Abstract
Objective
Pregnant women in a special physiological period, the body’s blood indicators will change to a certain extent. This study aims to explore the changes of serum immunoglobulin levels in healthy pregnant women and establish its reference interval (RI).
Methods
A total of 369 healthy pregnant women, who underwent pregnancy examination in the Department of Obstetrics, Second Xiangya Hospital of Central South University from August 2019 to October 2019, were enrolled for this study. They were divided into an early pregnancy group, a middle pregnancy group, and a late pregnancy group according to the pregnancy period, and 123 healthy non-pregnant women were selected as the controls. The levels of immunoglobulin G (IgG), immunoglobulin M (IgM), and immunoglobulin A (IgA) were determined by immune transmission turbidities. The level of immunoglobulin E (IgE) was determined by electrochemiluminescence. The differences in immunoglobulin levels between pregnant women and non-pregnant women and among different gestational periods were analyzed, and the RI of serum immunoglobulin level during pregnancy was established.
Results
Compared to the non-pregnant women, the levels of serum IgG, IgM, IgA, and IgE in pregnant women were significantly decreased (all P<0.01), with 51.81% for IgG, 43.84% for IgM, 55.80% for IgA, and 49.80% for IgE. Except that the IgG level of late pregnancy group was significantly lower than that of early pregnancy group (P<0.05), there were no significant differences in the IgG, IgM, IgA, and IgE levels among the other groups (all P>0.05). The RIs of serum IgG in early pregnancy, middle pregnancy, and late pregnancy were 6.02-7.70 g/L, 5.18-6.85 g/L, and 4.58-5.72 g/L, respectively, while the RIs of serum IgM, IgA, and IgE were 0.71-0.93 g/L, 0.90-1.09 g/L, and 68.30-107.69 ng/mL, respectively in pregnant women.
Conclusion
The levels of immunoglobulin in pregnant women are decreased significantly. The establishment of RIs of IgG, IgM, IgA and IgE in healthy pregnant women could provide scientific basis for clinical decision-making.
Keywords: pregnant women, immunoglobulin G, immunoglobulin M, immunoglobulin A, immunoglobulin E, reference interval
Abstract
目的
妊娠妇女处于一个特殊的生理时期,机体内的各项血液指标都会发生一定程度的改变,本研究旨在探讨健康妊娠妇女血清免疫球蛋白水平的变化,并建立其参考区间。
方法
选择2019年8月至2019年10月在中南大学湘雅二医院产科进行孕期检查的369例健康妊娠妇女,按照孕周将其分为早孕组、中孕组和晚孕组,并选取123例健康非孕期妇女作为对照组。利用免疫比浊法测定血清免疫球蛋白G(IgG)、免疫球蛋白M(IgM)、免疫球蛋白A(IgA)的含量,利用化学发光法测定血清免疫球蛋白E(IgE)的水平。比较妊娠妇女和非孕期妇女以及各孕期之间免疫球蛋白水平的差异,并建立相应的参考区间。
结果
相比健康非孕期妇女,健康妊娠妇女的血清免疫球蛋白水平均有明显的降低(均P<0.01),IgG、IgM、IgA及IgE分别下降51.81%、43.84%、55.80%及49.80%。比较早孕、中孕、晚孕各组之间血清IgG、IgM、IgA和IgE水平的变化,除IgG在晚孕和早孕之间差异有统计学意义(P<0.05)外,其余各类免疫球蛋白两组之间差异均无统计学意义(均P>0.05)。妊娠早期、中期及晚期血清IgG的参考区间分别为6.02~7.70 g/L、5.18~6.85 g/L及4.58~5.72 g/L,而在孕期血清IgM、IgA及IgE的参考区间分别为0.71~0.93 g/L、0.90~1.09 g/L及68.30~107.69 ng/mL。
结论
妊娠妇女的免疫球蛋白水平显著降低,本研究建立了健康妊娠妇女血清IgG、IgM、IgA及IgE水平95%的参考区间,可为临床决策提供科学依据。
Keywords: 妊娠妇女, 免疫球蛋白G, 免疫球蛋白M, 免疫球蛋白A, 免疫球蛋白E, 参考区间
Immunoglobulin is a tetrapeptide structure composed of two identical light chains (L chains) and two identical heavy chains (H chains) linked by an interchain disulfide bond. Each L chain and H chain can be divided into 2 parts: the variable region (V region) and the constant region (C region). V region is a specific site of antigen-antibody binding and the functions of C region include activation of complement and Fc receptor involved in opsonization and antibody-dependent cell-mediated cytotoxicity (ADCC). As an important immune molecule, immunoglobulin is mainly involved in the humoral immune response of our body and protects the body from antigens such as bacteria and viruses. Among them, IgG, IgM, IgA and IgE are the major immunoglobulins.
IgG is the serum immunoglobulin with the longest half-life and the highest content, accounting for 75%. There are 4 different subtypes of IgG: IgG1, IgG2, IgG3, IgG4. IgG is the main force involving in the anti-infective immune, and it is the only immunoglobulin that can cross the placental barrier. IgM accounts for 10% in serum, which is the first immunoglobulin expressed in humoral immunity. IgA accounts for 15% and is mainly present on the mucosal surface and in exocrine fluids such as saliva and colostrum. IgE accounts for the lowest (<0.01%). It is mainly associated with hypersensitivity, allergic reactions, and parasitic infections[1].
In the process of pregnancy, because the mother is in a very special physiological state, many physiological indicators will change, such as blood volume will increase by 20% and hormone levels will also change significantly during pregnancy[2]. At the same time the fetus is an allogenic antigen for the mother, it’s normal for the mother to make a series of immune response to ensure that the fetus can be safely exist rather than be ruled out. The disorders of immune system are closely related to a variety of pregnancy-related diseases[3]. Many studies have shown that when these diseases occur, the content of maternal immunoglobulins will be changed. Serum IgG, IgM and IgA levels of pregnant women with hypothyroidism are higher than those of the normal pregnant women and non-pregnant women[4]. Serum IgG, IgM and IgA levels of pregnant women with gestational hypertension were significantly lower than those of the normal pregnant women[5]. A significant decrease of serum IgG and IgM, and increase of IgA have been observed in women with gestational diabetes mellitus. In addition, 4 subtypes of IgG also show different change in these patients[6]. As an acute phase reaction protein, immunoglobulins can be elevated in preterm labor, preeclampsia, miscarriage and some other processes associated with the inflammatory response[7]. Therefore, the changes of serum immunoglobulin in pregnant women are of great significance for the early detection of some pregnancy-related diseases. Yet the premise for doctors to make accurate judgment is to establish an accurate and effective medical reference interval (RI). For the less relevant research at home and abroad, this study establishes standard reference sections in groups according to Definition, Establishment and Validation of Medical Laboratory Reference Section [8] and Medical Laboratory―Requirements for Quality and Competence [9], providing accurate information for medical staffs.
1. Material and methods
1.1. Subjects
According to the screening criteria[8],369 healthy pregnant women aged (29.47±3.84) years were selected from the Department of Obstetrics, Second Xiangya Hospital of Central South University from August 2019 to October 2019. They were divided into 3 groups according to different gestational age:123 were in early pregnancy (≤13+6 weeks), 123 in middle pregnancy (14-27+6 weeks),123 in late pregnancy (≥28 weeks). Inclusion criteria were as follows: 1) ≥18 years old; 2) no immune diseases; 3) hasn’t taken drugs that affect the detection indicator recently; 4) willing to support and cooperate with the research, and signed the informed consent. Exclusion criteria were as follows: 1) with heart, liver, or kidney diseases; 2) with mental illness such as depression; 3) has a history of diabetes and hypertension; 4) with diseases such as infection, hypersensitivity reaction, or hypothyroidism; 5) has alcohol abuse, smoking, or obesity; 6) has surgery during the previous 4 months of pregnancy or blood transfusion or donation within 6 months; 7) has a history of inherited diseases; 8) drug intake within 2 weeks or antibiotic abuse; 9) has viral hepatitis type B, viral hepatitis type C, AIDS, and other viral infectious diseases.
Simultaneously, 123 healthy non-pregnant women aged (29.52±3.77) years were selected from the same hospital for routine physical examinations during the same period as a control group. Non-pregnant women who had been pregnant and lactating within 1 year were excluded from the study.
1.2. Specimen collection and detection
All participants took the normal lifestyle and avoided strenuous exercise 3 days before drawing blood. After fasting for 8 h, venous blood was drawn in the morning.The specimens were collected in a vacuum blood collection tube containing biochemical separation gel (Guangzhou Yangpu Medical Products Co., Ltd.). After 30 minutes at room temperature, the samples were centrifuged at 3 000 g for 5 minutes and then measured within 2 hours. There was no hemolysis, blood lipid, and jaundice in the samples.
IgG, IgM, and IgA were detected by immune transmission turbidities using Beckman instruments and their complementary reagents. IgE was determined by electrochemiluminescence method using Roche E602 electrochemiluminescence immunoassay and its accessory reagents. For quality control, concentrations of serum immunoglobulin in 2 controls were tested. Precision and accuracy were demonstrated according to the document EP 15A recommended by Clinical Laboratory and Standards Institute (CLSI) and for participants in the comparison between laboratories organized by the National Center for Clinical Laboratory, the bias of serum immunoglobulin was <5%, and the results were satisfactory.
1.3. Statistical analyses
All data were analyzed by SPSS 22.0 statistical software. Prior to analysis, all data were outliers using the Dixon test. The distribution of data was judged by the Kolmogorov-Smirnov test. When P>0.05, the data were obeyed the Gaussian distribution, otherwise the data were non-Gaussian distributed. Multiple analysis of variance analysis (Turkey method) was used in multiple Gaussian distributed pregnant groups, and the differences among groups were analyzed by ANOVA analysis. If it does not meet the Gaussian distribution, use the non-parametric test of Mann-Whitney U test for comparison. The difference was statistically significant when P<0.05. At last,a nonparametric 95% percentile method was used to calculate the RI for serum immunoglobulin in pregnant women.
2. Results
2.1. Comparison of serum immunoglobulin levels in non-pregnant and pregnant women
Compared to the non-pregnant women, the levels of serum IgG, IgM, IgA, and IgE in pregnant women were significantly decreased (all P<0.01; Figure 1, 2), with 51.81% for IgG, 43.84% for IgM, 55.80% for IgA, and 49.80% for IgE.
Figure 1. Comparison of serum levels of IgG, IgM, and IgA between the pregnant group and the non-pregnant group.
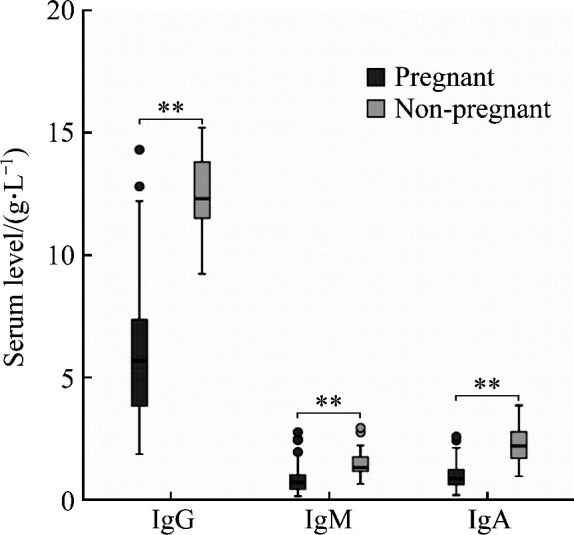
**P<0.01.
Figure 2. Comparison of serum IgE levels between pregnant and non-pregnant groups.
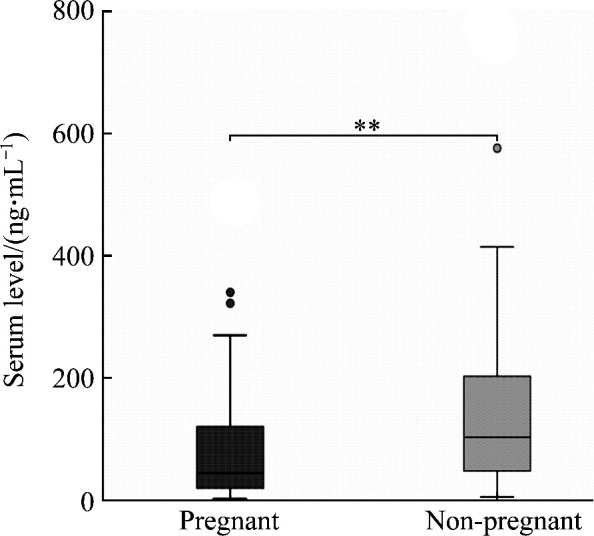
**P<0.01.
Calculated by 95% non-parametric method, the 95% RIs of serum levels of IgG, IgM, IgA, and IgE in non-pregnant women were 11.79-13.07 g/L, 1.27-1.65 g/L, 1.99-2.50 g/L, and 4.83-76.31 ng/mL, respectively.
2.2. Comparison of serum immunoglobulin levels in the different trimesters of pregnancy
Although there were some changes of serum levels of IgG, IgM, and IgA in the 3 periods of pregnancy, no significant difference was observed among the 3 periods (all P>0.05). There was significant difference in serum IgG level between the early pregnancy group and the late pregnancy group (P<0.05), but there was no significant difference in the serum levels of IgG, IgM, and IgA between other two groups (all P>0.05, Figure 3). No significant difference was found in serum IgE level among the 3 periods of pregnancy (P>0.05, Figure 4). Serum IgE level in the middle pregnancy increased, but it returned to the early equivalent level again in late pregnancy.
Figure 3. Comparison of serum IgG, IgM, and IgA levels in the various trimesters of pregnancy.
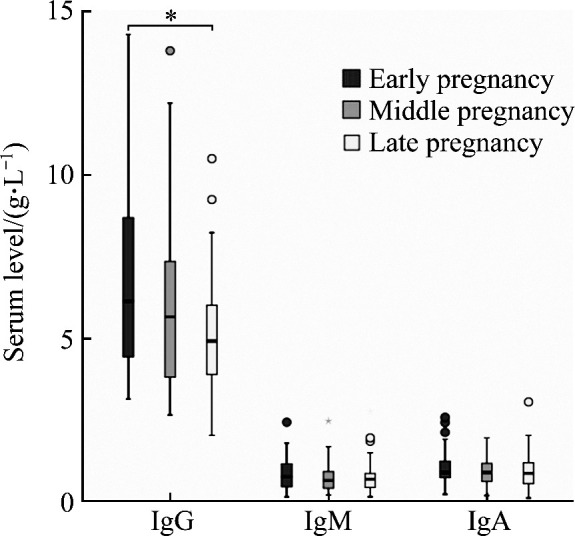
*P<0.05.
Figure 4. Comparison of serum IgE level in the various trimesters of pregnancy.
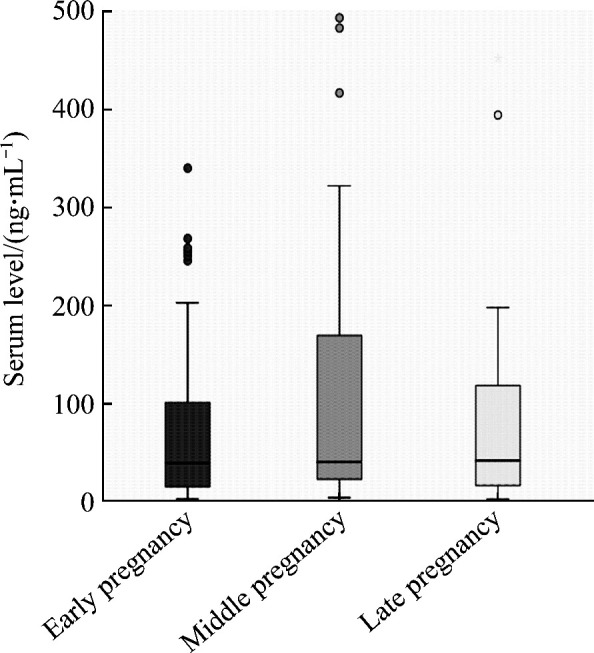
2.3. RI of serum immunoglobulin in the different trimesters of pregnancy
RI of the 3 pregnancy periods was established separately. The RI of serum IgG in early pregnancy, middle pregnancy, and late pregnancy were 6.02- 7.70, 5.18-6.85, and 4.58-5.72 g/L, respectively. The RIs of serum IgM, IgA, and IgE were established after combining data of different pregnancy periods. See Table 1 for details.
Table 1.
RI of serum immunoglobulin levels
| Groups | IgG/(g·L-1) | IgM/(g·L-1) | IgA/(g·L-1) | IgE/(ng·mL-1) |
|---|---|---|---|---|
| Early pregnancy | 6.02-7.70 | 0.71-0.93 | 0.90-1.09 | 68.30-107.69 |
| Middle pregnancy | 5.18-6.85 | 0.71-0.93 | 0.90-1.09 | 68.30-107.69 |
| Late pregnancy | 4.58-5.72 | 0.71-0.93 | 0.90-1.09 | 68.30-107.69 |
3. Discussion
In recent years, with the opening of the second child policy, more and more attention has been paid to the physiological health of pregnant women. Study[10] has shown that maternal serum immunoglobulin level can change significantly based on the special physiological condition of pregnant women, which can not only suggest the emergence of abnormal pregnancy, but also provide a scientific basis for early diagnosis, timely treatment, and prognosis of some pregnancy-related diseases. Moreover, intravenous immuno-globulin therapy is increasingly used in the field of obstetrics and neonatology[11], thence it is really important to pay attention to the changes of maternal serum immunoglobulin level. Scholars have done similar research before, but the result is quite controversial. Research[12] found that serum IgG, IgA, and IgM levels of pregnant women were decreased. Some found that pregnant women with decreased serum IgG and increased IgM had no significant difference in IgA levels[13]. Still others showed that there was a decrease in maternal IgG, IgM, IgA, and IgE during pregnancy[14]. There are 3 possible reasons for this circumstance. The first is the regional differences. There are regional differences in serum IgG level, which lead to different results in different countries or provinces. The second is the individual differences. Immunoglobulin especially the IgE changes dramatically in different individuals. Serum total IgE level is positively correlated with the pregnancy history of adult women. At the same time, the level of serum IgE during pregnancy seems to be related to the sex of the fetus.The third is due to the different detection principles, which is the most important reason[15-16]. At present, the commonly used methods are single immunodiffusion and immunoturbidimetry. Immunodiffusion method has more influencing factors,lower accuracy, and longer experimental time than immunoturbidimetry method, which has advantages of good precision and linear, high degree of automation, and strong anti-interference ability. The method of IgE used in our experiment is electrochemiluminescence, which has high sensitivity and specificity.
Although the test results for changes in serum immunoglobulin during pregnancy are controversial, it can be seen that the trend of IgG level is declining. The degree of IgG decrease has nothing to do with the age of the mother. So, what is the reason for the decrease in maternal immunoglobulin levels during pregnancy? Some scholars believe that serum IgG is consumed through antibody blocking effect during pregnancy; in addition, IgG can enter the fetus through the placental barrier, leading to the decrease of maternal IgG level[17].Meanwhile, immunoglobulin in pregnant women’s serum is diluted for an increase of body blood volume, resulting in the decreased final measured level. Some pregnant women have mild proteinuria, through which serum immunoglobulin is lost[18]. However, changes in serum immunoglobulin have nothing to do with hemodilution, as when increasing the volume of serum doesn’t result in an increase in the Ig content, that is, the production of IgG is constant. Finally, immunosuppression could be the main reason for the decline of immunoglobulin during pregnancy[19]. In addition, high IgE level may be associated with the occurrence of miscarriage.
Hormones play an important role in suppressing maternal immune function during pregnancy. Fetal trophoblast cells promote the production of IL-10 secreting B cells, which may be related to immune tolerance[20]. Villous trophoblast cells on the surface of decidual placenta, a functional immune tissue, interact with immune cells to play the role of immunosuppression and immune nutrition[21]. Simultaneously, the proportion of CD3+, CD4+, CD19+, CD4+/CD8+ and NK decreases[22], indicating that cellular immunity is significantly inhibited. Humoral immunity will be affected ascellular immunity and humoral immunity form an immune network that affects with each other. In addition to the immunosuppressive effects of regulatory T lymphocytes in the maternal body that specifically recognize the paternal component of fetal cells, there are many molecules that can exert immunosuppression in semen, such as TGF-β, which can induce the conversion of T lymphocytes into Treg cells[23].As a result, body’s immunoglobulin levels decline during pregnancy.
Although the mechanism of immunosuppression during pregnancy is not clear yet, this study found that there was a clear difference in the level of immunoglobulin between pregnant and non-pregnant women, and immunoglobulin levels can be used to monitor the pregnancy situation to some extent. Therefore, the 95% RIs of serum IgG, IgM, IgA and IgE of healthy pregnant women were established, providing a reliable basis for clinical decision-making.
Conflict of Interest
The authors declare that they have no conflicts of interest to disclose.
References
- 1.Xiong F, Tong Y, Li P, et al. Serum immunoglobulin E level and its impact on the pregnancy outcome associated with fetal growth restriction: a prospective cohort study[J]. Genet Mol Res, 2015, 14 (2): 3879-3888. [DOI] [PubMed] [Google Scholar]
- 2.Thomas N. Physiological changes during pregnancy and mode of delivery[J]. Invest Ophthalmol Vis Sci, 2016, 57(12): 273-278. [Google Scholar]
- 3.Bonney EA. Immune regulation in pregnancy: a matter of perspective?[J]. Obstet Gynecol Clin North Am, 2016, 43(4): 679-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ezzeddine D, Hamadi C, Abbas HA, et al. Prevalence and correlation of hypothyroidism with pregnancy outcomes among lebanese women[J]. J Endocr Soc, 2017, 1(5): 415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arinola G, Arowojolu A, Bamgboye A, et al. Serum concentrations of immunoglobulins and acute phase proteins in Nigerian women with preeclampsia[J]. Reprod Biol, 2006, 6(3): 265-274. [PubMed] [Google Scholar]
- 6.Yang X, Zhang CJ, Chen GZ, et al. Antibodies: The major participants in maternal-fetal interaction[J]. J Obstet Gynaecol Res, 2019, 45(1): 39-46. [DOI] [PubMed] [Google Scholar]
- 7.Gillespie SL, Porter K, Christian LM. Adaptation of the inflammatory immune response across pregnancy and postpartum in black and white women[J]. J Reprod Immunol, 2016, 114(2): 27-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.USA, Clinical Laboratory and Standards Institute . Definition, establishment and validation of medical laboratory reference section[R]. Wayne, PA, USA: CLSI, 2008. [Google Scholar]
- 9.International Standardisation Organisation . Medical laboratory―Requirements for quality and competence[R]. Geneva: ISO, 2012. [Google Scholar]
- 10.Yasuhara M, Tamaki H, Iyama S, et al. Reciprocal changes in serum levels of immunoglobulins (IgG, IgA, IgM) and complements (C3, C4) in normal pregnancy and after delivery[J]. J Clin Lab Immunol, 1992, 38(3): 137-41. [PubMed] [Google Scholar]
- 11.Kim DJ, Lee SK, Kim JY, et al. Intravenous immunoglobulin G modulates peripheral blood Th17 and Foxp3(+) regulatory T cells in pregnant women with recurrent pregnancy loss[J]. Am J Reprod Immunol, 2014, 71(5): 441-450. [DOI] [PubMed] [Google Scholar]
- 12.Gisella W, Katarina B, Pelle GL. Efficacy of treatment immune thrombocytopenic purpura in pregnancy with corticosteroids and intravenous immunoglobulin: a prospective follow-up of suggested practice[J]. Blood Coagul Fibrinolysis, 2018, 29(2): 141-147. [DOI] [PubMed] [Google Scholar]
- 13.Felix S, Friedrich H, Thorsten K. Off-label application of intravenous immunoglobulin (IVIG) for treatment of Cogan's syndrome during pregnancy[J]. BMJ Case Rep, 2019, 12(10): e227917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitington PF, Kelly S, Taylor SA, et al. Antenatal treatment with intravenous immunoglobulin to prevent gestational alloimmune liver disease: Comparative effectiveness of 14-week versus 18-week initiation[J]. Fetal Diagn Ther, 2018, 43(3): 218-225. [DOI] [PubMed] [Google Scholar]
- 15.Rivara AC, Miller EM. Pregnancy and immune stimulation: re-imagining the fetus as parasite to understand age-related immune system changes in US women[J]. Am J Hum Biol, 2017, 29(6): 418-426. [DOI] [PubMed] [Google Scholar]
- 16.Loken MO, Jeansson S, Jenum PA, et al. Serum level of immunoglobulin E during pregnancy―does offspring sex matter?[J]. Paediatr Perinat Epidemiol, 2010, 24(1): 75-78. [DOI] [PubMed] [Google Scholar]
- 17.Phillips JK, Mcbride CA, Hale SA, et al. Examination of prepregnancy and pregnancy urinary protein levels in healthy nulliparous women[J]. Reprod Sci, 2017, 24(3): 407-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calleja-Agius J, Jauniaux E, Pizzey AR, et al. Investigation of systemic inflammatory response in first trimester pregnancy failure[J]. Hum Reprod, 2012, 27(2): 349-357. [DOI] [PubMed] [Google Scholar]
- 19.Bisset LR, Fiddes TM, Gillett WR, et al. Challenges in vaccinating infants born to mothers taking immunoglobulin biologicals during pregnancy[J]. Expert Rev Vaccines, 2016, 15(2): 239-256. [DOI] [PubMed] [Google Scholar]
- 20.Fettke F, Schumacher A, Canellada A, et al. Maternal and fetal mechanisms of B cell regulation during pregnancy: human chorionic gonadotropin stimulates B cells to produce IL-10 while alpha-fetoprotein drives them into apoptosis[J]. Front Immunol, 2016, 7: 495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elizabeth CC, Gormley M, Kapidzic M, et al. Maternal decidual macrophages inhibit NK cell killing of invasive cytotrophoblasts during human pregnancy[J]. Biol Reprod, 2013, 88(6): 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.La Rocca C, Carbone F, Longobard S, et al. The immunology of pregnancy: regulatory T cells control maternal immune tolerance toward the fetus[J]. Immunol Lett, 2014, 162( 1 PtA): 41-48. [DOI] [PubMed] [Google Scholar]
- 23.庄旭, 陆漪婷, 陈云燕, 等. 子痫前期与妊娠合并慢性肾脏病孕妇血清及尿液β2-微球蛋白水平的比较[J]. 中华妇产科杂志, 2018, 53(2): 77-81. [Google Scholar]; ZHUANG Xu, LU Yiting, CHEN Yunyan, et al. Analysis of the difference of serum immunoglobulins, β2-microglobulin and transferrin in pre-eclampsia and pregnancies complicated with chronic kidney disease[J]. Chinese Journal of Obstetrics and Gynecology, 2018, 53(2): 77-81. [DOI] [PubMed] [Google Scholar]


