonomic neurons similarly demonstrates elevated acute viral DNA replication and infectious virus production following HSV-1 infection in combination with the stress hormone epinephrine (86). However, the impacts of stress during initial infection on later reactivation until now have not yet been investigated. Our findings suggest that additional stress that enhances c-Jun signaling during initial infection could exacerbate future reactivation. This has important implications for understanding the contributions to clinical HSV disease and why certain individuals may be more prone to reactivation than others. In addition, this observation has important experimental implications because it means that any manipulations performed on the virus or host during latency establishment could have an indirect effect on reactivation. Ideally, the contribution of viral and host factors should be studied solely during reactivation to draw meaningful conclusions on their direct effects.
How the viral genome or neuron itself retains a memory of the initial infection conditions remains unclear. We previously found that viral genomes could retain a memory of interferon signaling during initial infection to result in restricted reactivation mediated by the association with repressive promyelocytic leukemia nuclear bodies (57, 87). We now extend this study and show that cell stress has a converse effect and can prime future reactivation events. Given c-Jun’s ability to bind DNA and navigate chromatinized environments, it is tempting to speculate that stress signaling through c-Jun modifies the epigenetic nature of viral and/or host genomes to induce a form of silencing that is more primed for transcriptional activation. Outside of the context of viral infection, early life stress has been implicated in leaving such a “chromatin scar” in the central nervous system, with changes in epigenetic signatures particularly for H3K27 and H3K79 methylation (88, 89) and a dysregulated priming of genes. Further studies on the mechanism of HSV latent infection and changes in the host and viral epigenetics will be important to understand how both the virus and potentially neurons themselves have a memory of previous cell stress or immune events and the impact on future responses.
MATERIALS AND METHODS
Preparation of HSV-1 virus stocks
Stocks of Stayput Us11-GFP (strain SC16) for in vitro experiments were propagated and titrated on gH-complementing F6 cells (21, 90). Vero F6 cells were maintained in Dulbecco’s modified Eagle’s medium (Gibco) supplemented with 10% FetalPlex (Gemini Bio-Products) and 250 µg/mL of G418/Geneticin (Gibco).
Primary neuronal cultures
Sympathetic neurons from the SCG of post-natal day 0–2 (P0-P2) CD1 mice (Charles River Laboratories) were dissected as previously described (25). Rodent handling and husbandry were carried out under animal protocols approved by the Animal Care and Use Committee of the University of Virginia. Ganglia were briefly kept in Leibovitz’s L-15 media with 2.05 mM l-glutamine before dissociation in collagenase type IV (1 mg/mL) followed by trypsin (2.5 mg/mL) for 20 min; each dissociation step was at 37°C. Dissociated ganglia were triturated, and approximately 10,000 neurons per well were plated onto rat tail collagen in a 24-well plate. Sympathetic neurons were maintained in feeding media: Neurobasal Medium supplemented with PRIME-XV IS21 Neuronal Supplement (Irvine Scientific), 50 ng/mL Mouse NGF 2.5S (Alomone Labs), 2 mM l-glutamine, and 100 µg/mL Primocin (Invivogen). Aphidicolin (3.3 µg/mL) was added to the media for the first 5 days post-dissection to select against proliferating cells.
Lytic HSV-1 infection in primary neurons
P6-8 SCG neurons were infected with Stayput Us11-GFP at MOI 5 PFU/cell (assuming 10,000 cells per well) in Dulbecco’s phosphate-buffered saline (DPBS) + CaCl2 + MgCl2 supplemented with 1% fetal bovine serum and 4.5 g/L glucose for 3.5 hours at 37°C. The inoculum was replaced with feeding media (as described above). ACV was not utilized in these infections. Lytic infection was quantified by the numbers of GFP-positive neurons.
Establishment and reactivation of latent HSV-1 infection in primary neurons
P6-8 SCG neurons were infected with Stayput Us11-GFP at MOI 7.5 PFU/cell (assuming 10,000 cells per well) in DPBS + CaCl2 + MgCl2 supplemented with 1% fetal bovine serum, 4.5 g/L glucose, and 10 µM ACV for 3.5 hours at 37°C. The inoculum was replaced with feeding media (as described above) with 50 µM ACV. Six days post-infection, ACV was washed out and replaced with feeding media alone. Reactivation was reported by quantifying the numbers of GFP-positive neurons following the addition of 20–40 µM LY294002 (Tocris) or 60 µM forskolin (Tocris).
Analysis of viral DNA load and mRNA expression by reverse transcription–quantitative PCR
To assess the relative expression of HSV-1 lytic mRNA, total RNA was extracted from approximately 10,000 neurons using the Quick-RNA Miniprep Kit (Zymo Research) with on-column DNase I digestion. mRNA was converted to cDNA using the Maxima First Strand cDNA Synthesis Kit for RT-qPCR (Fisher Scientific), using random hexamers for first-strand synthesis and equal amounts of RNA (20–30 ng/reaction). To assess viral DNA load, total DNA was extracted from approximately 10,000 neurons using the Quick-DNA Miniprep Plus Kit (Zymo Research). qPCR was carried out using PowerUp SYBR Green Master Mix (ThermoFisher Scientific). The relative mRNA or DNA copy number was determined using the comparative CT (ΔΔCT) method normalized to mRNA or DNA levels in latently infected samples. Viral RNAs were normalized to mouse reference gene mGAPDH RNA. All samples were run in triplicate on an Applied Biosystems QuantStudio 6 Flex Real-Time PCR System, and the mean fold change was compared to the calculated reference gene. The sequence of both forward and reverse primers used has been published previously (28).
Preparation of lentiviral vectors
Lentiviruses expressing shRNA against c-Jun (c-Jun-1 = TRCN0000360511, c-Jun-2 = TRCN0000055205, and c-Jun-3 = TRCN0000042696) or a control lentivirus shRNA (pLKO.1 vector expressing a non-targeting shRNA control) were prepared by co-transfection using JetPRIME with psPAX2 and pCMV-VSV-G (91) into the 293LTV packaging cell line (Cell Biolabs). The supernatant was harvested at 40 and 64 hours post-transfection and filtered using a 45-µM PES filter. Sympathetic neurons were transduced overnight in neuronal media containing 8 µg/mL protamine sulfate and 50 µM ACV.
Western blotting
Neurons were lysed in RIPA buffer with cOmplete, Mini, EDTA-Free Protease Inhibitor Cocktail (Roche) and PhosSTOP Phosphatase Inhibitor Cocktail (Roche) on ice for 2 hours with regular vortexing to aid lysis. Insoluble proteins were removed via centrifugation, and the lysate protein concentration was determined using the Pierce Bicinchoninic Acid Protein Assay Kit (Invitrogen) using a standard curve created with bovine serum albumin (BSA) standards of known concentration. Equal quantities of protein (15–50 µg) were resolved on %–20% gradient SDS–polyacrylamide gels (Bio-Rad) and then transferred onto polyvinylidene difluoride membranes (Millipore Sigma). Membranes were blocked in PVDF Blocking Reagent for Can Get Signal (Toyobo) for 1 hour. Primary antibodies were diluted in Can Get Signal Immunoreaction Enhancer Solution 1 (Toyobo), and membranes were incubated overnight at 4°C. Horseradish peroxidase (HRP)-labeled secondary antibodies were diluted in Can Get Signal Immunoreaction Enhancer Solution 2 (Toyobo), and membranes were incubated for 1 hour at room temperature. Antibody usage is recorded in Table 1. Blots were developed using Western Lightning Plus-ECL Enhanced Chemiluminescence Substrate (PerkinElmer) and ProSignal ECL Blotting Film (Prometheus Protein Biology Products) according to the manufacturer’s instructions. Blots were stripped for reblotting using NewBlot PVDF Stripping Buffer (LI-COR). Band density was quantified in ImageJ.
TABLE 1.
Antibodies
| Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|
| Anti-c-Jun (mouse monoclonal) | BD Biosciences | Cat #610326 | WB (1:1,000) |
| Anti-α-tubulin (mouse monoclonal) | Millipore Sigma | Cat #T9026 | WB (1:2,500) |
| Anti-rabbit IgG antibody (H+L), peroxidase (goat polyclonal) | Vector Labs | Cat #PI-1000 | WB (1:10,000) |
| Anti-mouse IgG antibody (H+L), peroxidase (horse polyclonal) | Vector Labs | Cat #PI-2000 | WB (1:10,000) |
| Phospho-c-Jun (Ser63) II (rabbit polyclonal) | Cell Signaling Technology | Cat #9261 | IF (1:400) |
| Beta III tubulin (chicken polyclonal) | EMD Millipore | Cat #9354 | IF (1:500) |
Immunofluorescence
Neurons were fixed for 15 min in 4% formaldehyde and blocked for 1 hour in 5% bovine serum albumin and 0.3% Triton X-100 and incubated overnight in primary antibody. Following primary antibody treatment, neurons were incubated for 1 hour in Alexa Fluor 488-, 555-, and 647-conjugated secondary antibodies for multicolor imaging (Invitrogen). Nuclei were stained with Hoechst 33258 (Life Technologies). Images were acquired using an sCMOS charge-coupled device camera (pco.edge) mounted on a Nikon Eclipse Ti inverted epifluorescent microscope using NIS-Elements software (Nikon). Images were analyzed using ImageJ.
Click chemistry
To label replicating HSV-1 DNA, reactivated neuronal cultures were pulsed with 10-µM EdC for 1 hour prior to fixation and processing. Click chemistry was carried out as described previously (92) with some modifications to visualize EdC-incorporated replicating viral genomes. Neurons were washed with cytoskeletal (CSK) buffer (10 mM HEPES, 100 mM NaCl, 300 mM sucrose, 3 mM MgCl2, and 5 mM EGTA) and simultaneously fixed and permeabilized for 10 min in 1.8% methanol-free formaldehyde (0.5% Triton X-100, 1% phenylmethylsulfonyl fluoride) in CSK buffer, then washed twice with PBS before continuing to the click chemistry reaction and immunostaining. Samples were blocked with 3% BSA for 30 min, followed by click chemistry using EdC-labeled HSV-1 DNA and the Click-iT EdU Alexa Flour 555 Imaging Kit (ThermoFisher Scientific, C10638) according to the manufacturer’s instructions. For immunostaining, samples were incubated overnight with primary antibodies in 3% BSA. Following primary antibody treatment, neurons were incubated for 1 hour in Alexa Fluor 488-, 555-, and 647-conjugated secondary antibodies for multicolor imaging (Invitrogen). Nuclei were stained with Hoechst 33258 (Life Technologies). Images were acquired at 60× using an sCMOS charge-coupled device camera (pco.edge) mounted on a Nikon Eclipse Ti Inverted Epifluorescent microscope using NIS-Elements software (Nikon). Images were analyzed, and intensity was quantified using ImageJ.
Statistical analysis
Power analysis was used to determine the appropriate sample sizes for statistical analysis. All statistical analysis was performed using Prism V10. The normality of the data was determined with the Kolmogorov–Smirnov test. Specific analyses are included in the figure legends.
ACKNOWLEDGMENTS
We thank Gary Cohen at the University of Pennsylvania for the Vero F6 cells.
This work was supported by the National Institutes of Health grants NS105630 (A.R.C.), T32GM008136 (S.A.D. and A.K.F.), and T32AI007046 (A.L.W and S.C) and The Owens Family Foundation (A.R.C.).
Contributor Information
Anna R. Cliffe, Email: cliffe@virginia.edu.
Felicia Goodrum, The University of Arizona, Tucson, Arizona, USA.
REFERENCES
- 1. Knipe DM, Cliffe A. 2008. Chromatin control of herpes simplex virus lytic and latent infection. Nat Rev Microbiol 6:211–221. doi: 10.1038/nrmicro1794 [DOI] [PubMed] [Google Scholar]
- 2. Matthews SM, Groves IJ, O’Connor CM. 2023. Chromatin control of human cytomegalovirus infection. mBio 14:e0032623. doi: 10.1128/mbio.00326-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Collins-McMillen D, Buehler J, Peppenelli M, Goodrum F. 2018. Molecular determinants and the regulation of human cytomegalovirus latency and reactivation. Viruses 10:444. doi: 10.3390/v10080444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guo R, Gewurz BE. 2022. Epigenetic control of the Epstein-Barr lifecycle. Curr Opin Virol 52:78–88. doi: 10.1016/j.coviro.2021.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen HS, Lu F, Lieberman PM. 2013. Epigenetic regulation of EBV and KSHV latency. Curr Opin Virol 3:251–259. doi: 10.1016/j.coviro.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fröhlich J, Grundhoff A. 2020. Epigenetic control in kaposi sarcoma-associated herpesvirus infection and associated disease. Semin Immunopathol 42:143–157. doi: 10.1007/s00281-020-00787-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tovey MG, Lenoir G, Begon-Lours J. 1978. Activation of latent Epstein-Barr virus by antibody to human IgM. Nature 276:270–272. doi: 10.1038/276270a0 [DOI] [PubMed] [Google Scholar]
- 8. Reeves MB, MacAry PA, Lehner PJ, Sissons JGP, Sinclair JH. 2005. Latency, chromatin remodeling, and reactivation of human cytomegalovirus in the dendritic cells of healthy carriers. Proc Natl Acad Sci U S A 102:4140–4145. doi: 10.1073/pnas.0408994102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reeves MB, Lehner PJ, Sissons JGP, Sinclair JH. 2005. An in vitro model for the regulation of human cytomegalovirus latency and reactivation in dendritic cells by chromatin remodelling. J Gen Virol 86:2949–2954. doi: 10.1099/vir.0.81161-0 [DOI] [PubMed] [Google Scholar]
- 10. Söderberg-Nauclér C, Streblow DN, Fish KN, Allan-Yorke J, Smith PP, Nelson JA. 2001. Reactivation of latent human cytomegalovirus in CD14(+) monocytes is differentiation dependent. J Virol 75:7543–7554. doi: 10.1128/JVI.75.16.7543-7554.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taylor-Wiedeman J, Sissons P, Sinclair J. 1994. Induction of endogenous human cytomegalovirus gene expression after differentiation of monocytes from healthy carriers. J Virol 68:1597–1604. doi: 10.1128/JVI.68.3.1597-1604.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dooley AL, O’Connor CM. 2020. Regulation of the MIE locus during HCMV latency and reactivation. Pathogens 9:869. doi: 10.3390/pathogens9110869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cliffe AR, Garber DA, Knipe DM. 2009. Transcription of the herpes simplex virus latency-associated transcript promotes the formation of facultative heterochromatin on lytic promoters. J Virol 83:8182–8190. doi: 10.1128/JVI.00712-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kwiatkowski DL, Thompson HW, Bloom DC. 2009. The polycomb group protein Bmi1 binds to the herpes simplex virus 1 latent genome and maintains repressive histone marks during latency. J Virol 83:8173–8181. doi: 10.1128/JVI.00686-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bloom DC, Giordani NV, Kwiatkowski DL. 2010. Epigenetic regulation of latent HSV-1 gene expression. Biochim Biophys Acta 1799:246–256. doi: 10.1016/j.bbagrm.2009.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dochnal SA, Francois AK, Cliffe AR. 2021. De novo polycomb recruitment: lessons from latent herpesviruses. Viruses 13:1470. doi: 10.3390/v13081470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nicoll MP, Hann W, Shivkumar M, Harman LER, Connor V, Coleman HM, Proença JT, Efstathiou S. 2016. The HSV-1 latency-associated transcript functions to repress latent phase lytic gene expression and suppress virus reactivation from latently infected neurons. PLoS Pathog 12:e1005539. doi: 10.1371/journal.ppat.1005539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang QY, Zhou C, Johnson KE, Colgrove RC, Coen DM, Knipe DM. 2005. Herpesviral latency-associated transcript gene promotes assembly of Heterochromatin on viral Lytic-gene promoters in latent infection. Proc Natl Acad Sci U S A 102:16055–16059. doi: 10.1073/pnas.0505850102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Suzich JB, Cliffe AR. 2018. Strength in diversity: understanding the pathways to herpes simplex virus reactivation. Virology 522:81–91. doi: 10.1016/j.virol.2018.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilcox CL, Smith RL, Freed CR, Johnson EM. 1990. Nerve growth factor-dependence of herpes simplex virus latency in peripheral sympathetic and sensory neurons in vitro. J Neurosci 10:1268–1275. doi: 10.1523/JNEUROSCI.10-04-01268.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dochnal S, Merchant HY, Schinlever AR, Babnis A, Depledge DP, Wilson AC, Cliffe AR. 2022. DLK-dependent Biphasic reactivation of herpes Simplex virus latency established in the absence of Antivirals. J Virol 96:e0050822. doi: 10.1128/jvi.00508-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hill JM, Garza HH, Helmy MF, Cook SD, Osborne PA, Johnson EM, Thompson HW, Green LC, O’Callaghan RJ, Gebhardt BM. 1997. Nerve growth factor antibody stimulates reactivation of ocular herpes simplex virus type 1 in latently infected rabbits. J Neurovirol 3:206–211. doi: 10.3109/13550289709018295 [DOI] [PubMed] [Google Scholar]
- 23. Camarena V, Kobayashi M, Kim JY, Roehm P, Perez R, Gardner J, Wilson AC, Mohr I, Chao MV. 2010. Nature and duration of growth factor signaling through receptor tyrosine kinases regulates HSV-1 latency in neurons. Cell Host Microbe 8:320–330. doi: 10.1016/j.chom.2010.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Linderman JA, Kobayashi M, Rayannavar V, Fak JJ, Darnell RB, Chao MV, Wilson AC, Mohr I. 2017. Immune escape via a transient gene expression program enables productive replication of a latent pathogen. Cell Rep 18:1312–1323. doi: 10.1016/j.celrep.2017.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cliffe AR, Arbuckle JH, Vogel JL, Geden MJ, Rothbart SB, Cusack CL, Strahl BD, Kristie TM, Deshmukh M. 2015. Neuronal stress pathway mediating a histone methyl/phospho switch is required for herpes simplex virus reactivation. Cell Host Microbe 18:649–658. doi: 10.1016/j.chom.2015.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kobayashi M, Kim JY, Camarena V, Roehm PC, Chao MV, Wilson AC, Mohr I. 2012. A primary neuron culture system for the study of herpes simplex virus latency and reactivation. J Vis Exp:3823. doi: 10.3791/3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mattila RK, Harila K, Kangas SM, Paavilainen H, Heape AM, Mohr IJ, Hukkanen V. 2015. An investigation of herpes simplex virus type 1 latency in a novel mouse dorsal root ganglion model suggests a role for ICP34.5 in reactivation. J Gen Virol 96:2304–2313. doi: 10.1099/vir.0.000138 [DOI] [PubMed] [Google Scholar]
- 28. Cuddy SR, Schinlever AR, Dochnal S, Seegren PV, Suzich J, Kundu P, Downs TK, Farah M, Desai BN, Boutell C, Cliffe AR. 2020. Neuronal hyperexcitability is a DLK-dependent trigger of herpes simplex virus reactivation that can be induced by IL-1. Elife 9:e58037. doi: 10.7554/eLife.58037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goswami P, Ives AM, Abbott ARN, Bertke AS. 2022. Stress hormones epinephrine and corticosterone selectively reactivate HSV-1 and HSV-2 in sympathetic and sensory neurons. Viruses 14:1115. doi: 10.3390/v14051115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harrison KS, Zhu L, Thunuguntla P, Jones C. 2019. Antagonizing the glucocorticoid receptor impairs explant-induced reactivation in mice latently infected with herpes simplex virus 1. J Virol 93:e00418-19. doi: 10.1128/JVI.00418-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu HL, Shiflett LA, Kobayashi M, Chao MV, Wilson AC, Mohr I, Huang TT. 2019. TOP2beta-dependent nuclear DNA damage shapes extracellular growth factor responses via dynamic AKT phosphorylation to control virus latency. Mol Cell 74:466–480. doi: 10.1016/j.molcel.2019.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cliffe AR. 2019. DNA damage meets neurotrophin signaling: a delicate balancing AKT to maintain virus latency. Mol Cell 74:411–413. doi: 10.1016/j.molcel.2019.04.015 [DOI] [PubMed] [Google Scholar]
- 33. Kim JY, Mandarino A, Chao MV, Mohr I, Wilson AC. 2012. Transient reversal of Episome silencing precedes Vp16-dependent transcription during reactivation of latent HSV-1 in neurons. PLoS Pathog 8:e1002540. doi: 10.1371/journal.ppat.1002540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thompson RL, Preston CM, Sawtell NM. 2009. De novo synthesis of VP16 coordinates the exit from HSV latency in vivo. PLoS Pathog 5:e1000352. doi: 10.1371/journal.ppat.1000352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Campbell ME, Palfreyman JW, Preston CM. 1984. Identification of herpes simplex virus DNA sequences which encode a trans-acting polypeptide responsible for stimulation of immediate early transcription. J Mol Biol 180:1–19. doi: 10.1016/0022-2836(84)90427-3 [DOI] [PubMed] [Google Scholar]
- 36. Sawtell NM, Thompson RL. 2016. De novo herpes simplex virus VP16 expression gates a dynamic programmatic transition and sets the latent/Lytic balance during acute infection in trigeminal ganglia. PLoS Pathog 12:e1005877. doi: 10.1371/journal.ppat.1005877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Halford WP, Kemp CD, Isler JA, Davido DJ, Schaffer PA. 2001. ICP0, ICP4, or VP16 expressed from adenovirus vectors induces reactivation of latent herpes simplex virus type 1 in primary cultures of latently infected trigeminal ganglion cells. J Virol 75:6143–6153. doi: 10.1128/JVI.75.13.6143-6153.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Herrera FJ, Triezenberg SJ. 2004. VP16-dependent association of chromatin-modifying coactivators and underrepresentation of histones at immediate-early gene promoters during herpes simplex virus infection. J Virol 78:9689–9696. doi: 10.1128/JVI.78.18.9689-9696.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fan D, Wang M, Cheng A, Jia R, Yang Q, Wu Y, Zhu D, Zhao X, Chen S, Liu M, Zhang S, Ou X, Mao S, Gao Q, Sun D, Wen X, Liu Y, Yu Y, Zhang L, Tian B, Pan L, Chen X. 2020. The role of VP16 in the life cycle of alphaherpesviruses. Front Microbiol 11:1910. doi: 10.3389/fmicb.2020.01910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wysocka J, Herr W. 2003. The herpes simplex virus VP16-induced complex: the makings of a regulatory switch. Trends Biochem Sci 28:294–304. doi: 10.1016/S0968-0004(03)00088-4 [DOI] [PubMed] [Google Scholar]
- 41. Liang Y, Vogel JL, Arbuckle JH, Rai G, Jadhav A, Simeonov A, Maloney DJ, Kristie TM. 2013. Targeting the JMJD2 histone demethylases to epigenetically control herpesvirus infection and reactivation from latency. Sci Transl Med 5:167ra5. doi: 10.1126/scitranslmed.3005145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liang Y, Vogel JL, Narayanan A, Peng H, Kristie TM. 2009. Inhibition of the histone demethylase LSD1 blocks alpha-herpesvirus lytic replication and reactivation from latency. Nat Med 15:1312–1317. doi: 10.1038/nm.2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Narayanan A, Ruyechan WT, Kristie TM. 2007. The coactivator host cell factor-1 mediates set1 and MLL1 H3K4 trimethylation at herpesvirus immediate early promoters for initiation of infection. Proc Natl Acad Sci U S A 104:10835–10840. doi: 10.1073/pnas.0704351104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cliffe AR, Wilson AC. 2017. Restarting lytic gene transcription at the onset of herpes simplex virus reactivation. J Virol 91:e01419-16. doi: 10.1128/JVI.01419-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Whitford AL, Clinton CA, Kennedy EBL, Dochnal SA, Suzich JB, Cliffe AR. 2022. Ex vivo herpes simplex virus reactivation involves a dual leucine zipper kinase-dependent wave of lytic gene expression that is independent of histone demethylase activity and viral genome synthesis. J Virol 96:e0047522. doi: 10.1128/jvi.00475-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fischle W, Wang Y, Allis CD. 2003. Binary switches and modification cassettes in histone biology and beyond. Nature 425:475–479. doi: 10.1038/nature02017 [DOI] [PubMed] [Google Scholar]
- 47. Fischle W, Tseng BS, Dormann HL, Ueberheide BM, Garcia BA, Shabanowitz J, Hunt DF, Funabiki H, Allis CD. 2005. Regulation of HP1-chromatin binding by histone H3 methylation and phosphorylation. Nature 438:1116–1122. doi: 10.1038/nature04219 [DOI] [PubMed] [Google Scholar]
- 48. Noh KM, Maze I, Zhao D, Xiang B, Wenderski W, Lewis PW, Shen L, Li H, Allis CD. 2015. ATRX tolerates activity-dependent histone H3 methyl/phos switching to maintain repetitive element silencing in neurons. Proc Natl Acad Sci U S A 112:6820–6827. doi: 10.1073/pnas.1411258112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miller BR, Press C, Daniels RW, Sasaki Y, Milbrandt J, DiAntonio A. 2009. A dual leucine kinase-dependent axon self-destruction program promotes wallerian degeneration. Nat Neurosci 12:387–389. doi: 10.1038/nn.2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ghosh AS, Wang B, Pozniak CD, Chen M, Watts RJ, Lewcock JW. 2011. DLK induces developmental neuronal degeneration via selective regulation of proapoptotic JNK activity. J Cell Biol 194:751–764. doi: 10.1083/jcb.201103153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tedeschi A, Bradke F. 2013. The DLK signalling pathway--a double-edged sword in neural development and regeneration. EMBO Rep 14:605–614. doi: 10.1038/embor.2013.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Welsbie DS, Yang Z, Ge Y, Mitchell KL, Zhou X, Martin SE, Berlinicke CA, Hackler L Jr, Fuller J, Fu J, et al. 2013. Functional genomic screening identifies dual leucine zipper kinase as a key mediator of retinal ganglion cell death. Proc Natl Acad Sci U S A 110:4045–4050. doi: 10.1073/pnas.1211284110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Estus S, Zaks WJ, Freeman RS, Gruda M, Bravo R, Johnson EM. 1994. Altered gene expression in neurons during programmed cell death: identification of c-Jun as necessary for neuronal apoptosis. J Cell Biol 127:1717–1727. doi: 10.1083/jcb.127.6.1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Eilers A, Whitfield J, Babij C, Rubin LL, Ham J. 1998. Role of the Jun kinase pathway in the regulation of c-Jun expression and apoptosis in sympathetic neurons. J Neurosci 18:1713–1724. doi: 10.1523/JNEUROSCI.18-05-01713.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Malik AN, Vierbuchen T, Hemberg M, Rubin AA, Ling E, Couch CH, Stroud H, Spiegel I, Farh KK-H, Harmin DA, Greenberg ME. 2014. Genome-wide identification and characterization of functional neuronal activity-dependent enhancers. Nat Neurosci 17:1330–1339. doi: 10.1038/nn.3808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Su Y, Shin J, Zhong C, Wang S, Roychowdhury P, Lim J, Kim D, Ming GL, Song H. 2017. Neuronal activity modifies the chromatin accessibility landscape in the adult brain. Nat Neurosci 20:476–483. doi: 10.1038/nn.4494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Suzich JB, Cuddy SR, Baidas H, Dochnal S, Ke E, Schinlever AR, Babnis A, Boutell C, Cliffe AR. 2021. PML-NB-dependent type I interferon memory results in a restricted form of HSV latency. EMBO Rep 22:e52547. doi: 10.15252/embr.202152547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Deshmukh M, Kuida K, Johnson EM. 2000. Caspase inhibition extends the commitment to neuronal death beyond cytochrome C release to the point of mitochondrial depolarization. J Cell Biol 150:131–143. doi: 10.1083/jcb.150.1.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dhanasekaran DN, Reddy EP. 2008. JNK signaling in apoptosis. Oncogene 27:6245–6251. doi: 10.1038/onc.2008.301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hollville E, Romero SE, Deshmukh M. 2019. Apoptotic cell death regulation in neurons. FEBS J 286:3276–3298. doi: 10.1111/febs.14970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kristiansen M, Menghi F, Hughes R, Hubank M, Ham J. 2011. Global analysis of gene expression in NGF-deprived sympathetic neurons identifies molecular pathways associated with cell death. BMC Genomics 12:551. doi: 10.1186/1471-2164-12-551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Deshmane SL, Fraser NW. 1989. During latency, herpes simplex virus type 1 DNA is associated with nucleosomes in a chromatin structure. J Virol 63:943–947. doi: 10.1128/JVI.63.2.943-947.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kent JR, Zeng P-Y, Atanasiu D, Gardner J, Fraser NW, Berger SL. 2004. During lytic infection herpes simplex virus type 1 is associated with histones bearing modifications that correlate with active transcription. J Virol 78:10178–10186. doi: 10.1128/JVI.78.18.10178-10186.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kutluay SB, Triezenberg SJ. 2009. Regulation of histone deposition on the herpes simplex virus type 1 genome during lytic infection. J Virol 83:5835–5845. doi: 10.1128/JVI.00219-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cliffe AR, Knipe DM. 2008. Herpes simplex virus ICP0 promotes both histone removal and acetylation on viral DNA during lytic infection. J Virol 82:12030–12038. doi: 10.1128/JVI.01575-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cohen C, Corpet A, Roubille S, Maroui MA, Poccardi N, Rousseau A, Kleijwegt C, Binda O, Texier P, Sawtell N, Labetoulle M, Lomonte P. 2018. Promyelocytic leukemia (PML) nuclear bodies (NBS) induce latent/quiescent HSV-1 Genomes Chromatinization through a PML NB/Histone H3.3/H3.3 chaperone axis. PLoS Pathog 14:e1007313. doi: 10.1371/journal.ppat.1007313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hu H-L, Srinivas KP, Wang S, Chao MV, Lionnet T, Mohr I, Wilson AC, Depledge DP, Huang TT. 2022. Single-cell transcriptomics identifies Gadd45b as a regulator of herpesvirus-reactivating neurons. EMBO Rep 23:e53543. doi: 10.15252/embr.202153543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tiwari VK, Stadler MB, Wirbelauer C, Paro R, Schübeler D, Beisel C. 2011. A chromatin-modifying function of JNK during stem cell differentiation. Nat Genet 44:94–100. doi: 10.1038/ng.1036 [DOI] [PubMed] [Google Scholar]
- 69. Kushnir AS, Davido DJ, Schaffer PA. 2010. Role of nuclear factor Y in stress-induced activation of the herpes simplex virus type 1 ICP0 promoter. J Virol 84:188–200. doi: 10.1128/JVI.01377-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Workman A, Eudy J, Smith L, da Silva LF, Sinani D, Bricker H, Cook E, Doster A, Jones C. 2012. Cellular transcription factors induced in trigeminal ganglia during dexamethasone-induced reactivation from latency stimulate bovine herpesvirus 1 productive infection and certain viral promoters. J Virol 86:2459–2473. doi: 10.1128/JVI.06143-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ostler JB, Thunuguntla P, Hendrickson BY, Jones C. 2021. Transactivation of herpes simplex virus 1 (HSV-1) infected cell protein 4 enhancer by glucocorticoid receptor and stress-induced transcription factors requires overlapping krüppel-like transcription factor 4/Sp1 binding sites. J Virol 95:e01776-20. doi: 10.1128/JVI.01776-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ostler JB, Jones C. 2021. Stress induced transcription factors transactivate the herpes simplex virus 1 infected cell protein 27 (ICP27) transcriptional enhancer. Viruses 13:2296. doi: 10.3390/v13112296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wijesekera N, Hazell N, Jones C. 2022. Independent cis-regulatory modules within the herpes simplex virus 1 infected cell protein 0 (ICP0) promoter are transactivated by kruppel-like factor 15 and glucocorticoid receptor. Viruses 14:1284. doi: 10.3390/v14061284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Takahashi K, Yamanaka S. 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676. doi: 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- 75. Soufi A, Garcia MF, Jaroszewicz A, Osman N, Pellegrini M, Zaret KS. 2015. Pioneer transcription factors target partial DNA motifs on nucleosomes to initiate reprogramming. Cell 161:555–568. doi: 10.1016/j.cell.2015.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fonseca GJ, Tao J, Westin EM, Duttke SH, Spann NJ, Strid T, Shen Z, Stender JD, Sakai M, Link VM, Benner C, Glass CK. 2019. Diverse motif ensembles specify non-redundant DNA binding activities of AP-1 family members in macrophages. Nat Commun 10:414. doi: 10.1038/s41467-018-08236-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. McLean TI, Bachenheimer SL. 1999. Activation of cJun N-terminal kinase by herpes simplex virus type 1 enhances viral replication. J Virol 73:8415–8426. doi: 10.1128/JVI.73.10.8415-8426.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zachos G, Clements B, Conner J. 1999. Herpes simplex virus type 1 infection stimulates p38/c-Jun N-terminal mitogen-activated protein kinase pathways and activates transcription factor AP-1. J Biol Chem 274:5097–5103. doi: 10.1074/jbc.274.8.5097 [DOI] [PubMed] [Google Scholar]
- 79. Cassidy L, Meadows J, Catalán J, Barton S. 1997. Are reported stress and coping style associated with frequent recurrence of genital herpes? Genitourin Med 73:263–266. doi: 10.1136/sti.73.4.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Glaser R, Kiecolt-Glaser JK, Speicher CE, Holliday JE. 1985. Stress, loneliness, and changes in herpesvirus latency. J Behav Med 8:249–260. doi: 10.1007/BF00870312 [DOI] [PubMed] [Google Scholar]
- 81. Logan HL, Lutgendorf S, Hartwig A, Lilly J, Berberich SL. 1998. Immune, stress, and mood markers related to recurrent oral herpes outbreaks. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 86:48–54. doi: 10.1016/s1079-2104(98)90149-4 [DOI] [PubMed] [Google Scholar]
- 82. Padgett DA, Sheridan JF, Dorne J, Berntson GG, Candelora J, Glaser R. 1998. Social stress and the reactivation of latent herpes simplex virus type 1. Proc Natl Acad Sci U S A 95:7231–7235. doi: 10.1073/pnas.95.12.7231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Du T, Zhou G, Roizman B. 2012. Induction of apoptosis accelerates reactivation of latent HSV-1 in ganglionic organ cultures and replication in cell cultures. Proc Natl Acad Sci U S A 109:14616–14621. doi: 10.1073/pnas.1212661109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ashcraft KA, Bonneau RH. 2008. Psychological stress exacerbates primary vaginal herpes simplex virus type 1 (HSV-1) infection by impairing both innate and adaptive immune responses. Brain Behav Immun 22:1231–1240. doi: 10.1016/j.bbi.2008.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ashcraft KA, Hunzeker J, Bonneau RH. 2008. Psychological stress impairs the local CD8+ T cell response to mucosal HSV-1 infection and allows for increased pathogenicity via a glucocorticoid receptor-mediated mechanism. Psychoneuroendocrinology 33:951–963. doi: 10.1016/j.psyneuen.2008.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ives AM, Bertke AS. 2017. Stress hormones epinephrine and corticosterone selectively modulate herpes simplex virus 1 (HSV-1) and HSV-2 productive infections in adult sympathetic, but not sensory, neurons. J Virol 91:e00582-17. doi: 10.1128/JVI.00582-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cuddy SR, Cliffe AR. 2023. The intersection of innate immune pathways with the latent herpes Simplex virus genome. J Virol 97:e0135222. doi: 10.1128/jvi.01352-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Torres-Berrío A, Estill M, Ramakrishnan A, Kronman H, Patel V, Minier-Toribio A, Issler O, Browne CJ, Parise EM, van der Zee Y, Walker D, Martínez-Rivera FJ, Lardner CK, Durand-de Cuttoli R, Russo SJ, Shen L, Sidoli S, Nestler EJ. 2023. Monomethylation of lysine 27 at Histone 3 confers lifelong susceptibility to stress. bioRxiv. doi: 10.1101/2023.05.08.539829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kronman H, Torres-Berrío A, Sidoli S, Issler O, Godino A, Ramakrishnan A, Mews P, Lardner CK, Parise EM, Walker DM, van der Zee YY, Browne CJ, Boyce BF, Neve R, Garcia BA, Shen L, Peña CJ, Nestler EJ. 2021. Long-term behavioral and cell-type-specific molecular effects of early life stress are mediated by H3K79me2 dynamics in medium spiny neurons. Nat Neurosci 24:753–754. doi: 10.1038/s41593-021-00848-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Forrester A, Farrell H, Wilkinson G, Kaye J, Davis-Poynter N, Minson T. 1992. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J Virol 66:341–348. doi: 10.1128/JVI.66.1.341-348.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Stewart SA, Dykxhoorn DM, Palliser D, Mizuno H, Yu EY, An DS, Sabatini DM, Chen ISY, Hahn WC, Sharp PA, Weinberg RA, Novina CD. 2003. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA 9:493–501. doi: 10.1261/rna.2192803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Alandijany T, Roberts APE, Conn KL, Loney C, McFarlane S, Orr A, Boutell C. 2018. Distinct temporal roles for the promyelocytic leukaemia (PML) protein in the sequential regulation of intracellular host immunity to HSV-1 infection. PLoS Pathog 14:e1006769. doi: 10.1371/journal.ppat.1006769 [DOI] [PMC free article] [PubMed] [Google Scholar]